Lecture Presentation Chapter 13 Alcohols Phenols Thiols and







- Slides: 29

Lecture Presentation Chapter 13 Alcohols, Phenols, Thiols, and Ethers Karen C. Timberlake General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Chapter 13 Alcohols, Phenols, Thiols, and Ethers Anesthesia is typically administered by a nurse anesthetist who provides care before, during, and after a medical procedure. A nurse anesthetist gives medications to keep a patient asleep and pain-free while monitoring the patient’s vital signs. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Chapter 13 Readiness Core Chemistry Skills • Naming and Drawing Alkanes (12. 2) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

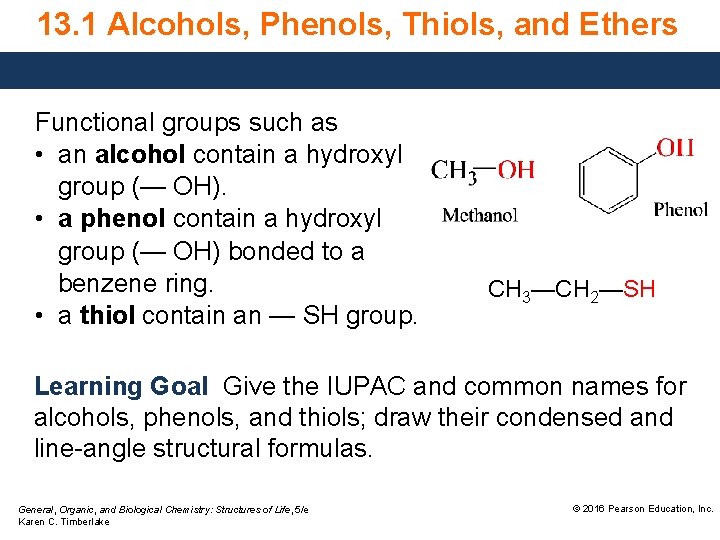
13. 1 Alcohols, Phenols, Thiols, and Ethers Functional groups such as • an alcohol contain a hydroxyl group (— OH). • a phenol contain a hydroxyl group (— OH) bonded to a benzene ring. • a thiol contain an — SH group. CH 3—CH 2—SH Learning Goal Give the IUPAC and common names for alcohols, phenols, and thiols; draw their condensed and line-angle structural formulas. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

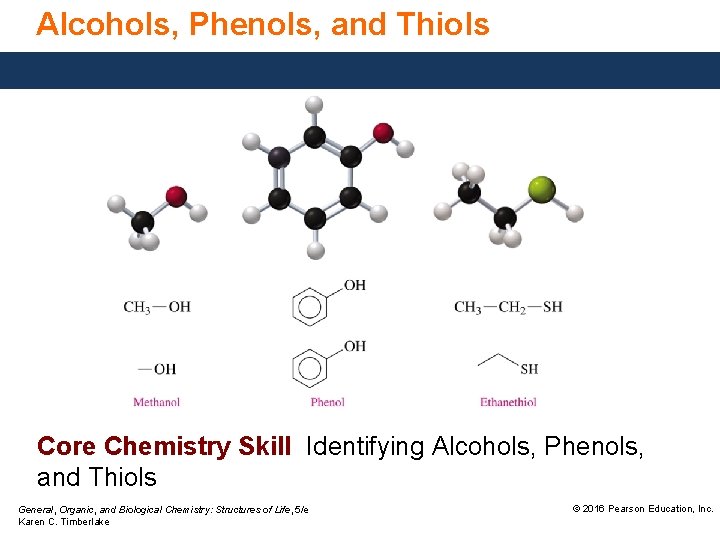
Alcohols, Phenols, and Thiols Core Chemistry Skill Identifying Alcohols, Phenols, and Thiols General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Naming Alcohols • In the IUPAC system, replace the e with ol. • With common names, use the name of the alkyl group followed by alcohol. Core Chemistry Skill Naming Alcohols and Phenols General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

