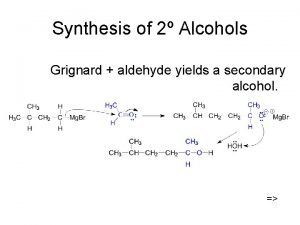
Synthesis of 2 Alcohols Grignard aldehyde yields a







- Slides: 18

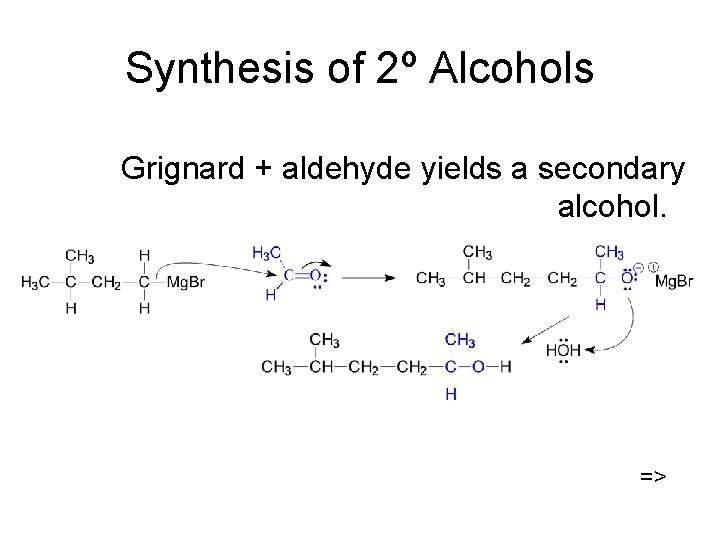
Synthesis of 2º Alcohols Grignard + aldehyde yields a secondary alcohol. =>

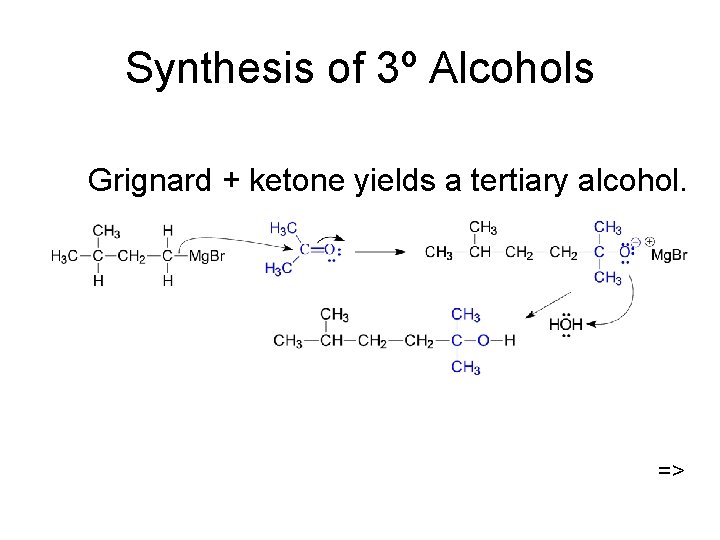
Synthesis of 3º Alcohols Grignard + ketone yields a tertiary alcohol. =>

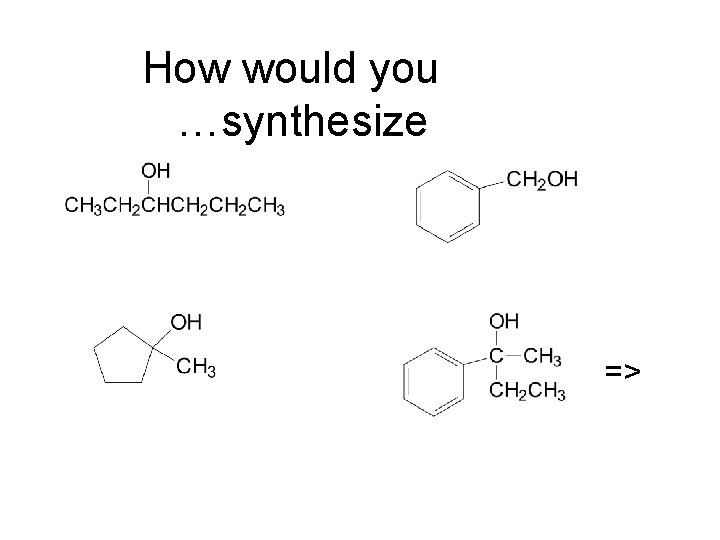
How would you …synthesize =>

Grignard Reactions with Acid Chlorides and Esters • Use two moles of Grignard reagent. • The product is a tertiary alcohol with two identical alkyl groups. • Reaction with one mole of Grignard reagent produces a ketone intermediate, which reacts with the second mole of Grignard reagent. =>

