Amines reactions Amines are similar to ammonia in



















- Slides: 37

Amines, reactions Amines are similar to ammonia in their reactions. Like ammonia, amines are basic. Like ammonia, amines are nucleophilic and react with alkyl halides, acid chlorides, and carbonyl compounds. The aromatic amines are highly reactive in electrophilic aromatic substitution.

Amine, reactions: 1. As bases 2. Alkylation 3. Reductive amination 4. Conversion into amides 5. EAS 6. Hofmann elimination from quarternary ammonium salts 7. Reactions with nitrous acid

1. As bases 2. a) with acids 3. b) relative base strength 4. c) Kb 5. d) effect of groups on base strength

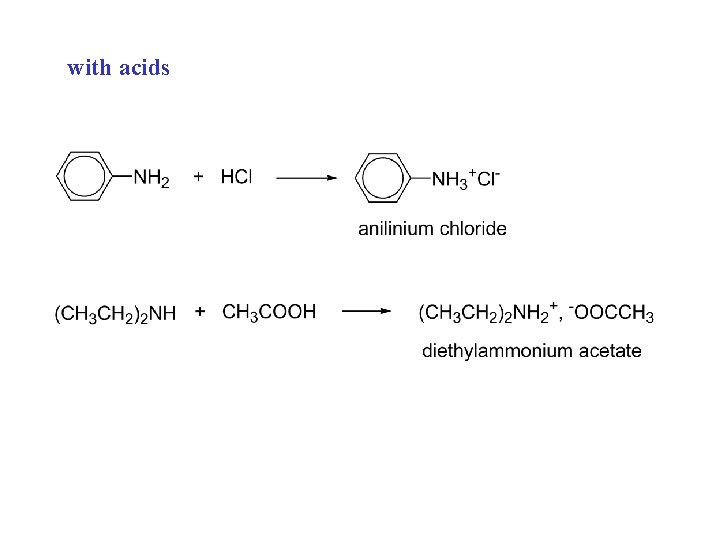
with acids

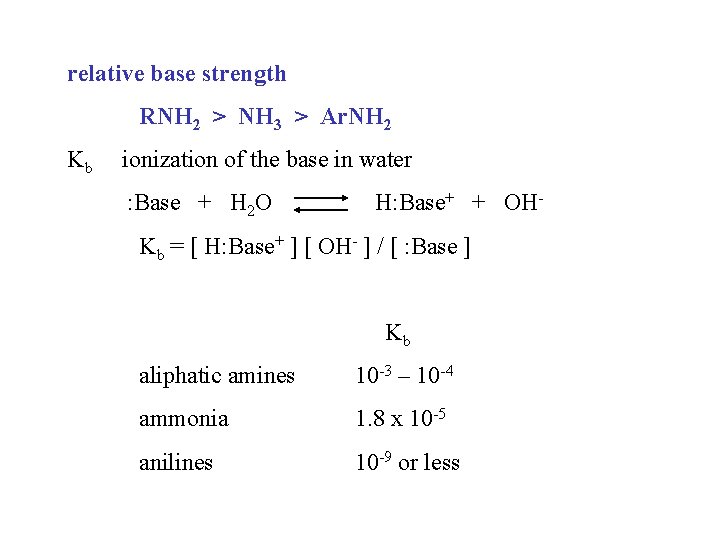
relative base strength RNH 2 > NH 3 > Ar. NH 2 Kb ionization of the base in water : Base + H 2 O H: Base+ + OH- Kb = [ H: Base+ ] [ OH- ] / [ : Base ] Kb aliphatic amines 10 -3 – 10 -4 ammonia 1. 8 x 10 -5 anilines 10 -9 or less

Why are aliphatic amines more basic than ammonia? NH 3 + H 2 O NH 4+ + OHR-NH 2 + H 2 O R-NH 3+ + OHThe alkyl group, -R, is an electron donating group. The donation of electrons helps to stabilize the ammonium ion by decreasing the positive charge, lowering the ΔH, shifting the ionization farther to the right and increasing the basicity.

