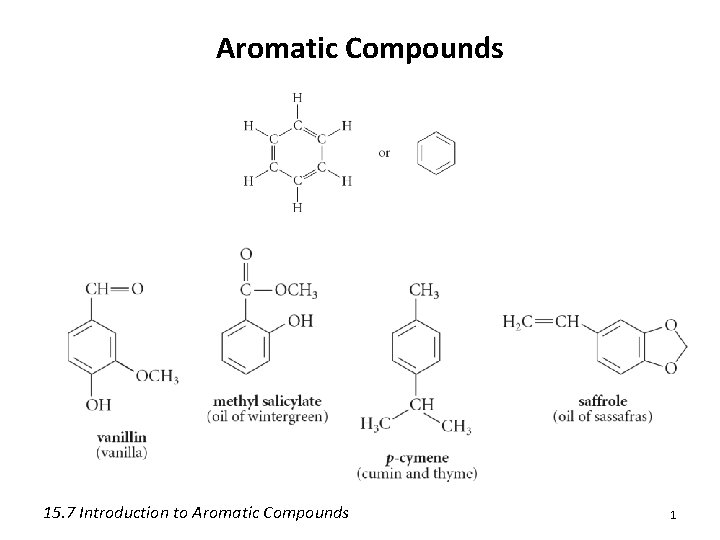
Aromatic Compounds 15 7 Introduction to Aromatic Compounds


- Slides: 29

Aromatic Compounds 15. 7 Introduction to Aromatic Compounds 1

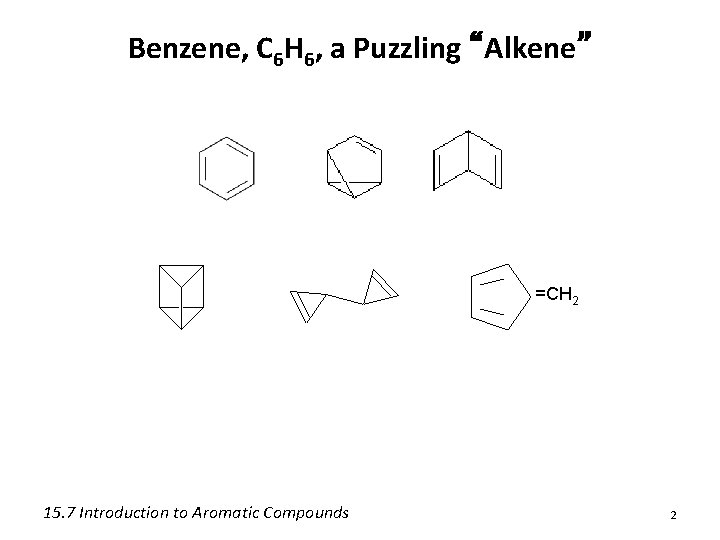
Benzene, C 6 H 6, a Puzzling “Alkene” =CH 2 15. 7 Introduction to Aromatic Compounds 2

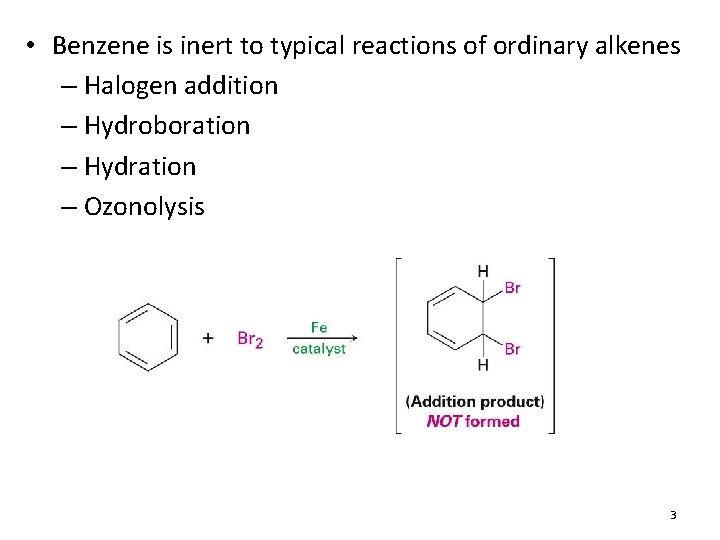
• Benzene is inert to typical reactions of ordinary alkenes – Halogen addition – Hydroboration – Hydration – Ozonolysis 3

