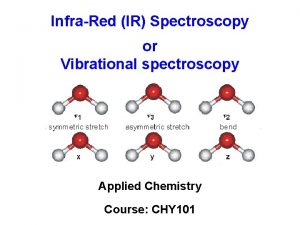
Quantitative aspects of IR spectroscopy as applied to
































![T = [1 - R 2] exp [- b s d] / 1 - T = [1 - R 2] exp [- b s d] / 1 -](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-48.jpg)























![DATA FOLLOW THE LANGMUIR MODEL 1. REGENERATED CARRIERS ≈ [B] (3 X 1019 atoms/cm DATA FOLLOW THE LANGMUIR MODEL 1. REGENERATED CARRIERS ≈ [B] (3 X 1019 atoms/cm](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-80.jpg)










![Temkin isotherm: Na = K 1 ln[1 + K 2 p] a variant of Temkin isotherm: Na = K 1 ln[1 + K 2 p] a variant of](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-95.jpg)










![More conveniently: ln [θ / p (1 – θ)] = ln [A / p More conveniently: ln [θ / p (1 – θ)] = ln [A / p](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-109.jpg)













![Use of: θ(CO)/θ(OC) = Kiso(T) A(CO)/A(OC) K(T) = exp [ΔS°/R] exp [-ΔH°/RT] allows the Use of: θ(CO)/θ(OC) = Kiso(T) A(CO)/A(OC) K(T) = exp [ΔS°/R] exp [-ΔH°/RT] allows the](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-130.jpg)



- Slides: 134

Quantitative aspects of IR spectroscopy as applied to adsorbed species Edoardo Garrone Dipartimento di Scienze dei Materiali ed Ingegneria Chimica, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Torino Italy

IR spectroscopy: mainly a qualitative technique, useful for recognising species Group frequencies: - carbonylic groups C=O at 1700 -1750 cm-1, - Si-H groups around 2200 cm-1, etc. [e. g. G. Socrates, Infrared and Raman characteristic group frequencies: tables and charts. (2001) Wiley, Chichester, United Kingdom]

Examples of qualitative use of IR Spectroscopy concerning adsorbed species - carbon dioxide on basic oxides: carbonate species may be formed, and/or species molecularly adsorbed onto cations (G. Ramis, G. Busca, V. Lorenzelli, Mater. Chem. Phys. 29 (1991) 425) - pyridine (or ammonia) adsorption: Brønsted or Lewis sites revealed by the formation of pyridinium (ammonium) species, or molecularly bound species (H. Knözinger, Adv. Catal. 25 (1976) 184)

A puzzling case concerning ethylene dissociative adsorption with extended metal surfaces: ethylidene species C-CH 3 (C. E. Anson, N. Sheppard, D. B. Powell, J. R. Norton, W. Fischer, R. L. Keiter, B. F. G. Johnson, J. Lewis, A. K. Bhattacharrya, S. A. R. Knox, M. L. Turner, J. Am. Chem. Soc. 116 (1994) 3058)

IR Spectroscopy also yields information on the local symmetry and the types of bonds - carbonate species (unidentate, bidentate, etc) - carbonylic species (CO on metals): “ontop”, bidentate, tridentate CO species, with different C-O bond order and frequencies, all below 2143 cm-1 (isolated molecule) - adsorption of CO on cations: C-O stretch with a frequency usually > 2143 cm-1

Much less developed the quantitative use of IR spectroscopy of surface species!

Modalities of measurement

Most common measurement type in the case of adsorbed species is transmission. Also available: • Diffuse Reflectance (DRIFT) techniques • Attenuated Total Reflection (ATR) or Grazing Angle • with metal ideal surfaces, the appropriate version of vibrational spectroscopy is the Electron Energy Loss Spectroscopy (EELS)

In the present survey, only transmission measurements are considered!

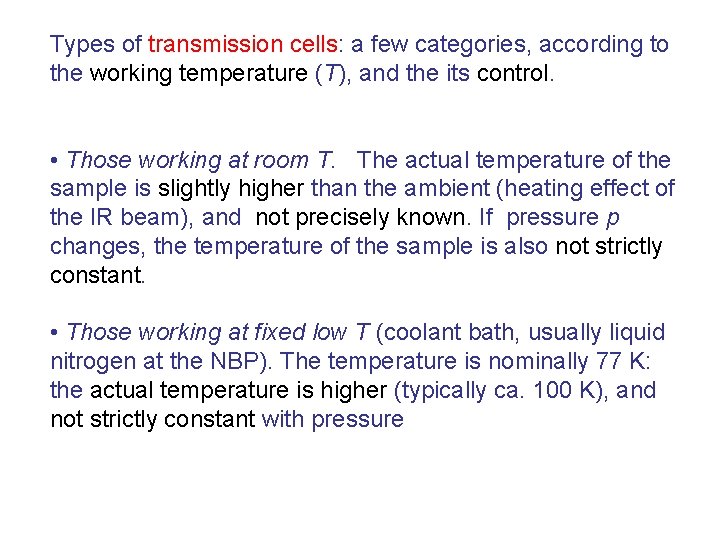
Types of transmission cells: a few categories, according to the working temperature (T), and the its control. • Those working at room T. The actual temperature of the sample is slightly higher than the ambient (heating effect of the IR beam), and not precisely known. If pressure p changes, the temperature of the sample is also not strictly constant. • Those working at fixed low T (coolant bath, usually liquid nitrogen at the NBP). The temperature is nominally 77 K: the actual temperature is higher (typically ca. 100 K), and not strictly constant with pressure

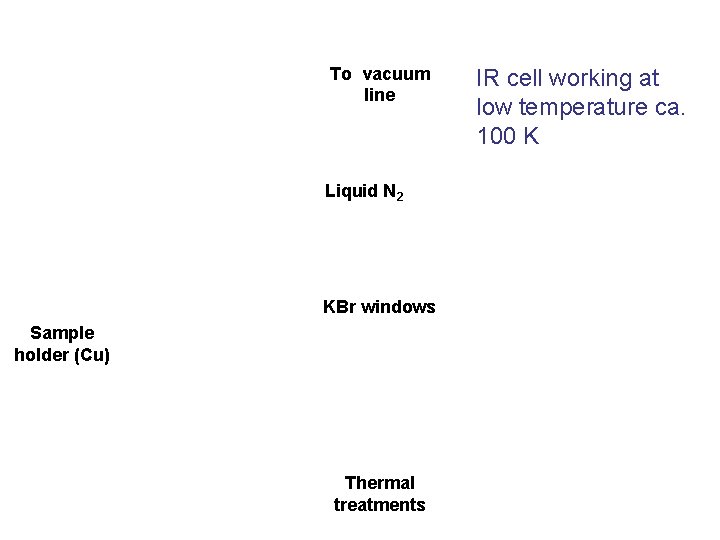
Cell with small optical path, working at RT To vacuum line thermal treatments KBr windows

To vacuum line Liquid N 2 KBr windows Sample holder (Cu) Thermal treatments IR cell working at low temperature ca. 100 K

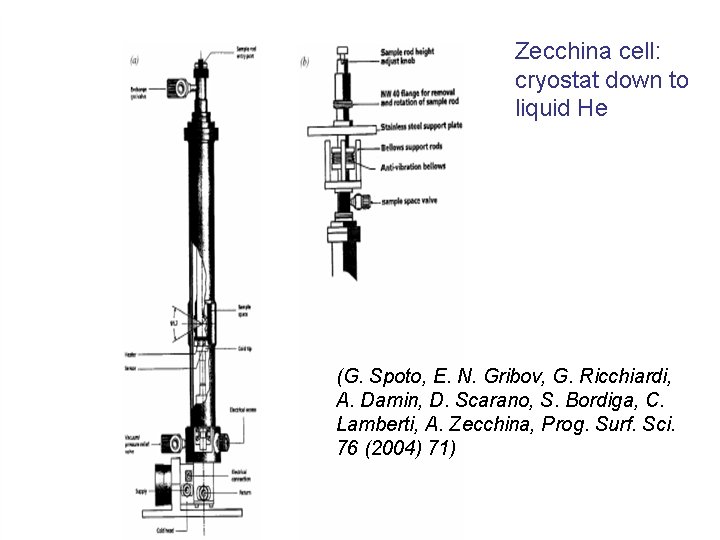
• A few cells work at variable T A) A. A. Tsyganenko: allows accurate measurement of T and p, but not their control. It works in a T range below ambient. Changes in T and p are slow enough so that equilibrium phenomena can be followed B) A. Zecchina and co-workers: equipped with a cryostat: strict control over T. It works below ambient T and may operate down to 4 K. C) A commercial cell (AABSPEC) working at controlled T and p also in a range above room T. Limited dead volume (few cubic centimetres)

Tsiganenko cell: T variable, but not controlled (C. Otero Areán, O. V. Manoilova, A. A. Tsyganenko, G. Turnes Palomino, M. Peñarroya Mentruit, G. Geobaldo, E. Garrone, Eur. J. Inorg. Chem. (2001) 1739)

Zecchina cell: cryostat down to liquid He (G. Spoto, E. N. Gribov, G. Ricchiardi, A. Damin, D. Scarano, S. Bordiga, C. Lamberti, A. Zecchina, Prog. Surf. Sci. 76 (2004) 71)

Commercial: also for T higher than ambient http: //www. aabspec. com

For simplicity, from now on an adsorbate showing only one band will be considered

Measurable quantities for an IR band: i) frequency (peak position) ii) intensity (either at the peak or integrated intensity) iii) half-width iv) other parameters entering the analytical representation of the band: e. g. , fraction of Lorentzian and Gaussian functions

Frequency (peak position) most readily measured quantity

Information on the adsorbing centre comes from the perturbation of a significant IR mode (e. g. , stretching mode of CO) from a reference value (unperturbed molecule) Usually, the stronger the interaction, the larger the perturbation. In simple cases, when considering a set of similar systems, the extent of perturbation has a quantitative meaning.

