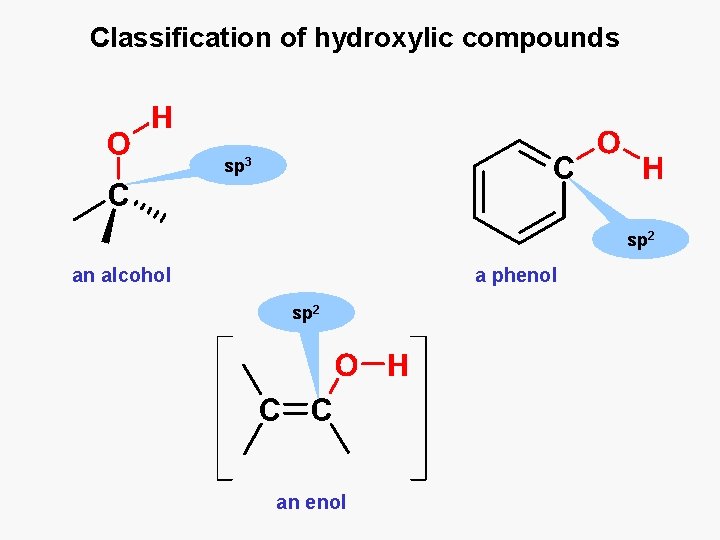
ALCOHOLS and PHENOLS Classification of hydroxylic compounds sp









- Slides: 56

ALCOHOLS and PHENOLS

Classification of hydroxylic compounds sp 3 sp 2 an alcohol a phenol sp 2 an enol

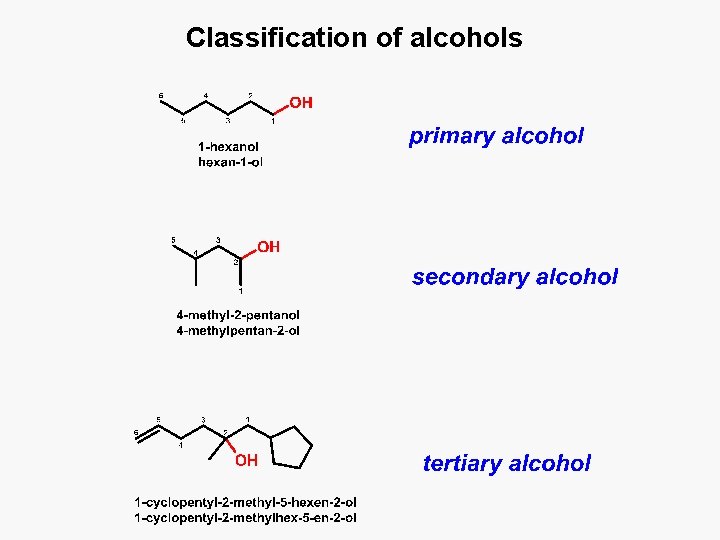
Classification of alcohols

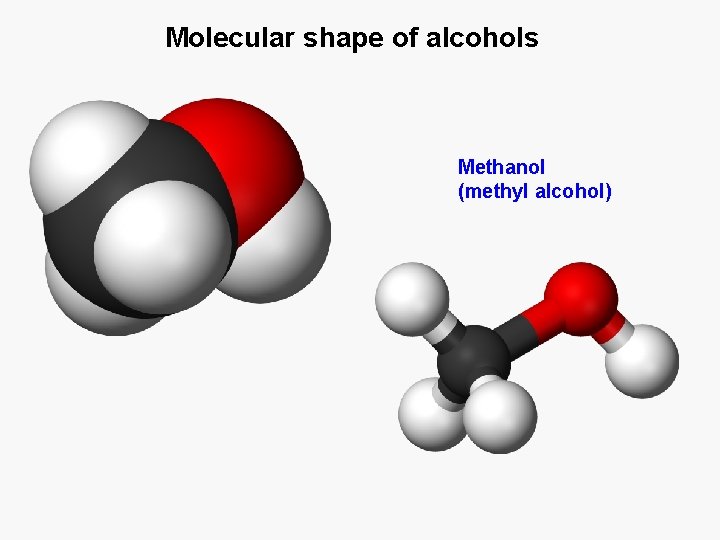
Molecular shape of alcohols Methanol (methyl alcohol)

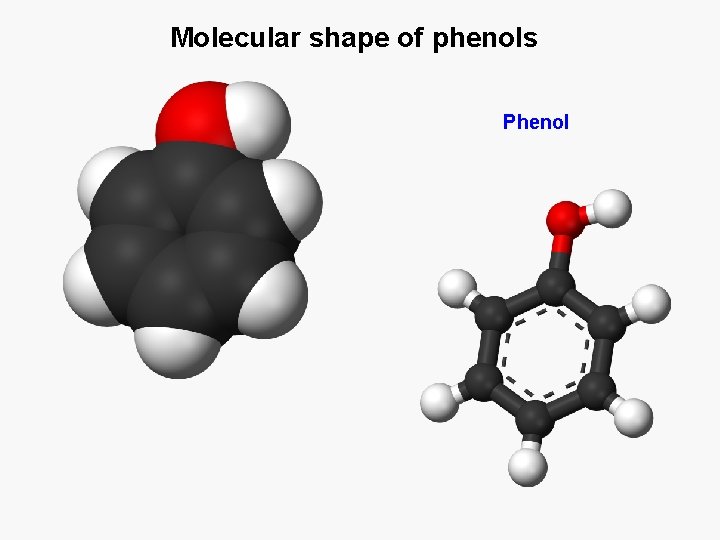
Molecular shape of phenols Phenol

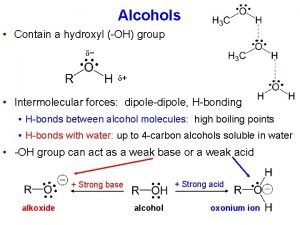
Properties of hydroxyl group • Polarity • Hydrogen bonding • Solubility in water • Basicity • Acidity

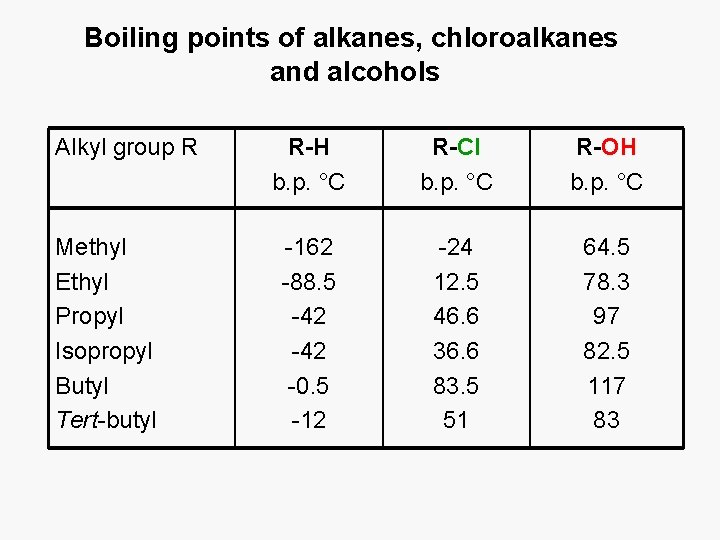
Boiling points of alkanes, chloroalkanes and alcohols Alkyl group R Methyl Ethyl Propyl Isopropyl Butyl Tert-butyl R-H b. p. °C R-Cl b. p. °C R-OH b. p. °C -162 -88. 5 -42 -0. 5 -12 -24 12. 5 46. 6 36. 6 83. 5 51 64. 5 78. 3 97 82. 5 117 83

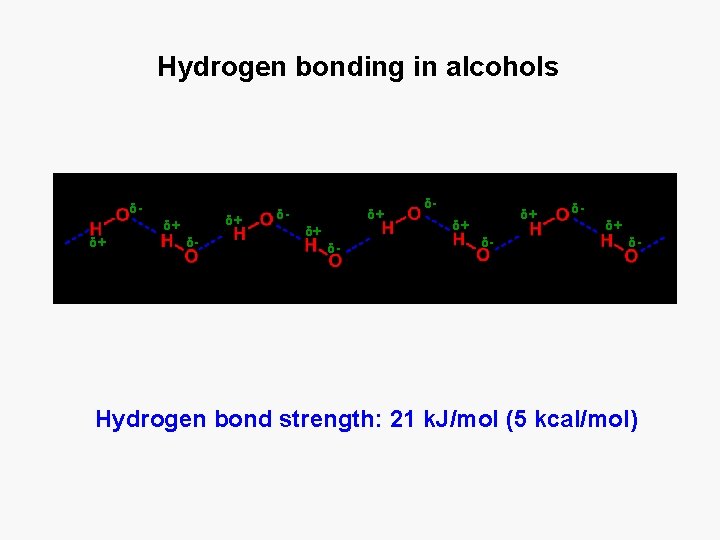
Hydrogen bonding in alcohols δ- δ+ δ+ δ+ δ- δ- δ+ δ+ δ- δδ+ δ- Hydrogen bond strength: 21 k. J/mol (5 kcal/mol)

