1 7 AMINES AND AMIDES AMINES Amines are






- Slides: 21

1. 7 AMINES AND AMIDES

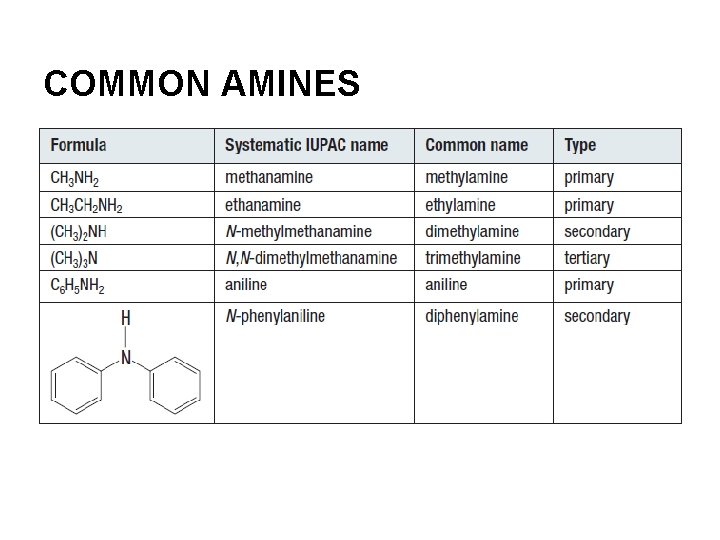
AMINES Amines are derivatives of ammonia molecules that contain a nitrogen atom bonded to one or more alkyl groups on each molecule. Many amines have strong, unpleasant odours. ex. putrescine cadaverine

COMMON AMINES

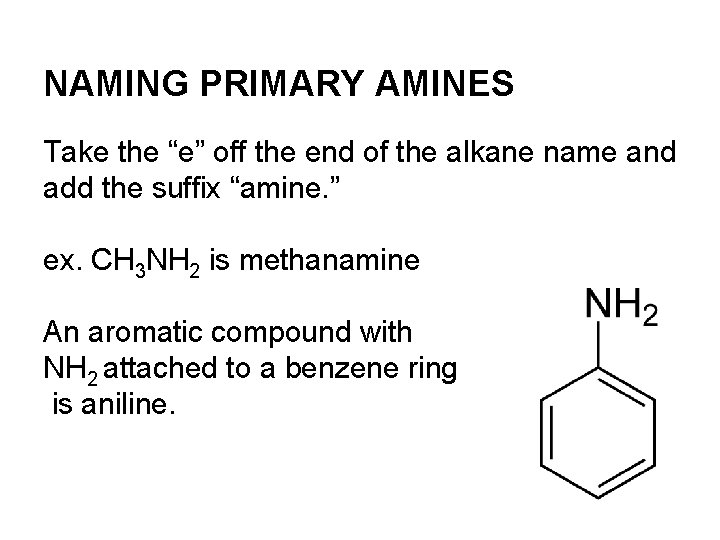
NAMING PRIMARY AMINES Take the “e” off the end of the alkane name and add the suffix “amine. ” ex. CH 3 NH 2 is methanamine An aromatic compound with NH 2 attached to a benzene ring is aniline.

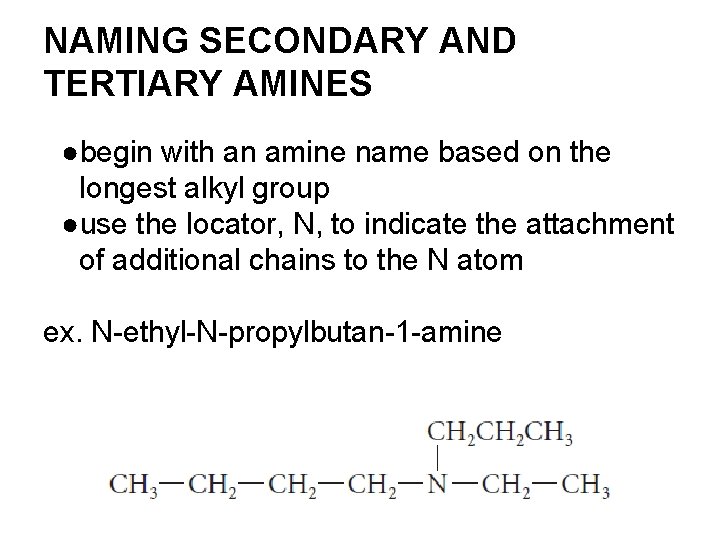
NAMING SECONDARY AND TERTIARY AMINES ●begin with an amine name based on the longest alkyl group ●use the locator, N, to indicate the attachment of additional chains to the N atom ex. N-ethyl-N-propylbutan-1 -amine