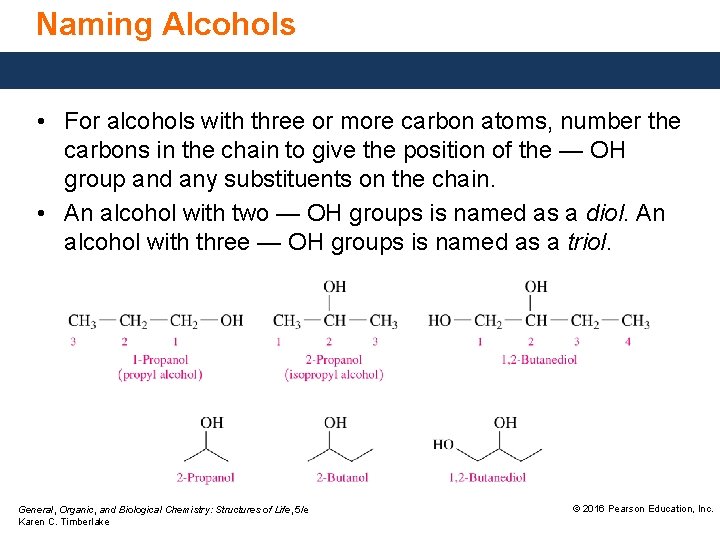
Naming Alcohols • For alcohols with three or more carbon atoms, number the carbons in the chain to give the position of the — OH group and any substituents on the chain. • An alcohol with two — OH groups is named as a diol. An alcohol with three — OH groups is named as a triol. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

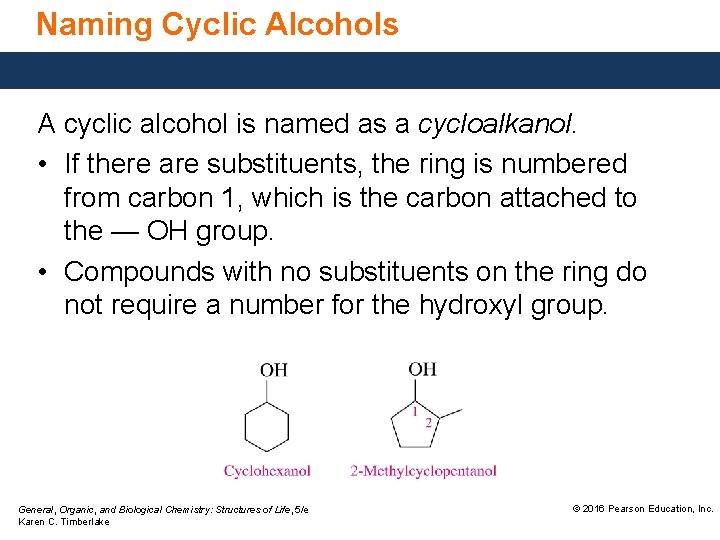
Naming Cyclic Alcohols A cyclic alcohol is named as a cycloalkanol. • If there are substituents, the ring is numbered from carbon 1, which is the carbon attached to the — OH group. • Compounds with no substituents on the ring do not require a number for the hydroxyl group. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Naming Phenols • The term phenol is the IUPAC name when a hydroxyl group ( — OH) is bonded to a benzene ring. • When there is a second substituent, the benzene ring is numbered starting with carbon 1, bonded to the — OH group. • The terms ortho, meta, and para are used for the common names of simple phenols. • The common name cresol is also used for methylphenols. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

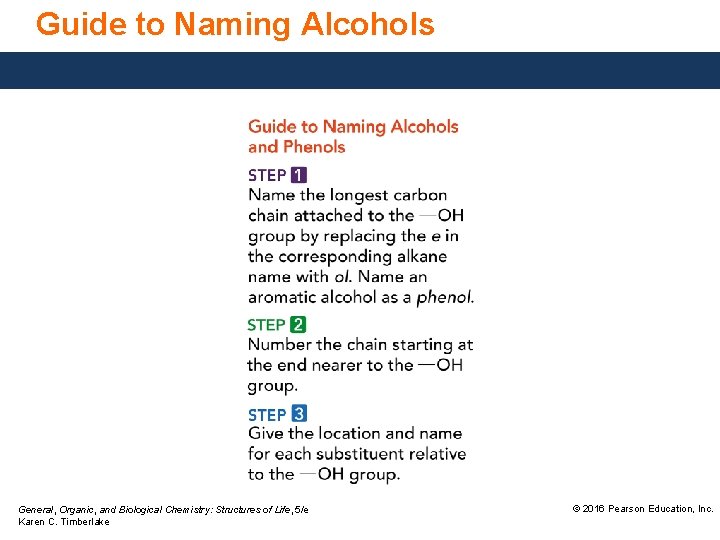
Guide to Naming Alcohols General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