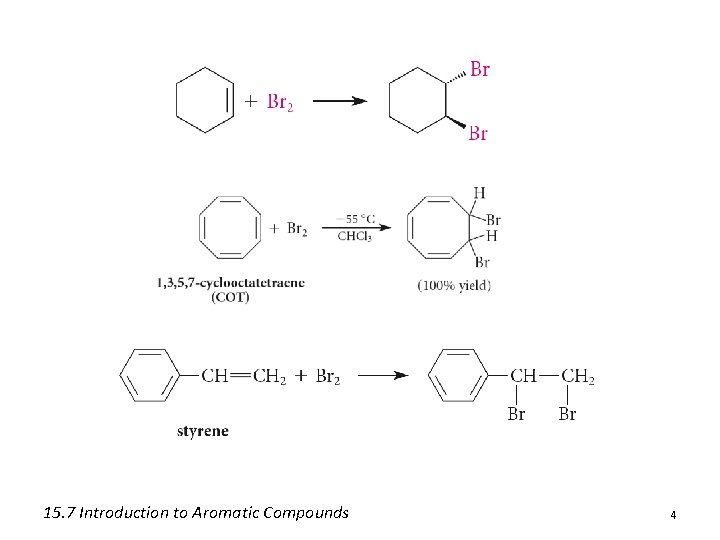
15. 7 Introduction to Aromatic Compounds 4

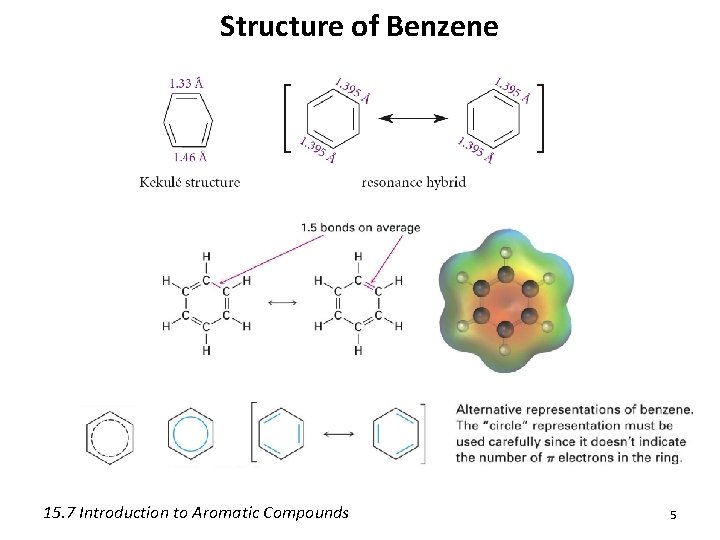
Structure of Benzene 15. 7 Introduction to Aromatic Compounds 5

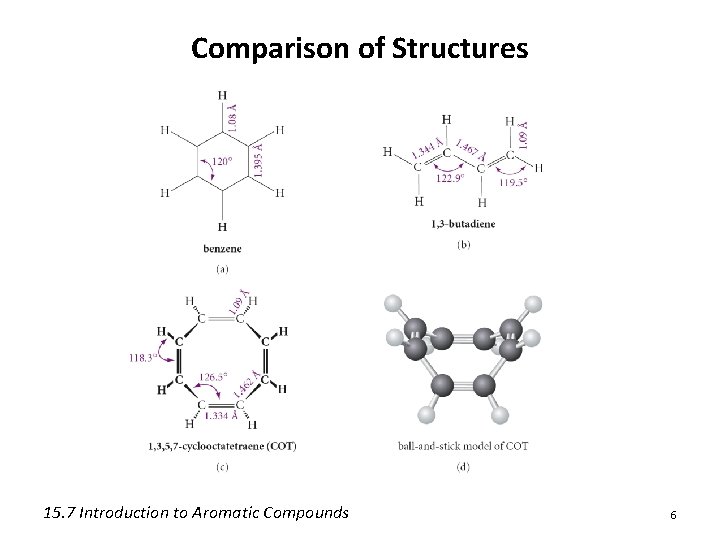
Comparison of Structures 15. 7 Introduction to Aromatic Compounds 6

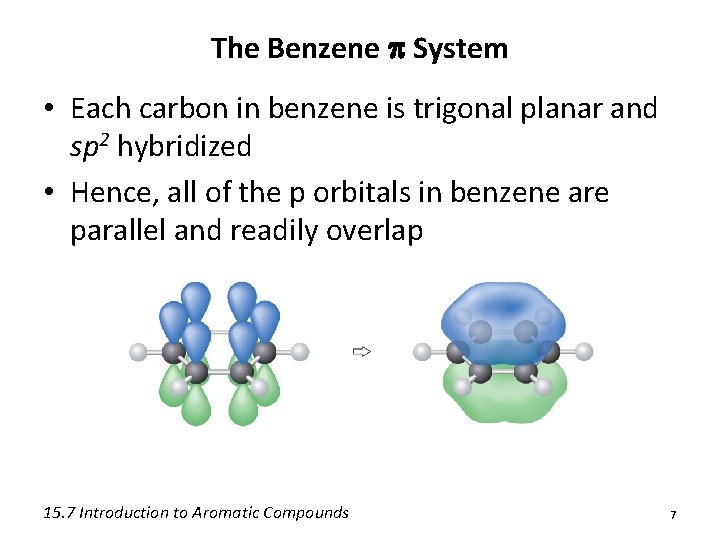
The Benzene p System • Each carbon in benzene is trigonal planar and sp 2 hybridized • Hence, all of the p orbitals in benzene are parallel and readily overlap 15. 7 Introduction to Aromatic Compounds 7