Grignard + Acid (Chloride (1 • Grignard attacks the carbonyl. • Chloride ion leaves. Ketone intermediate =>

(Grignard and Ester (1 • Grignard attacks the carbonyl. • Alkoxide ion leaves! ? ! Ketone intermediate =>

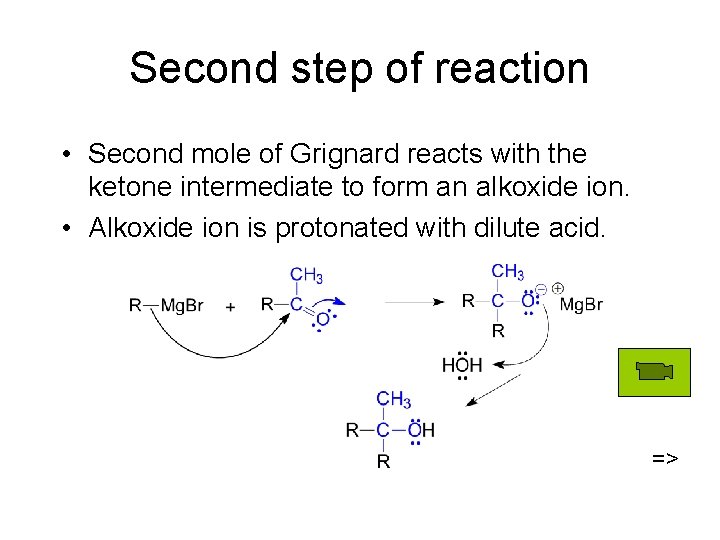
Second step of reaction • Second mole of Grignard reacts with the ketone intermediate to form an alkoxide ion. • Alkoxide ion is protonated with dilute acid. =>

How would you synthesize. . . Using an acid chloride or ester. =>

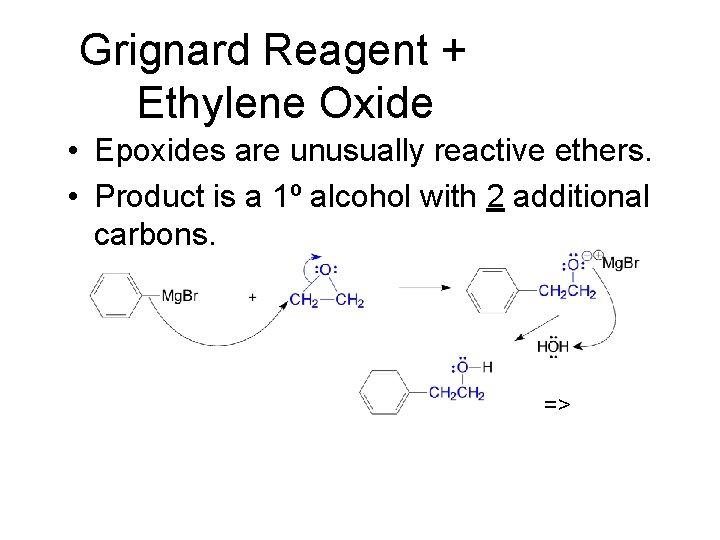
Grignard Reagent + Ethylene Oxide • Epoxides are unusually reactive ethers. • Product is a 1º alcohol with 2 additional carbons. =>

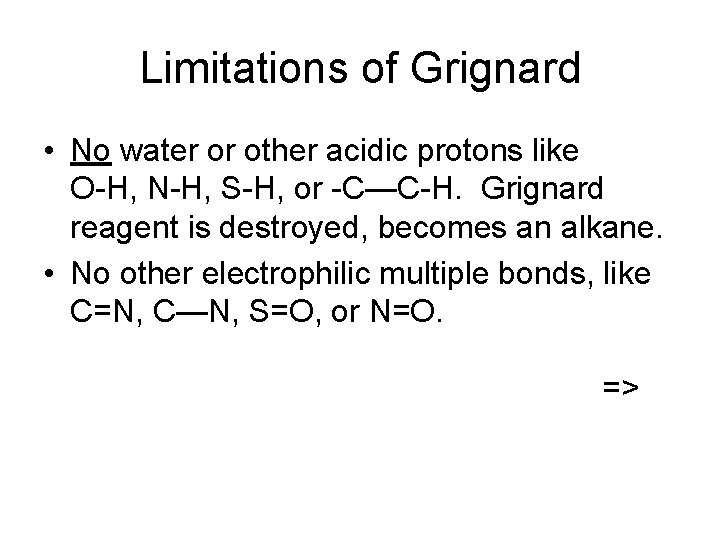
Limitations of Grignard • No water or other acidic protons like O-H, N-H, S-H, or -C—C-H. Grignard reagent is destroyed, becomes an alkane. • No other electrophilic multiple bonds, like C=N, C—N, S=O, or N=O. =>