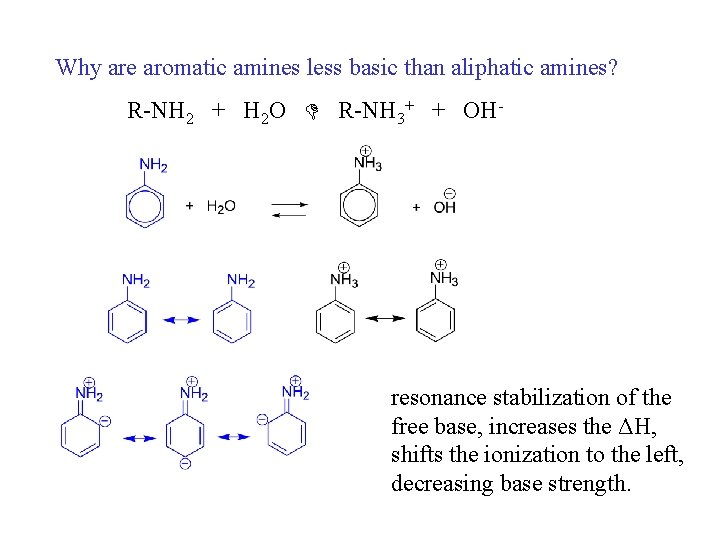
Why are aromatic amines less basic than aliphatic amines? R-NH 2 + H 2 O R-NH 3+ + OH- resonance stabilization of the free base, increases the ΔH, shifts the ionization to the left, decreasing base strength.

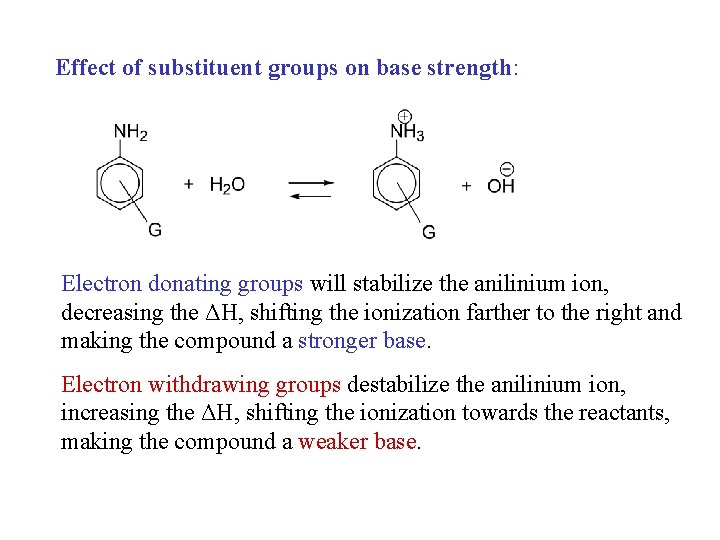
Effect of substituent groups on base strength: Electron donating groups will stabilize the anilinium ion, decreasing the ΔH, shifting the ionization farther to the right and making the compound a stronger base. Electron withdrawing groups destabilize the anilinium ion, increasing the ΔH, shifting the ionization towards the reactants, making the compound a weaker base.

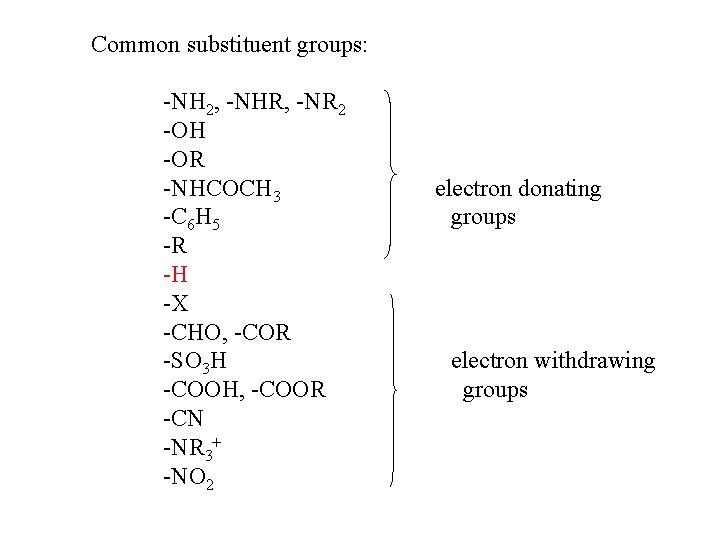
Common substituent groups: -NH 2, -NHR, -NR 2 -OH -OR -NHCOCH 3 -C 6 H 5 -R -H -X -CHO, -COR -SO 3 H -COOH, -COOR -CN -NR 3+ -NO 2 electron donating groups electron withdrawing groups