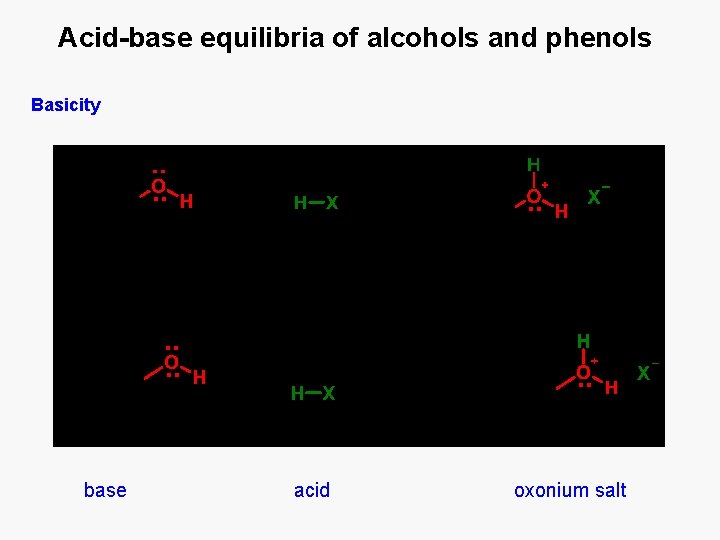
Acid-base equilibria of alcohols and phenols Basicity base acid oxonium salt

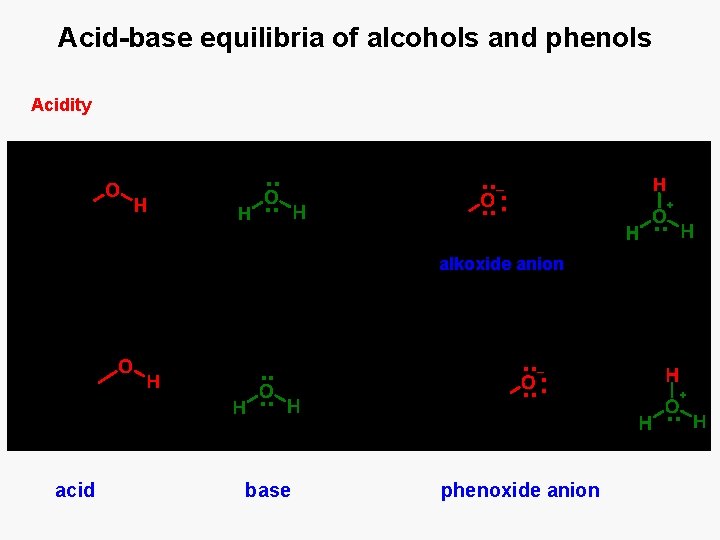
Acid-base equilibria of alcohols and phenols Acidity alkoxide anion acid base phenoxide anion

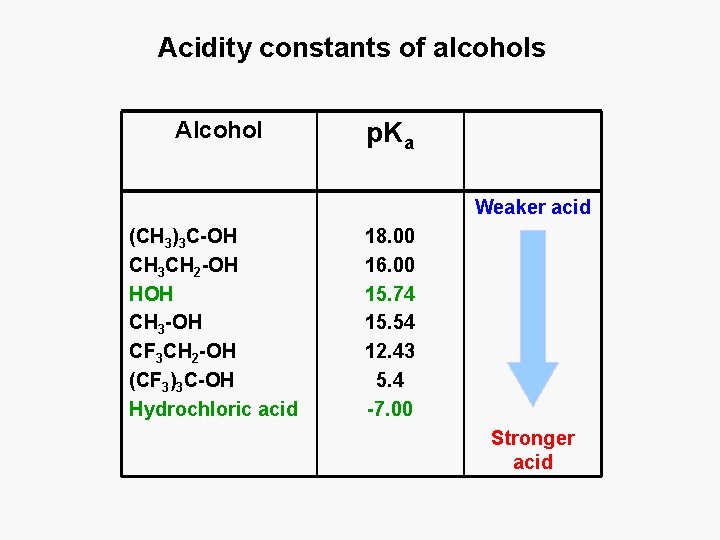
Acidity constants of alcohols Alcohol p. Ka Weaker acid (CH 3)3 C-OH CH 3 CH 2 -OH HOH CH 3 -OH CF 3 CH 2 -OH (CF 3)3 C-OH Hydrochloric acid 18. 00 16. 00 15. 74 15. 54 12. 43 5. 4 -7. 00 Stronger acid

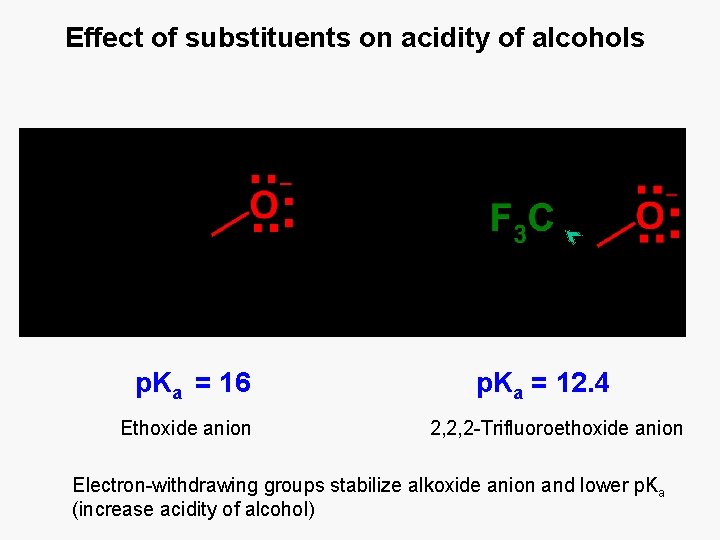
Effect of substituents on acidity of alcohols p. Ka = 16 Ethoxide anion p. Ka = 12. 4 2, 2, 2 -Trifluoroethoxide anion Electron-withdrawing groups stabilize alkoxide anion and lower p. Ka (increase acidity of alcohol)

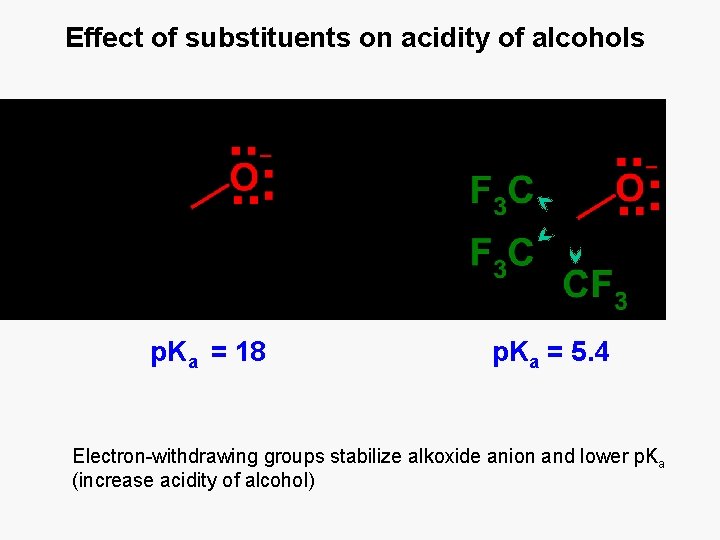
Effect of substituents on acidity of alcohols p. Ka = 18 p. Ka = 5. 4 Electron-withdrawing groups stabilize alkoxide anion and lower p. Ka (increase acidity of alcohol)

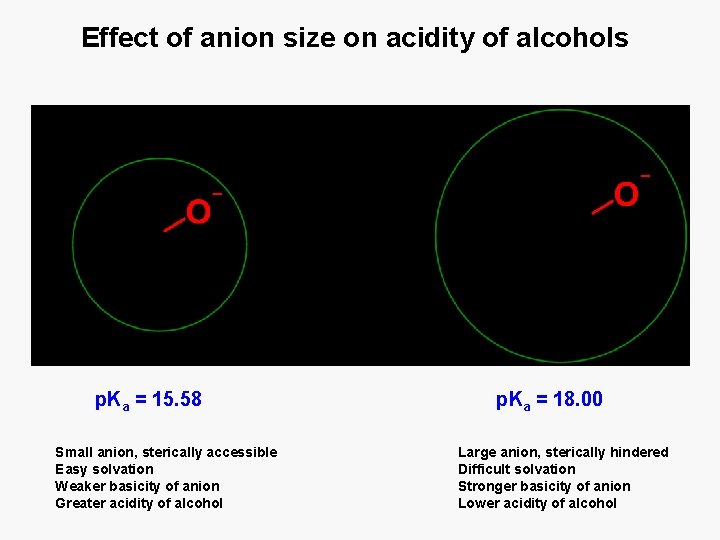
Effect of anion size on acidity of alcohols p. Ka = 15. 58 Small anion, sterically accessible Easy solvation Weaker basicity of anion Greater acidity of alcohol p. Ka = 18. 00 Large anion, sterically hindered Difficult solvation Stronger basicity of anion Lower acidity of alcohol