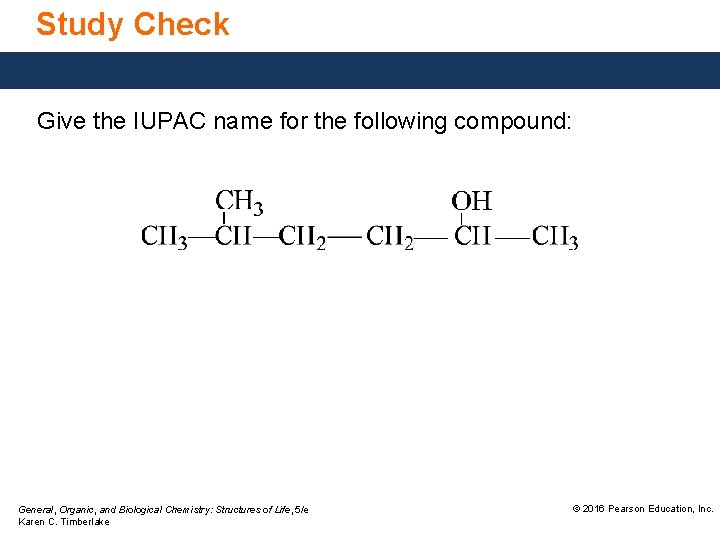
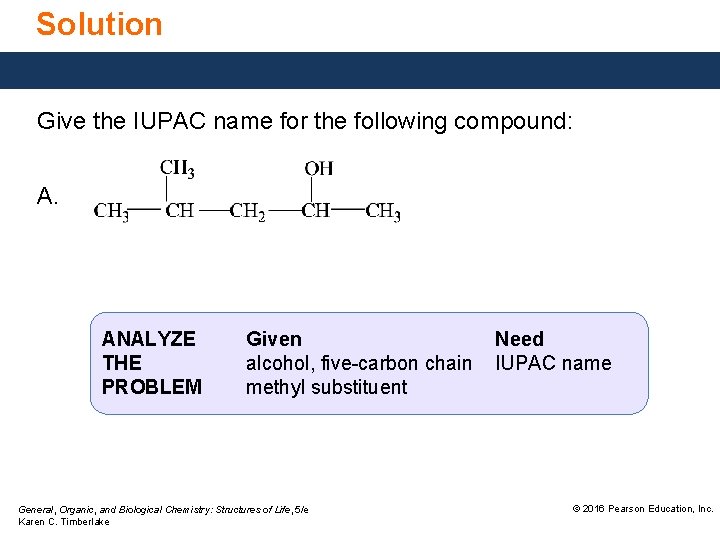
Study Check Give the IUPAC name for the following compound: General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

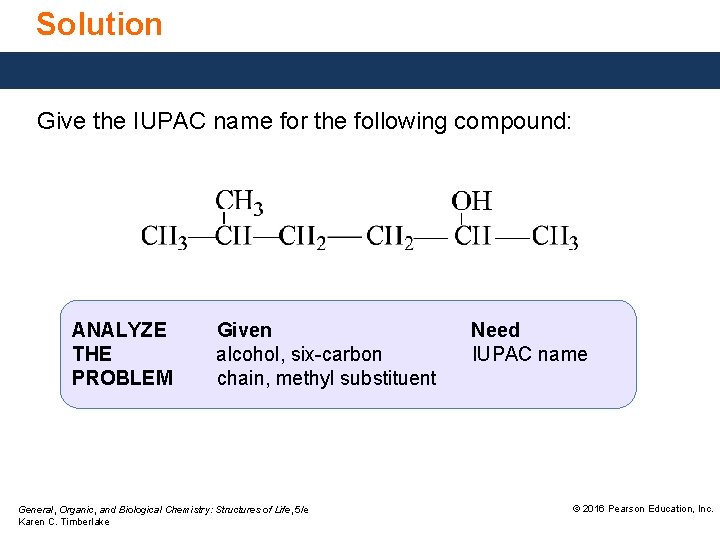
Solution Give the IUPAC name for the following compound: ANALYZE THE PROBLEM Given alcohol, six-carbon chain, methyl substituent General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Need IUPAC name © 2016 Pearson Education, Inc.