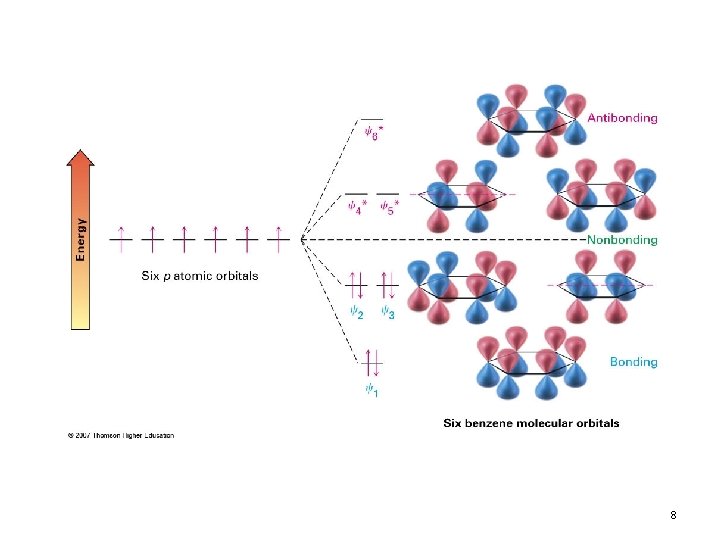
8

Stability of Benzene • Benzene: DH°f = 82. 93 k. J/mol – 13. 8 k. J/mol per CH group • COT: DH°f = 298. 0 k. J/mol – 37. 3 k. J/mol per CH group • Therefore, benzene is 23. 5 k. J/mol (37. 3 -13. 8) more stable than COT per every CH group • Empirical resonance energy of benzene – (23. 5 k. J/mol) x 6 CH groups = 141 k. J/mol – Estimate of energy by which benzene is stabilized by resonance 9

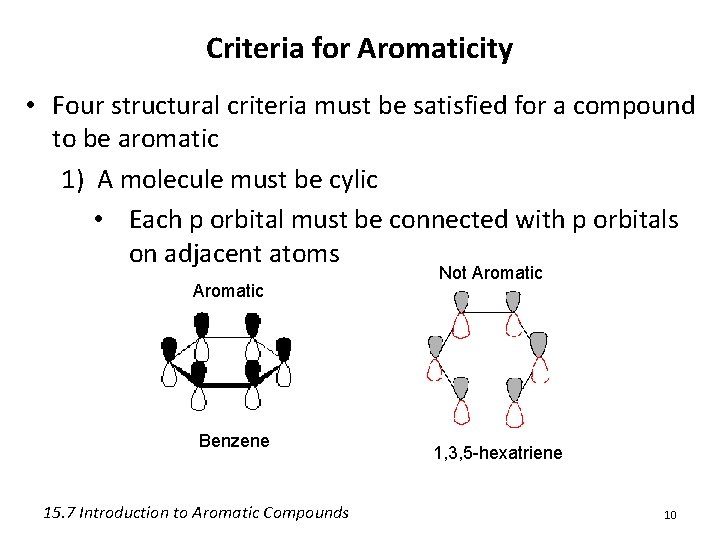
Criteria for Aromaticity • Four structural criteria must be satisfied for a compound to be aromatic 1) A molecule must be cylic • Each p orbital must be connected with p orbitals on adjacent atoms Aromatic Benzene 15. 7 Introduction to Aromatic Compounds Not Aromatic 1, 3, 5 -hexatriene 10

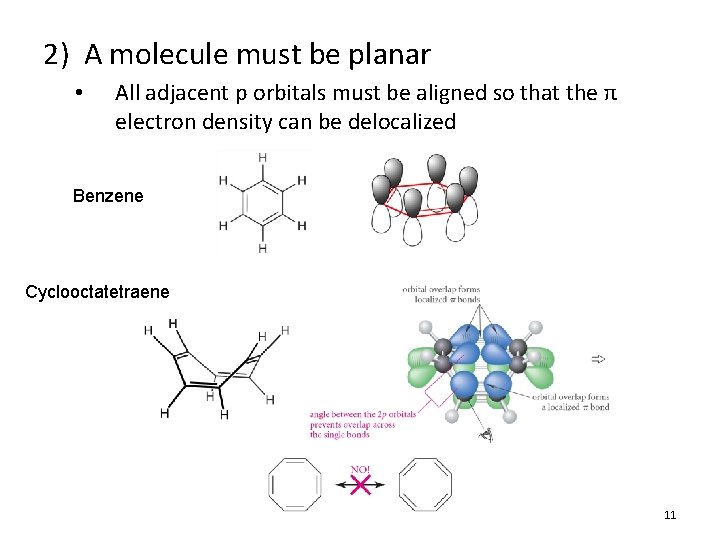
2) A molecule must be planar • All adjacent p orbitals must be aligned so that the π electron density can be delocalized Benzene Cyclooctatetraene 11

