Halides and Sulfides Halides Only 140 known species

![Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6] Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6]](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-4.jpg)



![Cs. Cl Structure • Cations and anions occupy cubic sites [CN = 8] – Cs. Cl Structure • Cations and anions occupy cubic sites [CN = 8] –](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-9.jpg)




![Sphalerite (Zn. S) Structure • Cations and anions occupy tetrahedral sites [CN = 4] Sphalerite (Zn. S) Structure • Cations and anions occupy tetrahedral sites [CN = 4]](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-19.jpg)


![Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6] Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6]](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-25.jpg)





- Slides: 37

Halides and Sulfides

Halides • Only 140 known species • F, Cl, Br, I as large anions • Strong, nearly exclusively ionic bonds • Gridwork, not discrete molecules • Generally isometric structure and form • Fairly low hardness • Many are water-soluble • Conductive via electrolysis (transport of ions), not by electron transport

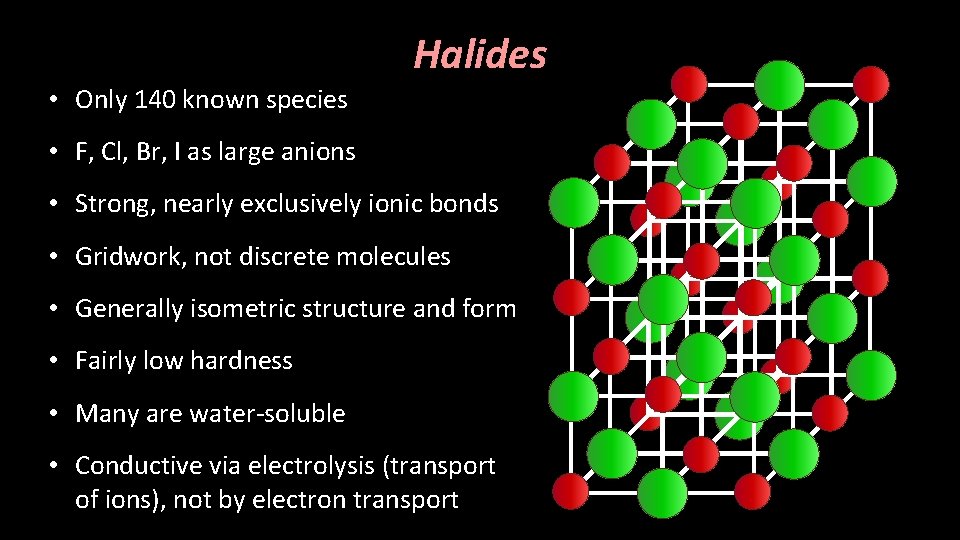
Halite (Na. Cl) Structure • Fundamentally defined by the formula AX, in which A is a cation with an equal and opposite charge to X, an anion. • There is an equal number of cations and anions, equally balancing charges
![Halite Na Cl Structure Cations and anions occupy octahedral sites CN 6 Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6]](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-4.jpg)
Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6] – RA : RX = 0. 41 – 0. 73

Halite (Na. Cl) Structure • Examples: – Halide Minerals: • Li. F, Li. Cl, Li. Br, Li. I • Na. F, Na. Cl, Na. Br, Na. I • KF, KCl, KBr, KI • Rb. F, Rb. Cl, Rb. Br, Rb. I – Oxide Minerals • Mg. O, Ca. O, Sr. O, Ba. O, Ni. O Sylvite - KCl Periclase - Mg. O – Sulfides • Mg. S, Ca. S, Mn. S, Pb. S Galena - Pb. S

Halite – Na. Cl • Abundant just about everywhere • Precipitates commonly found with gypsum, sylvite, anhydrite and calcite • Isometric habit, cubic cleavage • Obvious use as salt, but also used as ore for both sodium metal and chlorine gas

Sylvite – KCl • Precipitates commonly found with gypsum, halite, anhydrite and calcite, but as a very late precipitate • Distinctly bitter taste • Isometric habit, cubic cleavage • In N. Am. , found in SW US and Saskatchewan, Canada • Common use in fertilizers