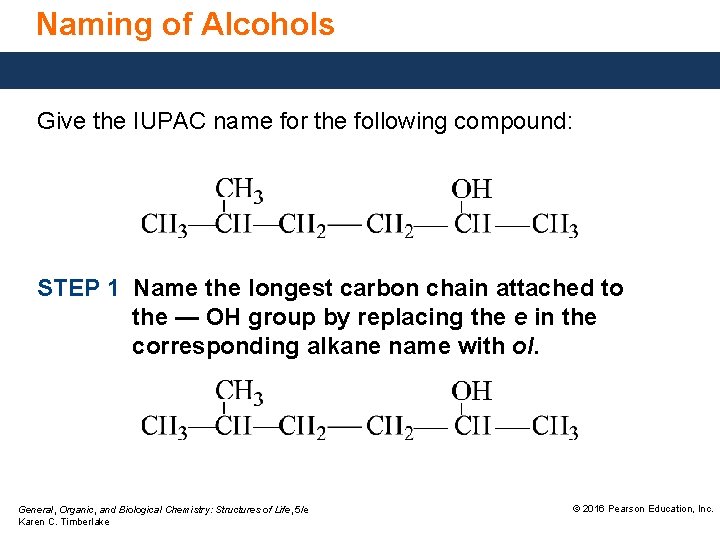
Naming of Alcohols Give the IUPAC name for the following compound: STEP 1 Name the longest carbon chain attached to the — OH group by replacing the e in the corresponding alkane name with ol. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

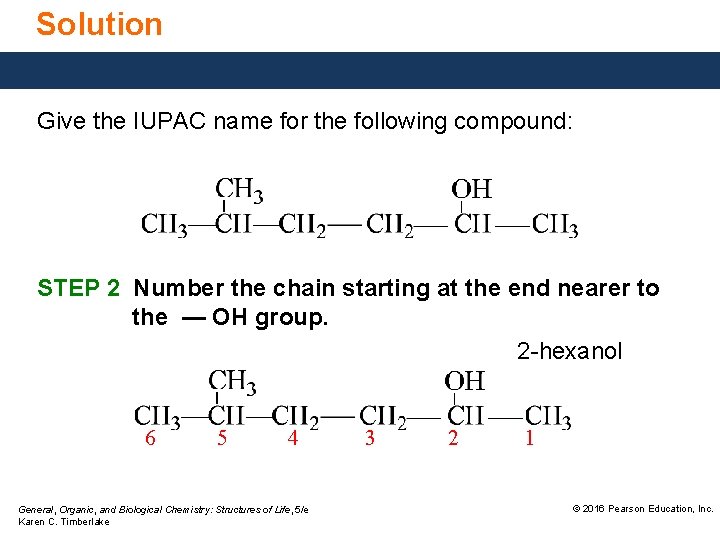
Solution Give the IUPAC name for the following compound: STEP 2 Number the chain starting at the end nearer to the — OH group. 2 -hexanol 6 5 4 General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake 3 2 1 © 2016 Pearson Education, Inc.