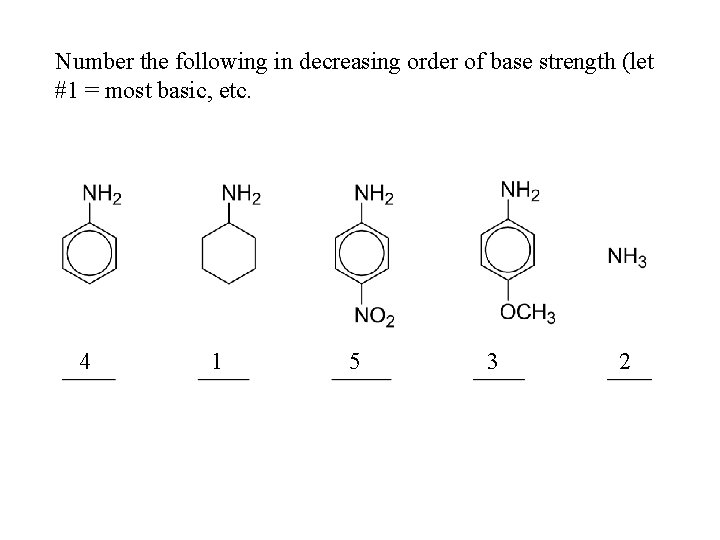
Number the following in decreasing order of base strength (let #1 = most basic, etc. 4 1 5 3 2

2. Alkylation (ammonolysis of alkyl halides)


3. Reductive amination


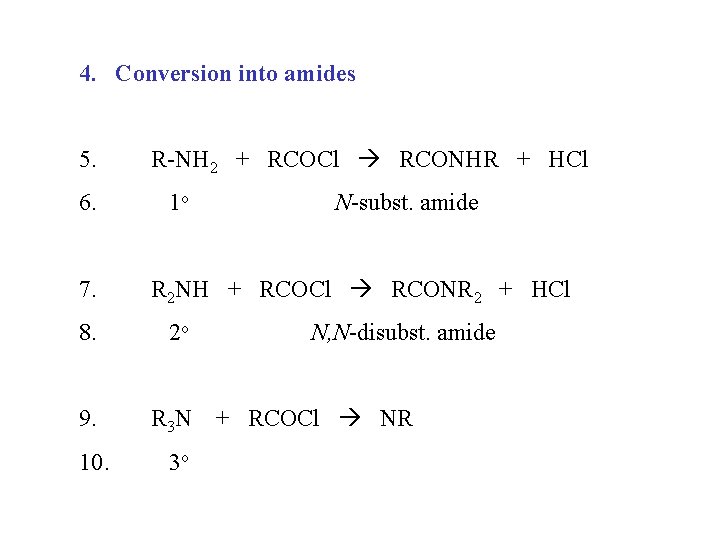
4. Conversion into amides 5. 6. 7. R-NH 2 + RCOCl RCONHR + HCl 1 o R 2 NH + RCOCl RCONR 2 + HCl 8. 2 o 9. R 3 N 10. N-subst. amide 3 o N, N-disubst. amide + RCOCl NR