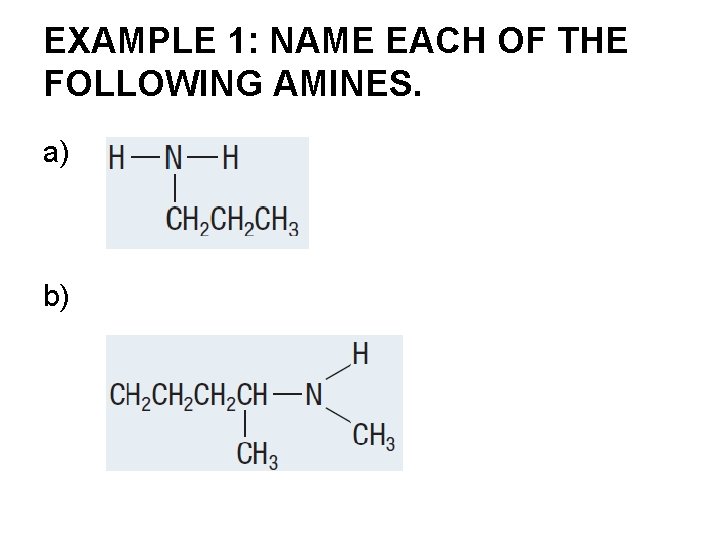
EXAMPLE 1: NAME EACH OF THE FOLLOWING AMINES. a) b)

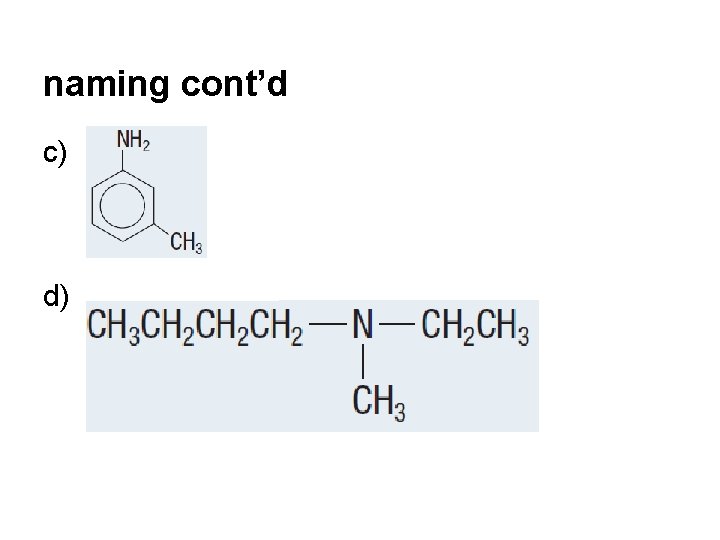
naming cont’d c) d)

EXAMPLE 2: Draw each of the following amines with ethyl as the attached alkyl groups. Then name each structure. a) a primary amine

EXAMPLE 2 cont’d b) a secondary amine c) a tertiary amine

PROPERTIES OF AMINES ●small amines are soluble in water ●N-C and N-H bonds are polar ●causes more VDW forces and more energy needed to break bonds ●higher MP and BP than their corresponding hydrocarbon compounds ●primary amines have higher BP’s than secondary bc they can form 2 H bonds. ●secondary amines can form 1 H bond and tertiary cannot form any

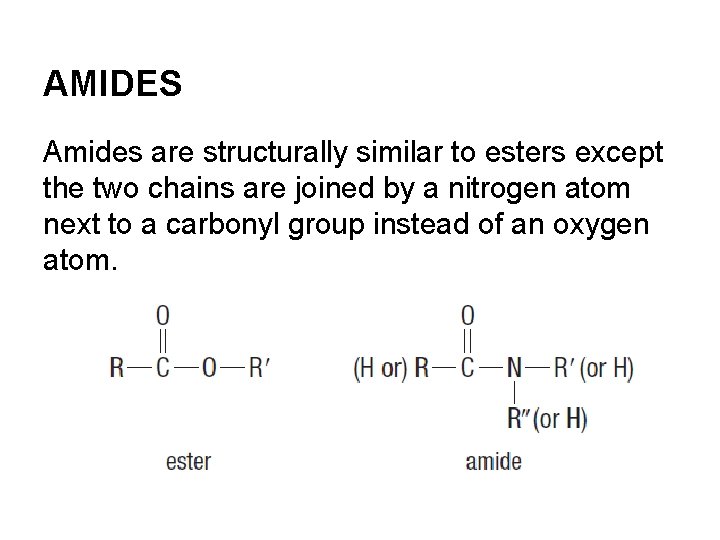
AMIDES Amides are structurally similar to esters except the two chains are joined by a nitrogen atom next to a carbonyl group instead of an oxygen atom.