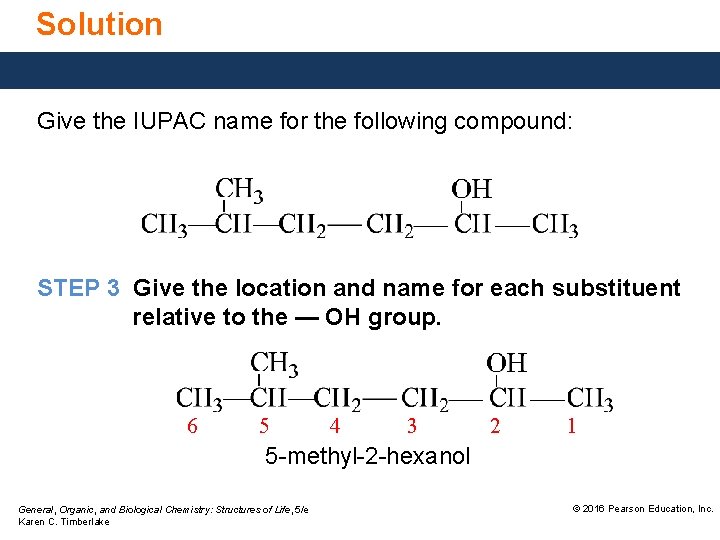
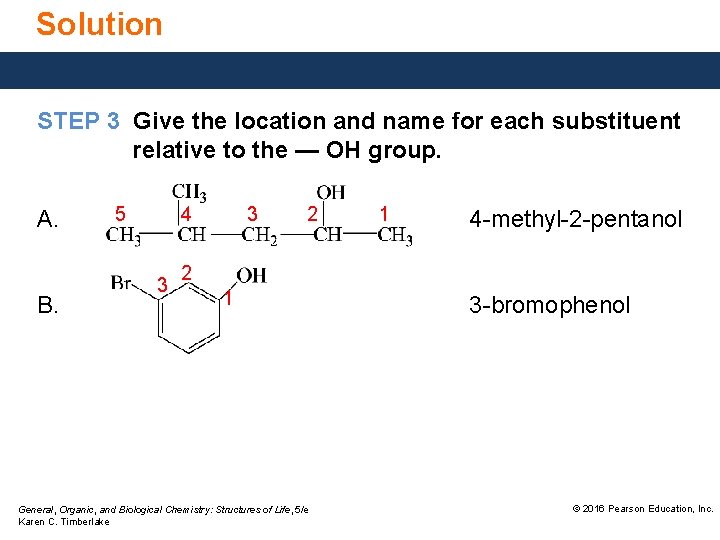
Solution Give the IUPAC name for the following compound: STEP 3 Give the location and name for each substituent relative to the — OH group. 6 5 4 3 2 1 5 -methyl-2 -hexanol General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

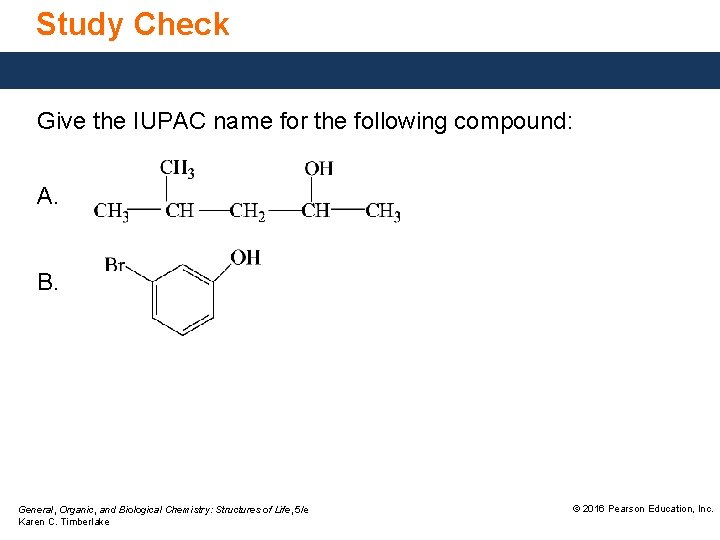
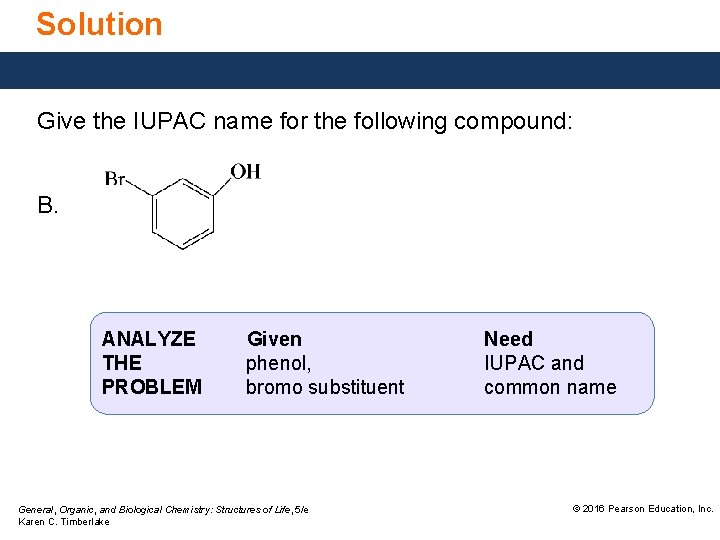
Study Check Give the IUPAC name for the following compound: A. B. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

