Mechanisms of organic reactions mirka rovenskalfmotol cuni cz


- Slides: 19

Mechanisms of organic reactions mirka. rovenska@lfmotol. cuni. cz

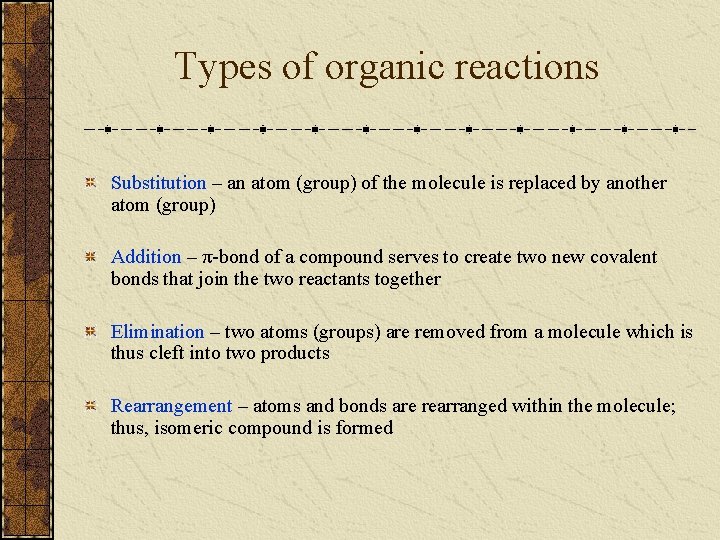
Types of organic reactions Substitution – an atom (group) of the molecule is replaced by another atom (group) Addition – π-bond of a compound serves to create two new covalent bonds that join the two reactants together Elimination – two atoms (groups) are removed from a molecule which is thus cleft into two products Rearrangement – atoms and bonds are rearranged within the molecule; thus, isomeric compound is formed

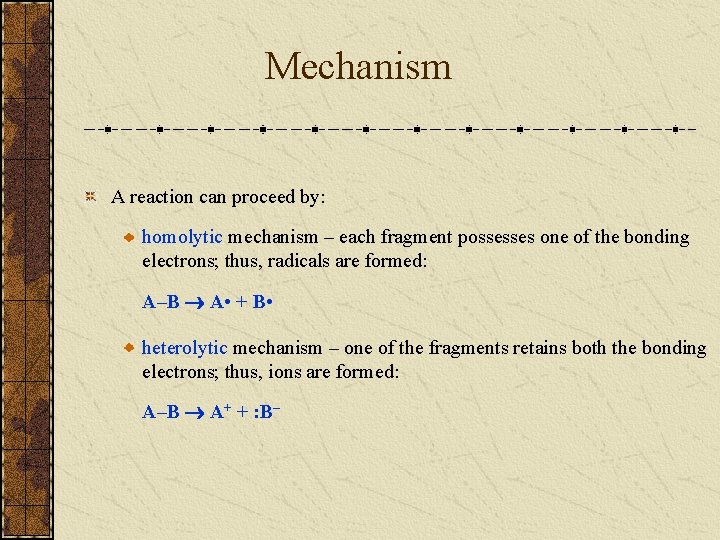
Mechanism A reaction can proceed by: homolytic mechanism – each fragment possesses one of the bonding electrons; thus, radicals are formed: A–B A • + B • heterolytic mechanism – one of the fragments retains both the bonding electrons; thus, ions are formed: A–B A+ + : B–