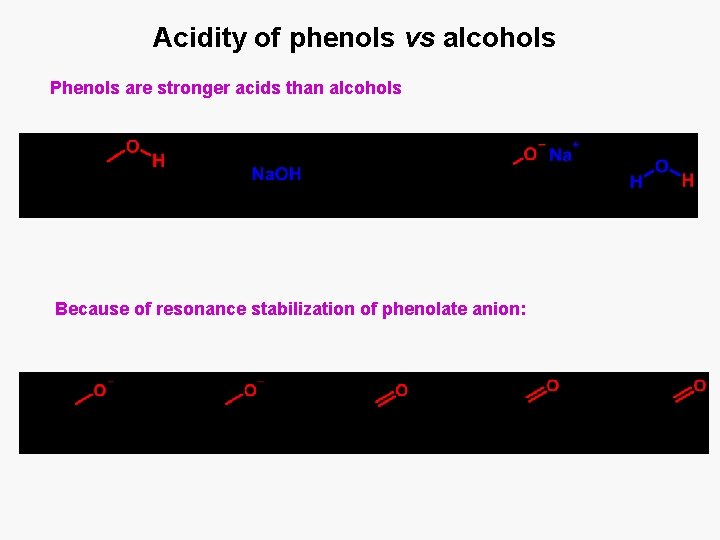
Acidity of phenols vs alcohols Phenols are stronger acids than alcohols Because of resonance stabilization of phenolate anion:

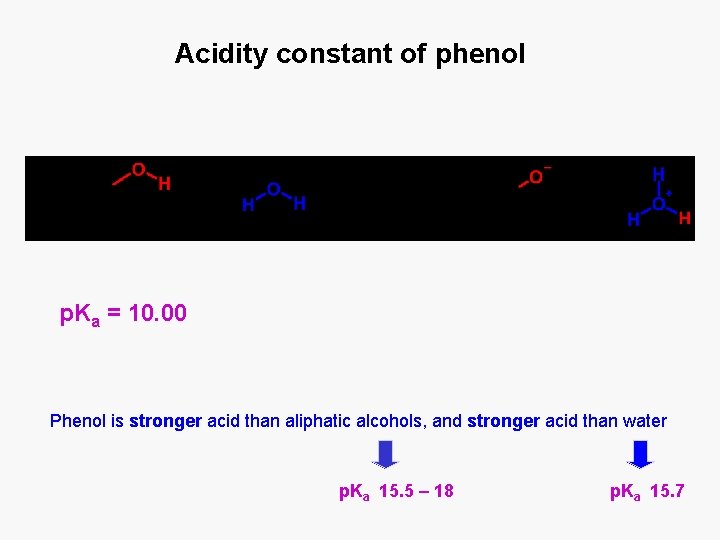
Acidity constant of phenol p. Ka = 10. 00 Phenol is stronger acid than aliphatic alcohols, and stronger acid than water p. Ka 15. 5 – 18 p. Ka 15. 7

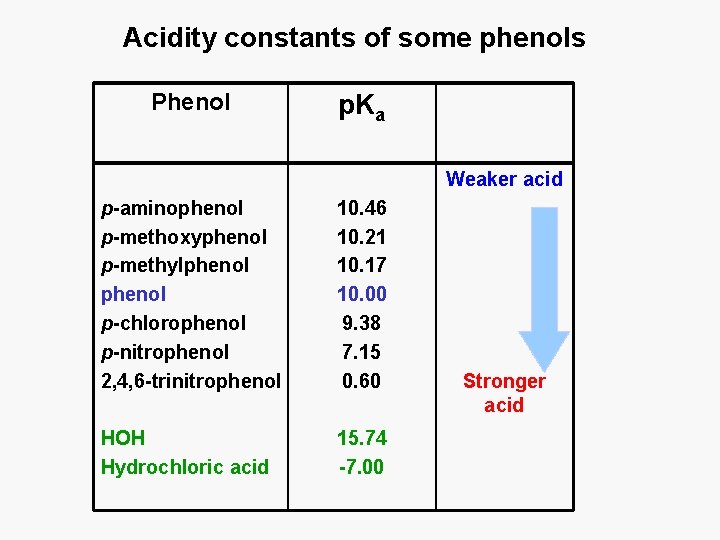
Acidity constants of some phenols Phenol p. Ka Weaker acid p-aminophenol p-methoxyphenol p-methylphenol p-chlorophenol p-nitrophenol 2, 4, 6 -trinitrophenol 10. 46 10. 21 10. 17 10. 00 9. 38 7. 15 0. 60 HOH Hydrochloric acid 15. 74 -7. 00 Stronger acid

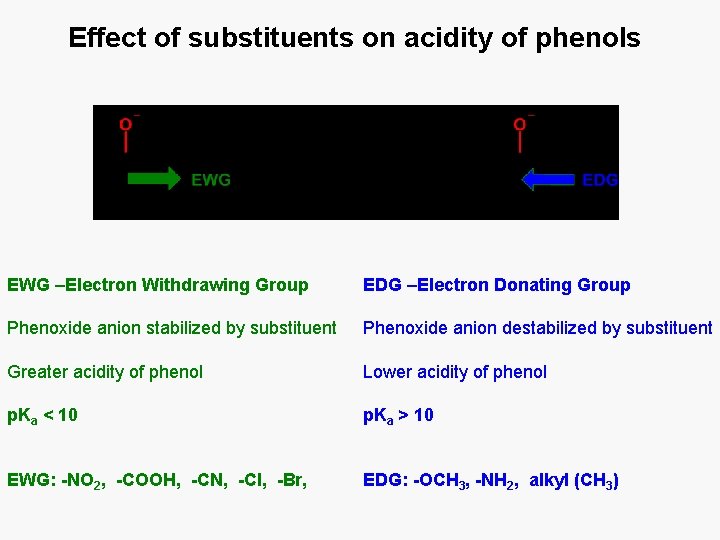
Effect of substituents on acidity of phenols EWG –Electron Withdrawing Group EDG –Electron Donating Group Phenoxide anion stabilized by substituent Phenoxide anion destabilized by substituent Greater acidity of phenol Lower acidity of phenol p. Ka < 10 p. Ka > 10 EWG: -NO 2, -COOH, -CN, -Cl, -Br, EDG: -OCH 3, -NH 2, alkyl (CH 3)

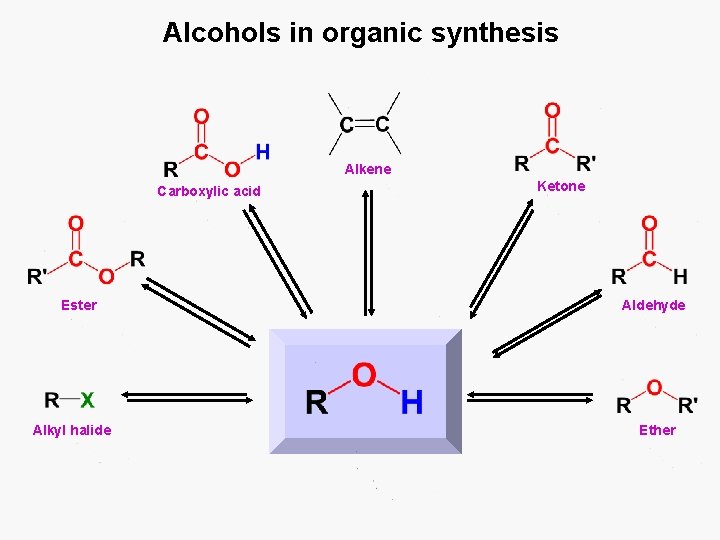
Alcohols in organic synthesis Alkene Carboxylic acid Ester Alkyl halide Ketone Aldehyde Ether

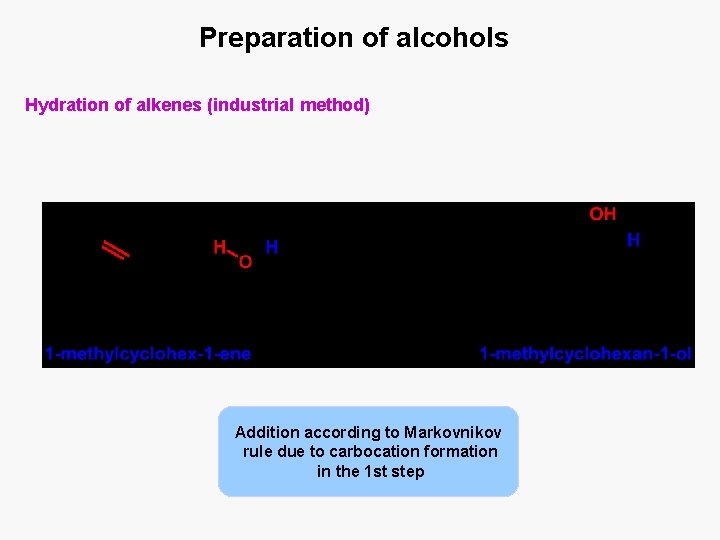
Preparation of alcohols Hydration of alkenes (industrial method) Addition according to Markovnikov rule due to carbocation formation in the 1 st step