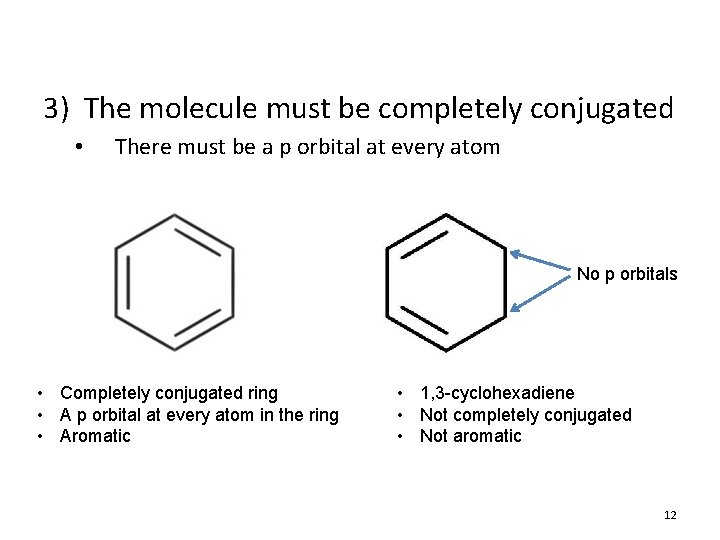
3) The molecule must be completely conjugated • There must be a p orbital at every atom No p orbitals • Completely conjugated ring • A p orbital at every atom in the ring • Aromatic • 1, 3 -cyclohexadiene • Not completely conjugated • Not aromatic 12

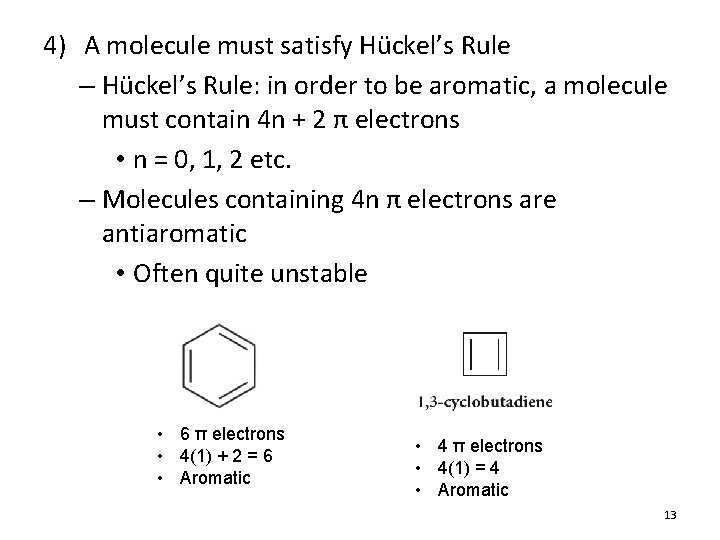
4) A molecule must satisfy Hückel’s Rule – Hückel’s Rule: in order to be aromatic, a molecule must contain 4 n + 2 π electrons • n = 0, 1, 2 etc. – Molecules containing 4 n π electrons are antiaromatic • Often quite unstable • 6 π electrons • 4(1) + 2 = 6 • Aromatic • 4 π electrons • 4(1) = 4 • Aromatic 13

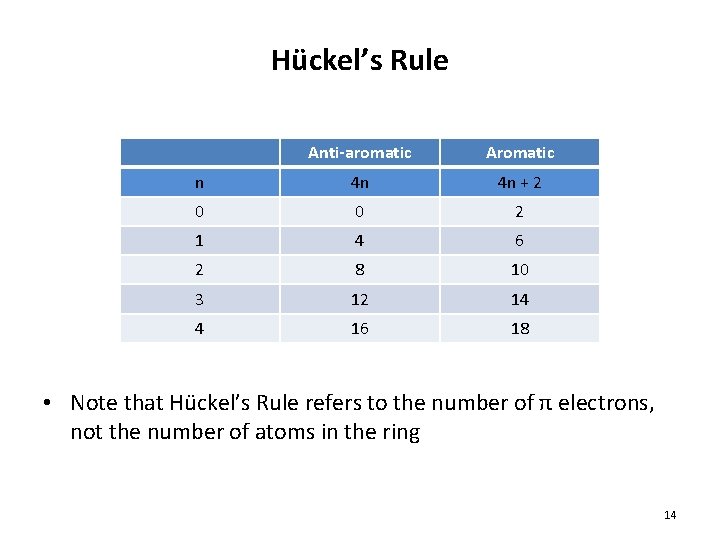
Hückel’s Rule Anti-aromatic Aromatic n 4 n 4 n + 2 0 0 2 1 4 6 2 8 10 3 12 14 4 16 18 • Note that Hückel’s Rule refers to the number of π electrons, not the number of atoms in the ring 14