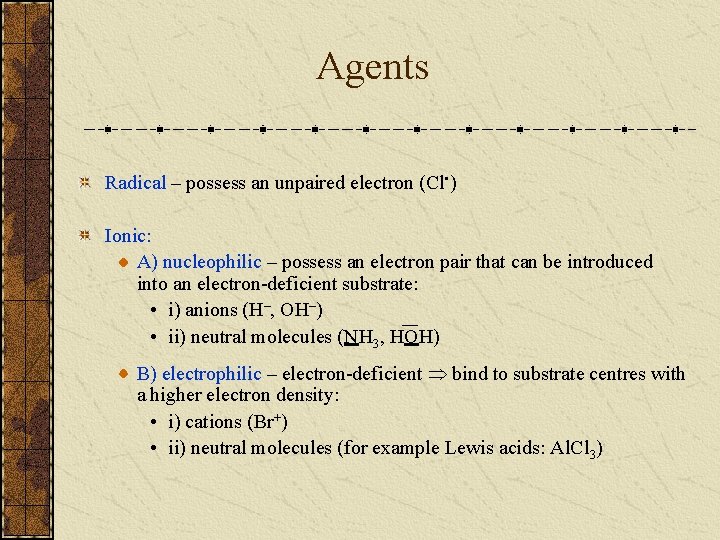
Agents Radical – possess an unpaired electron (Cl • ) Ionic: A) nucleophilic – possess an electron pair that can be introduced into an electron-deficient substrate: • i) anions (H–, OH–) • ii) neutral molecules (NH 3, HOH) B) electrophilic – electron-deficient bind to substrate centres with a higher electron density: • i) cations (Br+) • ii) neutral molecules (for example Lewis acids: Al. Cl 3)

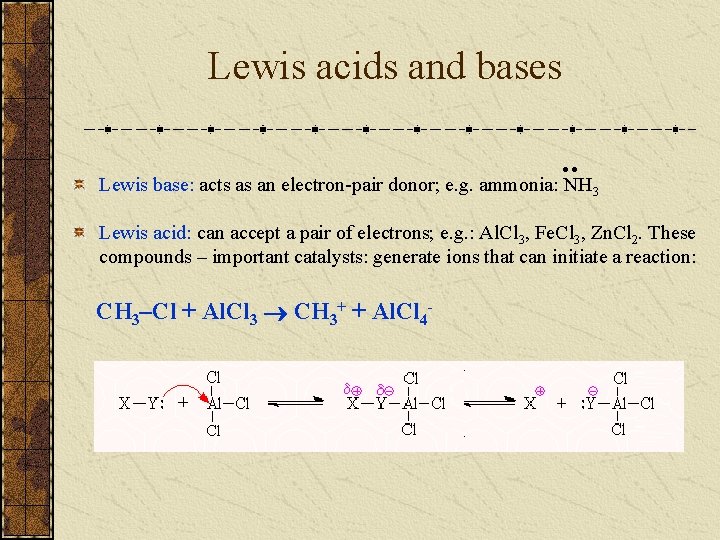
Lewis acids and bases • • Lewis base: acts as an electron-pair donor; e. g. ammonia: NH 3 Lewis acid: can accept a pair of electrons; e. g. : Al. Cl 3, Fe. Cl 3, Zn. Cl 2. These compounds – important catalysts: generate ions that can initiate a reaction: CH 3–Cl + Al. Cl 3 CH 3+ + Al. Cl 4 -

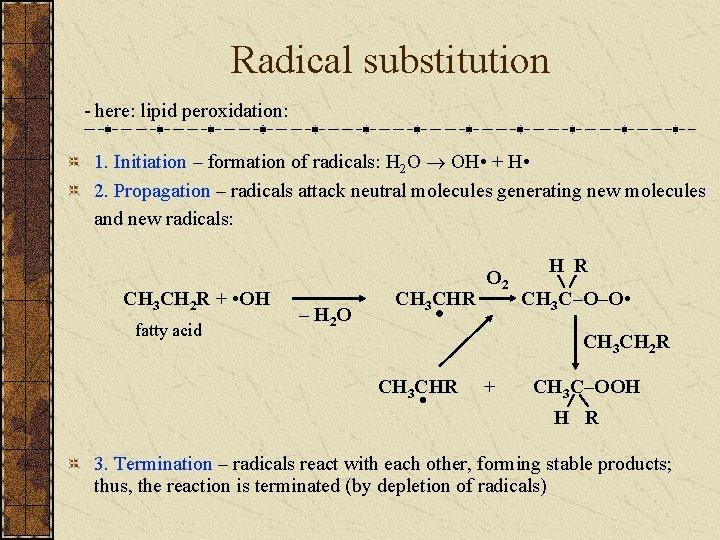
Radical substitution - here: lipid peroxidation: 1. Initiation – formation of radicals: H 2 O OH • + H • 2. Propagation – radicals attack neutral molecules generating new molecules and new radicals: CH 3 CH 2 R + • OH fatty acid – H 2 O CH 3 CHR O 2 • H R CH 3 C–O–O • CH 3 CH 2 R CH 3 CHR • + CH 3 C–OOH H R 3. Termination – radicals react with each other, forming stable products; thus, the reaction is terminated (by depletion of radicals)

