Organic Chemistry Second Edition David Klein Chapter 13




















- Slides: 93

Organic Chemistry Second Edition David Klein Chapter 13 Alcohols and Phenols Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

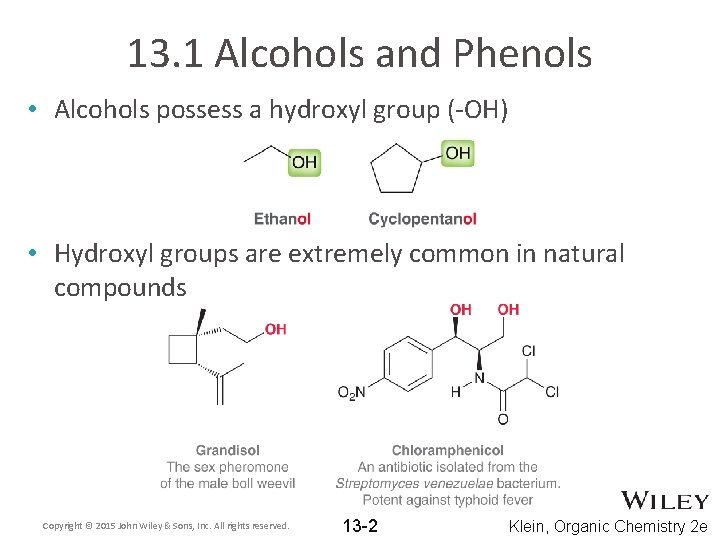
13. 1 Alcohols and Phenols • Alcohols possess a hydroxyl group (-OH) • Hydroxyl groups are extremely common in natural compounds Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -2 Klein, Organic Chemistry 2 e

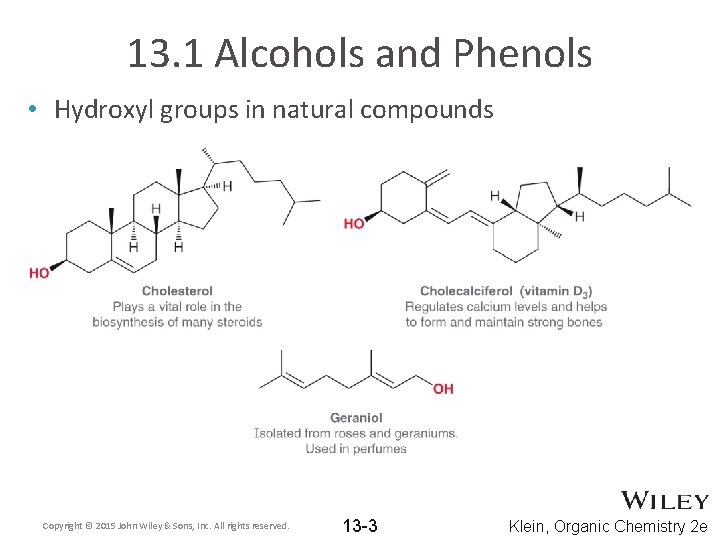
13. 1 Alcohols and Phenols • Hydroxyl groups in natural compounds Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -3 Klein, Organic Chemistry 2 e

13. 1 Alcohols and Phenols • Phenols possess a hydroxyl group directly attached to an aromatic ring Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -4 Klein, Organic Chemistry 2 e