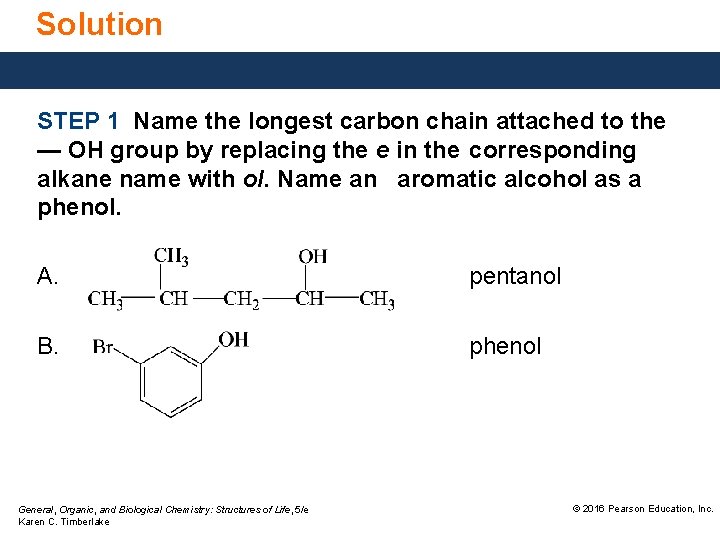
Solution Give the IUPAC name for the following compound: A. ANALYZE THE PROBLEM Given alcohol, five-carbon chain methyl substituent General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Need IUPAC name © 2016 Pearson Education, Inc.

Solution Give the IUPAC name for the following compound: B. ANALYZE THE PROBLEM Given phenol, bromo substituent General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Need IUPAC and common name © 2016 Pearson Education, Inc.

Solution STEP 1 Name the longest carbon chain attached to the — OH group by replacing the e in the corresponding alkane name with ol. Name an aromatic alcohol as a phenol. A. pentanol B. phenol General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

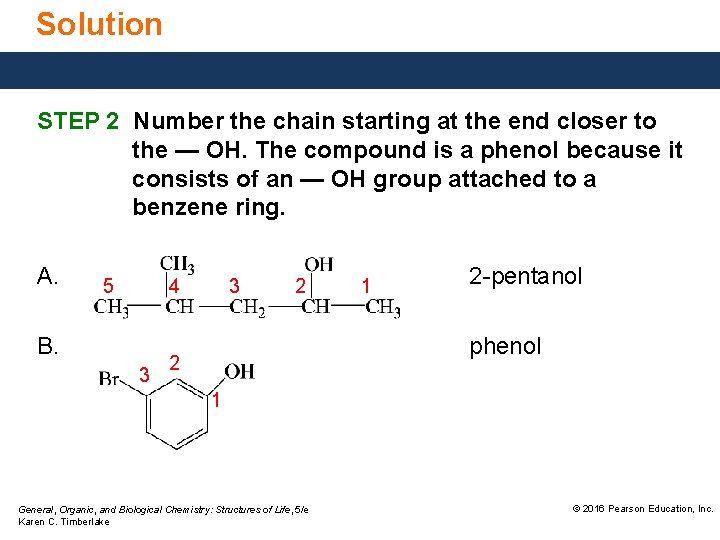
Solution STEP 2 Number the chain starting at the end closer to the — OH. The compound is a phenol because it consists of an — OH group attached to a benzene ring. A. 5 4 B. 3 3 2 1 2 -pentanol phenol 2 1 General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution STEP 3 Give the location and name for each substituent relative to the — OH group. A. B. 5 4 3 3 2 1 4 -methyl-2 -pentanol 2 1 General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake 3 -bromophenol © 2016 Pearson Education, Inc.