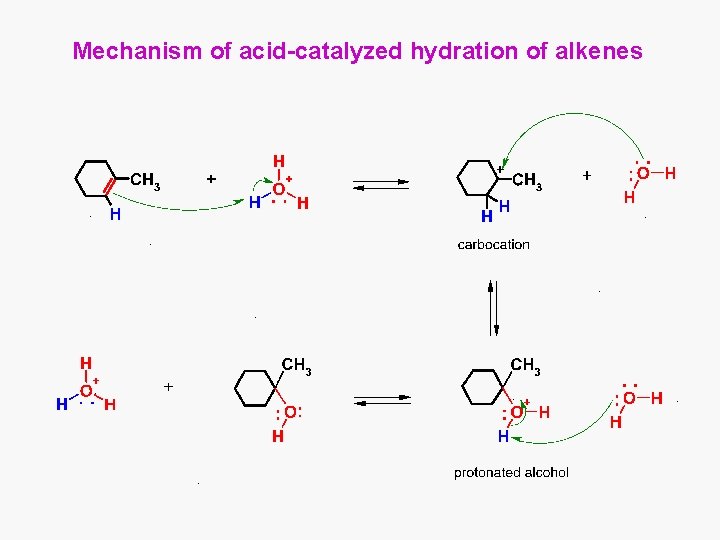
Mechanism of acid-catalyzed hydration of alkenes

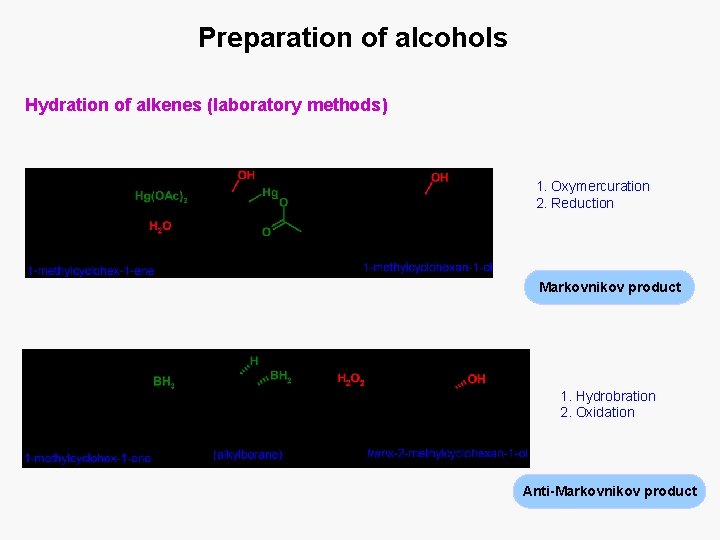
Preparation of alcohols Hydration of alkenes (laboratory methods) 1. Oxymercuration 2. Reduction Markovnikov product 1. Hydrobration 2. Oxidation Anti-Markovnikov product

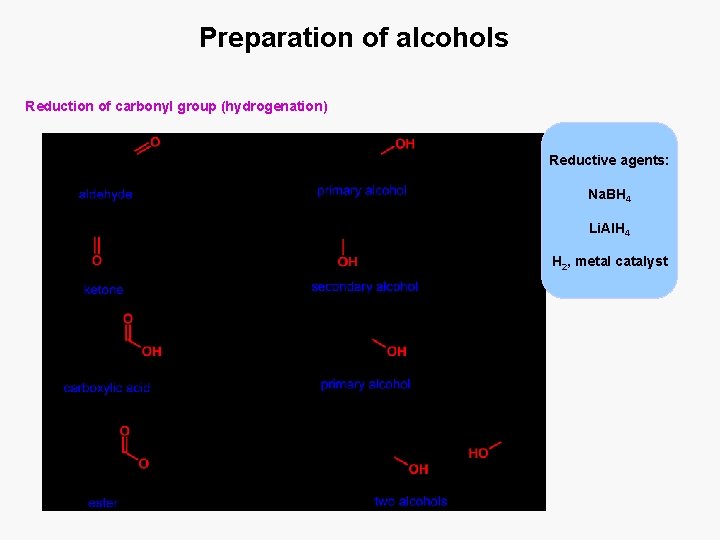
Preparation of alcohols Reduction of carbonyl group (hydrogenation) Reductive agents: Na. BH 4 Li. Al. H 4 H 2, metal catalyst

Preparation of alcohols Preparation of Grignard’s reagents from alkyl halides + Electrophile Nucleophile R = alkyl, aryl, alkenyl -

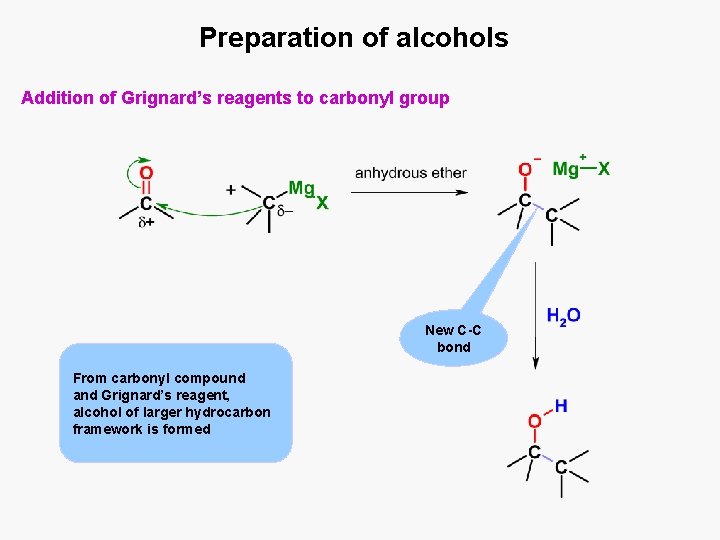
Preparation of alcohols Addition of Grignard’s reagents to carbonyl group New C-C bond From carbonyl compound and Grignard’s reagent, alcohol of larger hydrocarbon framework is formed

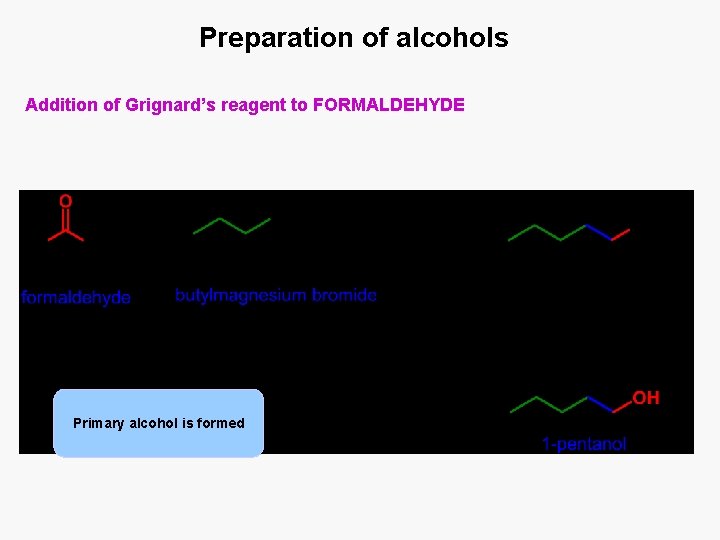
Preparation of alcohols Addition of Grignard’s reagent to FORMALDEHYDE Primary alcohol is formed

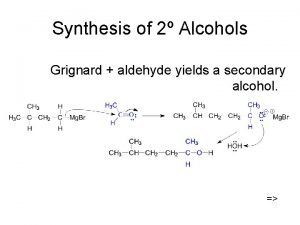
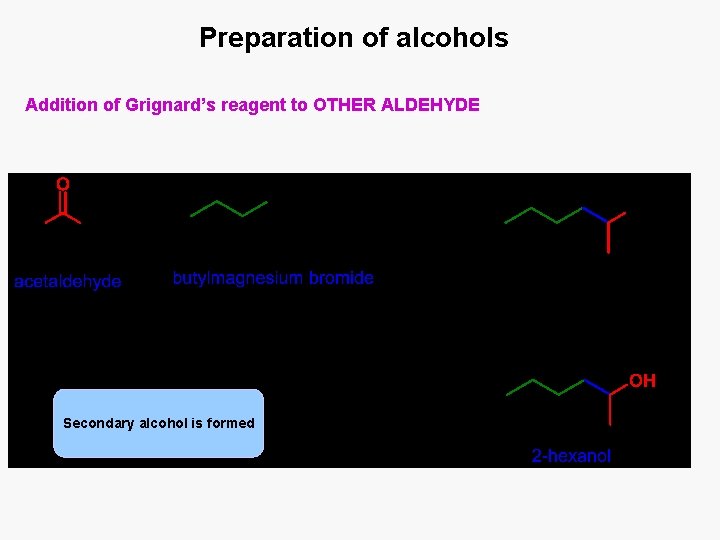
Preparation of alcohols Addition of Grignard’s reagent to OTHER ALDEHYDE Secondary alcohol is formed