Correlations of the frequency (more commonly, the shift) with: - the adsorption enthalpy, measured independently - another frequency of the same system - the frequency of another (similar) system - another feature of the same IR band (e. g. halfwidth)

- the adsorption enthalpy, measured independently - another frequency of the same system - the frequency of another (similar) system - another feature of the same IR band (e. g. halfwidth)

Two examples: - CO adsorbed on cations - H-bonding

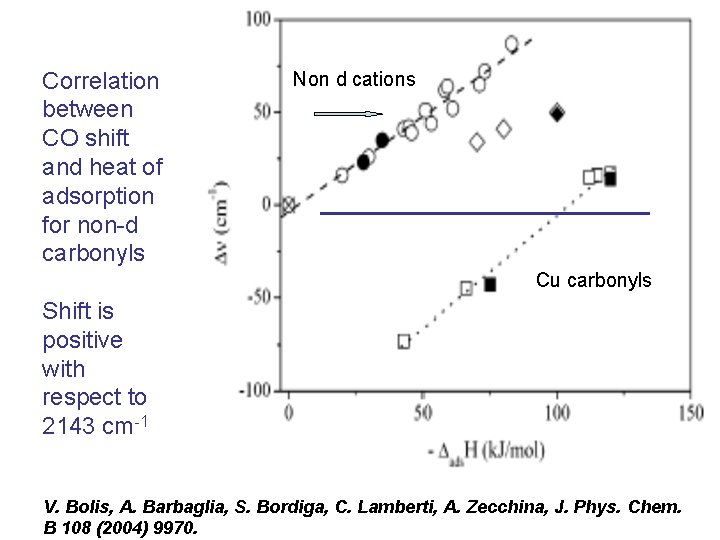
CO adsorbed on cations either non d, d 0 or d 10, (non classical carbonyls, with stretching frequencies higher than the isolated molecule) Linear dependence between calorimetrically measured heats of adsorption and the hypsochromic shift:

Correlation between CO shift and heat of adsorption for non-d carbonyls Non d cations Cu carbonyls Shift is positive with respect to 2143 cm-1 V. Bolis, A. Barbaglia, S. Bordiga, C. Lamberti, A. Zecchina, J. Phys. Chem. B 108 (2004) 9970.

Only electrostatics involved, no proper chemical bond If double interactions take place with both ends of the CO molecule as in Al-rich zeolites, the linear relationship does not hold lower frequency and larger interaction enthalpy (C. Otero Areán, M. Rodriguez Delgado, C. Lopez Bauçà, L. Vrbka, P. Nachtigall, Phys. Chem. Phys. 9 (2007) 457)

H-bonding: the shift of the O-H stretch Δν(O-H) measures the strength of Hbond A few formulas proposed: electrostatics basically involved! Classical work by N. Sheppard and G. C. Pimentel. (N. Sheppard, in Hydrogen Bonding, ed. D. Hadzi, Pergamon Press, London, 1959, p. 85. G. C. Pimentel and A. L. Mc. Clellan, in The Hydrogen Bond, W. H. Freeman and Co. , San Francisco, 1960).

- the adsorption enthalpy, measured independently - another frequency of the same system - the frequency of another (similar) system - another feature of the same IR band (e. g. halfwidth)

Correlation between two different modes of the same type of adduct. CO H-bonded to different acidic hydroxyls: the C -O frequency linearly correlated to Δν (O-H) (O. Cairon, T. Chevreau, J. C. Lavalley, J. Chem. Soc. Faraday Trans. 94 (1998) 3039)

- the adsorption enthalpy, measured independently - another frequency of the same system - the frequency of another (similar) system - another feature of the same IR band (e. g. halfwidth)

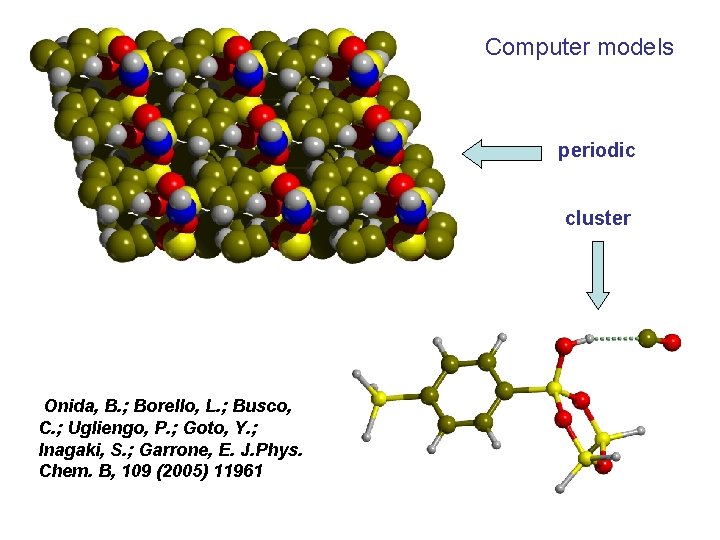
Example: the silanol in phenylene Periodic Mesoporous Organosilica (PMO) H-bonded to molecules with increasingly basic character

1, 4 diphenylene PMO Inagaki, S. ; Guan, S. ; Ohsuna, T. ; Terasaki, O. Nature 2002, 416, 304.

Computer models periodic cluster Onida, B. ; Borello, L. ; Busco, C. ; Ugliengo, P. ; Goto, Y. ; Inagaki, S. ; Garrone, E. J. Phys. Chem. B, 109 (2005) 11961

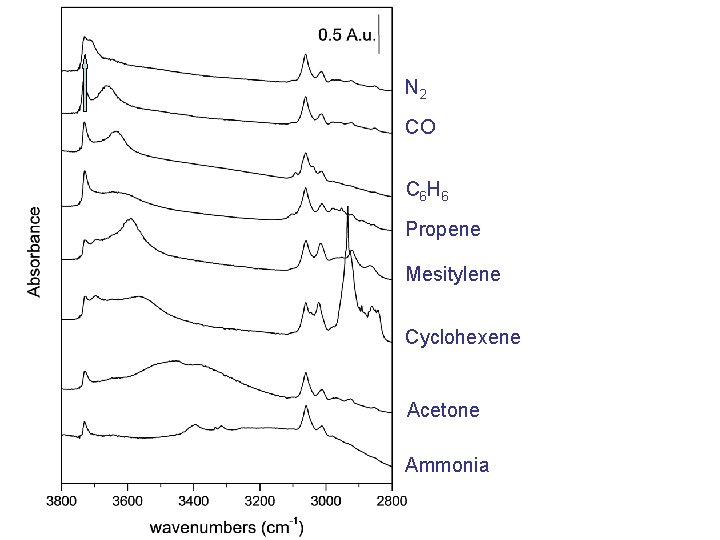
N 2 CO C 6 H 6 Propene Mesitylene Cyclohexene Acetone Ammonia Increasing basicity

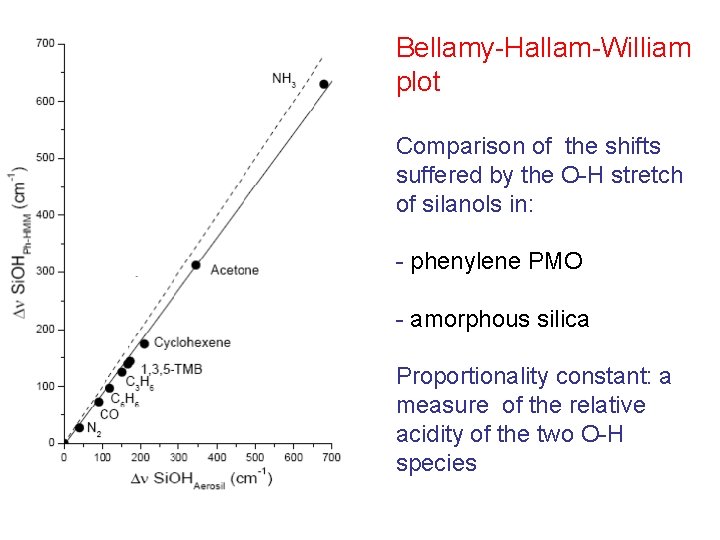
Bellamy-Hallam-William plot Comparison of the shifts suffered by the O-H stretch of silanols in: - phenylene PMO - amorphous silica Proportionality constant: a measure of the relative acidity of the two O-H species

Proportionality constant ca. 0. 96 Silanol in PMO slightly less acidic than in silica For bridged OH species in zeolites proportionality constant ca. 3 (much more acidic!) Deviations may occur!

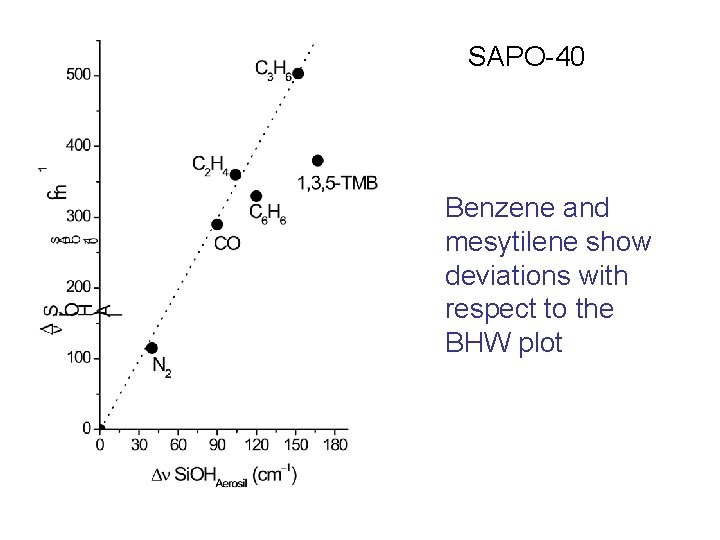
SAPO-40 Benzene and mesytilene show deviations with respect to the BHW plot