Summary • Aromatic: cyclic, planar, completely conjugated compound with 4 n + 2 π electrons • Anti-aromatic: cyclic, planar, completely conjugated compound with 4 n π electrons • Non-aromatic: a compound that lacks one or more of the following requirements: being cyclic, planar, or completely conjugated. 15

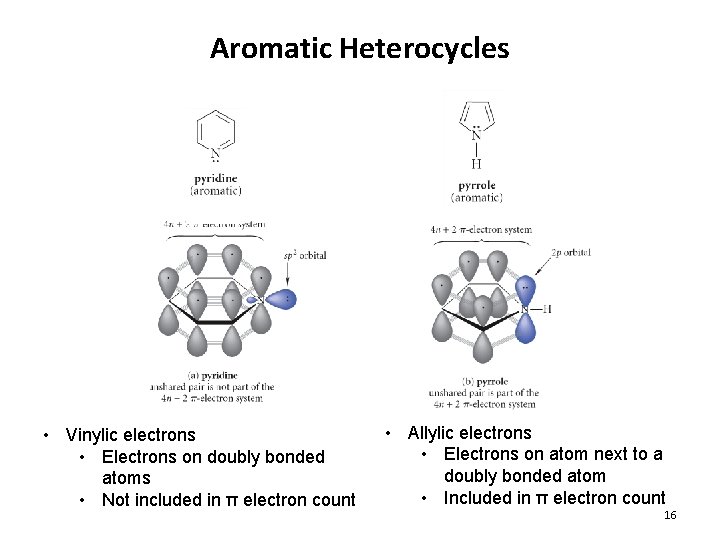
Aromatic Heterocycles • Vinylic electrons • Electrons on doubly bonded atoms • Not included in π electron count • Allylic electrons • Electrons on atom next to a doubly bonded atom • Included in π electron count 16

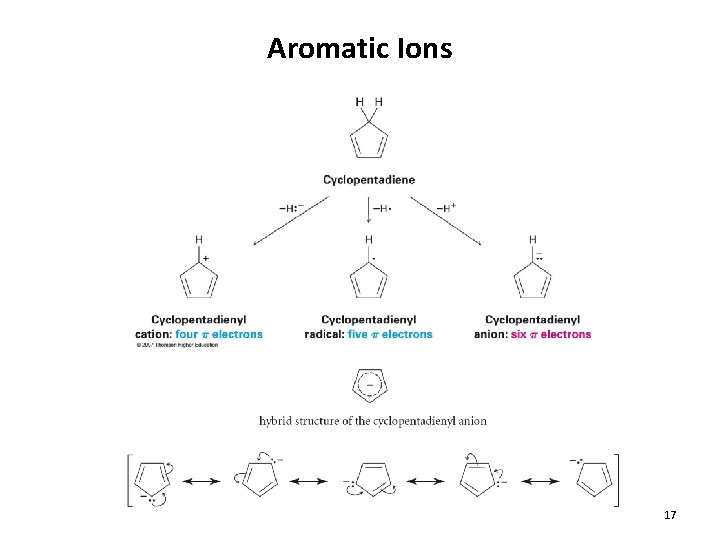
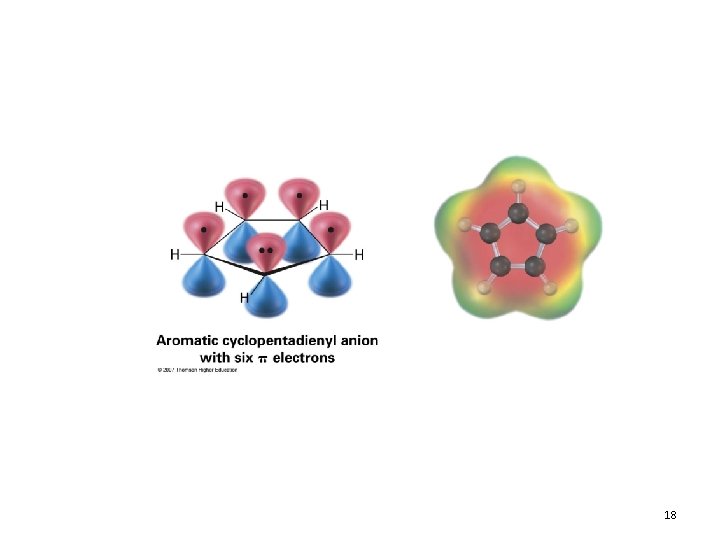
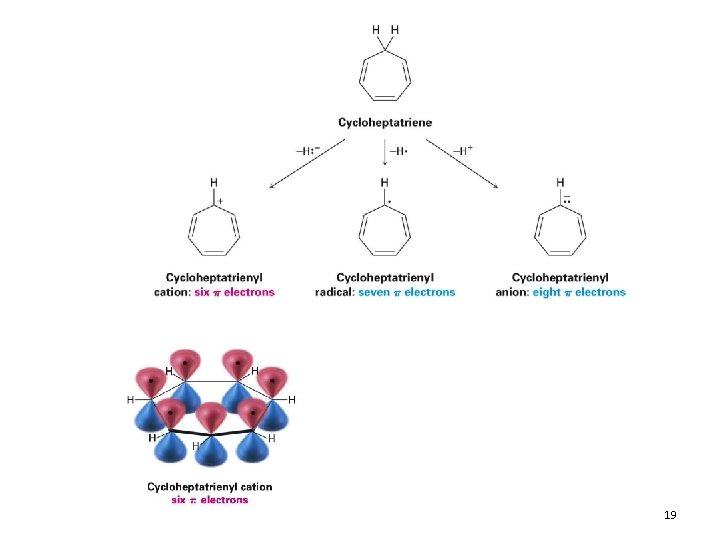
Aromatic Ions 17