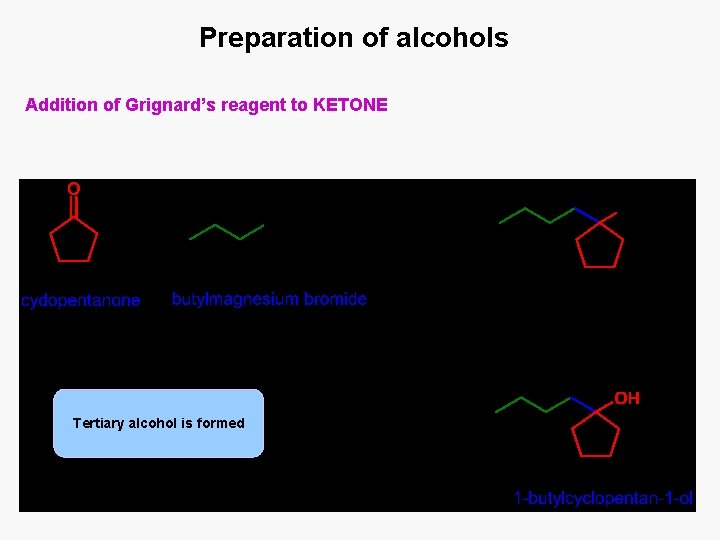
Preparation of alcohols Addition of Grignard’s reagent to KETONE Tertiary alcohol is formed

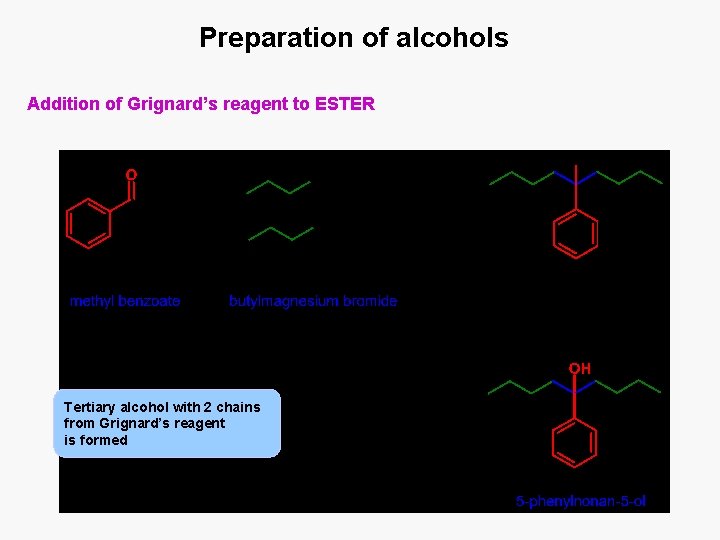
Preparation of alcohols Addition of Grignard’s reagent to ESTER Tertiary alcohol with 2 chains from Grignard’s reagent is formed

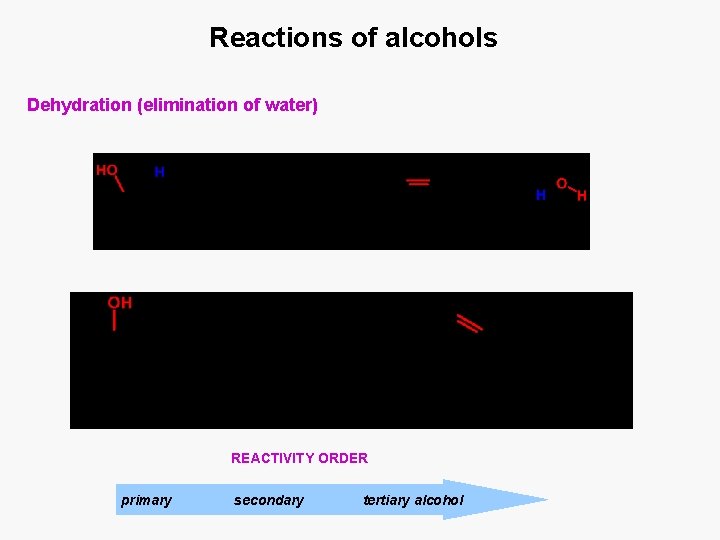
Reactions of alcohols Dehydration (elimination of water) REACTIVITY ORDER primary secondary tertiary alcohol

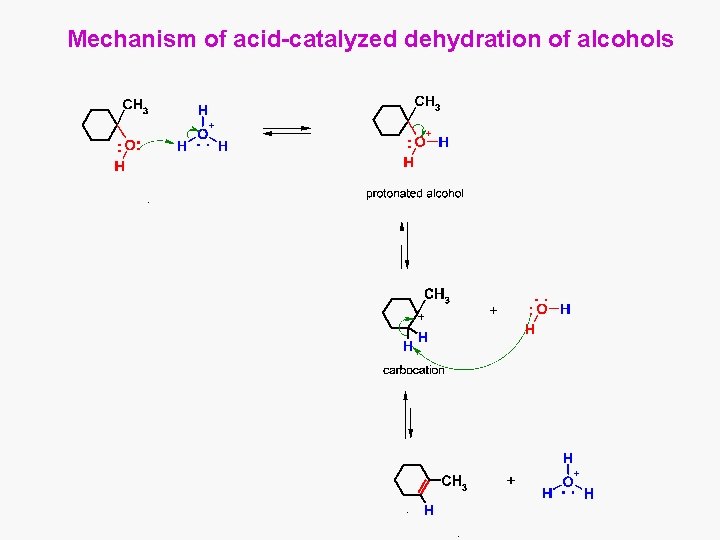
Mechanism of acid-catalyzed dehydration of alcohols

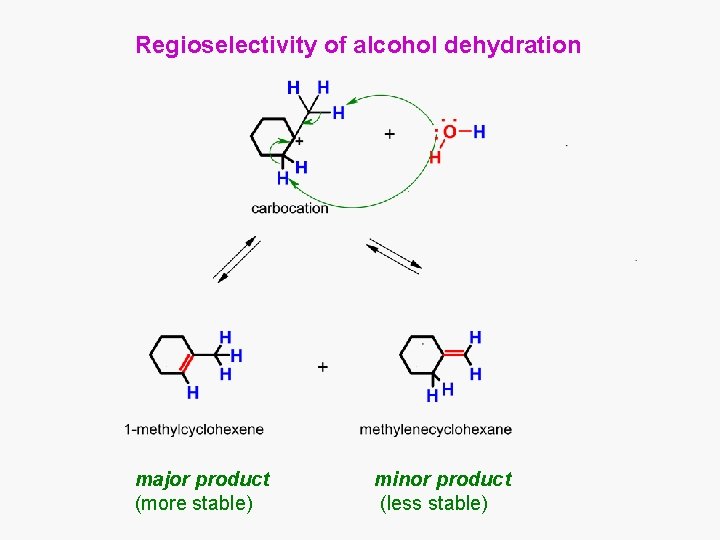
Regioselectivity of alcohol dehydration major product (more stable) minor product (less stable)

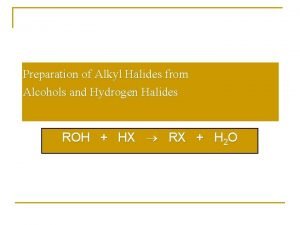
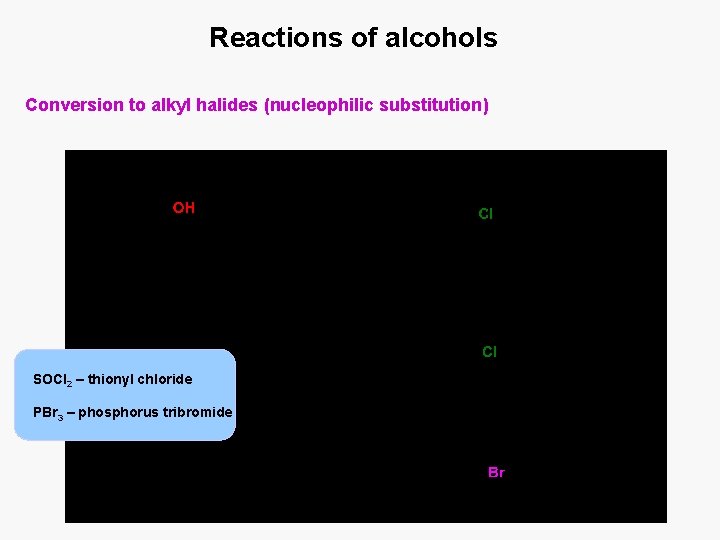
Reactions of alcohols Conversion to alkyl halides (nucleophilic substitution) SOCl 2 – thionyl chloride PBr 3 – phosphorus tribromide