Electrophilic substitution An electron-deficient agent reacts with an electron-rich substrate; the substrate retains the bonding electron pair, a cation (proton) is removed: R–X + E+ R–E + X+ Typical of aromatic hydrocarbons: chlorination nitration etc.

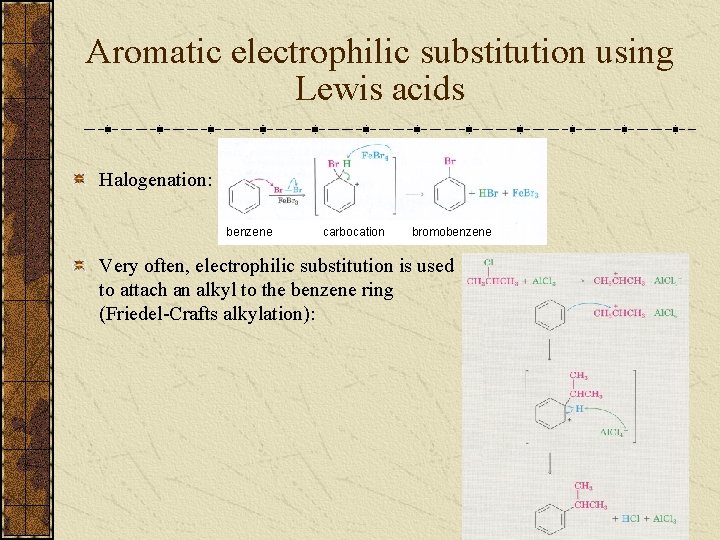
Aromatic electrophilic substitution using Lewis acids Halogenation: benzene carbocation bromobenzene Very often, electrophilic substitution is used to attach an alkyl to the benzene ring (Friedel-Crafts alkylation):

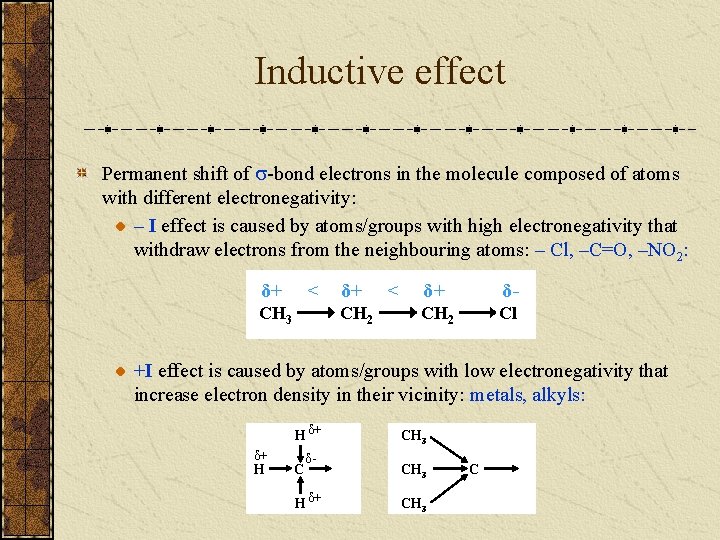
Inductive effect Permanent shift of -bond electrons in the molecule composed of atoms with different electronegativity: – I effect is caused by atoms/groups with high electronegativity that withdraw electrons from the neighbouring atoms: – Cl, –C=O, –NO 2: δ+ < CH 3 δ+ CH 2 < δ+ δ- CH 2 Cl +I effect is caused by atoms/groups with low electronegativity that increase electron density in their vicinity: metals, alkyls: H δ+ δ+ H C δ- H δ+ CH 3 C