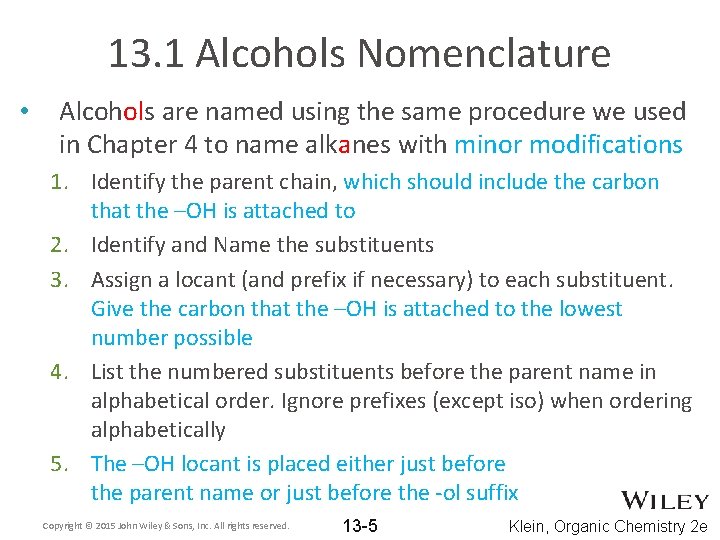
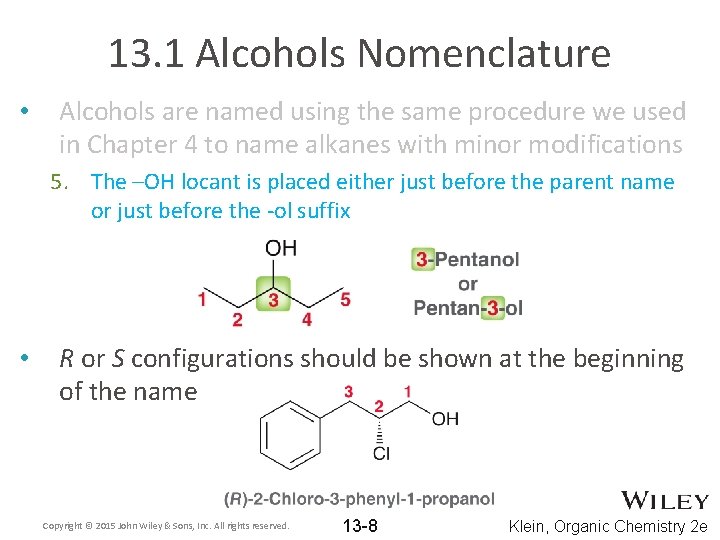
13. 1 Alcohols Nomenclature • Alcohols are named using the same procedure we used in Chapter 4 to name alkanes with minor modifications 1. Identify the parent chain, which should include the carbon that the –OH is attached to 2. Identify and Name the substituents 3. Assign a locant (and prefix if necessary) to each substituent. Give the carbon that the –OH is attached to the lowest number possible 4. List the numbered substituents before the parent name in alphabetical order. Ignore prefixes (except iso) when ordering alphabetically 5. The –OH locant is placed either just before the parent name or just before the -ol suffix Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -5 Klein, Organic Chemistry 2 e

13. 1 Alcohols Nomenclature • Alcohols are named using the same procedure we used in Chapter 4 to name alkanes with minor modifications 1. Identify the parent chain Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -6 Klein, Organic Chemistry 2 e

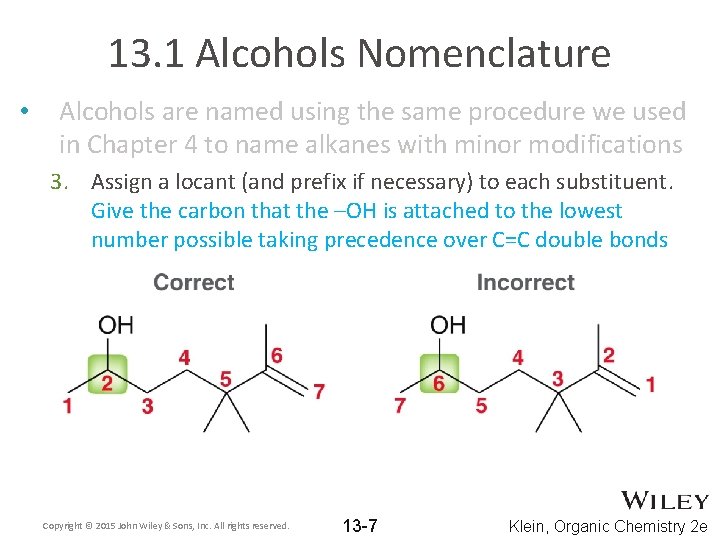
13. 1 Alcohols Nomenclature • Alcohols are named using the same procedure we used in Chapter 4 to name alkanes with minor modifications 3. Assign a locant (and prefix if necessary) to each substituent. Give the carbon that the –OH is attached to the lowest number possible taking precedence over C=C double bonds Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -7 Klein, Organic Chemistry 2 e