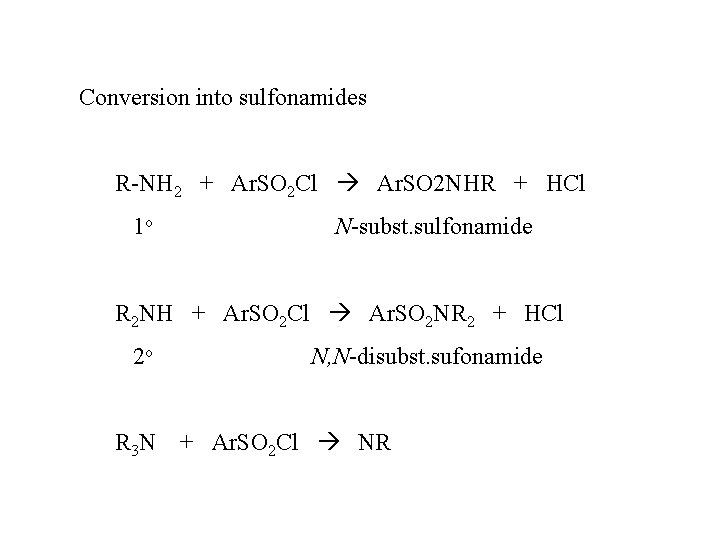
Conversion into sulfonamides R-NH 2 + Ar. SO 2 Cl Ar. SO 2 NHR + HCl 1 o N-subst. sulfonamide R 2 NH + Ar. SO 2 Cl Ar. SO 2 NR 2 + HCl 2 o R 3 N N, N-disubst. sufonamide + Ar. SO 2 Cl NR

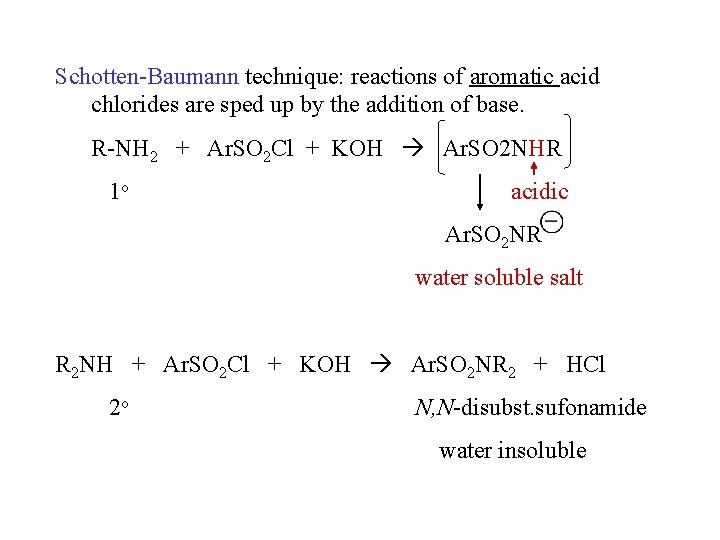
Schotten-Baumann technique: reactions of aromatic acid chlorides are sped up by the addition of base. R-NH 2 + Ar. SO 2 Cl + KOH Ar. SO 2 NHR 1 o acidic Ar. SO 2 NR water soluble salt R 2 NH + Ar. SO 2 Cl + KOH Ar. SO 2 NR 2 + HCl 2 o N, N-disubst. sufonamide water insoluble

Hinsberg Test: unknown amine + benzenesulfonyl chloride, KOH (aq) Reacts to produce a clear solution and then gives a ppt upon acidification primary amine. Reacts to produce a ppt secondary amine. Doesn’t react tertiary amine.


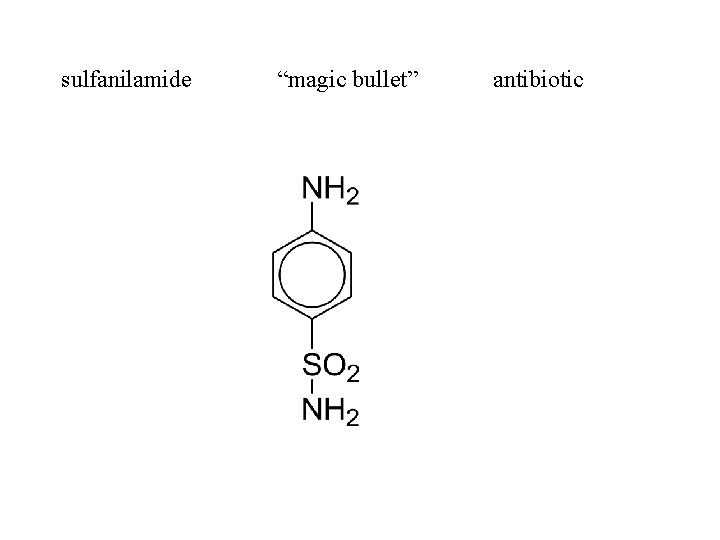
sulfanilamide “magic bullet” antibiotic


5. EAS 6. -NH 2, -NHR, -NR 2 are powerful activating groups and ortho/para directors 7. a) nitration 8. b) sulfonation 9. c) halogenation 10. d) Friedel-Crafts alkylation 11. e) Friedel-Crafts acylation 12. f) coupling with diazonium salts 13. g) nitrosation