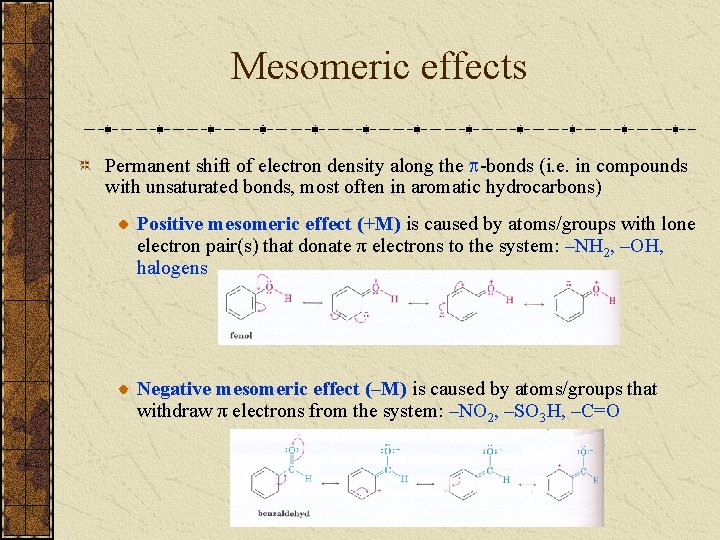
Mesomeric effects Permanent shift of electron density along the -bonds (i. e. in compounds with unsaturated bonds, most often in aromatic hydrocarbons) Positive mesomeric effect (+M) is caused by atoms/groups with lone electron pair(s) that donate π electrons to the system: –NH 2, –OH, halogens Negative mesomeric effect (–M) is caused by atoms/groups that withdraw π electrons from the system: –NO 2, –SO 3 H, –C=O

Activating/deactivating groups If inductive and mesomeric effects are contradictory, then the stronger one predominates Consequently, the group bound to the aromatic ring is: activating – donates electrons to the aromatic ring, thus facilitating the electrophilic substitution: • a) +M > – I… –OH, –NH 2 • b) only +I…alkyls deactivating – withdraws electrons from the aromatic ring, thus making the electrophilic substitution slower: • a) –M and –I… –C=O, –NO 2 • b) – I > +M…halogens

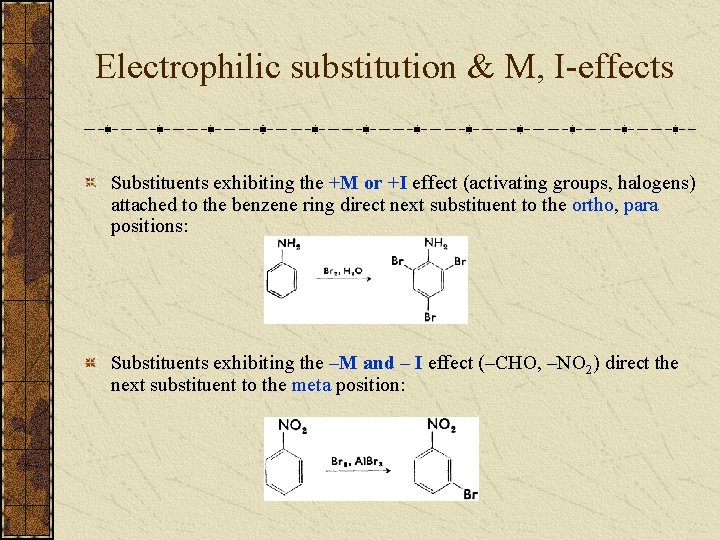
Electrophilic substitution & M, I-effects Substituents exhibiting the +M or +I effect (activating groups, halogens) attached to the benzene ring direct next substituent to the ortho, para positions: Substituents exhibiting the –M and – I effect (–CHO, –NO 2) direct the next substituent to the meta position:

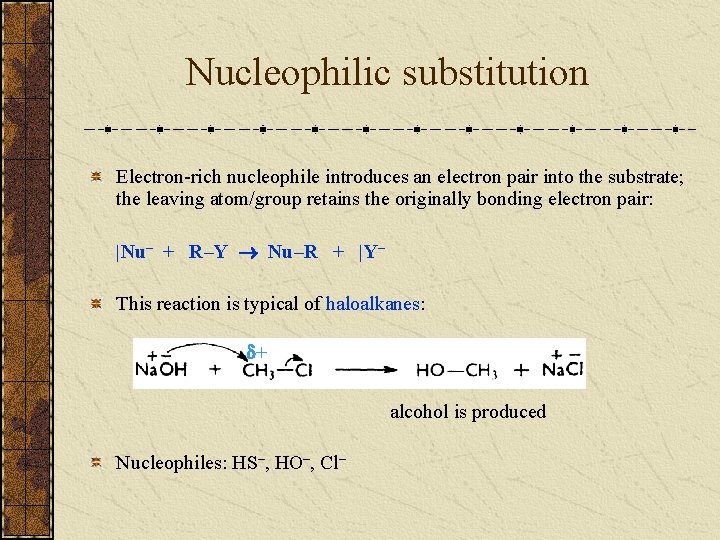
Nucleophilic substitution Electron-rich nucleophile introduces an electron pair into the substrate; the leaving atom/group retains the originally bonding electron pair: |Nu– + R–Y Nu–R + |Y– This reaction is typical of haloalkanes: + alcohol is produced Nucleophiles: HS–, HO–, Cl–

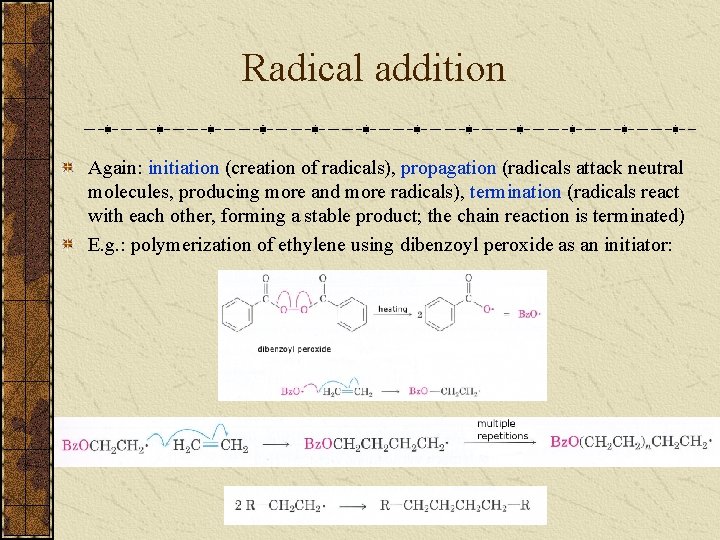
Radical addition Again: initiation (creation of radicals), propagation (radicals attack neutral molecules, producing more and more radicals), termination (radicals react with each other, forming a stable product; the chain reaction is terminated) E. g. : polymerization of ethylene using dibenzoyl peroxide as an initiator:

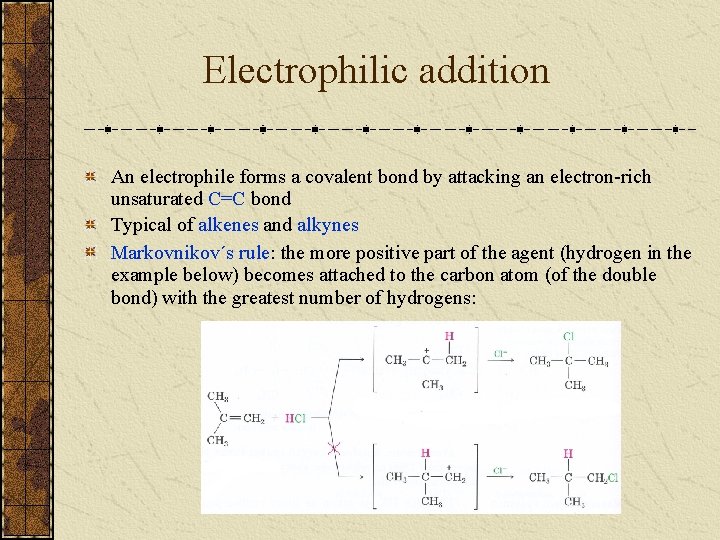
Electrophilic addition An electrophile forms a covalent bond by attacking an electron-rich unsaturated C=C bond Typical of alkenes and alkynes Markovnikov´s rule: the more positive part of the agent (hydrogen in the example below) becomes attached to the carbon atom (of the double bond) with the greatest number of hydrogens:

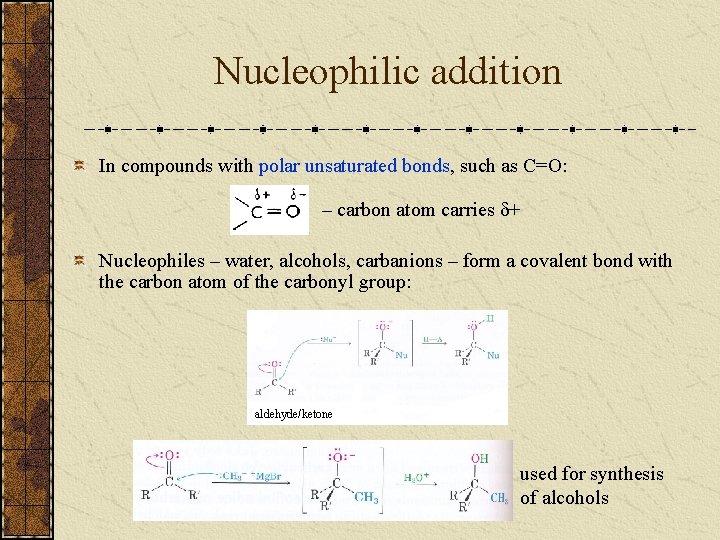
Nucleophilic addition In compounds with polar unsaturated bonds, such as C=O: – carbon atom carries + Nucleophiles – water, alcohols, carbanions – form a covalent bond with the carbon atom of the carbonyl group: aldehyde/ketone used for synthesis of alcohols

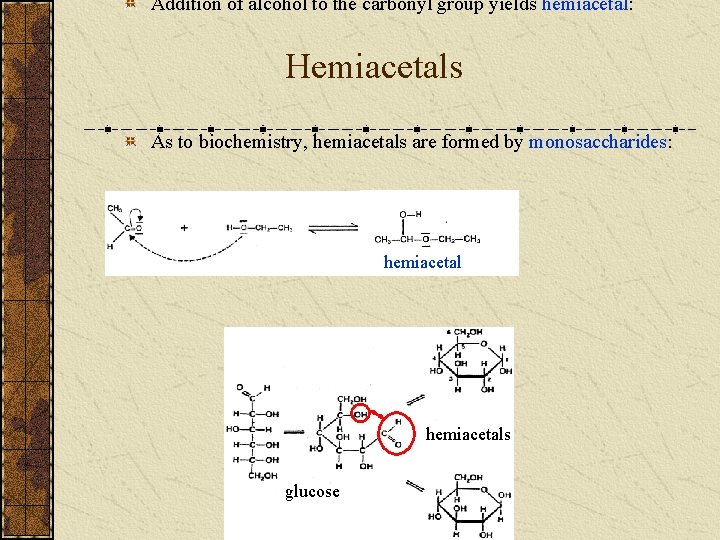
Addition of alcohol to the carbonyl group yields hemiacetal: Hemiacetals As to biochemistry, hemiacetals are formed by monosaccharides: hemiacetals glucose