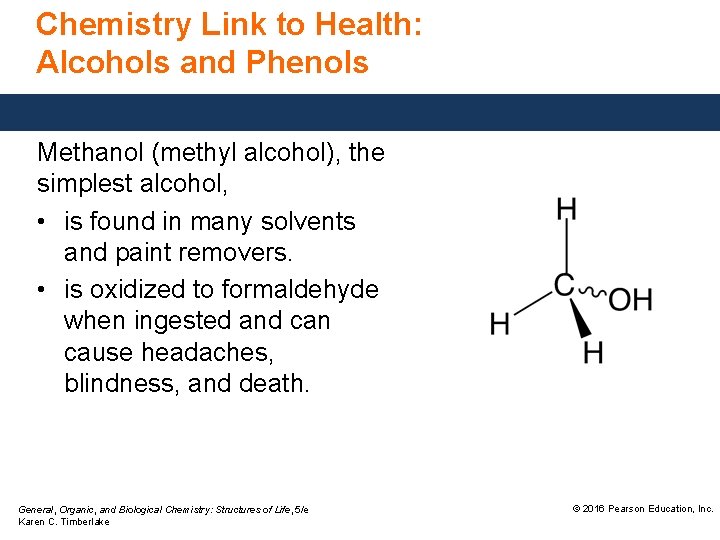
Chemistry Link to Health: Alcohols and Phenols Methanol (methyl alcohol), the simplest alcohol, • is found in many solvents and paint removers. • is oxidized to formaldehyde when ingested and can cause headaches, blindness, and death. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Chemistry Link to Health: Alcohols and Phenols Ethanol (ethyl alcohol) • has been known since prehistoric times as an intoxicating product. • is formed by the fermentation of grains, sugars, and starches. Fermentation C 6 H 12 O 6 2 CH 3—CH 2—OH + 2 CO 2 • is used as a solvent for perfumes, varnishes, and some medicines, such as tincture of iodine. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Chemistry Link to Health: Alcohols and Phenols 1, 2 -ethanediol (ethylene glycol) • is used as an antifreeze in heating and cooling systems. • is also a solvent for paints, inks, and plastics. • is used in the production of synthetic fibers such as Dacron. If ingested, it is extremely toxic. In the body, it is oxidized to oxalic acid, which forms insoluble salts in the kidneys that cause renal damage, convulsions, and death. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

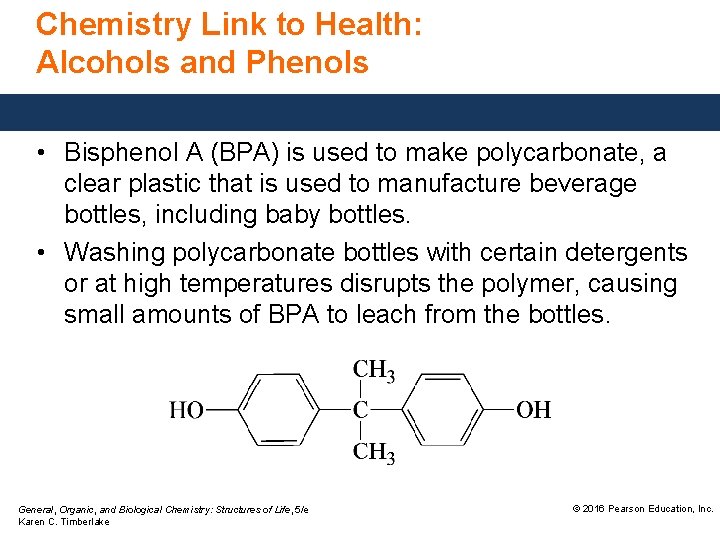
Chemistry Link to Health: Alcohols and Phenols • Bisphenol A (BPA) is used to make polycarbonate, a clear plastic that is used to manufacture beverage bottles, including baby bottles. • Washing polycarbonate bottles with certain detergents or at high temperatures disrupts the polymer, causing small amounts of BPA to leach from the bottles. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