18

19

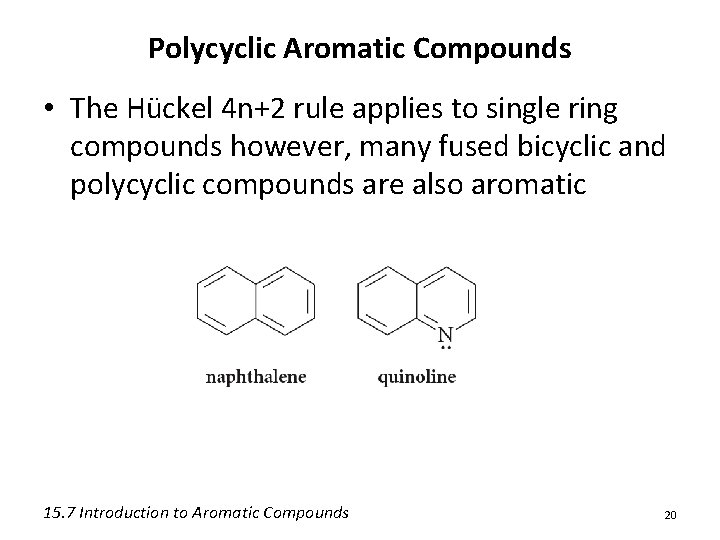
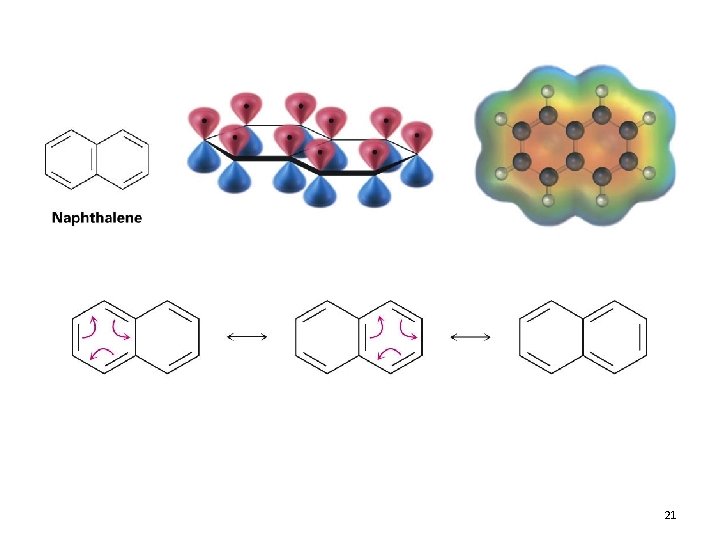
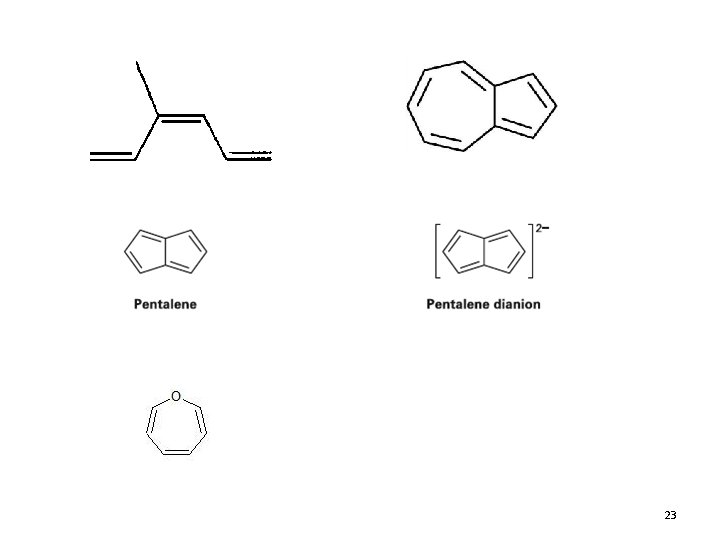
Polycyclic Aromatic Compounds • The Hückel 4 n+2 rule applies to single ring compounds however, many fused bicyclic and polycyclic compounds are also aromatic 15. 7 Introduction to Aromatic Compounds 20