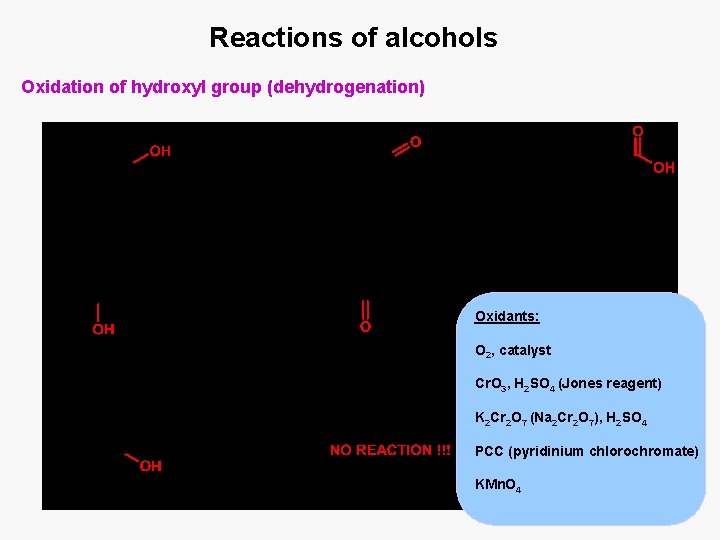
Reactions of alcohols Oxidation of hydroxyl group (dehydrogenation) Oxidants: O 2, catalyst Cr. O 3, H 2 SO 4 (Jones reagent) K 2 Cr 2 O 7 (Na 2 Cr 2 O 7), H 2 SO 4 PCC (pyridinium chlorochromate) KMn. O 4

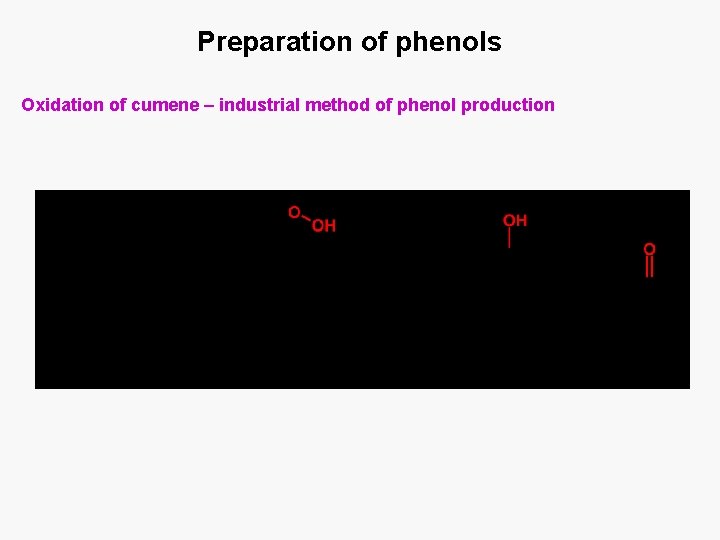
Preparation of phenols Oxidation of cumene – industrial method of phenol production

Preparation of phenols Thermal decomposition of arylsufonic acids

Reactivity of phenols • Aromatic ring susceptible for electrophilic substitution (alkylation, nitration, halogenation etc. ) • Hydroxyl group does not react in nucleophilic substitution • Hydroxyl proton more acidic than in aliphatic alcohols • Different way of oxidation

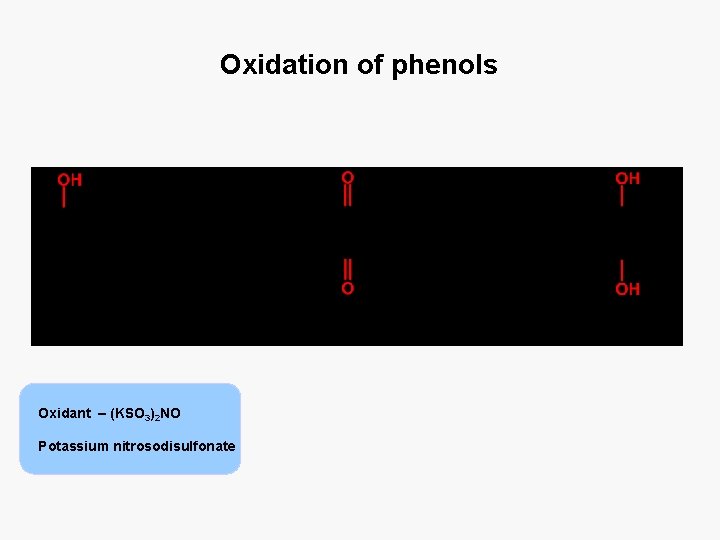
Oxidation of phenols Oxidant – (KSO 3)2 NO Potassium nitrosodisulfonate

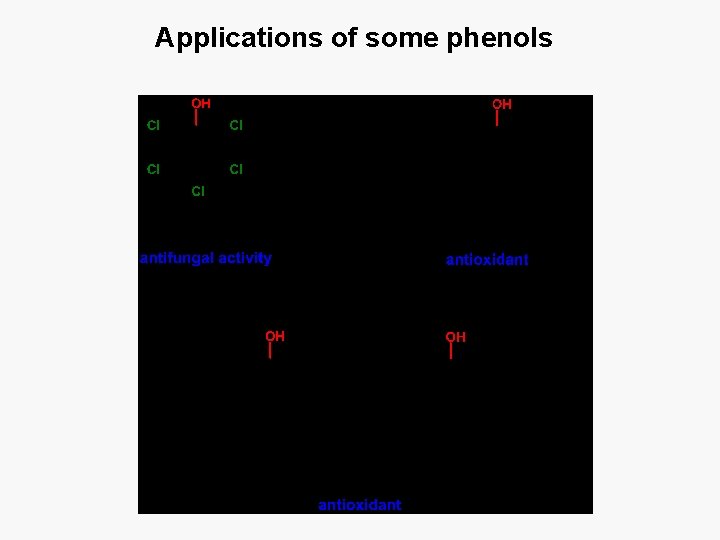
Applications of some phenols

ETHERS

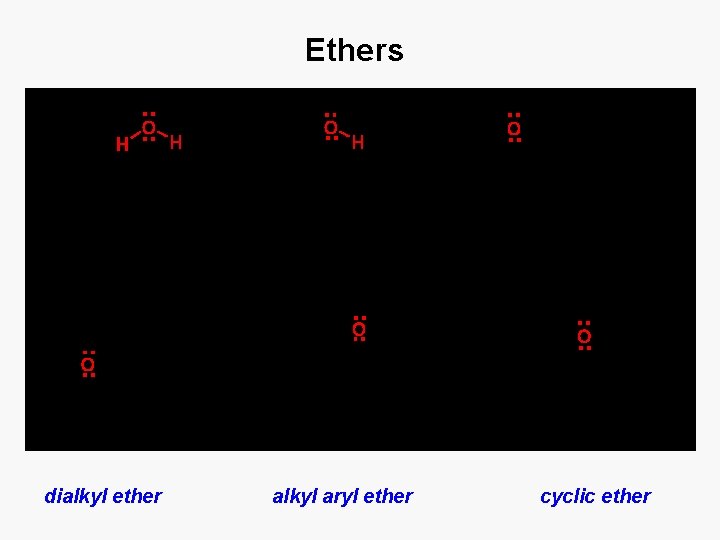
Ethers dialkyl ether alkyl aryl ether cyclic ether

Ethers diethyl ether anisole tetrahydrofuran

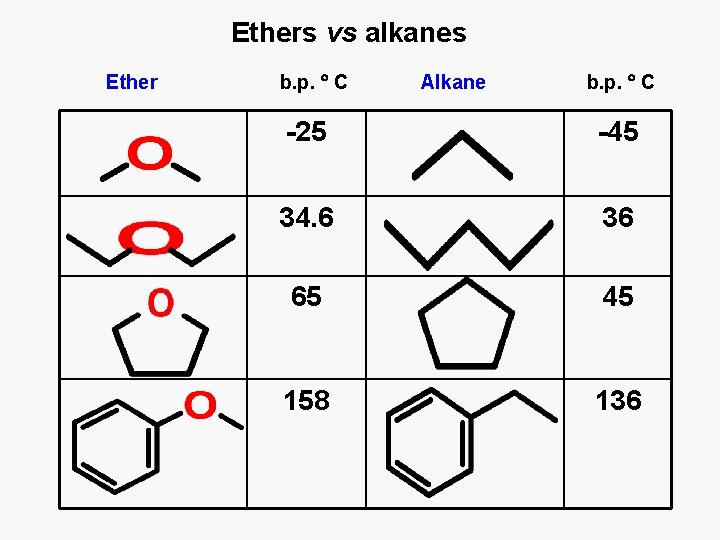
Ethers vs alkanes Ether b. p. C Alkane b. p. C -25 -45 34. 6 36 65 45 158 136