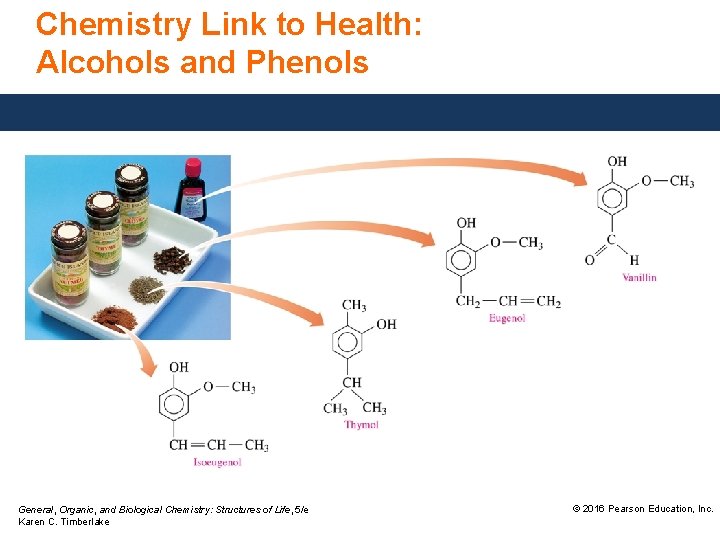
Chemistry Link to Health: Alcohols and Phenols General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Thiols • contain a thiol (— SH) group. • often have strong and sometimes disagreeable odors. • are found in cheese, onions, garlic, and oysters. • are used to detect gas leaks. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

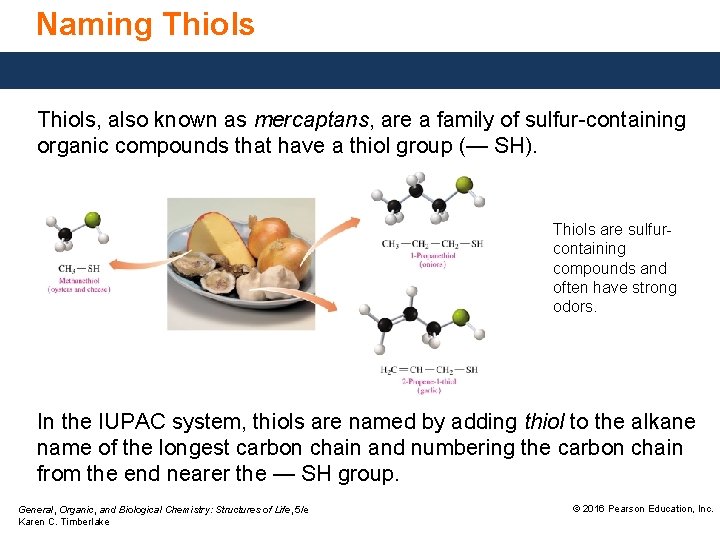
Naming Thiols, also known as mercaptans, are a family of sulfur-containing organic compounds that have a thiol group (— SH). Thiols are sulfurcontaining compounds and often have strong odors. In the IUPAC system, thiols are named by adding thiol to the alkane name of the longest carbon chain and numbering the carbon chain from the end nearer the — SH group. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

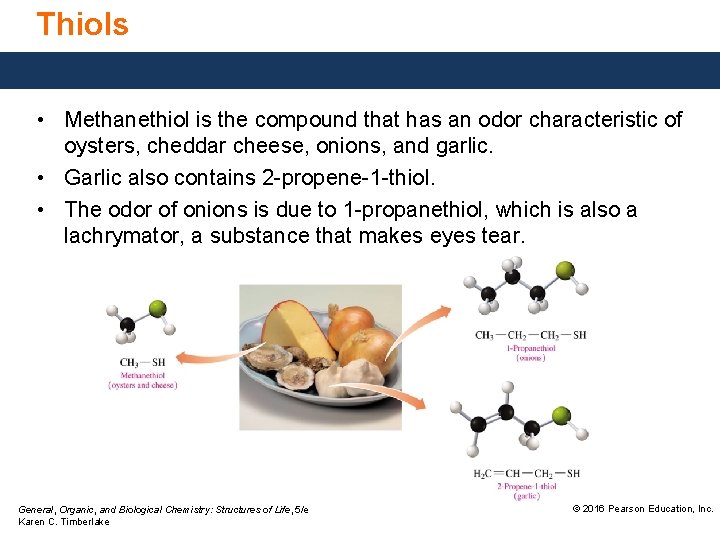
Thiols • Methanethiol is the compound that has an odor characteristic of oysters, cheddar cheese, onions, and garlic. • Garlic also contains 2 -propene-1 -thiol. • The odor of onions is due to 1 -propanethiol, which is also a lachrymator, a substance that makes eyes tear. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.