21

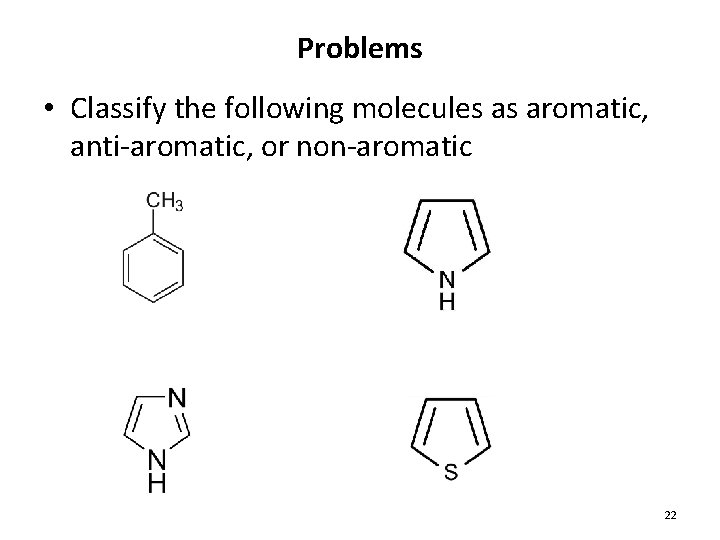
Problems • Classify the following molecules as aromatic, anti-aromatic, or non-aromatic 22

23

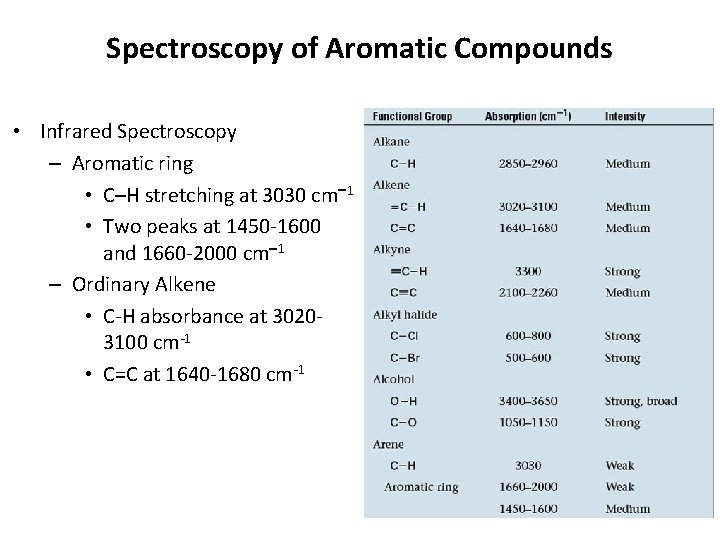
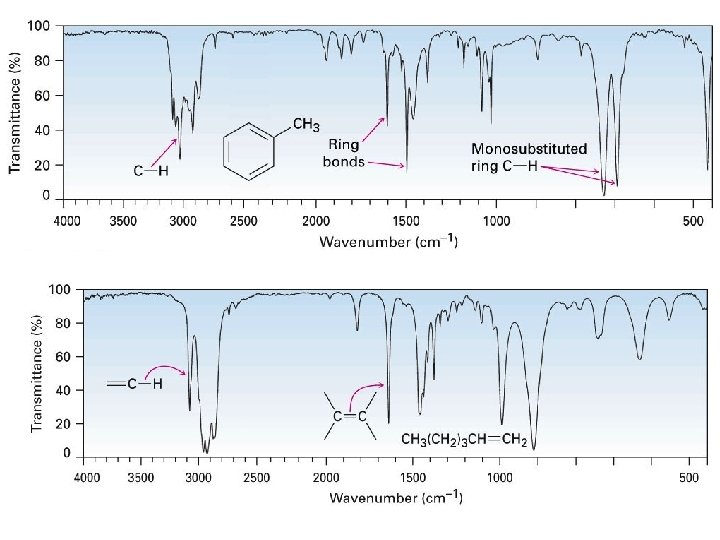
Spectroscopy of Aromatic Compounds • Infrared Spectroscopy – Aromatic ring • C–H stretching at 3030 cm 1 • Two peaks at 1450 -1600 and 1660 -2000 cm 1 – Ordinary Alkene • C-H absorbance at 30203100 cm-1 • C=C at 1640 -1680 cm-1