13. 1 Alcohols Nomenclature • Alcohols are named using the same procedure we used in Chapter 4 to name alkanes with minor modifications 5. The –OH locant is placed either just before the parent name or just before the -ol suffix • R or S configurations should be shown at the beginning of the name Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -8 Klein, Organic Chemistry 2 e

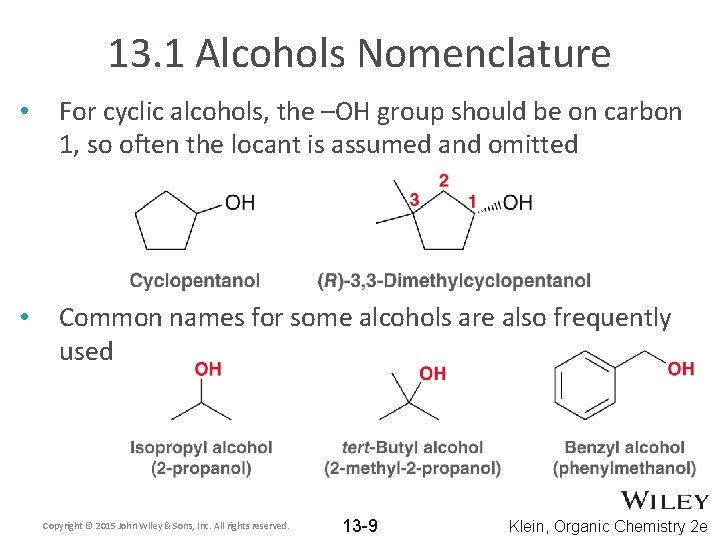
13. 1 Alcohols Nomenclature • For cyclic alcohols, the –OH group should be on carbon 1, so often the locant is assumed and omitted • Common names for some alcohols are also frequently used Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -9 Klein, Organic Chemistry 2 e

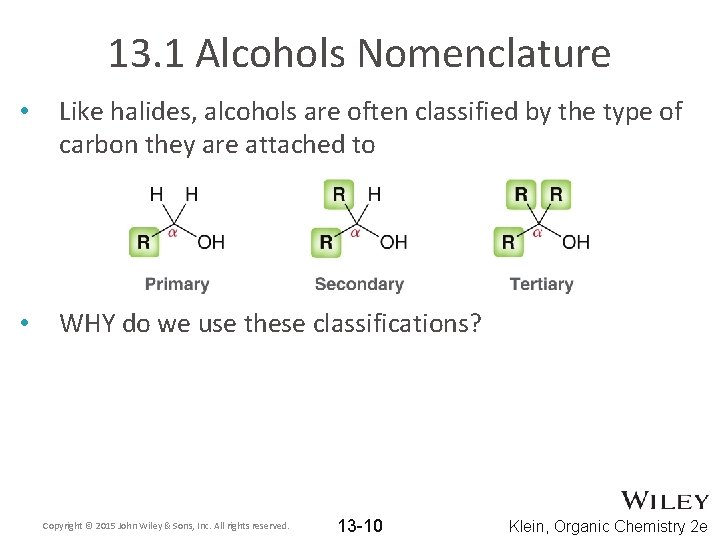
13. 1 Alcohols Nomenclature • Like halides, alcohols are often classified by the type of carbon they are attached to • WHY do we use these classifications? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -10 Klein, Organic Chemistry 2 e

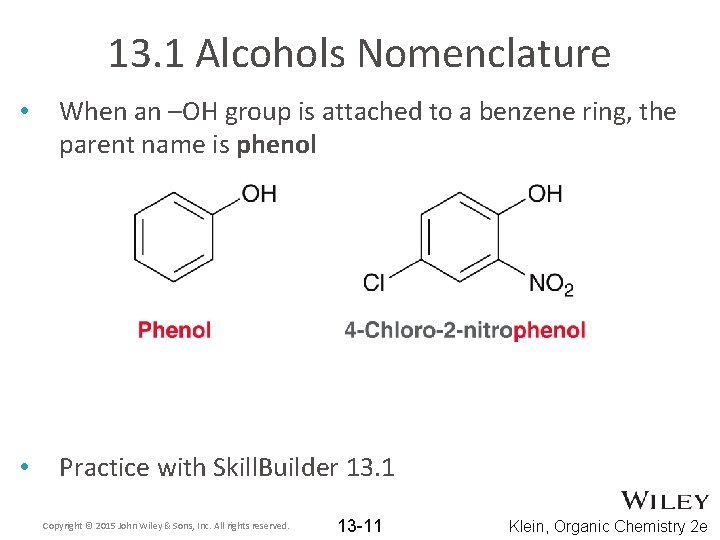
13. 1 Alcohols Nomenclature • When an –OH group is attached to a benzene ring, the parent name is phenol • Practice with Skill. Builder 13. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -11 Klein, Organic Chemistry 2 e