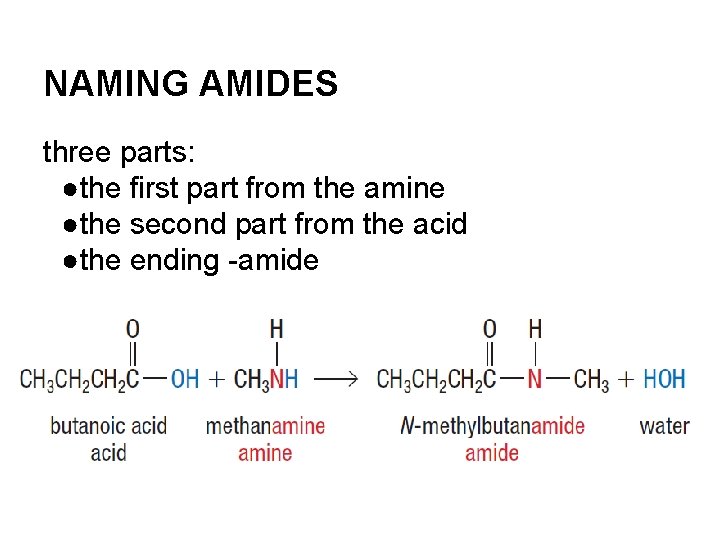
NAMING AMIDES three parts: ●the first part from the amine ●the second part from the acid ●the ending -amide

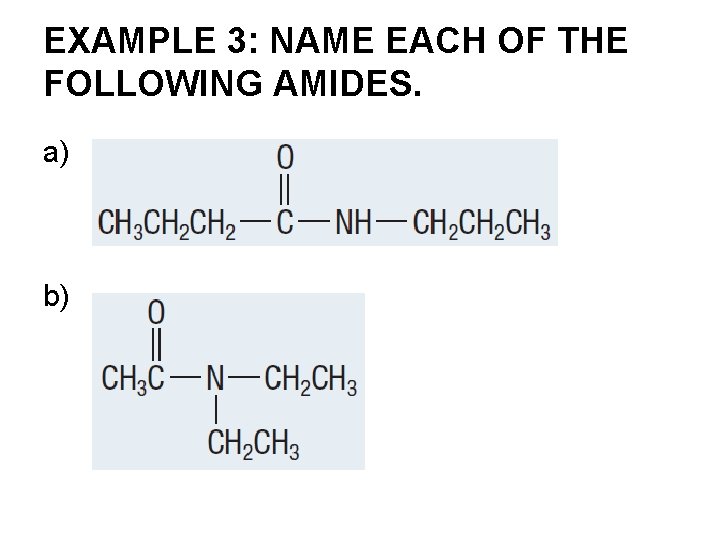
EXAMPLE 3: NAME EACH OF THE FOLLOWING AMIDES. a) b)

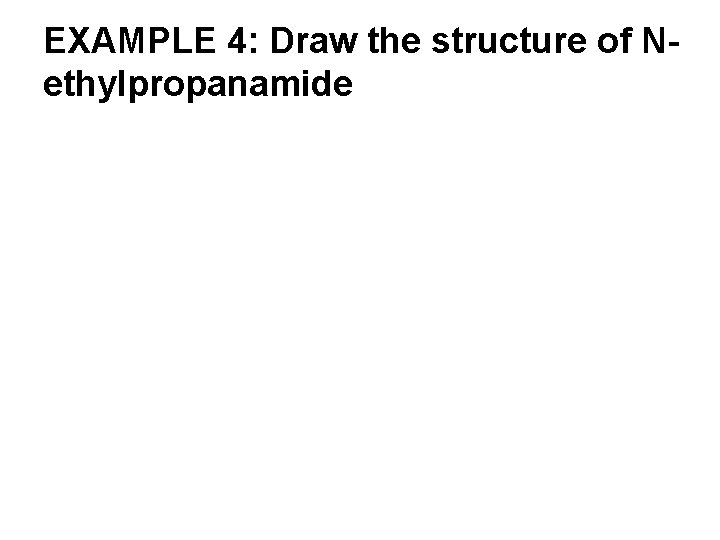
EXAMPLE 4: Draw the structure of Nethylpropanamide