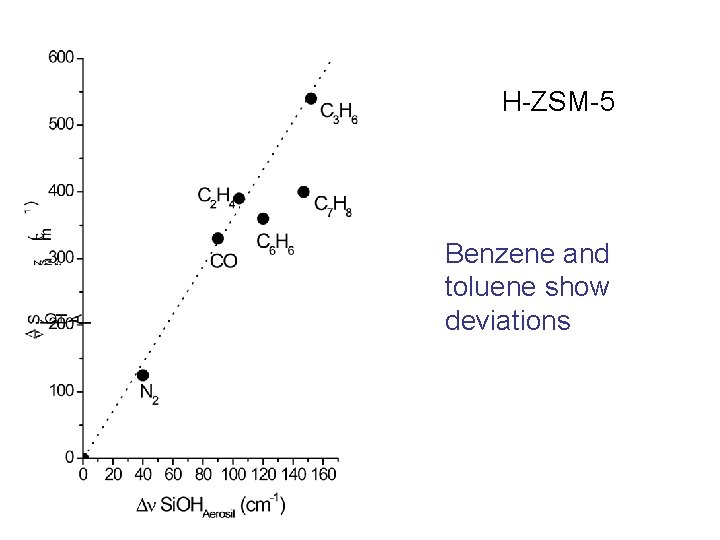
H-ZSM-5 Benzene and toluene show deviations

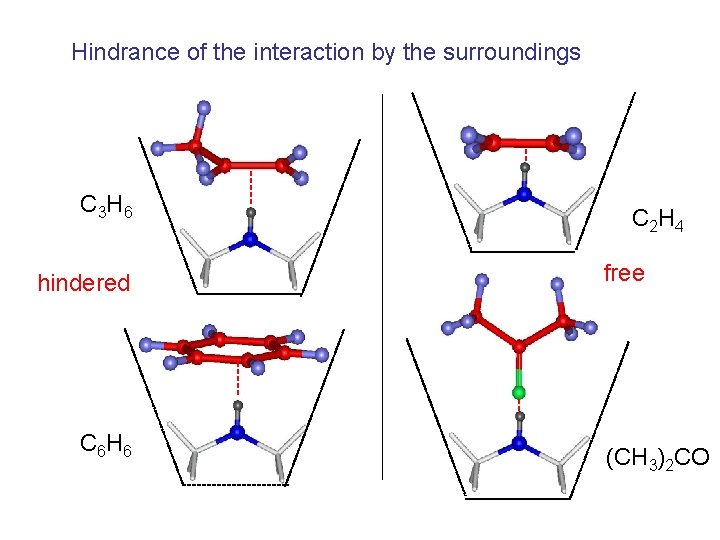
Hindrance of the interaction by the surroundings C 3 H 6 hindered C 6 H 6 C 2 H 4 free (CH 3)2 CO

- the adsorption enthalpy, measured independently - another frequency of the same system - the frequency of another (similar) system - another feature of the same IR band (e. g. halfwidth, intensity)

In H-bonding, the larger the shift, the wider and the more intense the band of the stretching mode of the O-H species engaged Quantitative relationships are known

N 2 CO C 6 H 6 Propene Mesitylene Cyclohexene Acetone Ammonia

Intensity More troublesome quantity

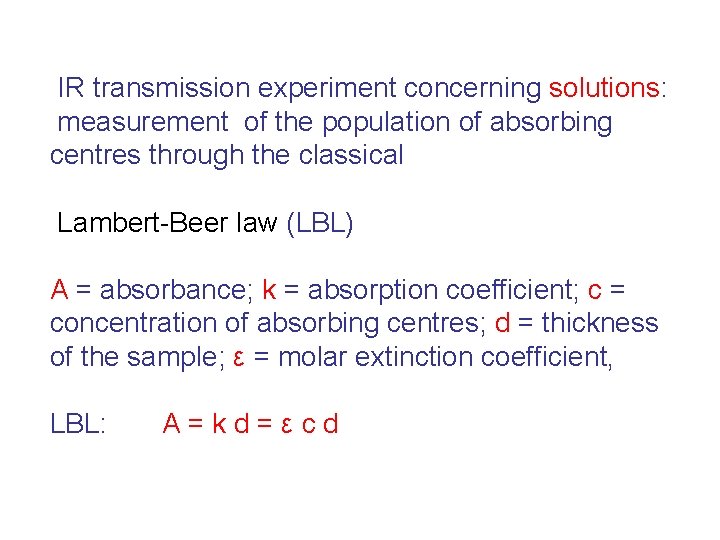
IR transmission experiment concerning solutions: measurement of the population of absorbing centres through the classical Lambert-Beer law (LBL) A = absorbance; k = absorption coefficient; c = concentration of absorbing centres; d = thickness of the sample; ε = molar extinction coefficient, LBL: A=kd=εcd

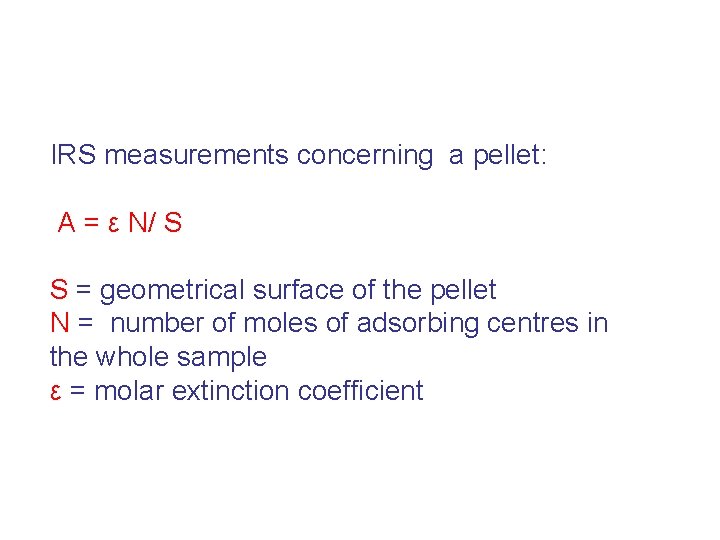
IRS measurements concerning a pellet: A = ε N/ S S = geometrical surface of the pellet N = number of moles of adsorbing centres in the whole sample ε = molar extinction coefficient

Absorbance measures the number of moles in the sample! quantitative aspects! Two reasons could impede the applicability of LBL: i) the presence of scattering because of the powder structure of the samples; ii) a change in the environment of the absorbing centres due to a change in pressure, in reversible adsorptions (not really important)

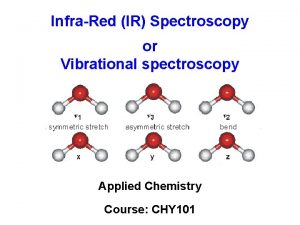
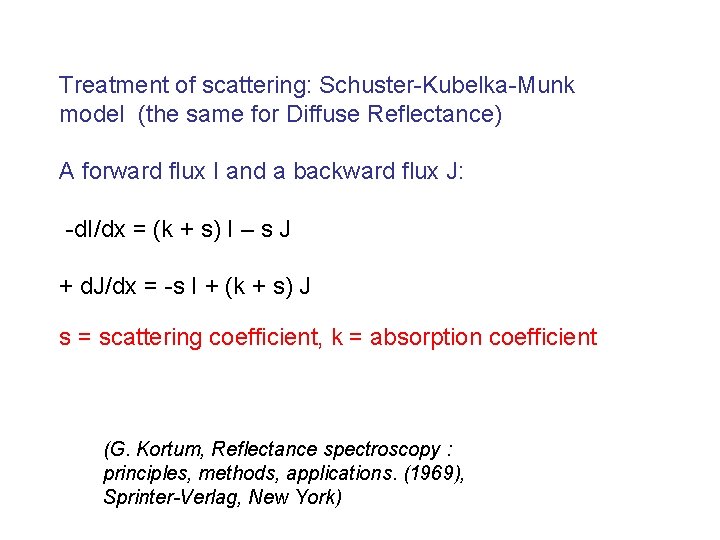
Treatment of scattering: Schuster-Kubelka-Munk model (the same for Diffuse Reflectance) A forward flux I and a backward flux J: -d. I/dx = (k + s) I – s J + d. J/dx = -s I + (k + s) J s = scattering coefficient, k = absorption coefficient (G. Kortum, Reflectance spectroscopy : principles, methods, applications. (1969), Sprinter-Verlag, New York)
![T 1 R 2 exp b s d 1 T = [1 - R 2] exp [- b s d] / 1 -](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-48.jpg)
T = [1 - R 2] exp [- b s d] / 1 - R 2 exp [- 2 b s d] b = [(1 + k/s)2 - 1]1/2; d = sample thickness, R = reflectance of the sample at infinite thickness R = 1 + k/s - [(k/s)2 + 2 k/s] 1/2 In case of moderate scattering (s < 10% k), - ln T = Aapp sd + kd + (s/k) 2 [1 – kd]

k depends on the concentration c, s does not. k = k 0 + ε N/S s = s 0 (k 0 = absorption of the solid alone): k is growing with coverage Result: in case of moderate scattering (s < 10% k), LBL holds (small offset, the term sd). For larger values of s/k, deviations may occur. Note: scatter of radiation is more often due to voids in the sample than to the actual particles. Silica samples, white when powdered, tend to become transparent when pelleted. The condition s << k is more readily fulfilled for pelleted samples than for loose powders

Example showing that the intensity of a band has to be considered with care: Porous silicon Also shows a peculiar way of making a quantitative use of IR spectroscopy!