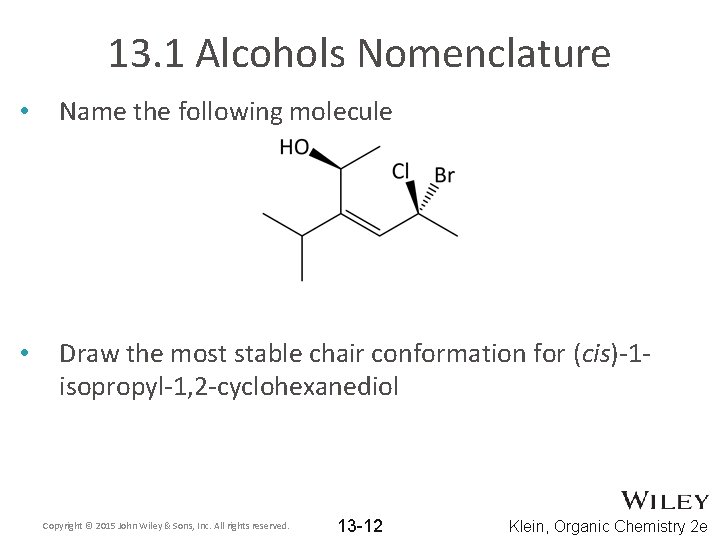
13. 1 Alcohols Nomenclature • Name the following molecule • Draw the most stable chair conformation for (cis)-1 isopropyl-1, 2 -cyclohexanediol Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -12 Klein, Organic Chemistry 2 e

13. 1 Commercially Important Alcohols • • • Methanol (CH 3 OH) is the simplest alcohol With a suitable catalyst, about 2 billion gallons of methanol is made industrially from CO 2 and H 2 every year Methanol is poisonous, but it has many uses 1. Solvent 2. Precursor for chemical syntheses 3. Fuel Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -13 Klein, Organic Chemistry 2 e

13. 1 Commercially Important Alcohols • • • Ethanol (CH 3 CH 2 OH) has been produced by fermentation for thousands of years. HOW? About 5 billion gallons of ethanol is made industrially from the acid-catalyzed hydration of ethylene every year Ethanol has many uses 1. Solvent, precursor for chemical syntheses, fuel 2. Human consumption – ethanol suitable for drinking is heavily taxed. Ethanol used for purposes other than drinking is often denatured. WHY? • Is it poisonous? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -14 Klein, Organic Chemistry 2 e

13. 1 Commercially Important Alcohols • • • Isopropanol is rubbing alcohol. Draw its structure Isopropanol is made industrially from the acid-catalyzed hydration of propylene Isopropanol is poisonous, but it has many uses 1. Industrial solvent 2. Antiseptic 3. Gasoline additive Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -15 Klein, Organic Chemistry 2 e