Properties of ethers Comparison to alcohols and alkanes • More polar than alkanes, less than alcohols • No hydrogen bonding • Boiling points – higher than for alkanes, lower than for alcohols • Solubility in water limited • Neutral - nor basic nor acidic • Used frequently as solvents

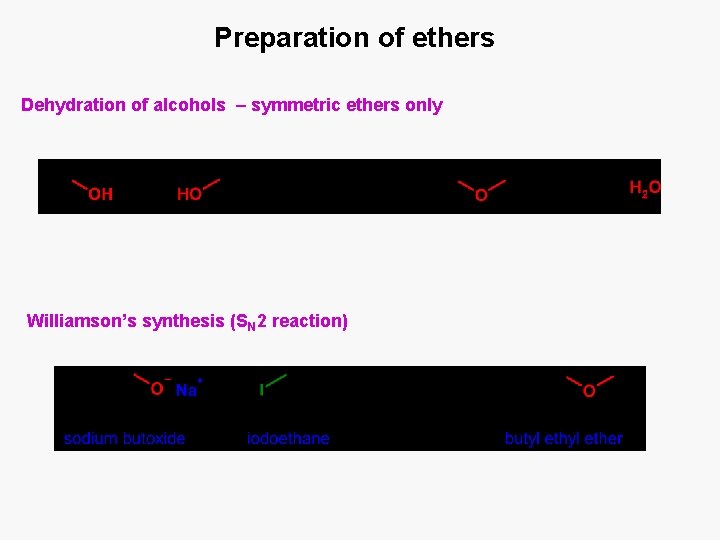
Preparation of ethers Dehydration of alcohols – symmetric ethers only Williamson’s synthesis (SN 2 reaction)

Other example of Williamson’s synthesis

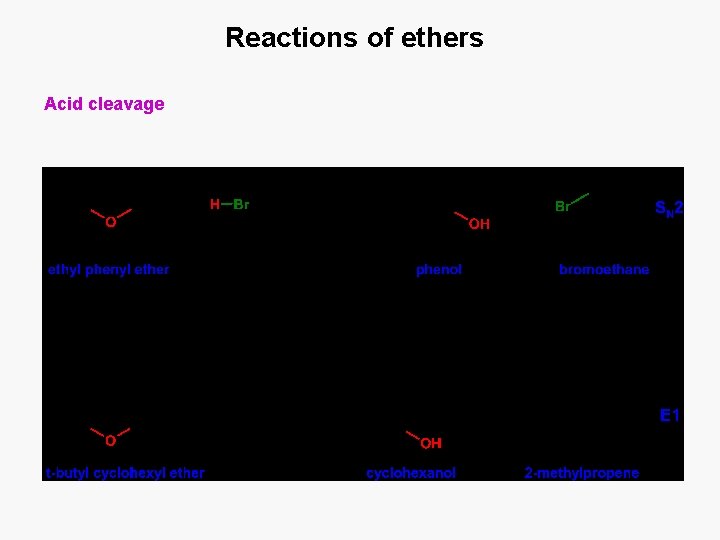
Reactions of ethers Acid cleavage

Claisen rearrangement of allyl ethers

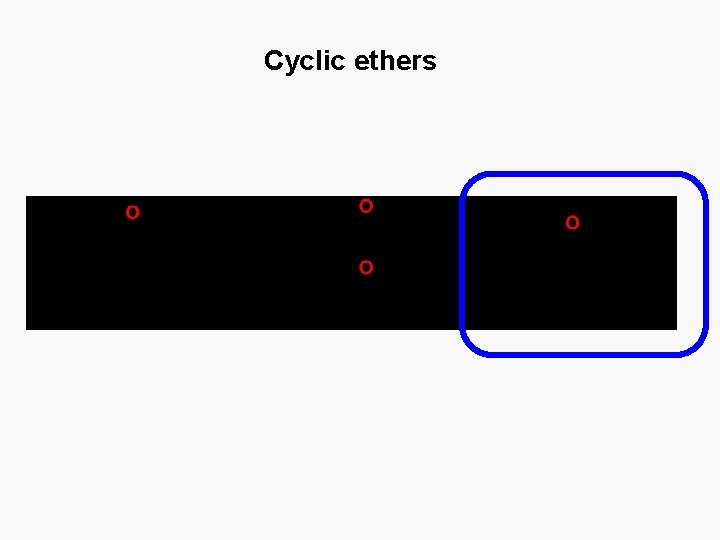
Cyclic ethers

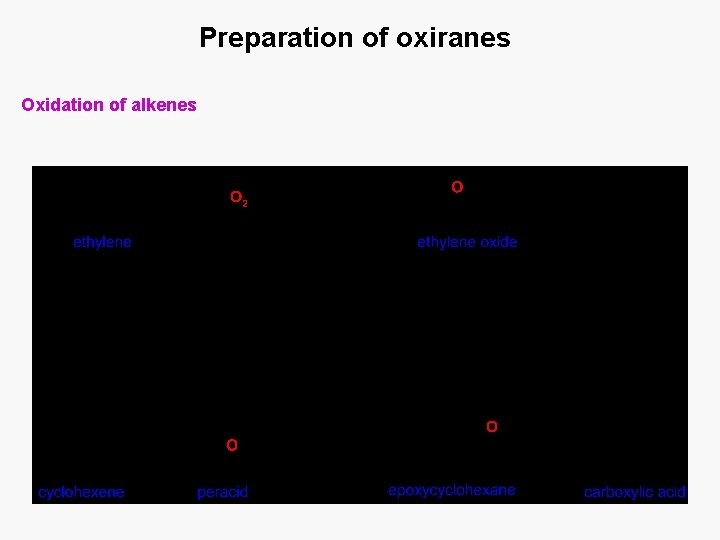
Preparation of oxiranes Oxidation of alkenes

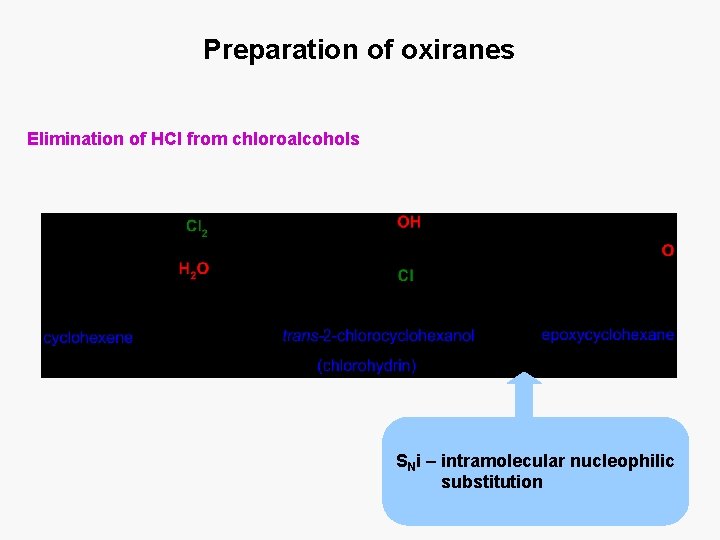
Preparation of oxiranes Elimination of HCl from chloroalcohols SNi – intramolecular nucleophilic substitution

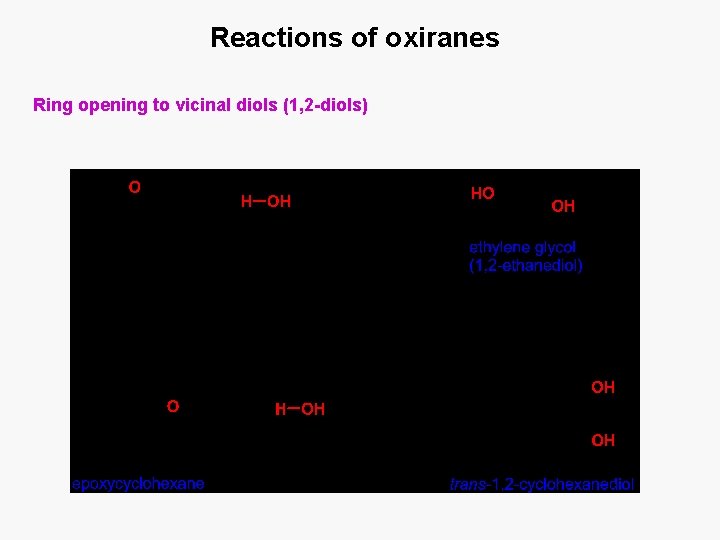
Reactions of oxiranes Ring opening to vicinal diols (1, 2 -diols)