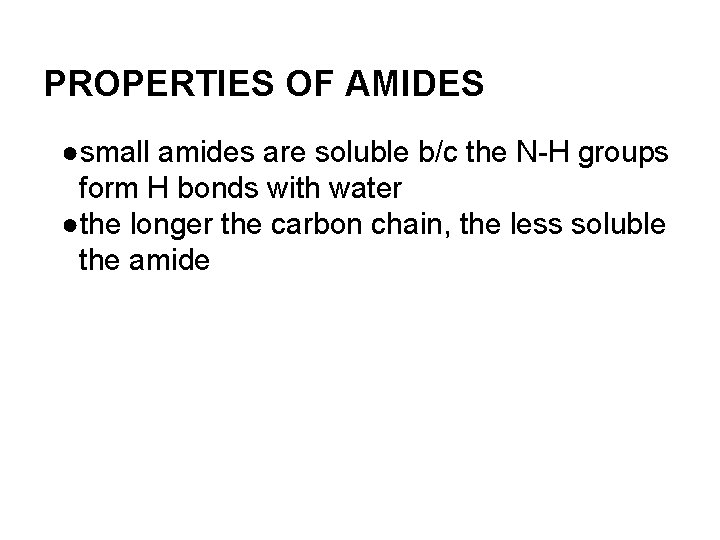
PROPERTIES OF AMIDES ●small amides are soluble b/c the N-H groups form H bonds with water ●the longer the carbon chain, the less soluble the amide

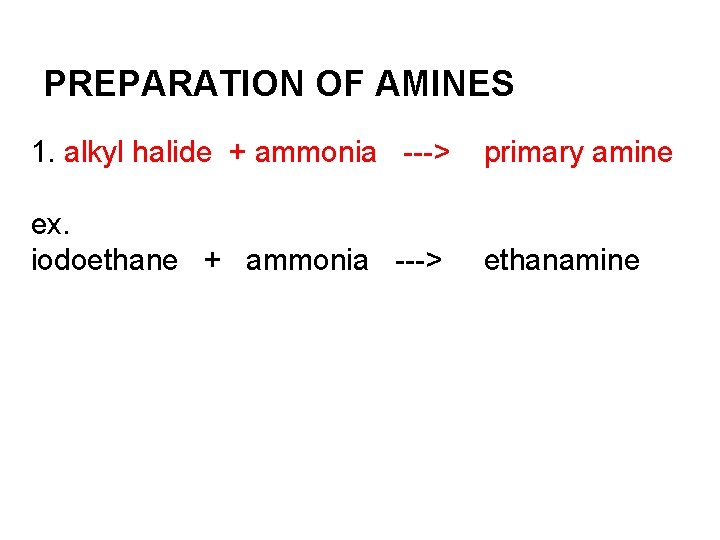
PREPARATION OF AMINES 1. alkyl halide + ammonia ---> primary amine ex. iodoethane + ammonia ---> ethanamine

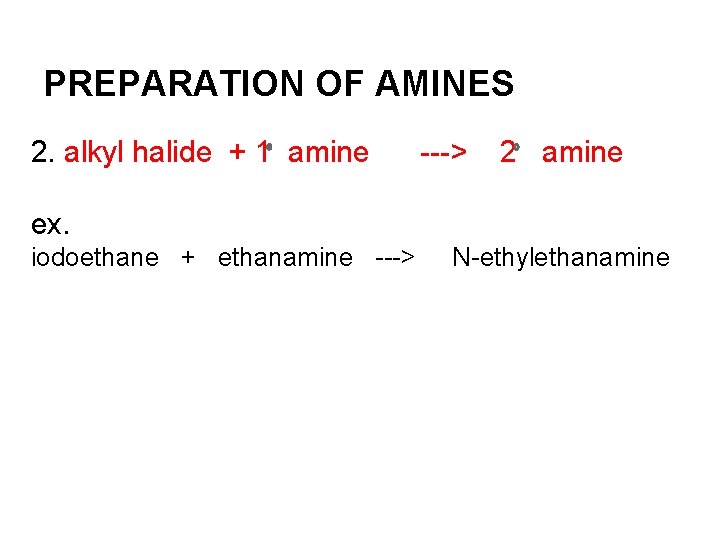
PREPARATION OF AMINES 2. alkyl halide + 1 amine ---> 2 amine ex. iodoethane + ethanamine ---> N-ethylethanamine