Reduction of Carbonyl • Reduction of aldehyde yields 1º alcohol. • Reduction of ketone yields 2º alcohol. • Reagents: – Sodium borohydride, Na. BH 4 – Lithium aluminum hydride, Li. Al. H 4 – Raney nickel =>

Sodium Borohydride • Hydride ion, H , attacks the carbonyl carbon, forming an alkoxide ion. • Then the alkoxide ion is protonated by dilute acid. • Only reacts with carbonyl of aldehyde or ketone, not with carbonyls of esters or carboxylic acids. =>

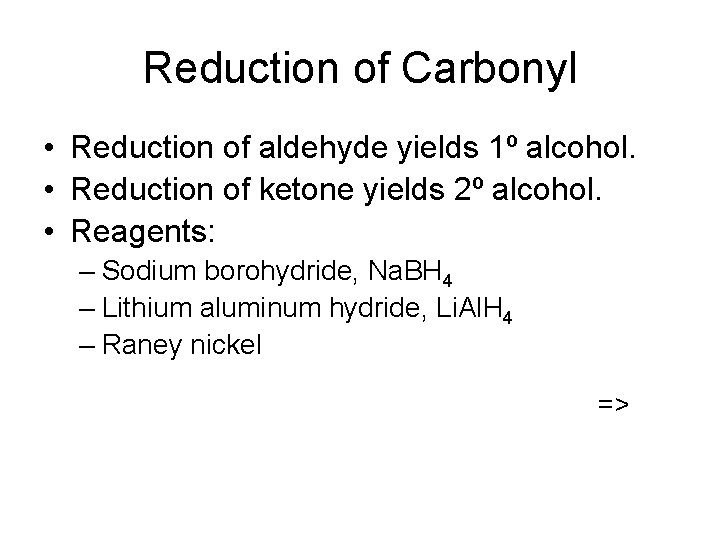
Lithium Aluminum Hydride • Stronger reducing agent than sodium borohydride, but dangerous to work with. • Converts esters and acids to 1º alcohols. =>

Comparison of Reducing Agents • Li. Al. H 4 is stronger. • Li. Al. H 4 reduces more stable compounds which are resistant to reduction. =>

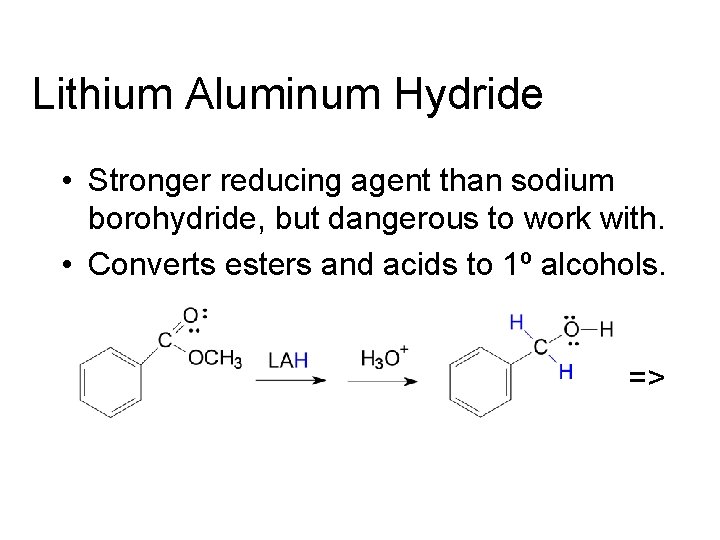
Catalytic Hydrogenation • Add H 2 with Raney nickel catalyst. • Also reduces any C=C bonds. =>

(Thiols (Mercaptans • • • Sulfur analogues of alcohols, -SH. Named by adding -thiol to alkane name. The -SH group is called mercapto. Complex with heavy metals: Hg, As, Au. More acidic than alcohols, react with Na. OH to form thiolate ion. Stinks! => •