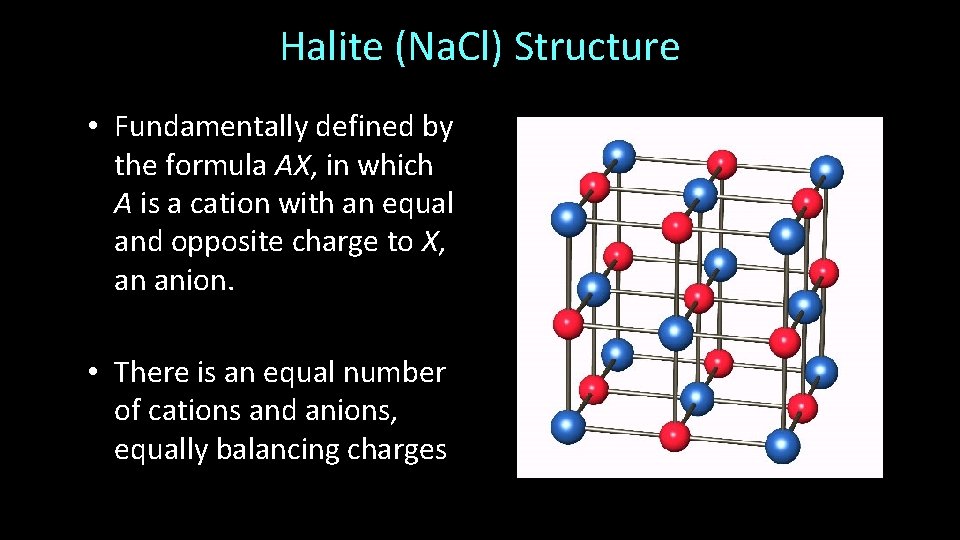
Cs. Cl Structure • Fundamentally defined by the formula AX, in which A is a cation with an equal and opposite charge to X, an anion. • There is an equal number of cations and anions, equally balancing charges
![Cs Cl Structure Cations and anions occupy cubic sites CN 8 Cs. Cl Structure • Cations and anions occupy cubic sites [CN = 8] –](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-9.jpg)
Cs. Cl Structure • Cations and anions occupy cubic sites [CN = 8] – RA : RX = 0. 73 – 1

Cs. Cl Structure • Examples: – Not Commonly Found in Nature – Halide Compounds: • Cs. Cl, Cs. Br, Cs. I – Ammonium Halide Compounds • (NH 4)Cl, (NH 4)Br – (NH 4)1+ group occupies A-site Salammoniac – NH 4 Cl

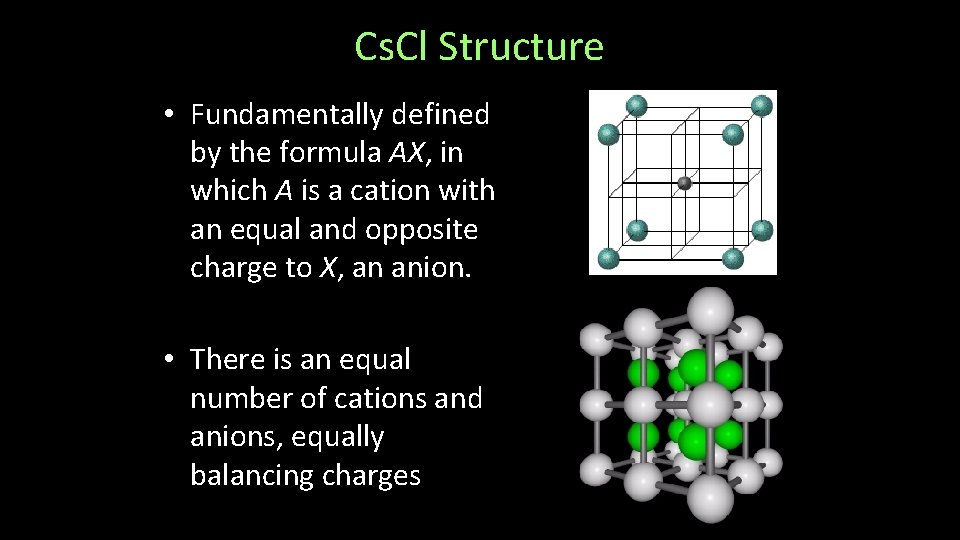
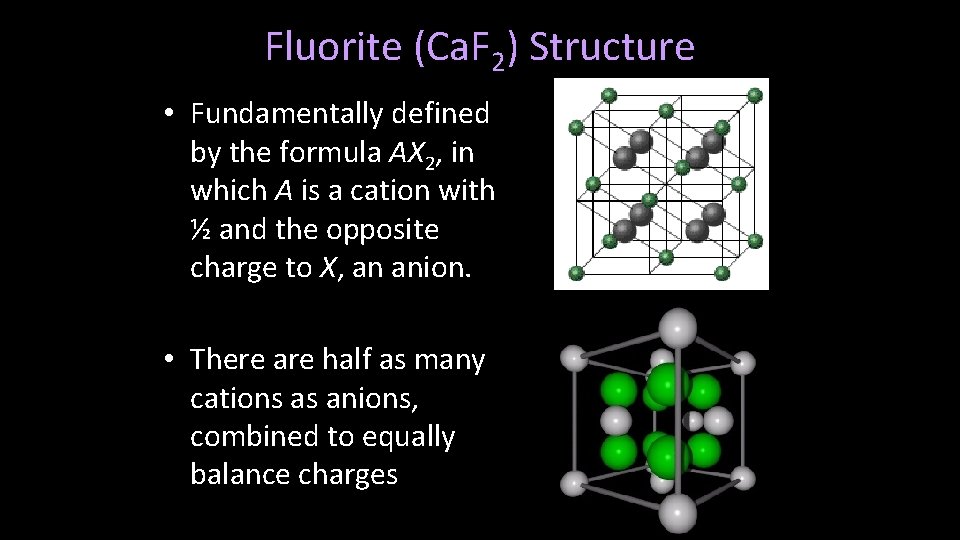
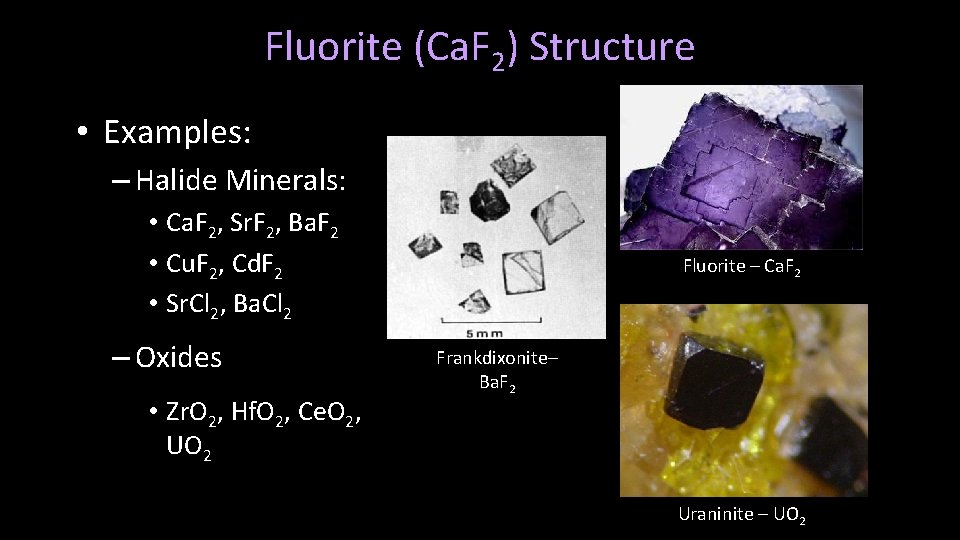
Fluorite (Ca. F 2) Structure • Fundamentally defined by the formula AX 2, in which A is a cation with ½ and the opposite charge to X, an anion. • There are half as many cations as anions, combined to equally balance charges