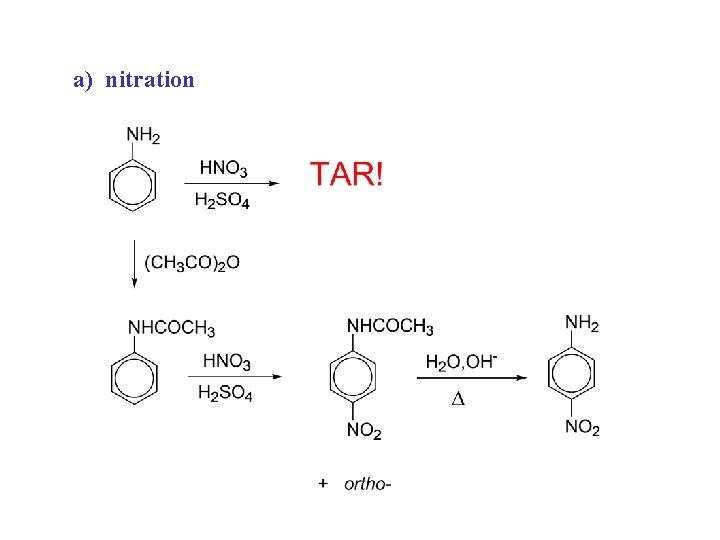
a) nitration

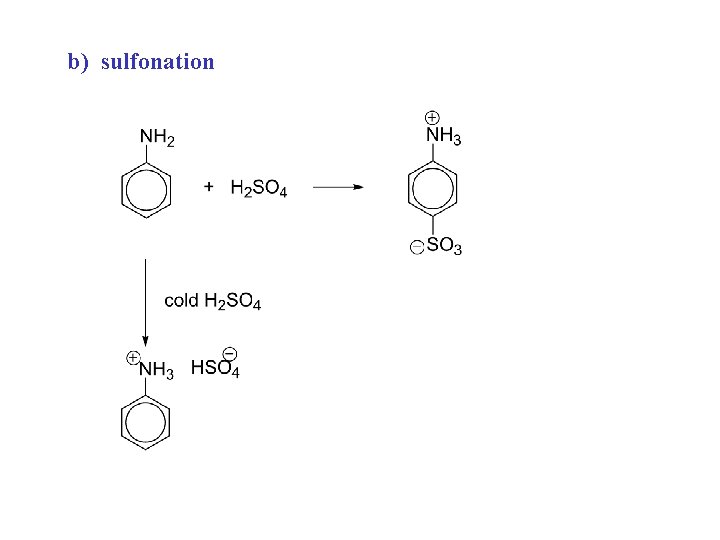
b) sulfonation

c) halogenation

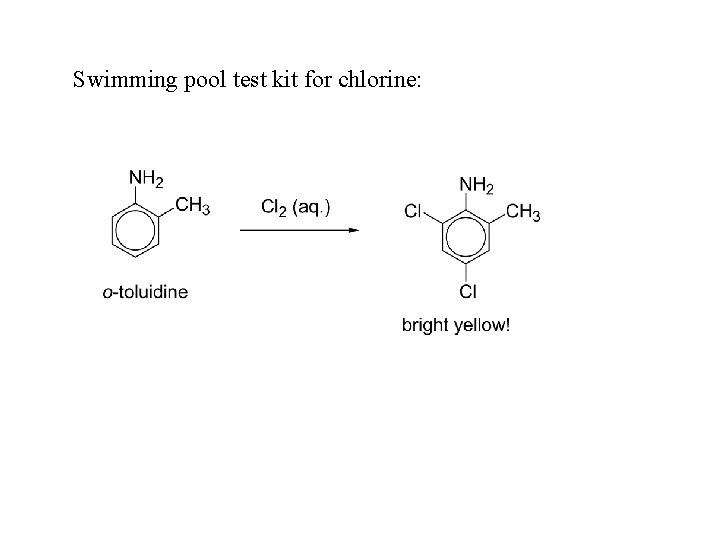
Swimming pool test kit for chlorine:

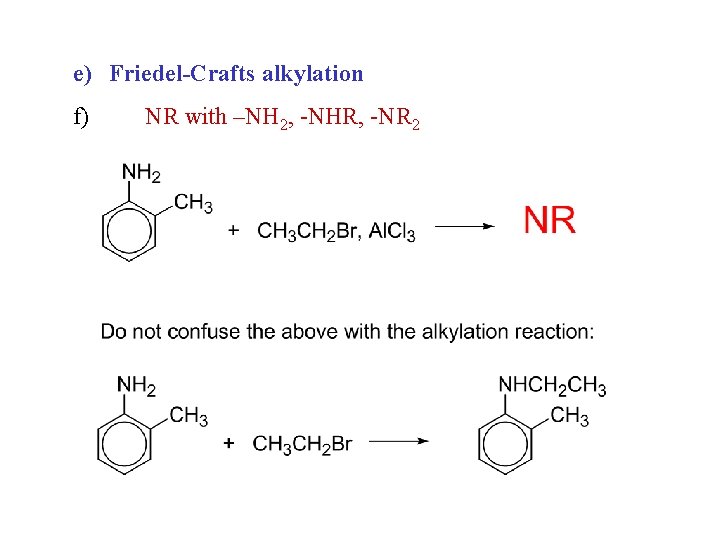
e) Friedel-Crafts alkylation f) NR with –NH 2, -NHR, -NR 2

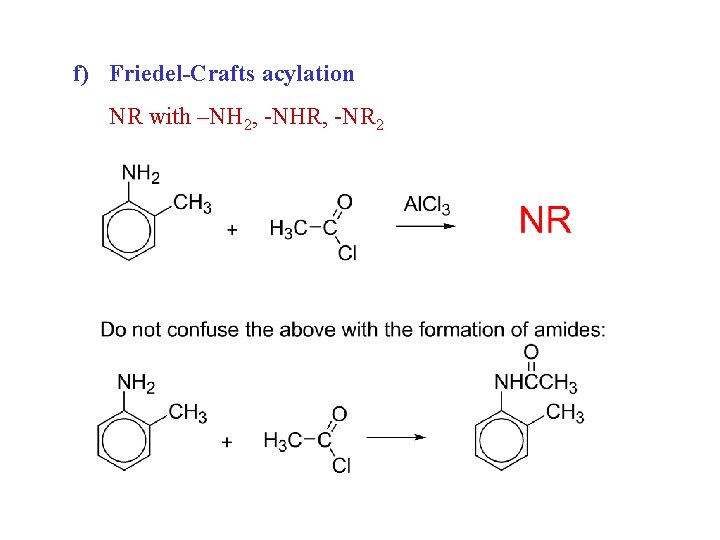
f) Friedel-Crafts acylation NR with –NH 2, -NHR, -NR 2