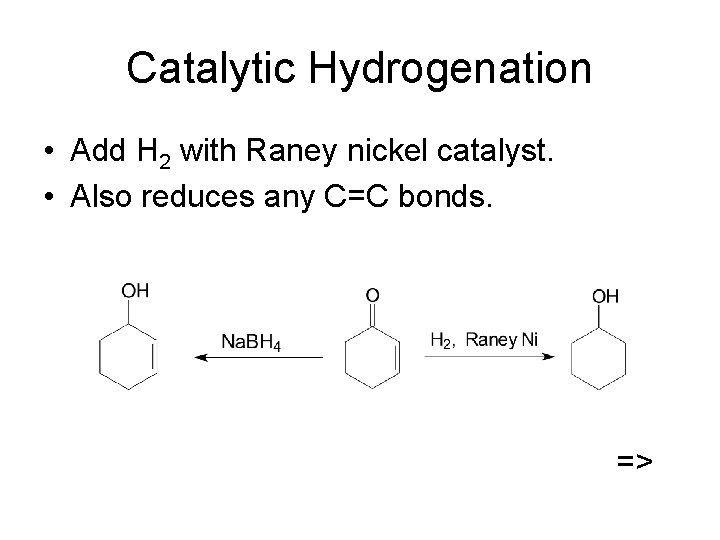
Thiol Synthesis Use a large excess of sodium hydrosulfide with unhindered alkyl halide to prevent dialkylation to R-S-R. =>

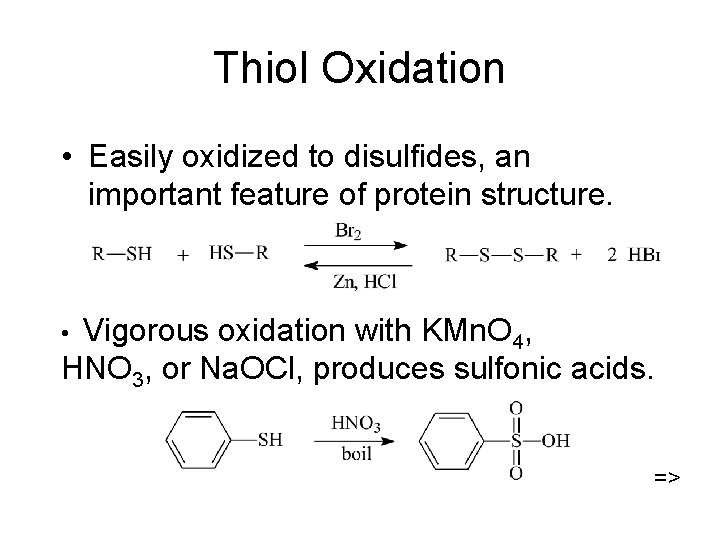
Thiol Oxidation • Easily oxidized to disulfides, an important feature of protein structure. Vigorous oxidation with KMn. O 4, HNO 3, or Na. OCl, produces sulfonic acids. • =>
 Synthesis of secondary alcohol
Synthesis of secondary alcohol Grignard reagent formula
Grignard reagent formula What is secondary alcohol
What is secondary alcohol Grignard reaksiyonu mekanizması
Grignard reaksiyonu mekanizması Acidity of phenol
Acidity of phenol Methyl cyanide with grignard reagent
Methyl cyanide with grignard reagent Acid chloride + grignard reagent
Acid chloride + grignard reagent Epoxide plus grignard
Epoxide plus grignard Grignard reagent structure
Grignard reagent structure Imine formation
Imine formation Preparation of alcohol from grignard reagent
Preparation of alcohol from grignard reagent Lucas reagent is
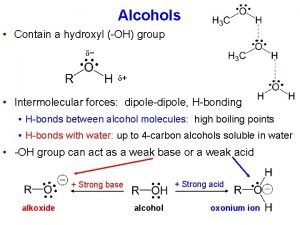
Lucas reagent is Acidity of alcohols
Acidity of alcohols Ethers naming
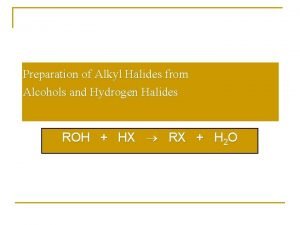
Ethers naming Hydrogen halide
Hydrogen halide Primary alcohol oxidation
Primary alcohol oxidation Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Erythritol production process
Erythritol production process Pentanal intermolecular forces
Pentanal intermolecular forces