Fluorite (Ca. F 2) Structure • Examples: – Halide Minerals: • Ca. F 2, Sr. F 2, Ba. F 2 • Cu. F 2, Cd. F 2 • Sr. Cl 2, Ba. Cl 2 – Oxides • Zr. O 2, Hf. O 2, Ce. O 2, UO 2 Fluorite – Ca. F 2 Frankdixonite– Ba. F 2 Uraninite – UO 2

Fluorite– Ca. F 2 • Isometric crystal form, classic octahedral cleavage • Fluoresces under black-light • Typically yellow, purple, or green, also clear, red, brown, blue, etc. • Usually hydrothermal, also as a gangue mineral with Ag/Pb ores • Commonly associated with dolomite and calcite

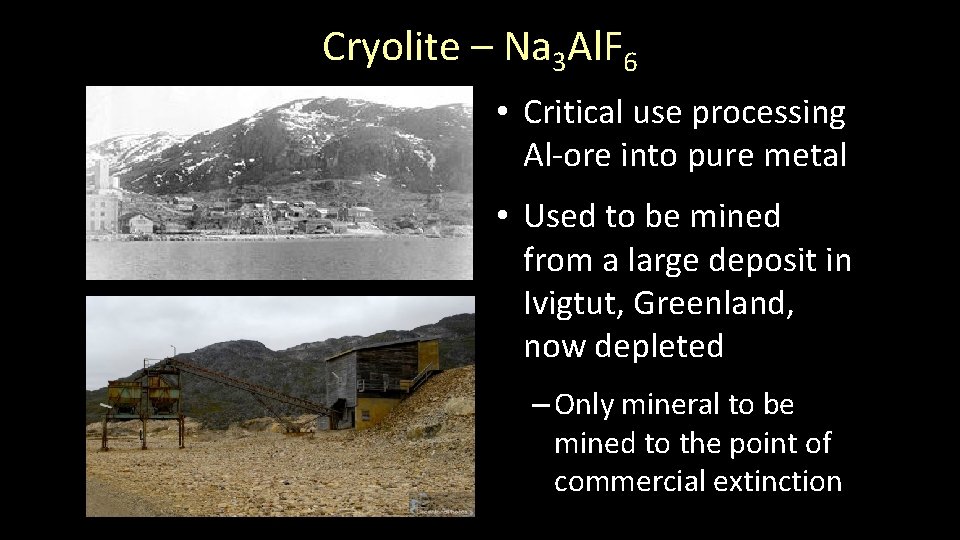
Cryolite – Na 3 Al. F 6 • Monoclinic: 2/m • Crystals are rare • Hardness: 2 ½ • Colorless-to-white • Powdered cryolite will disappear when submerged in water