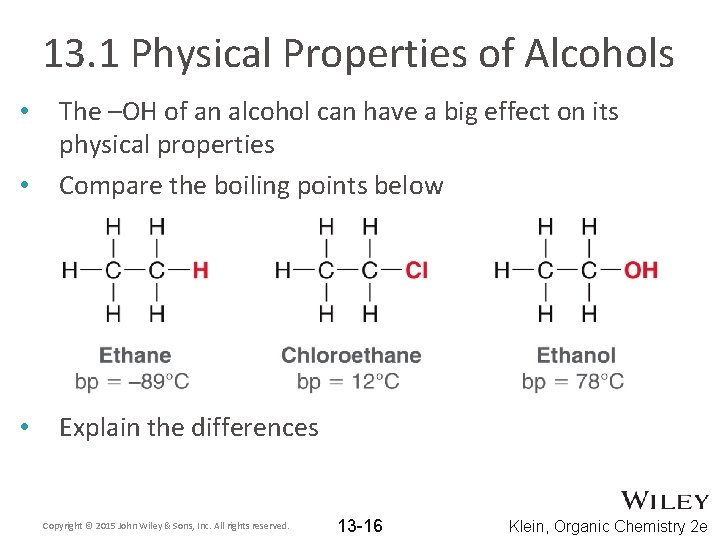
13. 1 Physical Properties of Alcohols • The –OH of an alcohol can have a big effect on its physical properties Compare the boiling points below • Explain the differences • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -16 Klein, Organic Chemistry 2 e

13. 1 Physical Properties of Alcohols • Because they can H-bond, hydroxyl groups can attract water molecules strongly • Alcohols with small carbon chains are miscible in water (they mix in any ratio). WHY? • Alcohols with large carbon chains do not readily mix with water Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -17 Klein, Organic Chemistry 2 e

13. 1 Physical Properties of Alcohols • Do hydrophobic groups repel or attract water? • WHY are molecules with large hydrophobic groups generally insoluble in water? Alcohols with 3 or less carbons are generally water miscible Alcohols with more than 3 carbons are not miscible, and their solubility decreases as the size of the hydrophobic group increases • • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -18 Klein, Organic Chemistry 2 e

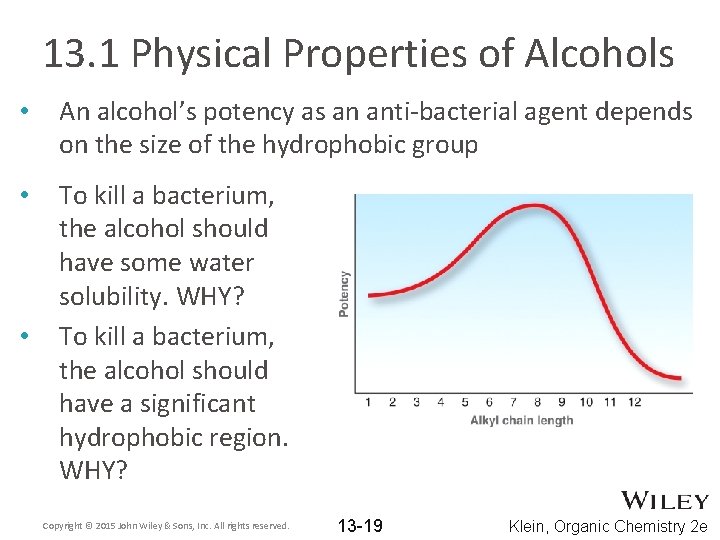
13. 1 Physical Properties of Alcohols • An alcohol’s potency as an anti-bacterial agent depends on the size of the hydrophobic group • To kill a bacterium, the alcohol should have some water solubility. WHY? To kill a bacterium, the alcohol should have a significant hydrophobic region. WHY? • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -19 Klein, Organic Chemistry 2 e

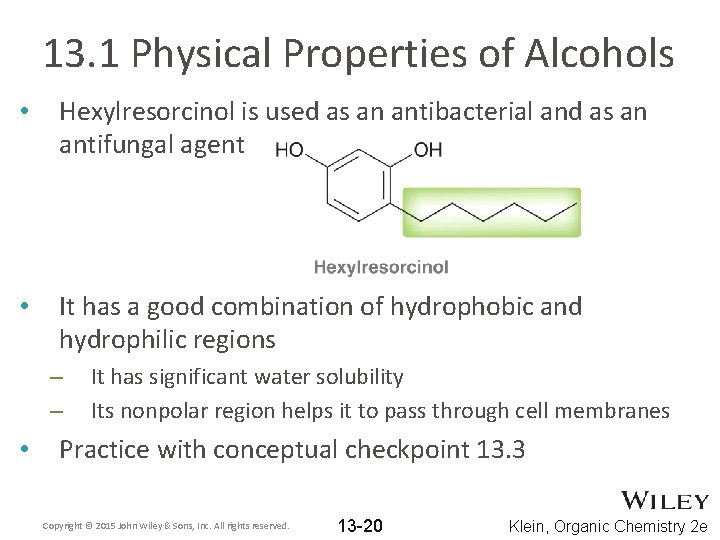
13. 1 Physical Properties of Alcohols • Hexylresorcinol is used as an antibacterial and as an antifungal agent • It has a good combination of hydrophobic and hydrophilic regions – – • It has significant water solubility Its nonpolar region helps it to pass through cell membranes Practice with conceptual checkpoint 13. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -20 Klein, Organic Chemistry 2 e