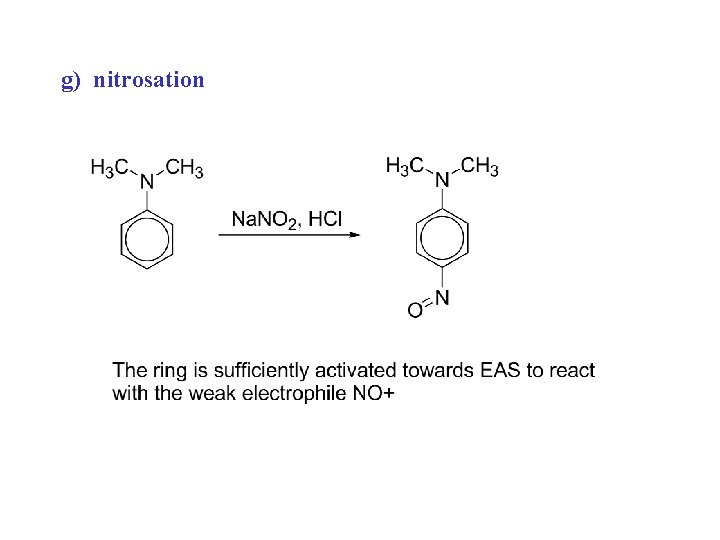
g) nitrosation

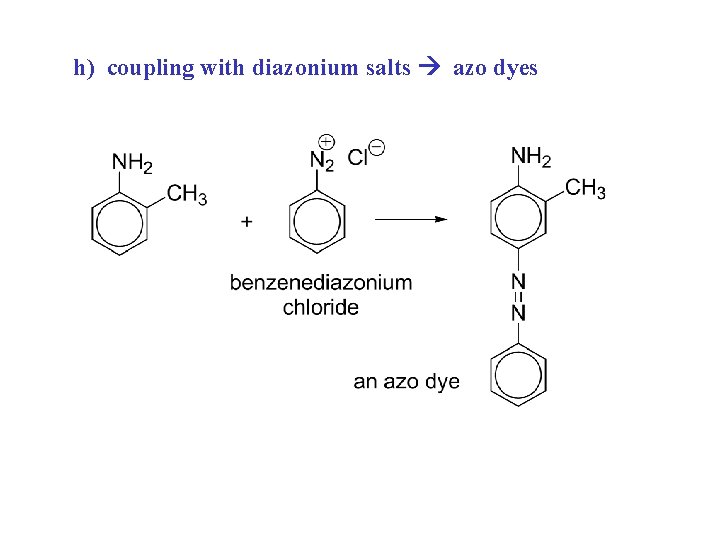
h) coupling with diazonium salts azo dyes

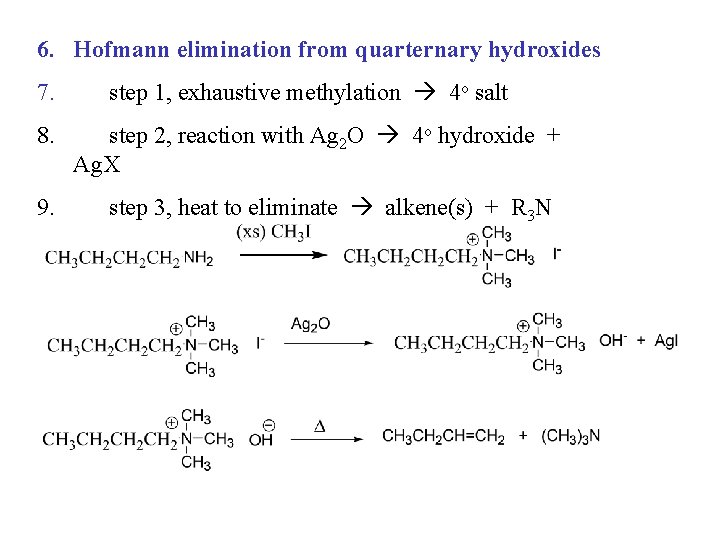
6. Hofmann elimination from quarternary hydroxides 7. 8. 9. step 1, exhaustive methylation 4 o salt step 2, reaction with Ag 2 O 4 o hydroxide + Ag. X step 3, heat to eliminate alkene(s) + R 3 N


7. Reactions with nitrous acid

note: 90% of all tested nitrosamines are carcinogenic in man. Many nitrosamine cancers are organ specific. For example, dimethylnitrosamine causes liver cancer while the nitrosamines in tobacco smoke cause lung cancer. Sodium nitrite (“cure”) is used as a preservative in meats such as bacon, bologna, hot dogs, etc. to kill the organism responsible for botulism poisoning. In the stomach, the nitrous acid produced from sodium nitrite can react with secondary and tertiary amines to form nitrosamines. To reduce the formation of nitrosamines, ascorbic acid (Vitamin C) is now added to foods cured with sodium nitrite. Nitrosamines are also found in beer!

Amines, reactions Amines are similar to ammonia in their reactions. Like ammonia, amines are basic. Like ammonia, amines are nucleophilic and react with alkyl halides, acid chlorides, and carbonyl compounds. The aromatic amines are highly reactive in electrophilic aromatic substitution.

Amine, reactions: 1. As bases 2. Alkylation 3. Reductive amination 4. Conversion into amides 5. EAS 6. Hofmann elimination from quarternary ammonium salts 7. Reactions with nitrous acid