Amines are derivatives of ammonia NH 3 Amines
![Preparation of Aromatic Amines + 6[H] + 2 H 2 O Aromatic amines are Preparation of Aromatic Amines + 6[H] + 2 H 2 O Aromatic amines are](https://slidetodoc.com/presentation_image_h/3439eadd778e3647469eb6fcd31cae87/image-4.jpg)
- Slides: 4

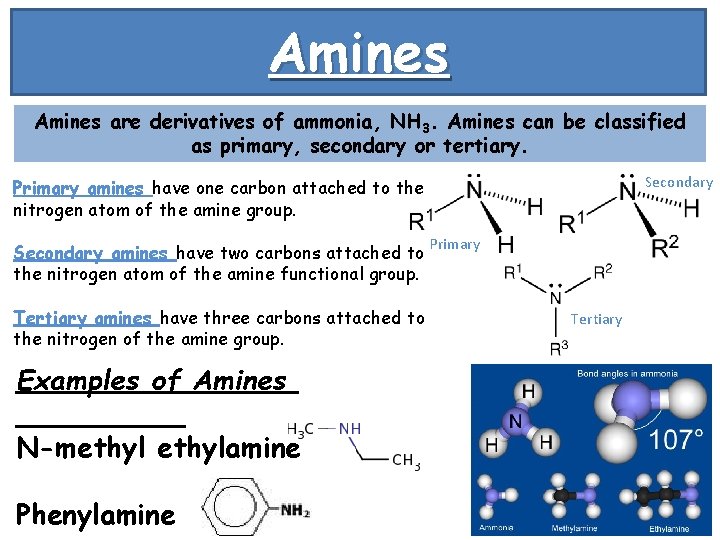
Amines are derivatives of ammonia, NH 3. Amines can be classified as primary, secondary or tertiary. Secondary Primary amines have one carbon attached to the nitrogen atom of the amine group. Secondary amines have two carbons attached to the nitrogen atom of the amine functional group. Tertiary amines have three carbons attached to the nitrogen of the amine group. Examples of Amines N-methylamine Phenylamine Primary Tertiary

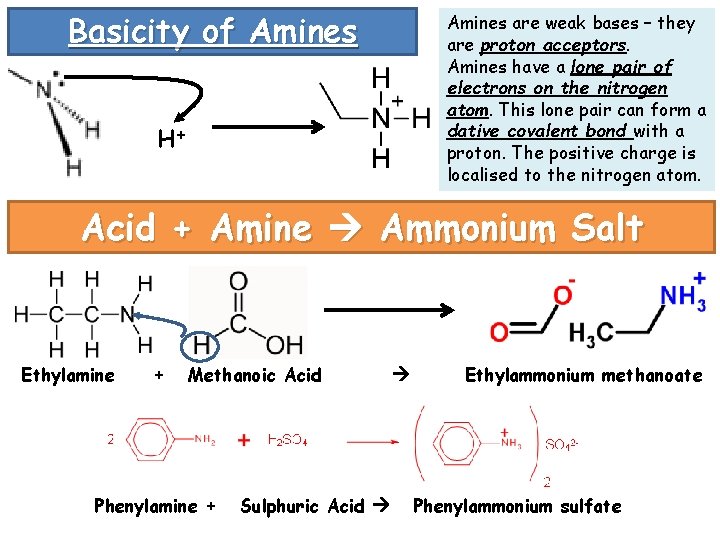
Basicity of Amines are weak bases – they are proton acceptors. Amines have a lone pair of electrons on the nitrogen atom. This lone pair can form a dative covalent bond with a proton. The positive charge is localised to the nitrogen atom. H+ Acid + Amine Ammonium Salt Ethylamine + Methanoic Acid Phenylamine + Sulphuric Acid Ethylammonium methanoate Phenylammonium sulfate

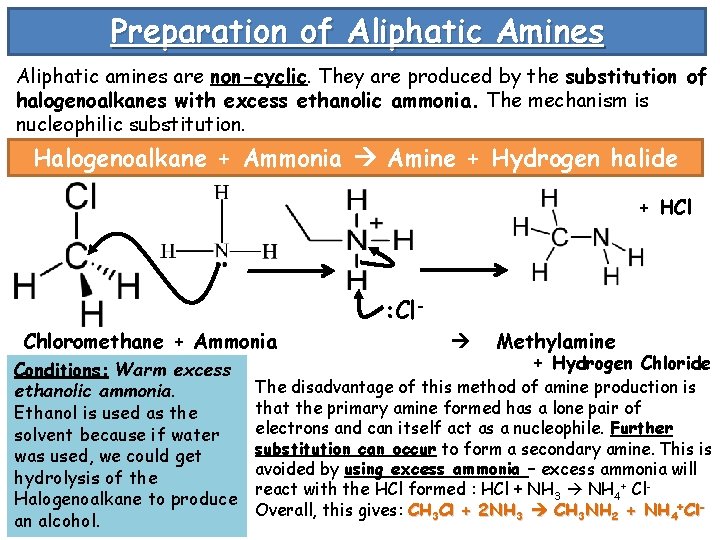
Preparation of Aliphatic Amines Aliphatic amines are non-cyclic. They are produced by the substitution of halogenoalkanes with excess ethanolic ammonia. The mechanism is nucleophilic substitution. Halogenoalkane + Ammonia Amine + Hydrogen halide + HCl : Cl. Chloromethane + Ammonia Conditions: Warm excess ethanolic ammonia. Ethanol is used as the solvent because if water was used, we could get hydrolysis of the Halogenoalkane to produce an alcohol. Methylamine + Hydrogen Chloride The disadvantage of this method of amine production is that the primary amine formed has a lone pair of electrons and can itself act as a nucleophile. Further substitution can occur to form a secondary amine. This is avoided by using excess ammonia – excess ammonia will react with the HCl formed : HCl + NH 3 NH 4+ Cl. Overall, this gives: CH 3 Cl + 2 NH 3 CH 3 NH 2 + NH 4+Cl-
![Preparation of Aromatic Amines 6H 2 H 2 O Aromatic amines are Preparation of Aromatic Amines + 6[H] + 2 H 2 O Aromatic amines are](https://slidetodoc.com/presentation_image_h/3439eadd778e3647469eb6fcd31cae87/image-4.jpg)
Preparation of Aromatic Amines + 6[H] + 2 H 2 O Aromatic amines are formed by the reduction of nitrobenzene, nitrobenzene in the presence of concentrated HCl and Sn. Synthesis of Azo Dyes 1. Diazotisation – aromatic amine transformed to diazonium salt. Conditions: Below 10°; Na. NO 2 ; Excess HCl. The Na. NO 2 and HCl react to form nitrous acid, HNO 2, in situ. 2. Coupling reaction with phenol – diazonium salt is coupled with a phenolic compound under alkaline conditions. The benzene rings are linked together through an azo (N=N) functional group. Na. NO 2 + HCl HNO 2 + Na. Cl Under alkaline conditions, so the HCl formed reacts with the Na. OH to form sodium chloride + water: Na. OH + HCl Na. Cl + H 2 O