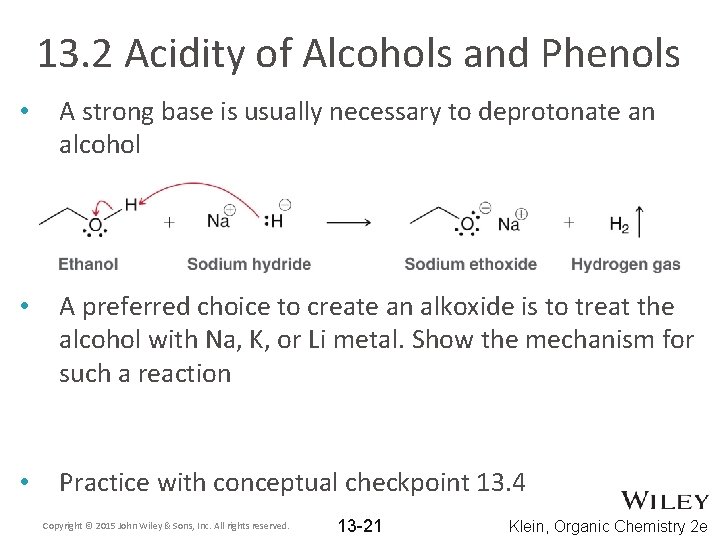
13. 2 Acidity of Alcohols and Phenols • A strong base is usually necessary to deprotonate an alcohol • A preferred choice to create an alkoxide is to treat the alcohol with Na, K, or Li metal. Show the mechanism for such a reaction • Practice with conceptual checkpoint 13. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -21 Klein, Organic Chemistry 2 e

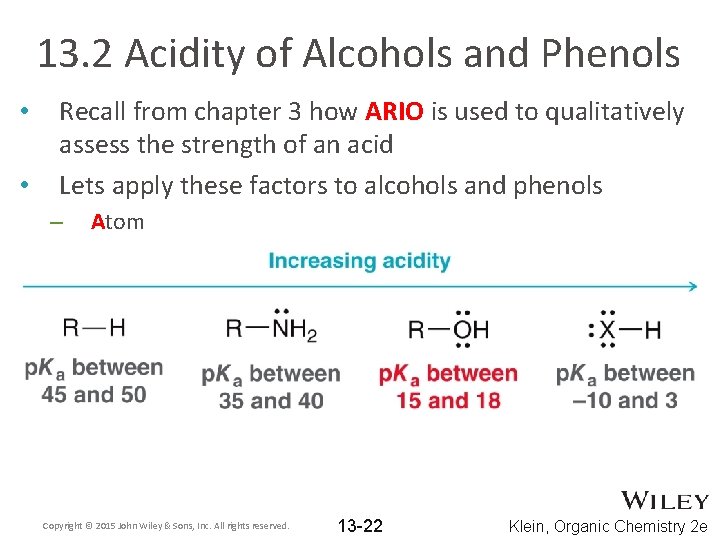
13. 2 Acidity of Alcohols and Phenols • • Recall from chapter 3 how ARIO is used to qualitatively assess the strength of an acid Lets apply these factors to alcohols and phenols – Atom Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -22 Klein, Organic Chemistry 2 e