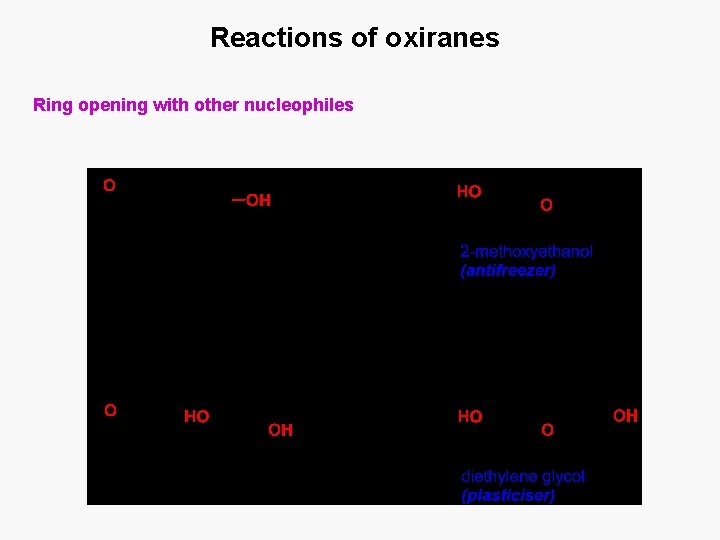
Reactions of oxiranes Ring opening with other nucleophiles

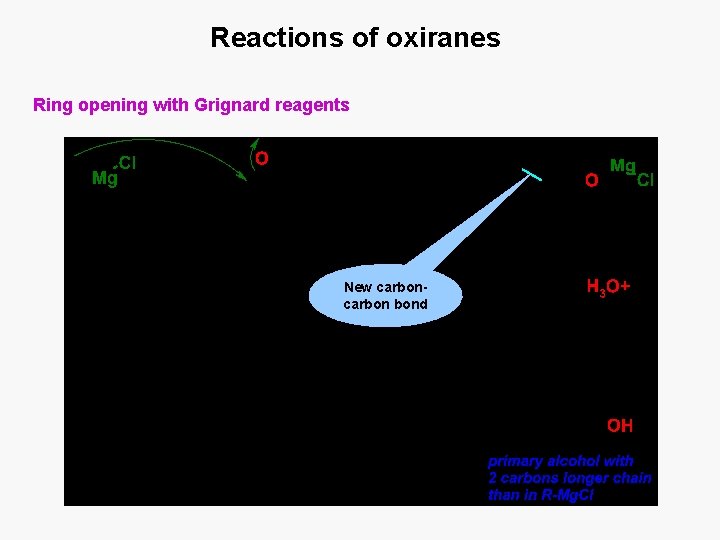
Reactions of oxiranes Ring opening with Grignard reagents New carbon bond

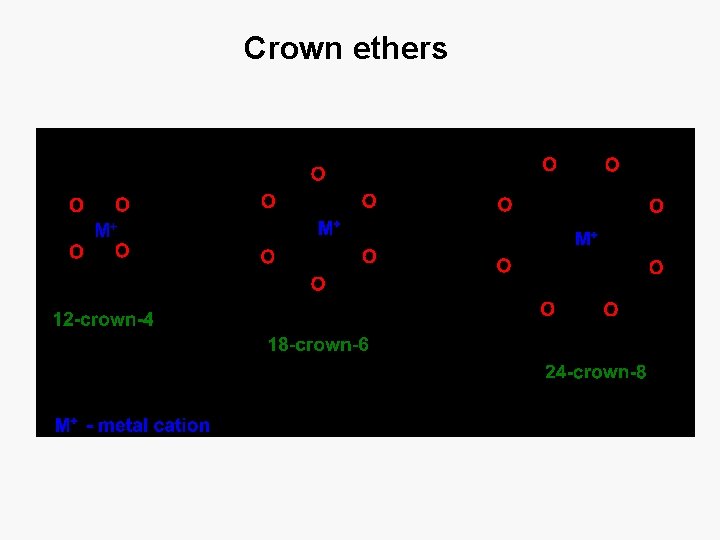
Crown ethers

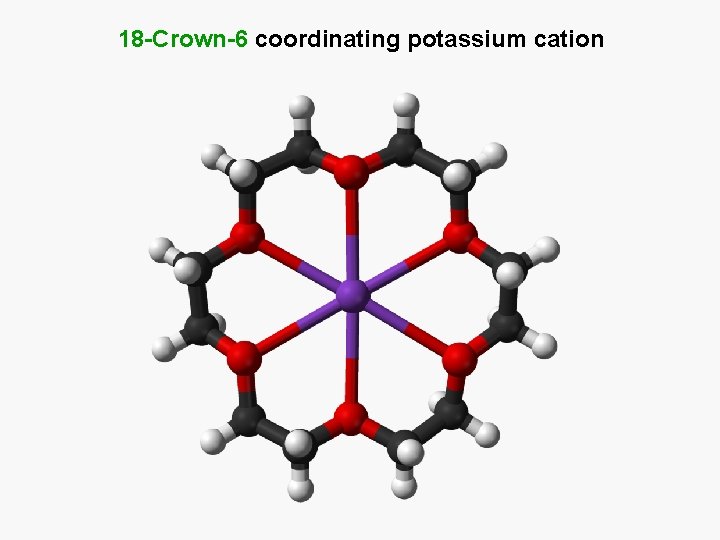
18 -Crown-6 coordinating potassium cation
 Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Ester naming
Ester naming Polyphenols classification
Polyphenols classification Venn diagram of ionic covalent and metallic bonds
Venn diagram of ionic covalent and metallic bonds Primary aldehyde
Primary aldehyde Secondary alcohol to aldehyde
Secondary alcohol to aldehyde Alcohols containing cppp nucleus
Alcohols containing cppp nucleus High boiling point alcohols
High boiling point alcohols Ch3ch2ch2oh oxidation
Ch3ch2ch2oh oxidation Lucas reagent equation
Lucas reagent equation Preparing haloalkanes from alcohols
Preparing haloalkanes from alcohols Reactions of alcohols 1 chemsheets answers
Reactions of alcohols 1 chemsheets answers Tscl py reaction
Tscl py reaction Acidity of alcohols
Acidity of alcohols Relative sweetness chart
Relative sweetness chart Alcohols nomenclature
Alcohols nomenclature Naming alkyl halides
Naming alkyl halides Naming ethers
Naming ethers Isopropyl methyl ether
Isopropyl methyl ether Alcohol and hbr
Alcohol and hbr Lucas test
Lucas test What is the classification of organic compounds
What is the classification of organic compounds Differential extraction
Differential extraction Types of organometallic compounds
Types of organometallic compounds Sextext of electron
Sextext of electron Six membered heterocyclic compound
Six membered heterocyclic compound Manifold classification in statistics
Manifold classification in statistics Eager learner and lazy learner
Eager learner and lazy learner Traditional classification vs modern classification
Traditional classification vs modern classification Is maple syrup a homogeneous mixture
Is maple syrup a homogeneous mixture Elements and compounds examples
Elements and compounds examples Pure substances and mixtures worksheet answers
Pure substances and mixtures worksheet answers Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Naming compounds and writing formulas
Naming compounds and writing formulas Atoms elements molecules and compounds worksheet
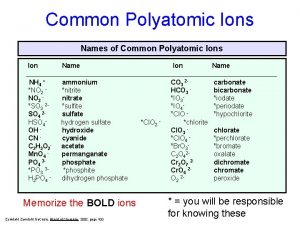
Atoms elements molecules and compounds worksheet Types of polyatomic ions
Types of polyatomic ions Elements and compounds examples
Elements and compounds examples Elements and compounds examples
Elements and compounds examples Purification and characterization of organic compounds
Purification and characterization of organic compounds Empirical formula pogil
Empirical formula pogil Prefixes for hydrates
Prefixes for hydrates Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Sulphur and its compounds klb notes
Sulphur and its compounds klb notes What is the chemical formula for tetranitrogen heptoxide?
What is the chemical formula for tetranitrogen heptoxide? Polyatomic compounds
Polyatomic compounds Why do ionic compounds have high melting and boiling points
Why do ionic compounds have high melting and boiling points Difference between mixture and compund
Difference between mixture and compund Types of matter elements compounds and mixtures
Types of matter elements compounds and mixtures Chemsheets
Chemsheets Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 7 ionic compounds and metals
Chapter 7 ionic compounds and metals Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Klb chemistry book 3 nitrogen and its compounds
Klb chemistry book 3 nitrogen and its compounds Indefinite pronouns structure
Indefinite pronouns structure Laboratory equipment
Laboratory equipment Mixtures images
Mixtures images Compounds formed between metal and nonmetals
Compounds formed between metal and nonmetals