Cryolite – Na 3 Al. F 6 • Critical use processing Al-ore into pure metal • Used to be mined from a large deposit in Ivigtut, Greenland, now depleted – Only mineral to be mined to the point of commercial extinction

Atacamite – Cu 2 Cl(OH)3 • Orthorhombic – 2/m 2/m • Hardness = 3 - 3 ½ • Found only in extremely arid, copper-enriched regions – Chile, Australia, and Arizona • Used as a minor copper ore – Copper-concentration makes it one of the very few stronglycolored halide minerals

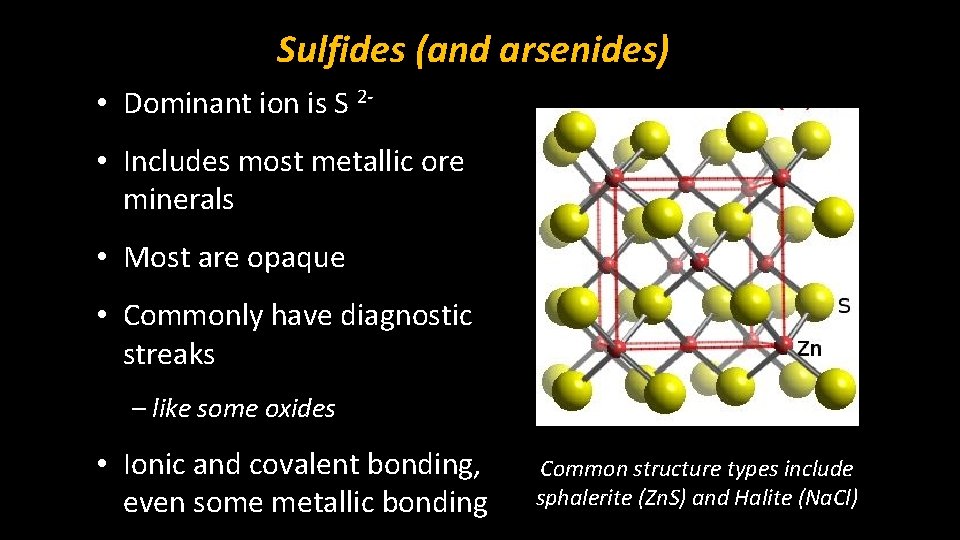
Sulfides (and arsenides) • Dominant ion is S 2 • Includes most metallic ore minerals • Most are opaque • Commonly have diagnostic streaks – like some oxides • Ionic and covalent bonding, even some metallic bonding Common structure types include sphalerite (Zn. S) and Halite (Na. Cl)

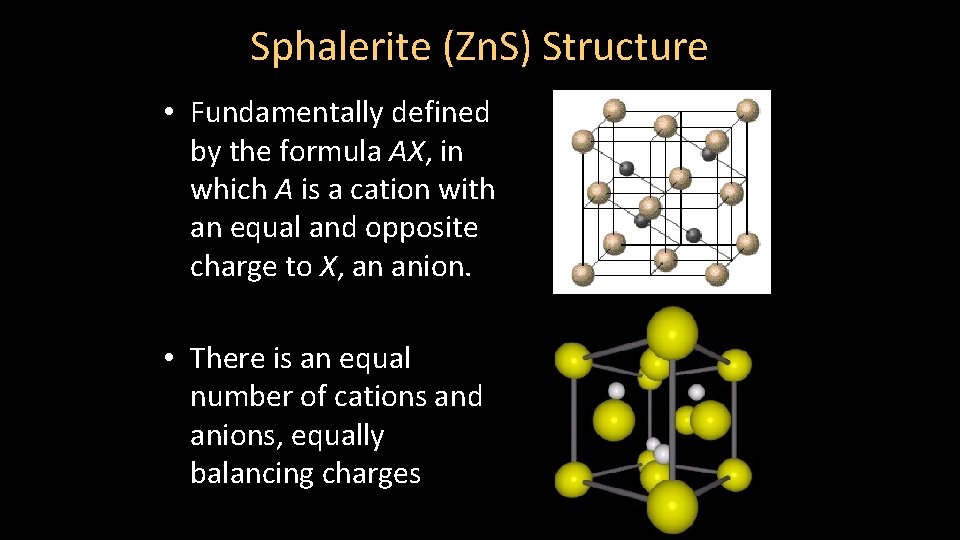
Sphalerite (Zn. S) Structure • Fundamentally defined by the formula AX, in which A is a cation with an equal and opposite charge to X, an anion. • There is an equal number of cations and anions, equally balancing charges
![Sphalerite Zn S Structure Cations and anions occupy tetrahedral sites CN 4 Sphalerite (Zn. S) Structure • Cations and anions occupy tetrahedral sites [CN = 4]](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-19.jpg)
Sphalerite (Zn. S) Structure • Cations and anions occupy tetrahedral sites [CN = 4] – RA : RX = 0. 32