PREPARATION The electrochemical cell, in figure, is made of Teflon, resisting to HF. The cathode is a platinum Silicon piece (1. 1 x 1. 1 cm) rod, whereas the anode is the silicon itself. The electrolyte is an ethanoic HF solution. Teflon Cell

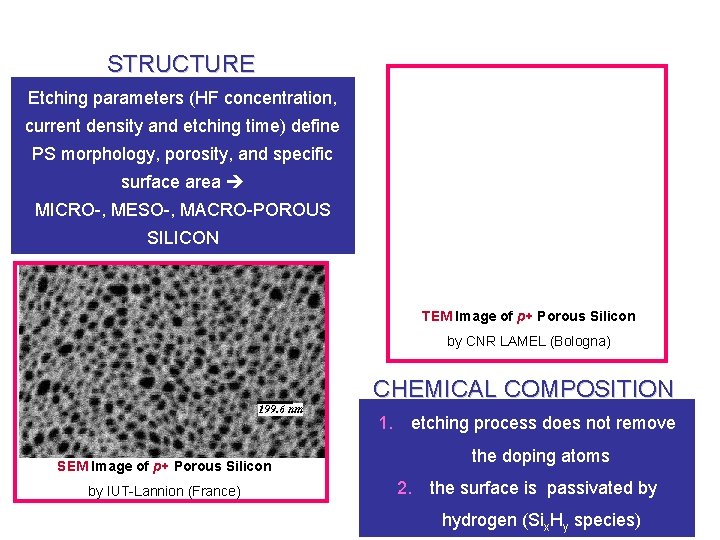
STRUCTURE Etching parameters (HF concentration, current density and etching time) define PS morphology, porosity, and specific surface area MICRO-, MESO-, MACRO-POROUS SILICON TEM Image of p+ Porous Silicon by CNR LAMEL (Bologna) CHEMICAL COMPOSITION 1. etching process does not remove SEM Image of p+ Porous Silicon by IUT-Lannion (France) the doping atoms 2. the surface is passivated by hydrogen (Six. Hy species)

Morphology of Porous Silicon p+

1. Loss of conductivity due to etching process nearly insulating material 2. IR TRANSPARENT 3. Electrical reactivation in presence of NO 2 TRACES

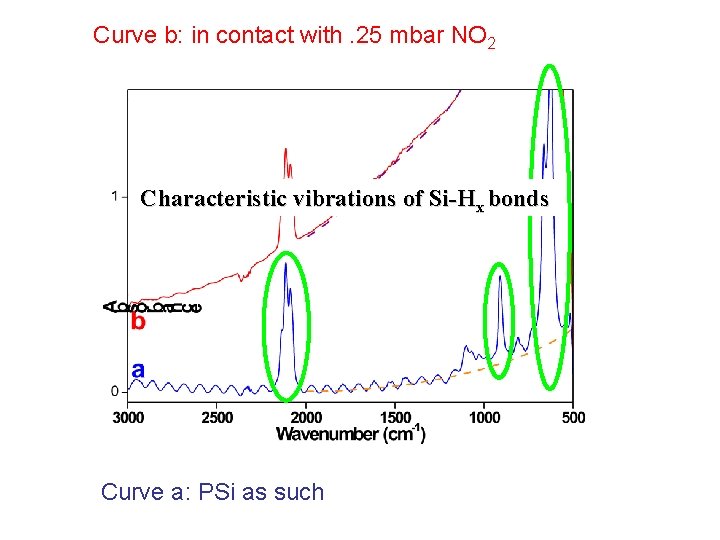
Curve b: in contact with. 25 mbar NO 2 Characteristic vibrations of Si-Hx bonds Curve a: PSi as such

Two effects: - marked increase in the background - decrease in the intensity of Si-H bands (not their location) Cause: injection of charge carriers Affects intensity, not frequency! (F. Geobaldo, P. Rivolo, S. Borini, L. Boarino, G. Amato, M. Chiesa and E. Garrone J. Phys. Chem. B 108 (2004) 18306)

Same phenomenon observed with reducible oxides (Zn. O, Sn. O 2) Reduction converts an insulator oxide into a semiconductor!

In conclusion, is LBL valid?

LBL validated a posteriori (several examples in this talk) • LBL probably holds in the vast majority of cases. • The problem with LBL, though, is that determination of ε is difficult, because very seldom A and N are simultaneously determined. • More often, A and N are measured in separate experiments, one spectroscopic, one volumetric: identity of temperature not assured.

Example of the uncertainties on molar extinction coefficients: non classical carbonyls on cationic centres. Generally believed that ε increases with the frequency, though moderately, e. g. ε = 0. 7 + b (ν -2143) when ε is given in 106 cm/mol, b = 0. 050.

A. A. Tsyganenko has recently proposed a decreasing behaviour of ε with frequency for a set of carbonyls in zeolitic cationic centres E. V. Kondriateva, O. V. Manoilova and A. A. Tsyganenko, Kinetics and Catalysis 49 (2008) 451. Entirely different measurement of ε through the vibrational polarizability αv (CO) = 4 3 v 2

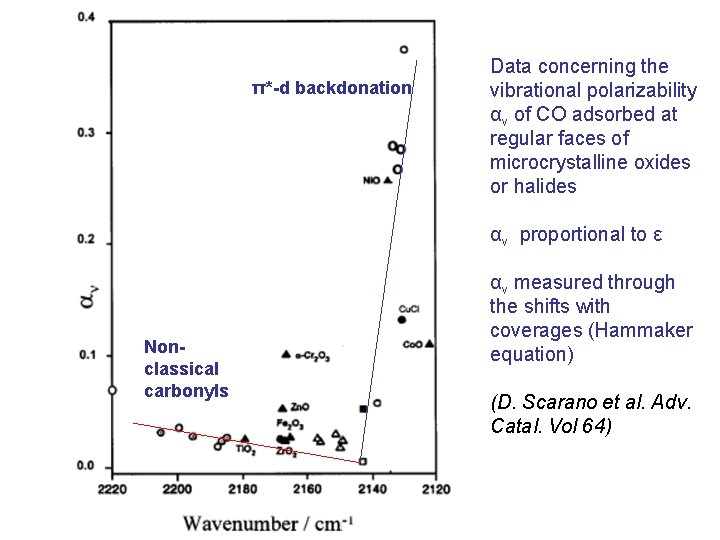
π*-d backdonation Data concerning the vibrational polarizability αν of CO adsorbed at regular faces of microcrystalline oxides or halides αν proportional to ε Nonclassical carbonyls αν measured through the shifts with coverages (Hammaker equation) (D. Scarano et al. Adv. Catal. Vol 64)

Concerning ε, CO adsorbed on zeolitic isolated cationic centres is different from CO adsorbed at regular faces of oxides?

In the following: cases of quantitative use of IR spectra, relying on LBL, not requiring the knowledge of ε.

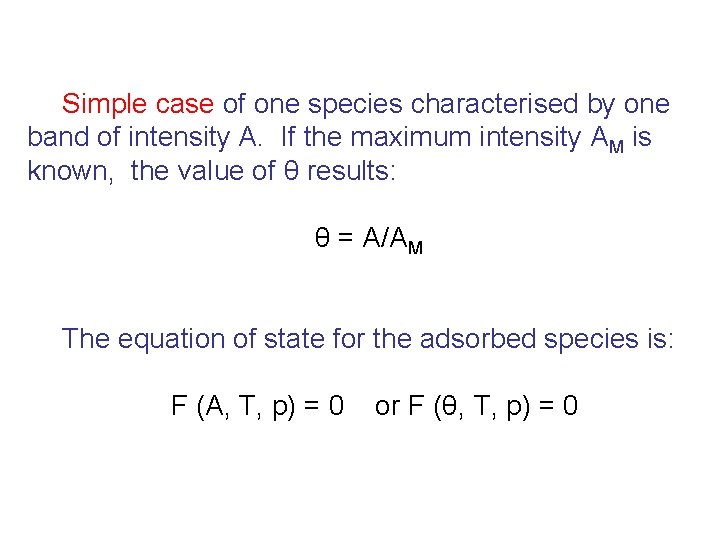
Simple case of one species characterised by one band of intensity A. If the maximum intensity AM is known, the value of θ results: θ = A/AM The equation of state for the adsorbed species is: F (A, T, p) = 0 or F (θ, T, p) = 0

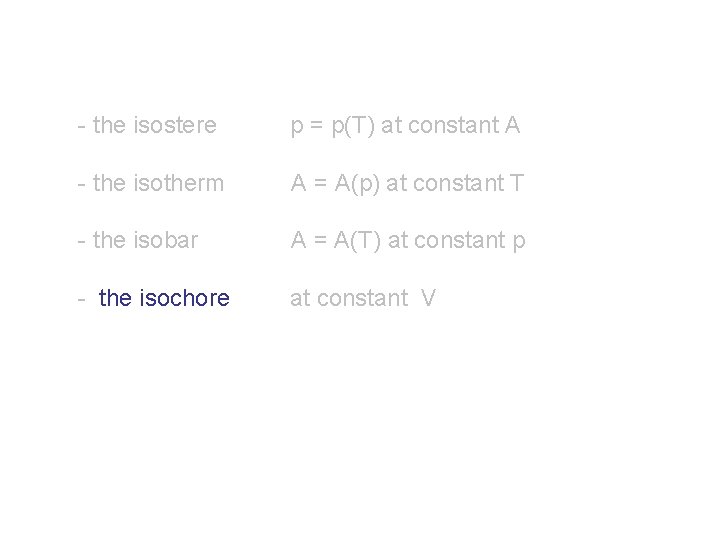
By keeping constant one observable at a time, one obtains: - the isostere p = p(T) at constant A - the isotherm A = A(p) at constant T - the isobar A = A(T) at constant p Also possible: - the isochore at constant overall V volume

- the isostere p = p(T) at constant A - the isotherm A = A(p) at constant T - the isobar A = A(T) at constant p - the isochore at constant V

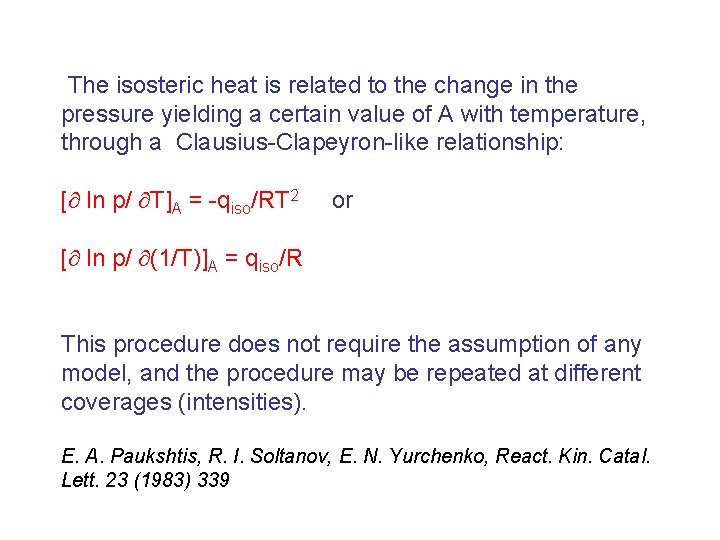
The isosteric heat is related to the change in the pressure yielding a certain value of A with temperature, through a Clausius-Clapeyron-like relationship: [ ln p/ T]A = -qiso/RT 2 or [ ln p/ (1/T)]A = qiso/R This procedure does not require the assumption of any model, and the procedure may be repeated at different coverages (intensities). E. A. Paukshtis, R. I. Soltanov, E. N. Yurchenko, React. Kin. Catal. Lett. 23 (1983) 339

In principle: in the presence of several species, isosteric heat may be calculated for each species: advantage over direct calorimetry! Same for isotherm, etc… Separation into several contributions!