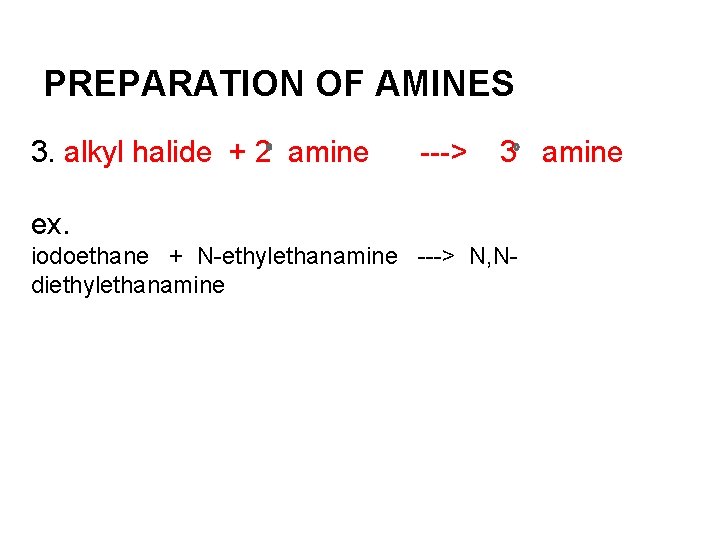
PREPARATION OF AMINES 3. alkyl halide + 2 amine ---> 3 amine ex. iodoethane + N-ethylethanamine ---> N, Ndiethylethanamine

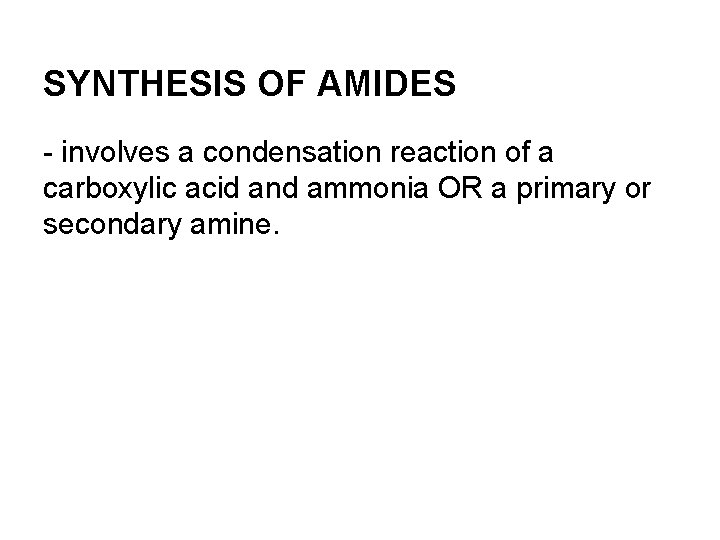
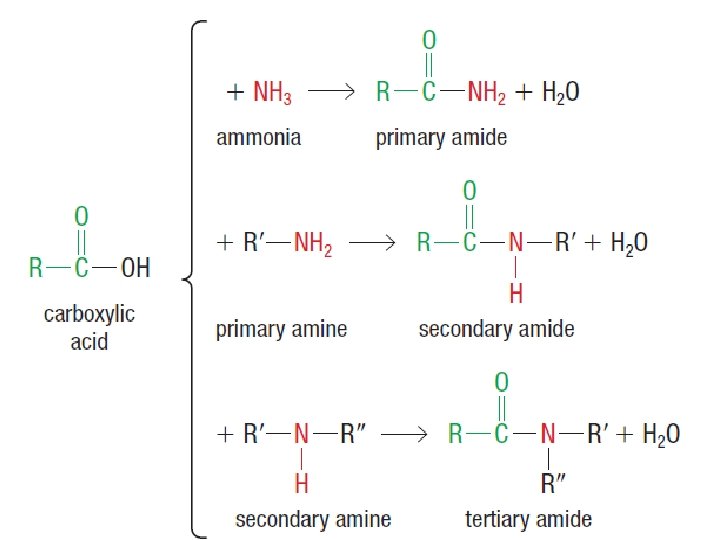
SYNTHESIS OF AMIDES - involves a condensation reaction of a carboxylic acid and ammonia OR a primary or secondary amine.


HOMEWORK: p. 58 #1, 2 p. 60 #1, 2 p. 62 #1 - 5