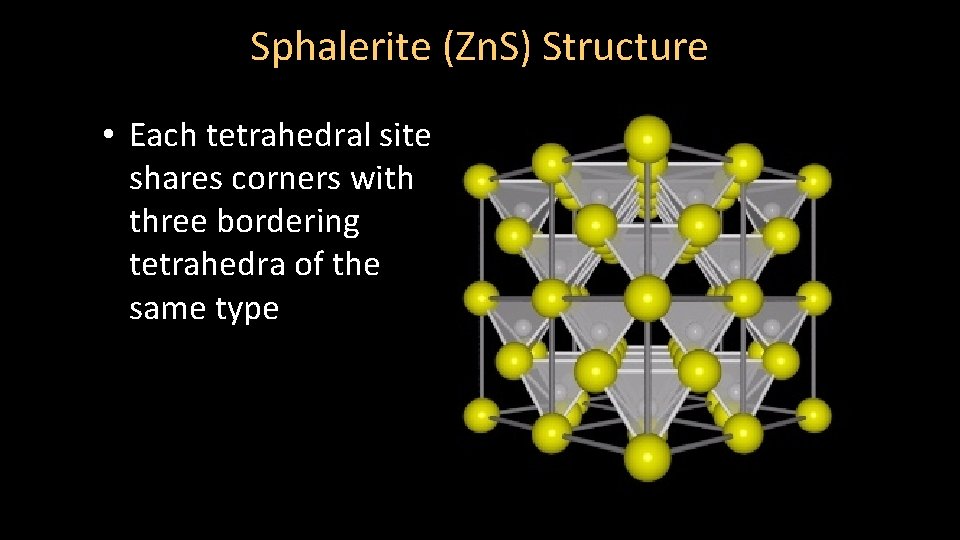
Sphalerite (Zn. S) Structure • Each tetrahedral site shares corners with three bordering tetrahedra of the same type

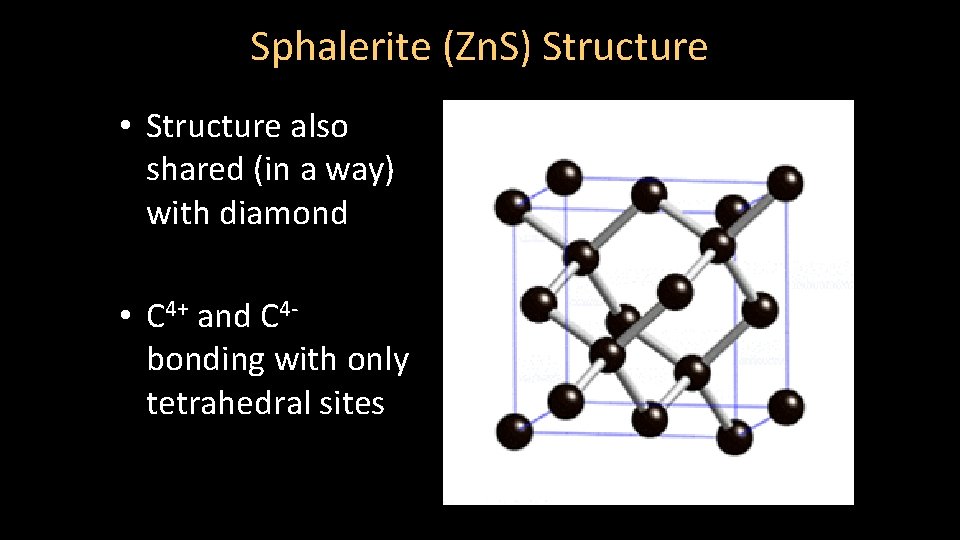
Sphalerite (Zn. S) Structure • Structure also shared (in a way) with diamond • C 4+ and C 4 bonding with only tetrahedral sites