- the isostere p = p (T) at constant A - the isotherm A = A (p) at constant T - the isobar A = A (T) at constant p - the isochore at constant V

Two cases: - Ideal adsorption (Langmuir model) - non-ideal (UNILAN, Temkin model)

Ideal (Langmuir) model: sites all alike and non-interacting Langmuir equation: A / AM = θ = K(T) p/[1 + K(T) p] K(T) = exp [ΔS°/R] exp [-ΔH°/RT] (van’t Hoff equation)

IRS provides a priori indications on the ideal nature of the adsorption Related IR band is expected: - to be narrow - to have a Lorentzian shape - not to shift with coverage. Definite evidence comes from a constant heat of adsorption, as measured independently.

Identity among sites is provided by the structure in some cases. Requirements for non-interaction among sites: - the solid constitutes an insulator matrix - a low density of sites

Examples of ideal adsorbing systems: - Boron atoms at the surface of PSi - isolated silanols on amorphous silica (Aerosil, MCM- 41, etc. ) - carboxylic groups on functionalized silica - cationic sites on zeolites

- Boron atoms at the surface of PSi - isolated silanols on amorphous silica (Aerosil, MCM- 41, etc. ) - carboxylic groups on functionalized silica - cationic sites on zeolites

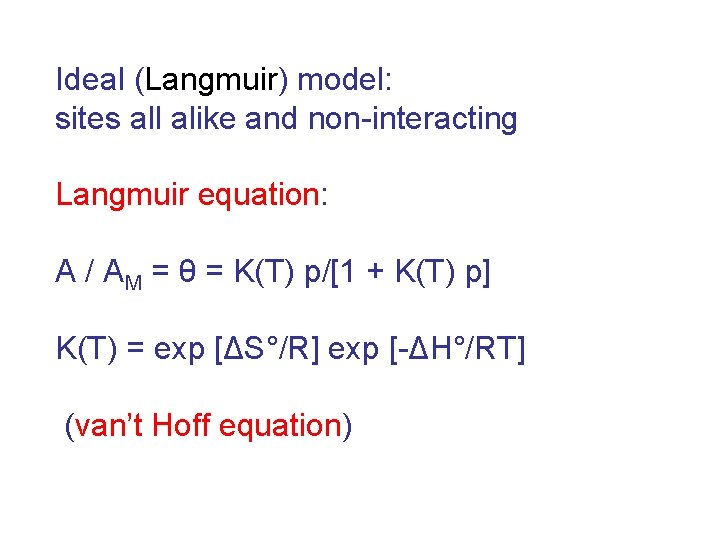
Mechanism of interaction of NO 2 with PSi (hole injection)

Each adsorbed molecule injects a carrier (hole) the background of IR spectrum rises increase in volume concentration of carriers (Drude formula) adsorbed amount isotherm

Spectra at increasing equilibrium pressures of NO 2
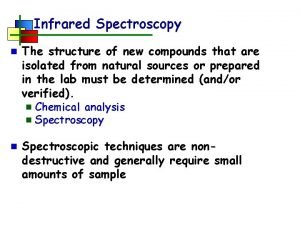
![DATA FOLLOW THE LANGMUIR MODEL 1 REGENERATED CARRIERS B 3 X 1019 atomscm DATA FOLLOW THE LANGMUIR MODEL 1. REGENERATED CARRIERS ≈ [B] (3 X 1019 atoms/cm](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-80.jpg)
DATA FOLLOW THE LANGMUIR MODEL 1. REGENERATED CARRIERS ≈ [B] (3 X 1019 atoms/cm 3) almost all carriers have been reactivated 2. Carriers increase describable by a REVERSIBLE CHEMISORPTION PROCESS 3. ADSORPTION ISOTHERM applicable

Sites for NO 2 adsorption (surface B atoms): • isolated • non interacting because of the low concentration of B atoms in the pristine sample Langmuir conditions

- Boron atoms at the surface of PSi - isolated silanols on amorphous silica (Aerosil, MCM- 41, etc. ) - carboxylic groups on functionalized silica - cationic sites on zeolites

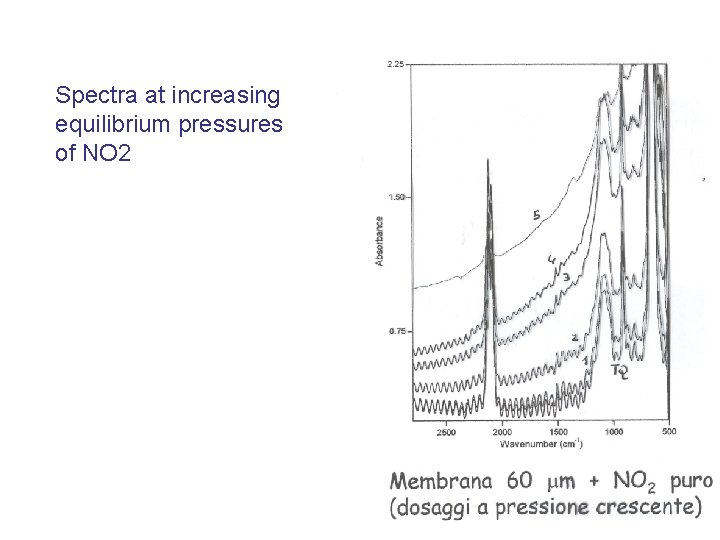
O-H band at 3750 cm-1: - very thin, all sites equivalent - low density (ca. 1 OH/100 Å2) Methylcyclohexene on Silica (B. Onida, M. Allian, E. Borello, P. Ugliengo, E. Garrone Langmuir 13 (1997) 5107) The coverage θ is calculated from the ratio between the actual intensity and the maximum intensity

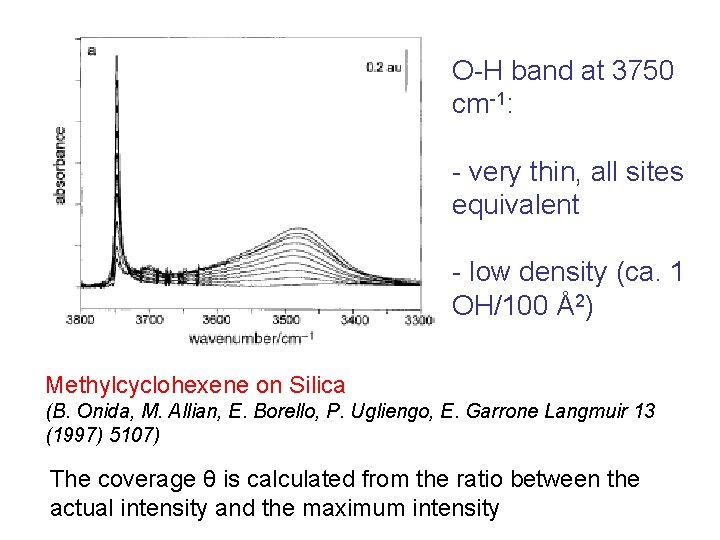
Langmuir isotherm ammonia Check of the isotherm and evaluation of K Absence of solvation

Deviation from ideality because of solvation Benzene, methylcyclohexene, acetone, etc. Check of the isotherm and evaluation of K

- Boron atoms at the surface of PSi - isolated silanols on amorphous silica (Aerosil, MCM- 41, etc. ) - carboxylic groups on functionalized silica - cationic sites on zeolites

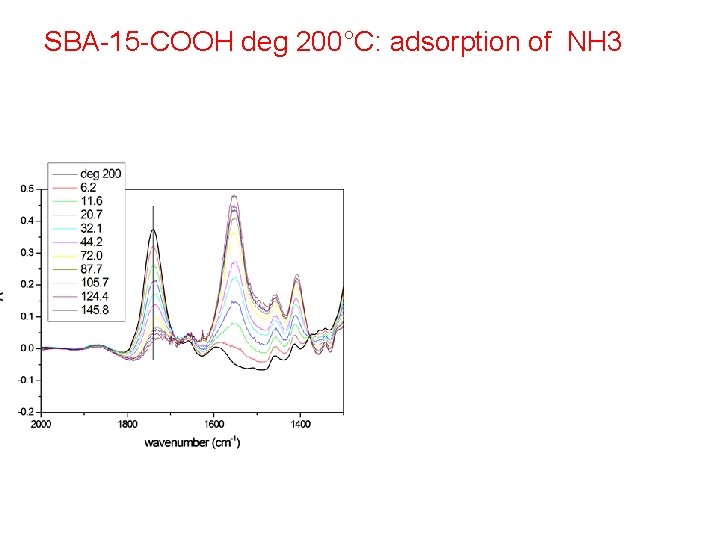
SBA-15 -COOH deg 200°C: adsorption of NH 3

Reversible formation of ammonium species: R-COOH + NH 3(g) R-COO- + NH 4+ Carbonyl, ammonium and carboxylate modes all present in the IR spectrum! Equilibrium constant: K = θ/ [ (1 – θ) p] as in Langmuir adsorption

Check of the isotherm: equilibrium constant evaluated! Deviations because of solvation of ammonium species by molecular ammonia