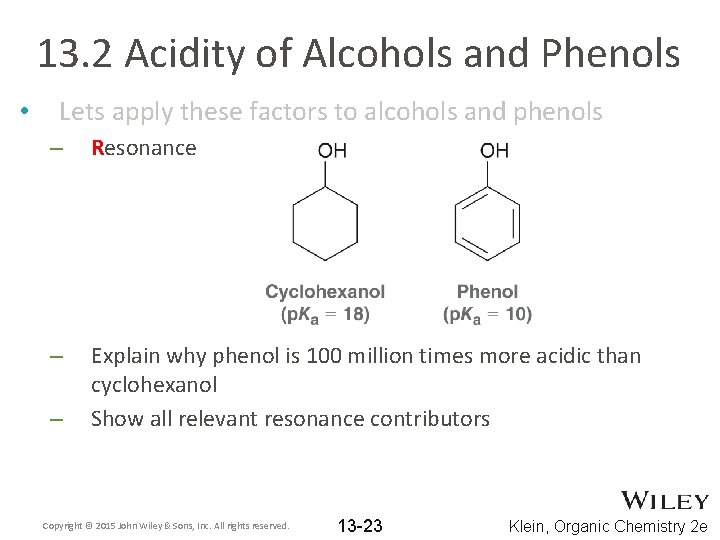
13. 2 Acidity of Alcohols and Phenols • Lets apply these factors to alcohols and phenols – Resonance – Explain why phenol is 100 million times more acidic than cyclohexanol Show all relevant resonance contributors – Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -23 Klein, Organic Chemistry 2 e

13. 2 Acidity of Alcohols and Phenols • Given the relatively low p. Ka of phenols, will Na. OH be a strong enough base to deprotonate a phenol? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -24 Klein, Organic Chemistry 2 e

13. 2 Acidity of Alcohols and Phenols • Lets apply these factors to alcohols and phenols – Induction: unless there is an electronegative group nearby, induction won’t be very significant – Orbital: in what type of orbital do the alkoxide electrons reside? How does that effect acidity? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -25 Klein, Organic Chemistry 2 e

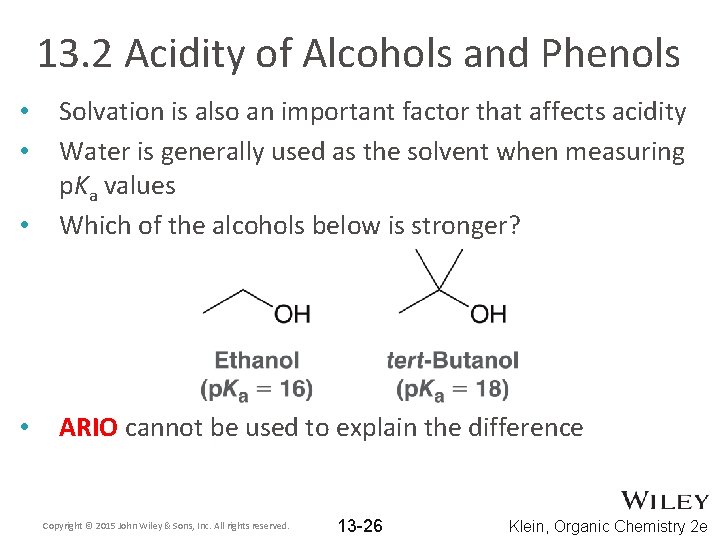
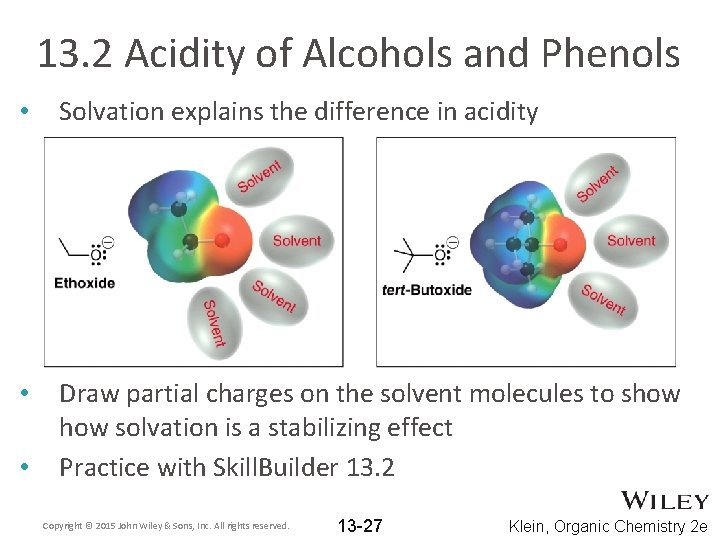
13. 2 Acidity of Alcohols and Phenols • Solvation is also an important factor that affects acidity Water is generally used as the solvent when measuring p. Ka values Which of the alcohols below is stronger? • ARIO cannot be used to explain the difference • • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -26 Klein, Organic Chemistry 2 e