Sphalerite (Zn. S) Structure • Examples: – Sulfide Minerals: • Zn. S, Cu. Fe. S 2 – Silicon Carbide (Si. C) Sphalerite - Zn. S – Diamond Chalcopyrite – Cu. Fe. S 2 Diamond - C

Sphalerite– Zn. S • Isometric mineral: 43 m • Structure is based around four-fold coordination of both cations and anions • Unique dodecahedral {011} cleavage • Distinctive range of yellow-brown streaks • Low-T polymorph vs. high-T structure: Wurtzite • Typically used as zinc ore

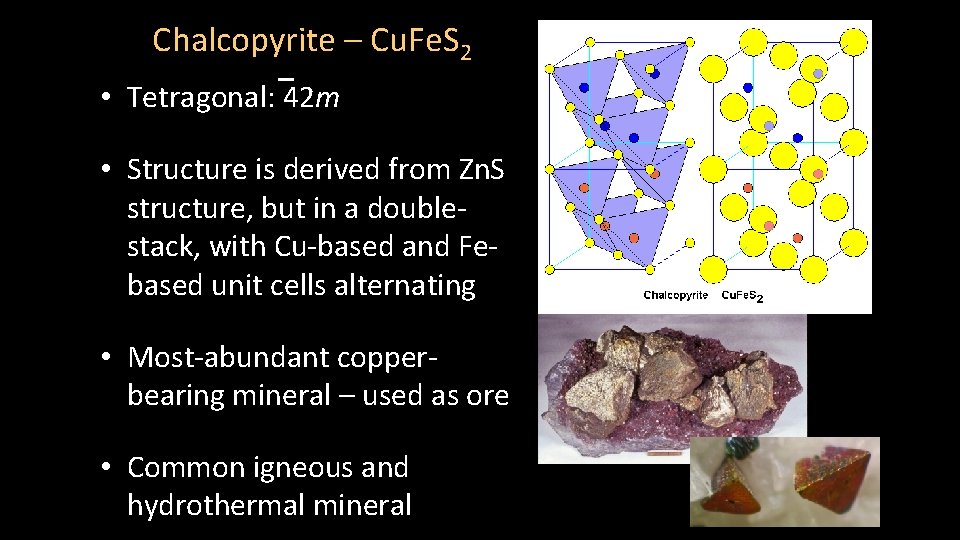
Chalcopyrite – Cu. Fe. S 2 • Tetragonal: 42 m • Structure is derived from Zn. S structure, but in a doublestack, with Cu-based and Febased unit cells alternating • Most-abundant copperbearing mineral – used as ore • Common igneous and hydrothermal mineral
![Halite Na Cl Structure Cations and anions occupy octahedral sites CN 6 Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6]](https://slidetodoc.com/presentation_image_h2/44229f96331d060de57946c39b9d6d05/image-25.jpg)
Halite (Na. Cl) Structure • Cations and anions occupy octahedral sites [CN = 6] – RA : RX = 0. 41 – 0. 73 • Sulfide Examples: – Galena (Pb. S) – Pyrite (Fe. S 2)

Galena – Pb. S • Isometric: 4/m 32/m • Halite structure, with Pb 2+ substituting for Na 1+, and S 2 - substituting for Cl 1 • Very high density: SG ~7. 5 • Often in hydrothermal veins with silver-based minerals • Commonly found in MVT deposits with sphalerite (Zn. S) • Used as lead ore

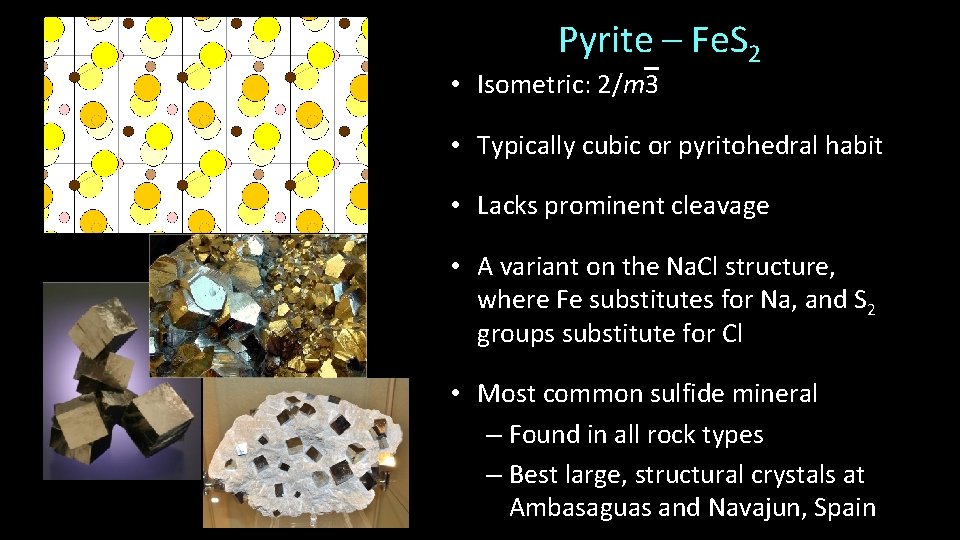
Pyrite – Fe. S 2 • Isometric: 2/m 3 • Typically cubic or pyritohedral habit • Lacks prominent cleavage • A variant on the Na. Cl structure, where Fe substitutes for Na, and S 2 groups substitute for Cl • Most common sulfide mineral – Found in all rock types – Best large, structural crystals at Ambasaguas and Navajun, Spain