- Boron atoms at the surface of PSi - isolated silanols on amorphous silica (Aerosil, MCM- 41, etc. ) - carboxylic groups on functionalized silica - cationic sites on zeolites

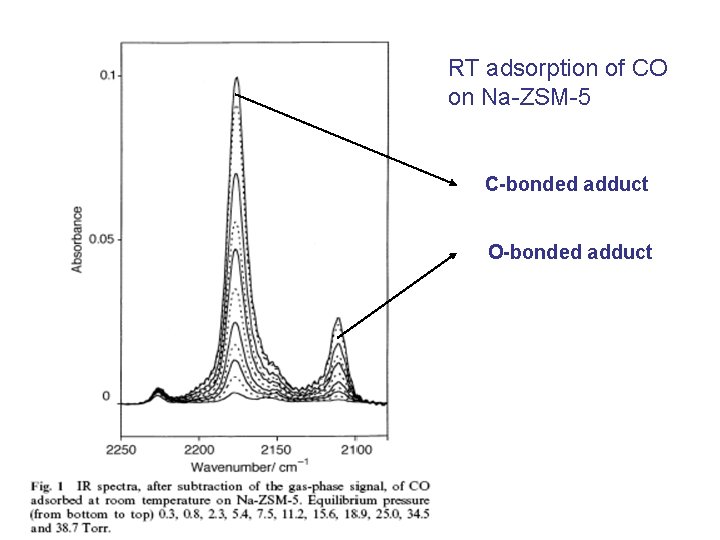
CO/Na-ZSM-5 blue: Na; green: C; red: O

RT adsorption of CO on Na-ZSM-5 C-bonded adduct O-bonded adduct

Three types of isotherms: - volumetric - calorimetric - optical (sum of the intensities of C-down and O-down bands) Optical isotherm as good as the others!

Non-ideal case: the Temkin (UNILAN) model
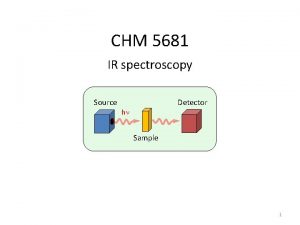
![Temkin isotherm Na K 1 ln1 K 2 p a variant of Temkin isotherm: Na = K 1 ln[1 + K 2 p] a variant of](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-95.jpg)
Temkin isotherm: Na = K 1 ln[1 + K 2 p] a variant of the UNILAN model: rectangular distribution of energies between two values E 1 and E 2, with E 2 –E 1 = 2 s. Kh = equilibrium constant for E = (E 1 + E 2) / 2. The Temkin isotherm generally assumed for structural heterogeneity: valid also for induced heterogeneity in a regular array of adsorption sites, all structurally equal.

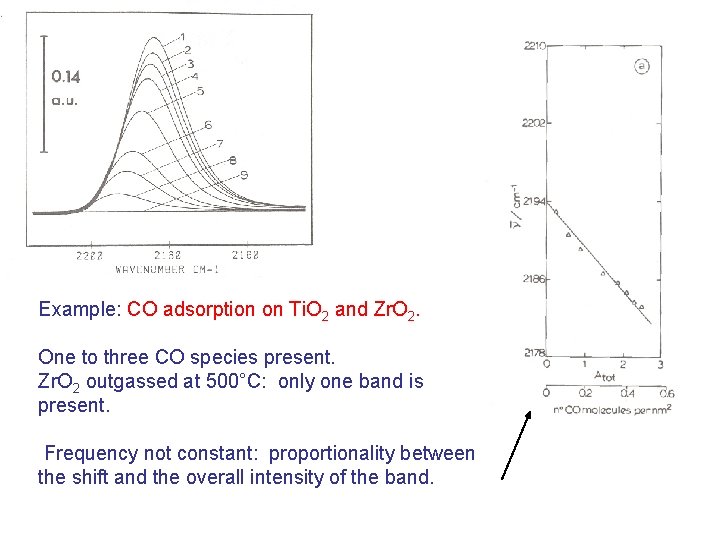
Example: CO adsorption on Ti. O 2 and Zr. O 2. One to three CO species present. Zr. O 2 outgassed at 500°C: only one band is present. Frequency not constant: proportionality between the shift and the overall intensity of the band.

The differential heat of adsorption, calorimetrically determined, has a nearly linear decrease (V. Bolis, B. Fubini, E. Garrone, C. Morterra, J. Chem. Soc. , Faraday Trans. 85 (1989) 1383)

- the isostere p = p(T) at constant A - the isotherm A = A(p) at constant T - the isobar A = A(T) at constant p - the isochore at constant V

Rather rare in the literature on oxides. Example: adsorption of CO at the (100) face of Mg. O followed at temperatures below 60 K (G. Spoto, E. Gribov, A. Damin, G. Ricchiardi, A. Zecchina, Surf. Sci. Lett. 540 (2003) 605. ). As the adsorption has ideal features, the elaboration is straightforward: the equation (see below) Ln [θ / p (1 – θ)] = ln [A / p (AM – A)] = ΔS° / R – ΔH° / RT is used dropping the constant term in pressure. Information on ΔS° is lost!

Spectroscopic determination of thermodynamic features of CO adsorption on metal particles (substantial electronic effects): Bianchi and associates Constancy of pressure is obtained by flowing the adsorbate gas in a dynamic system at a constant pressure. Temkin model adopted (A. Bourane, O. Dulaurent, D. Bianchi J. Catal. 196 (2000) 115)

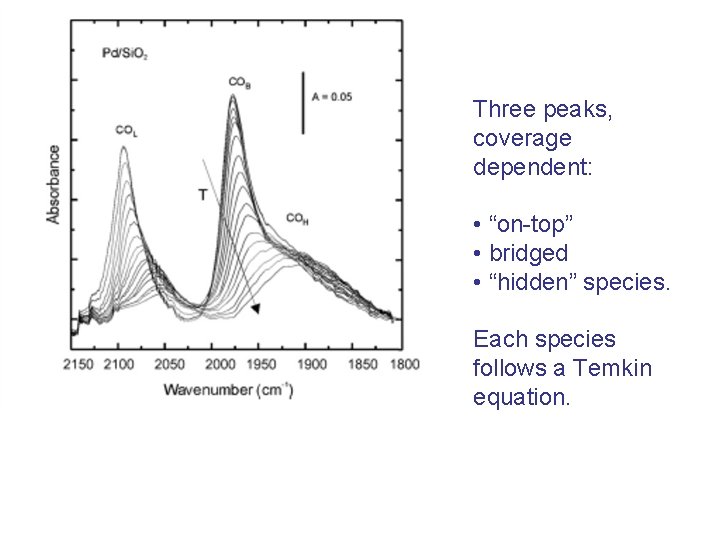
Three peaks, coverage dependent: • “on-top” • bridged • “hidden” species. Each species follows a Temkin equation.

Analysis of the behaviour of the intensities with temperature through the Temkin equation allows the determination of E 1 and E 2.

- the isostere p = p(T) at constant A - the isotherm A = A(p) at constant T - the isobar A = A(T) at constant p - the isochore at constant V

Constancy of volume: obtained by closing the cell after gas admission, then varying T and, consequently, p. Desorption counterbalanced by the increase in pressure Design of the cell: A. A. Tsiganenko Methods: E. Garrone, C. O. Arean (E. Garrone and C. Otero Areán, Chem. Soc. Rev. 34 (2005) 1) Acronym: VTIR

Three types of process followed so far: i) Langmuir-type adsorption on a cationic site (or hydroxyl species); ii) isomerism between two forms of adsorbate (e. g. carbonyl/isocarbonyl); iii) formation of dicarbonyls from monocarbonyls.

Langmuir-type adsorption on a cationic site (or hydroxyl species) isomerism between two forms of adsorbate (e. g. carbonyl/isocarbonyl); formation of dicarbonyls from monocarbonyls.

Suppose only one band is present, of absorbance A. The three quantities A, T and p are related by a mass balance equation, e. g. of the type: Nt = p Vg/RT + A/(ε S) if the gas phase has an ideal behaviour (Nt = total number of moles and Vg = volume of the cell). It is, however, convenient to treat T and p as independent variables, and to study the function A = A(T, p).

Van’t Hoff equation, under the assumption of entropy and enthalpy of adsorption constant with temperature: θ = A/AM = exp [ S°/R] exp[- H°/RT] p / {1 + exp [ S°/R]exp[- H°/RT] p} AM = a parameter to be determined
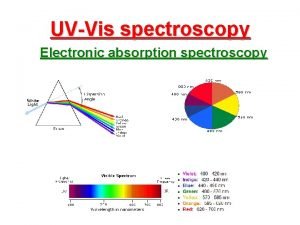
![More conveniently ln θ p 1 θ ln A p More conveniently: ln [θ / p (1 – θ)] = ln [A / p](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-109.jpg)
More conveniently: ln [θ / p (1 – θ)] = ln [A / p (AM – A)] = ΔS° / R – ΔH° / RT At very low coverages it results: Ln [A / T)] = const – ΔH° / RT does not require AM !

Two examples: CO on protonic zeolites H 2 on cationic zeolites

Two examples: CO on protonic zeolites H 2 on cationic zeolites

Coverage measured directly from the ratio of intensities HY zeolite, interaction with CO

Results: ΔH° = -25. 6 k. J/mol and ΔS° = -161 J mol-1 K-1 Excellent agreement with the calorimetric value for H-ZSM-5 of - 27 k. J/mol (more acidic!) (S. Savitz, A. L. Myers and J. R. Gorte J. Phys. Chem. B 103 (1999) 3687)

Two examples: CO on protonic zeolites H 2 on cationic zeolites

VTIR spectra around 100 K of dihydrogen on Na. FER Increasing T H 0 = - 6. 0 k. J/mol S 0 = -78 J/(mol K)

Result not readily obtained in other ways! Low T calorimetry is difficult, volumetric isosteric methods as alternative The study of H 2 adsorption on several zeolitic systems has shown that ΔS 0 values are correlated to the corresponding ΔH 0 values. Compensation effect. Result relevant in storage problem

Limiting value for ΔS°, corresponding to loss of translational modes (E. Garrone, B. Bonelli and C. Otero Areán Chem. Phys. Letters 456 (2008) 68)

Langmuir-type adsorption on a cationic site (or hydroxyl species); isomerism between two forms of adsorbate (e. g. carbonyl/isocarbonyl); formation of dicarbonyls from monocarbonyls.