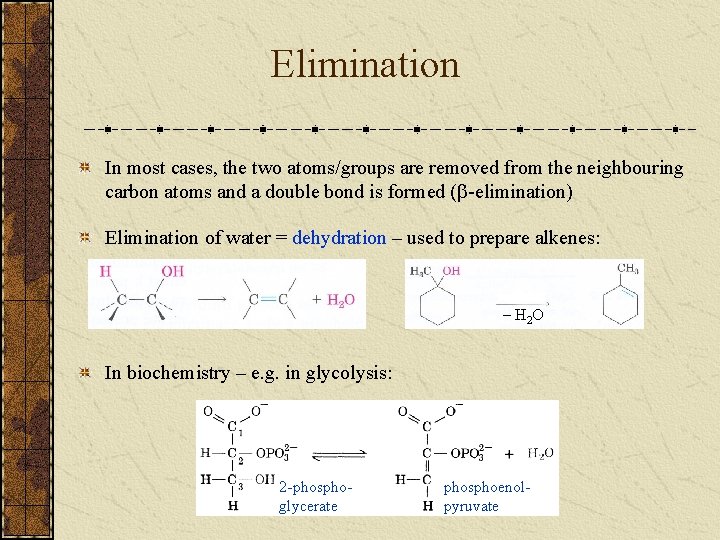
Elimination In most cases, the two atoms/groups are removed from the neighbouring carbon atoms and a double bond is formed ( -elimination) Elimination of water = dehydration – used to prepare alkenes: – H 2 O In biochemistry – e. g. in glycolysis: 2 -phosphoglycerate phosphoenolpyruvate

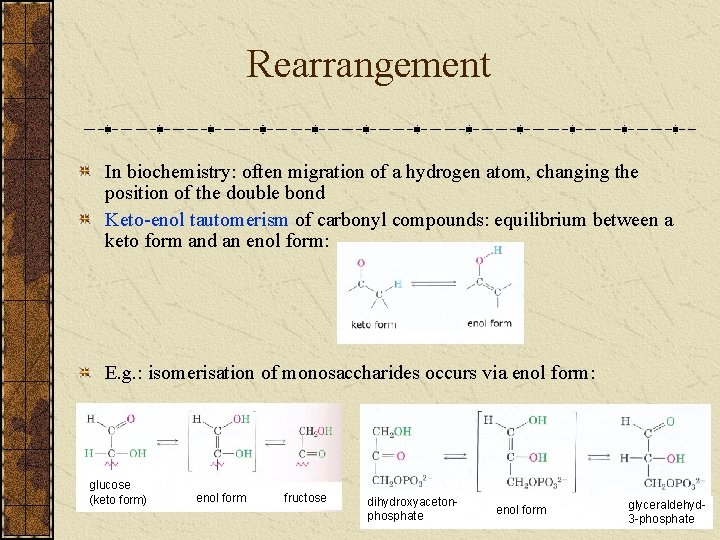
Rearrangement In biochemistry: often migration of a hydrogen atom, changing the position of the double bond Keto-enol tautomerism of carbonyl compounds: equilibrium between a keto form and an enol form: E. g. : isomerisation of monosaccharides occurs via enol form: glucose (keto form) enol form fructose dihydroxyacetonphosphate enol form glyceraldehyd 3 -phosphate
 Organic chemistry
Organic chemistry Mirka nisula
Mirka nisula Mirka moisio
Mirka moisio Mirka jalonen
Mirka jalonen Osnovna škola mirka pereša kapela
Osnovna škola mirka pereša kapela Reactions of organic compounds
Reactions of organic compounds Rehos cuni
Rehos cuni Charles university faculty of humanities
Charles university faculty of humanities Shoah
Shoah Cuni sap
Cuni sap Pez cuni
Pez cuni How to write half reactions
How to write half reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Ego defenses
Ego defenses Vlad hosu
Vlad hosu Example of regression defense mechanism
Example of regression defense mechanism Primary embedding mechanisms adalah
Primary embedding mechanisms adalah