Marcasite – Fe. S 2 • Orthorhombic: 2/m 2/m • Polymorph of pyrite – Arrangement of ions is skewed, similar to rutile (Ti. O 2) structure • Unstable, very easily oxidized to limonite as coating • Deposited from low-T, acidic solutions • Often found with lead and zinc ores in veins

Arsenopyrite– Fe. As. S • Monoclinic: 2/m, – Pseudo-orthorhombic • Similar structure to marcasite (Fe. S 2), but with ½ of the S 2 - replaced by As 3 • Most-common arsenic-bearing mineral • Found with Sn and W ores in high-T hydrothermal deposits – Also found with ores of silver, copper, gold, and other common sulfides (Pb. S, Zn. S, Fe. S 2, etc. )

Pyrrhotite – Fe 1 -x. S • Monoclinic: 2/m at low-T • Hexagonal: 6/m 2/m at high-T • Structural deficiency of iron relative to sulfur – x in formula is between 0 and 0. 2 – Cation layer is in hexagonal closest packing, alternately filled or partially-filled • Found in alkali-poor igneous rocks (ex: norites)

Chalcocite – Cu 2 S • Monoclinic, Pseudo-orthorhombic: 2/m or m below 105°C • Distinct crystals are quite rare – Tabular habit when observable – Usually fine-grained or massive • Hexagonal packing of sulfur atoms at low-T – High-T form, high chalcocite, allows structural gaps to be filled • Crucial copper ore mineral

Bornite– Cu 5 Fe. S 4 • Tetragonal: 42 m • Usually massive, rarely pseudocubic crystals • Extensive solid-solution within Cu-Fe-S system • Known for its purple-blue-green tarnish – Peacock ore

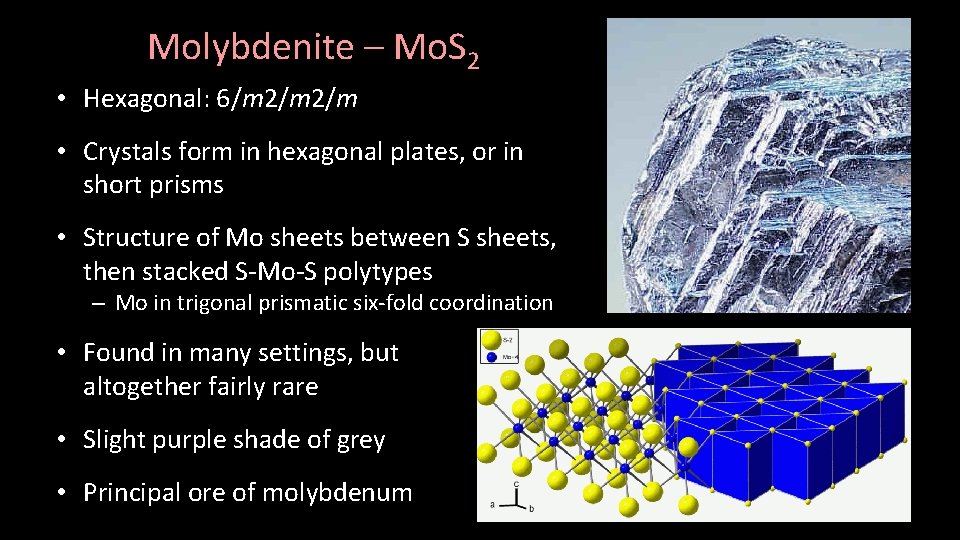
Molybdenite – Mo. S 2 • Hexagonal: 6/m 2/m • Crystals form in hexagonal plates, or in short prisms • Structure of Mo sheets between S sheets, then stacked S-Mo-S polytypes – Mo in trigonal prismatic six-fold coordination • Found in many settings, but altogether fairly rare • Slight purple shade of grey • Principal ore of molybdenum