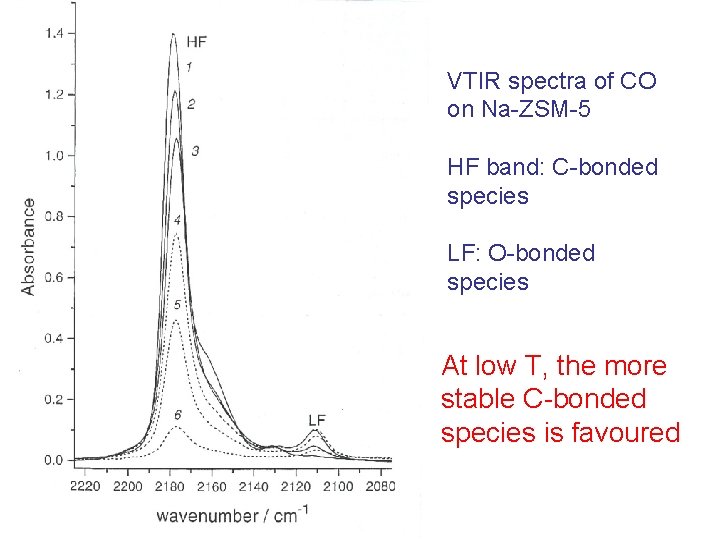
RT adsorption of CO on Na-ZSM-5 C-bonded adduct O-bonded adduct At relatively high T, both species are favoured, regardless their energy!

VTIR spectra of CO on Na-ZSM-5 HF band: C-bonded species LF: O-bonded species At low T, the more stable C-bonded species is favoured

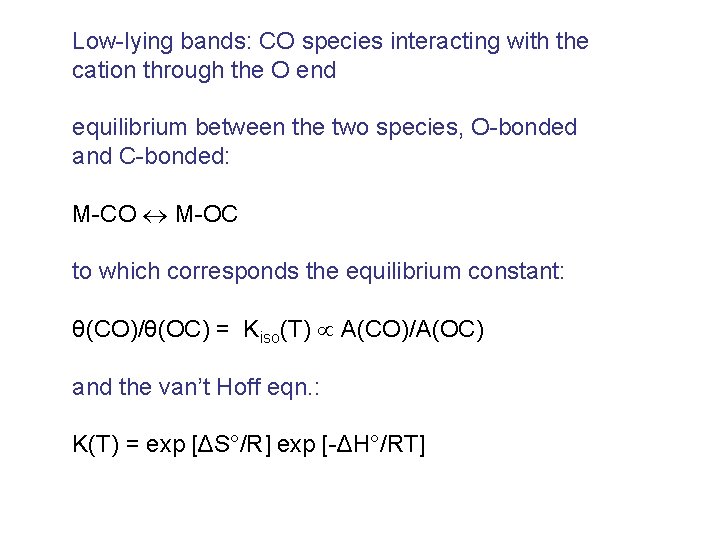
Low-lying bands: CO species interacting with the cation through the O end equilibrium between the two species, O-bonded and C-bonded: M-CO M-OC to which corresponds the equilibrium constant: θ(CO)/θ(OC) = Kiso(T) A(CO)/A(OC) and the van’t Hoff eqn. : K(T) = exp [ΔS°/R] exp [-ΔH°/RT]

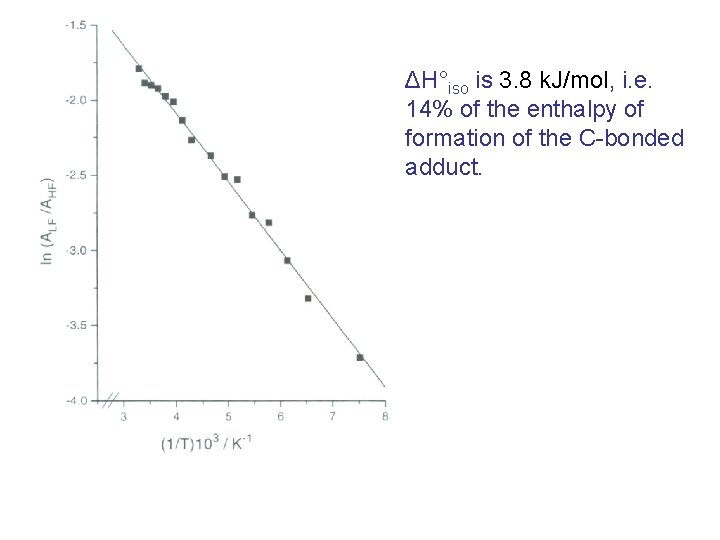
ΔH°iso is 3. 8 k. J/mol, i. e. 14% of the enthalpy of formation of the C-bonded adduct.

Langmuir-type adsorption on a cationic site (or hydroxyl species); isomerism between two forms of adsorbate (e. g. carbonyl/isocarbonyl); formation of dicarbonyls from monocarbonyls.

Several cations in zeolites may coordinate more than one CO molecule. Example: CO adsorbed on Ca(Na)Y Up to tri-carbonyls formed with increasing coverage at 77 K (K. I. Hadjiivanov, H. Knozinger Chem. Phys. Lett. 303 (1999) 513)

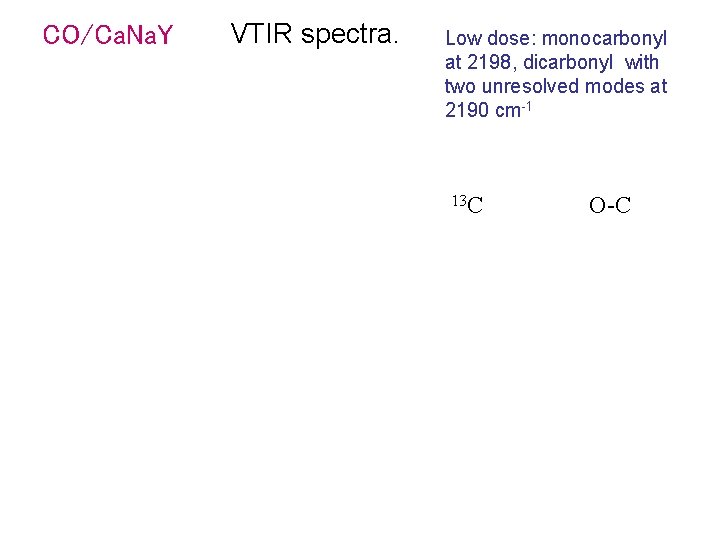
CO/Ca. Na. Y VTIR spectra. Low dose: monocarbonyl at 2198, dicarbonyl with two unresolved modes at 2190 cm-1 13 C O-C

IR spectra at variable temperature concerning the adsorption of CO at variable temperature on Sr. Y

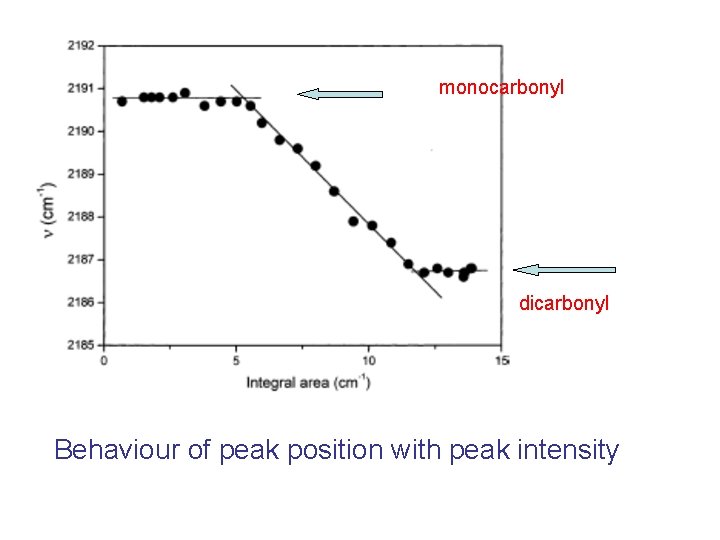
One major band shifting with decreasing temperature (i. e. increasing coverage) from 2191 cm-1 to 2187 cm-1. - monocarbonyl Sr(CO) 2191 cm-1 - dicarbonyl Sr(CO)2, two unresolved modes at 2187 cm-1 One minor band shifting from 2098 to 2095 cm-1 (O-bonded complexes) (E. Garrone, B. Bonelli, A. A. Tsiganenko, M. Rodriguez Delgado, G. Turnes Palomino, O. V. Manoilova, C. Otero Areán J. Phys. Chem. B 107 (2003) 2537)

monocarbonyl dicarbonyl Behaviour of peak position with peak intensity

Opposite ends of the diagram: regions where the monocarbonyl or the dicarbonyl predominate. This allows, through the use of θ = A/AM = exp [ΔS°/R] exp[-ΔH°/RT] p/{1 + exp [ΔS°/R] exp[-ΔH°/RT] p}, to measure the enthalpy changes of: - monocarbonyl formation (adsorption on the naked cation) - dicarbonyl formation (coordination of a second CO molecule).
![Use of θCOθOC KisoT ACOAOC KT exp ΔSR exp ΔHRT allows the Use of: θ(CO)/θ(OC) = Kiso(T) A(CO)/A(OC) K(T) = exp [ΔS°/R] exp [-ΔH°/RT] allows the](https://slidetodoc.com/presentation_image/1936f1ac1b1c7a9a07cec72464aa748e/image-130.jpg)
Use of: θ(CO)/θ(OC) = Kiso(T) A(CO)/A(OC) K(T) = exp [ΔS°/R] exp [-ΔH°/RT] allows the evaluation of the enthalpy changes related to the isomerisms Sr(CO)++ Sr(OC)++ Sr(CO)++2 Sr(CO, OC)++

Enthalpies of formation of the various CO adducts formed with Sr. Y zeolite Fairly complete energetic characterisation of the possible adducts!