26

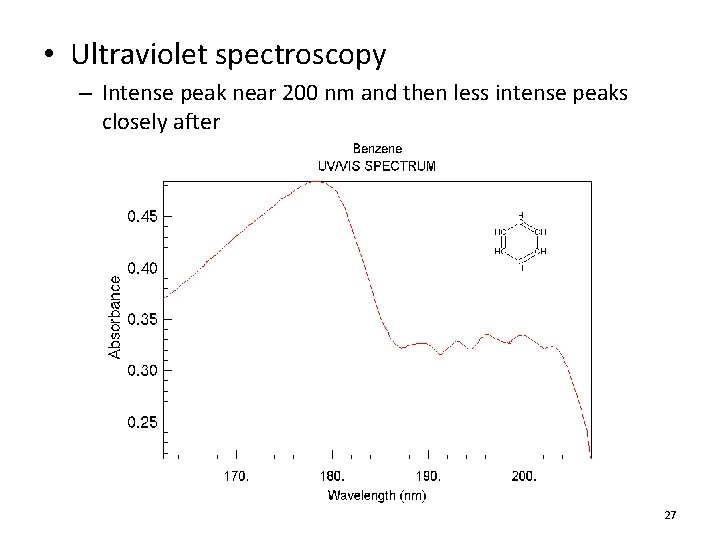
• Ultraviolet spectroscopy – Intense peak near 200 nm and then less intense peaks closely after 27

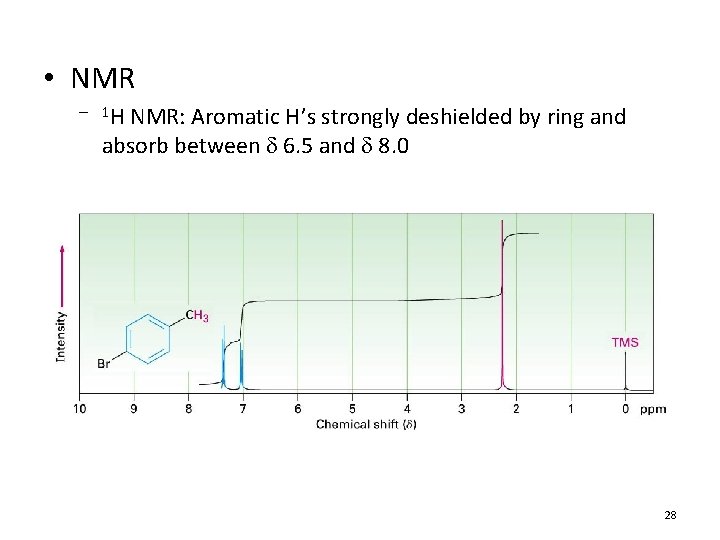
• NMR – 1 H NMR: Aromatic H’s strongly deshielded by ring and absorb between 6. 5 and 8. 0 28

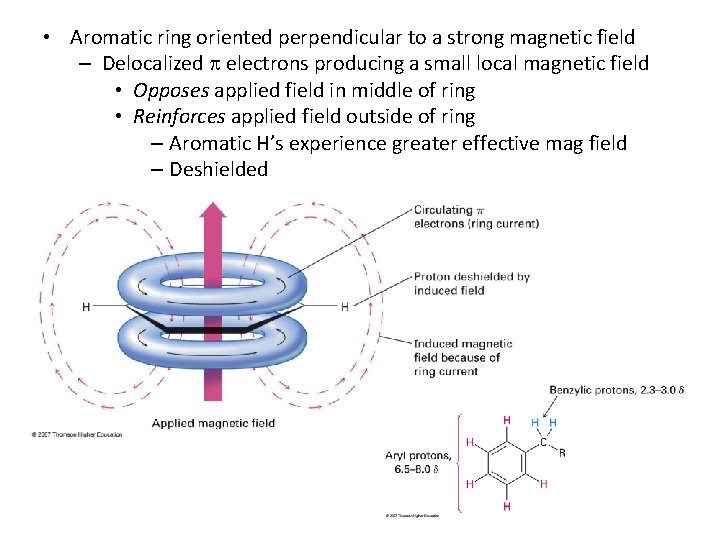
• Aromatic ring oriented perpendicular to a strong magnetic field – Delocalized electrons producing a small local magnetic field • Opposes applied field in middle of ring • Reinforces applied field outside of ring – Aromatic H’s experience greater effective mag field – Deshielded