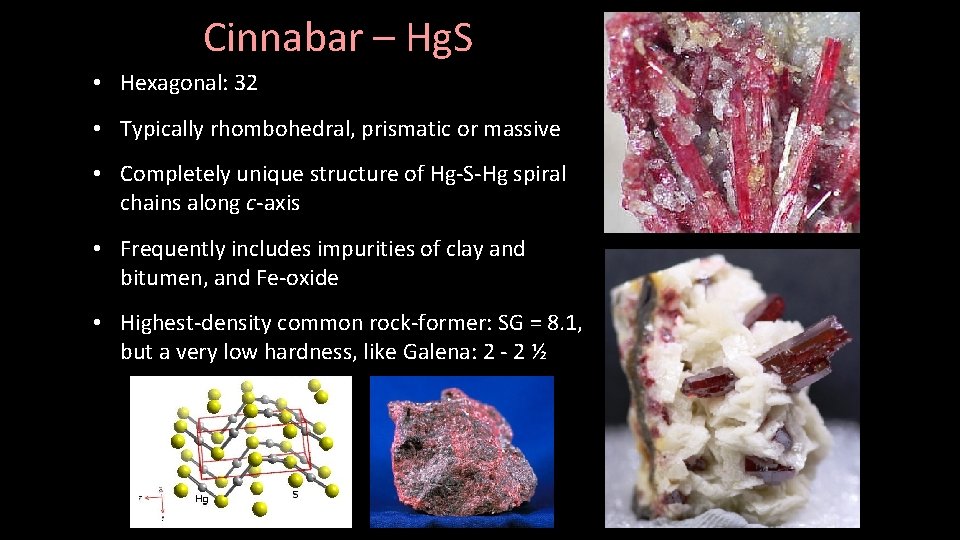
Cinnabar – Hg. S • Hexagonal: 32 • Typically rhombohedral, prismatic or massive • Completely unique structure of Hg-S-Hg spiral chains along c-axis • Frequently includes impurities of clay and bitumen, and Fe-oxide • Highest-density common rock-former: SG = 8. 1, but a very low hardness, like Galena: 2 - 2 ½

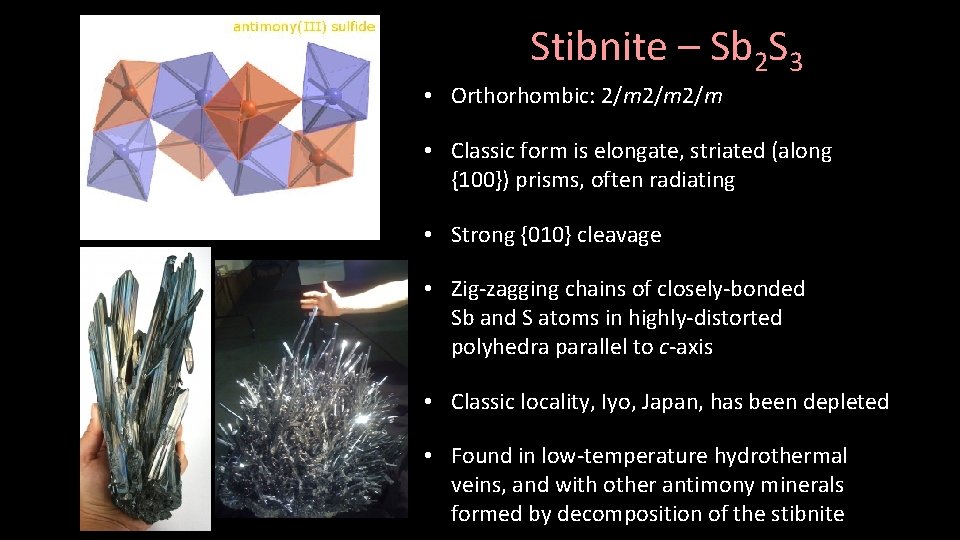
Stibnite – Sb 2 S 3 • Orthorhombic: 2/m 2/m • Classic form is elongate, striated (along {100}) prisms, often radiating • Strong {010} cleavage • Zig-zagging chains of closely-bonded Sb and S atoms in highly-distorted polyhedra parallel to c-axis • Classic locality, Iyo, Japan, has been depleted • Found in low-temperature hydrothermal veins, and with other antimony minerals formed by decomposition of the stibnite

Realgar – As. S Orpiment – As 2 S 3 • Both are monoclinic: 2/m • Realgar forms in ringlike groups of As 4 S 4, like S 8 – Each As bonds to two sulfurs, and to another As • In Orpiment, trigonal As. S 3 pyramids link corners to make six-member, corrugated rings, As 2 S 3 • Both were used in pigments until their poisonous nature was widely recognized

Next Time… Oxides and Hydroxides