Conclusions Frequency readily measured: quantitative correlations possible with either, other IR features or quantities independently measured Intensity troublesome: i) validity of LBL not always assured; ii) ε known with poor accuracy (band intensity and adsorbed amounts not measured simultaneously!)

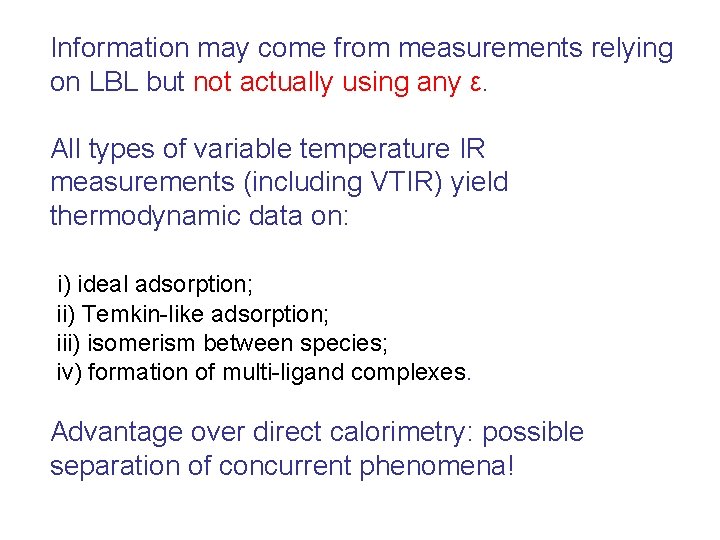
Information may come from measurements relying on LBL but not actually using any ε. All types of variable temperature IR measurements (including VTIR) yield thermodynamic data on: i) ideal adsorption; ii) Temkin-like adsorption; iii) isomerism between species; iv) formation of multi-ligand complexes. Advantage over direct calorimetry: possible separation of concurrent phenomena!

Thanks for your attention
 Economic aspects of applied plant anatomy
Economic aspects of applied plant anatomy Quantitative chemistry grade 11
Quantitative chemistry grade 11 What is spectroscopy?
What is spectroscopy? Mossbauer spectroscopy
Mossbauer spectroscopy Photoelectron spectroscopy pogil
Photoelectron spectroscopy pogil Uv spectra of dienes
Uv spectra of dienes Atomic emission spectroscopy lecture notes
Atomic emission spectroscopy lecture notes Beryllium pes spectrum
Beryllium pes spectrum Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy Principle of fluorescence spectroscopy
Principle of fluorescence spectroscopy![Cat] Cat]](data:image/svg+xml,%3Csvg%20xmlns=%22http://www.w3.org/2000/svg%22%20viewBox=%220%200%20200%20200%22%3E%3C/svg%3E) Cat]
Cat] Spectroscopy
Spectroscopy Ir spectroscopy instrumentation
Ir spectroscopy instrumentation How is beer-lambert law used in spectroscopy
How is beer-lambert law used in spectroscopy Isms spectroscopy
Isms spectroscopy Selection rule for raman spectroscopy
Selection rule for raman spectroscopy Gross selection rules
Gross selection rules Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Characterzation def
Characterzation def Mossbauer spectroscopy
Mossbauer spectroscopy Selection rules for raman spectroscopy
Selection rules for raman spectroscopy Spectroscopy
Spectroscopy Spatially resolved acoustic spectroscopy
Spatially resolved acoustic spectroscopy Nebulizer in aas
Nebulizer in aas Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Principles of fluorescence spectroscopy
Principles of fluorescence spectroscopy Difference between ir and raman spectroscopy
Difference between ir and raman spectroscopy Ir spectra chart
Ir spectra chart Dispersive ir spectroscopy
Dispersive ir spectroscopy Infrared spectroscopy theory
Infrared spectroscopy theory Selection rule
Selection rule Applications of uv and visible spectroscopy
Applications of uv and visible spectroscopy Selection rule for electronic transition
Selection rule for electronic transition Application of atomic emission spectroscopy
Application of atomic emission spectroscopy Raman spectroscopy disadvantages
Raman spectroscopy disadvantages Stretching and bending vibrations in ir spectroscopy
Stretching and bending vibrations in ir spectroscopy Principle of mossbauer spectroscopy
Principle of mossbauer spectroscopy Spectrochemical series of ligands
Spectrochemical series of ligands Auxochrome in uv spectroscopy
Auxochrome in uv spectroscopy Mossbauer spectroscopy
Mossbauer spectroscopy Reflectance spectroscopy
Reflectance spectroscopy Fluorescence spectroscopy - ppt
Fluorescence spectroscopy - ppt Advantages of aas over fes
Advantages of aas over fes Ionization atomic absorption spectrophotomery
Ionization atomic absorption spectrophotomery Nir spectroscopy instrumentation
Nir spectroscopy instrumentation Ir sample preparation
Ir sample preparation Ir spectroscopy
Ir spectroscopy Vernier spectral analysis
Vernier spectral analysis Spectroscopy equations
Spectroscopy equations Gas separation
Gas separation Erzeng
Erzeng Rotational spectral lines
Rotational spectral lines Introduction to spectrophotometry
Introduction to spectrophotometry Props wikipedia
Props wikipedia Photoluminescence spectroscopy
Photoluminescence spectroscopy Ir spectroscopy
Ir spectroscopy Slit spectroscopy
Slit spectroscopy International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Chromophore examples
Chromophore examples Advantages and disadvantages of spectroscopy
Advantages and disadvantages of spectroscopy Rotational spectroscopy notes
Rotational spectroscopy notes Nitro group ir peak
Nitro group ir peak Ir spectroscopy provides information about
Ir spectroscopy provides information about Interference of atomic absorption spectroscopy
Interference of atomic absorption spectroscopy Nmr spectroscopy diagram
Nmr spectroscopy diagram Raman spectroscopy basics
Raman spectroscopy basics Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Microwave spectroscopy definition
Microwave spectroscopy definition Ir spectroscopy
Ir spectroscopy Mass spec principle
Mass spec principle Rotational spectroscopy
Rotational spectroscopy Beer lambert
Beer lambert Lambert's law definition
Lambert's law definition Spectroscopy
Spectroscopy Uv visible spectroscopy introduction
Uv visible spectroscopy introduction Atomic emission spectroscopy principle
Atomic emission spectroscopy principle Inline raman spectroscopy
Inline raman spectroscopy Aas adalah
Aas adalah Ir spectroscopy
Ir spectroscopy Interferogram
Interferogram Mass spectrometry problem set
Mass spectrometry problem set What is spectroscopy
What is spectroscopy Hooke's law in ir spectroscopy
Hooke's law in ir spectroscopy Upcdms
Upcdms Ir spectroscopy
Ir spectroscopy Draw a photoelectron spectrum for aluminum
Draw a photoelectron spectrum for aluminum Uv vis spectroscopy in pharmaceutical analysis
Uv vis spectroscopy in pharmaceutical analysis Spectroscopy
Spectroscopy Xps instrument
Xps instrument Slit spectroscopy
Slit spectroscopy Photoelectron spectroscopy ap chemistry
Photoelectron spectroscopy ap chemistry Infrared spectroscopy ppt
Infrared spectroscopy ppt Atomic fluorescence spectroscopy principle
Atomic fluorescence spectroscopy principle Objectives of spectroscopy
Objectives of spectroscopy Application of fluorescence spectroscopy
Application of fluorescence spectroscopy Total consumption burner
Total consumption burner Flame aas
Flame aas Factors affecting chemical shift
Factors affecting chemical shift Gamma spectroscopy software
Gamma spectroscopy software Definition of ir spectroscopy
Definition of ir spectroscopy What are the different types of spectroscopy
What are the different types of spectroscopy P-diethylbenzene nmr
P-diethylbenzene nmr Advantages and disadvantages of spectroscopy
Advantages and disadvantages of spectroscopy Rotational spectroscopy
Rotational spectroscopy Mossbauer spectroscopy
Mossbauer spectroscopy Woodward fieser rules for polyenes
Woodward fieser rules for polyenes Absorbtion spectroscopy
Absorbtion spectroscopy Deep learning spectroscopy
Deep learning spectroscopy Advantages and disadvantages of spectroscopy
Advantages and disadvantages of spectroscopy Amine group ir spectrum
Amine group ir spectrum Why are raman and ir complementary
Why are raman and ir complementary Spectroscopy
Spectroscopy Advantages of flame photometry
Advantages of flame photometry Use of raman spectroscopy
Use of raman spectroscopy Dept nmr spectroscopy
Dept nmr spectroscopy Chirped pulse fourier transform microwave spectroscopy
Chirped pulse fourier transform microwave spectroscopy What is spectroscopy
What is spectroscopy Impedance definition
Impedance definition Principle of absorption
Principle of absorption Spectroscopy
Spectroscopy Laser diffraction spectroscopy
Laser diffraction spectroscopy Discourse aspects of interlanguage
Discourse aspects of interlanguage American dream concept
American dream concept Cultural aspects
Cultural aspects Symmetric key
Symmetric key 4 aspects of research
4 aspects of research Chapter 18 visual merchandising and display
Chapter 18 visual merchandising and display Triadic model pe
Triadic model pe Aspects of responsibility
Aspects of responsibility Psychosocial aspects of living with diabetes
Psychosocial aspects of living with diabetes Building and sustaining relationships in retailing
Building and sustaining relationships in retailing Technical element of drama
Technical element of drama Crucial aspects of preparing digital audio files
Crucial aspects of preparing digital audio files Legal aspects of doing business in canada
Legal aspects of doing business in canada Aspects of romanticism
Aspects of romanticism










![Cat] Cat]](https://slidetodoc.com/wp-content/uploads/2021/03/4140887_17f7687481329e2d488ef1872cadc836-300x225.jpg)