13. 2 Acidity of Alcohols and Phenols • Solvation explains the difference in acidity • Draw partial charges on the solvent molecules to show solvation is a stabilizing effect Practice with Skill. Builder 13. 2 • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -27 Klein, Organic Chemistry 2 e

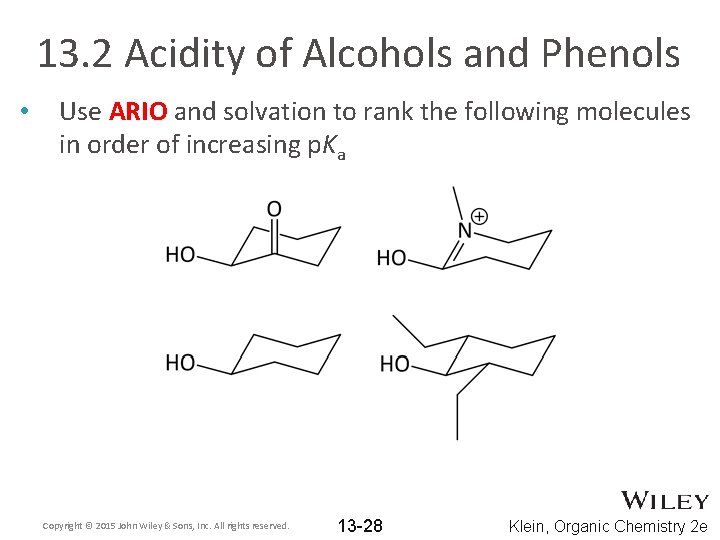
13. 2 Acidity of Alcohols and Phenols • Use ARIO and solvation to rank the following molecules in order of increasing p. Ka Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -28 Klein, Organic Chemistry 2 e

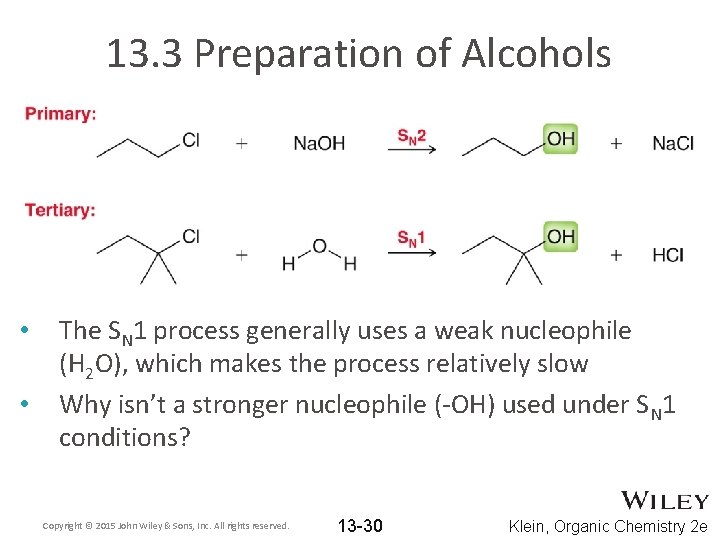
13. 3 Preparation of Alcohols • • • We saw in chapter 7 that substitution reactions can yield an alcohol What reagents did we use to accomplish this transformation? We saw that the substitution can occur by SN 1 or SN 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -29 Klein, Organic Chemistry 2 e

13. 3 Preparation of Alcohols • • The SN 1 process generally uses a weak nucleophile (H 2 O), which makes the process relatively slow Why isn’t a stronger nucleophile (-OH) used under SN 1 conditions? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -30 Klein, Organic Chemistry 2 e

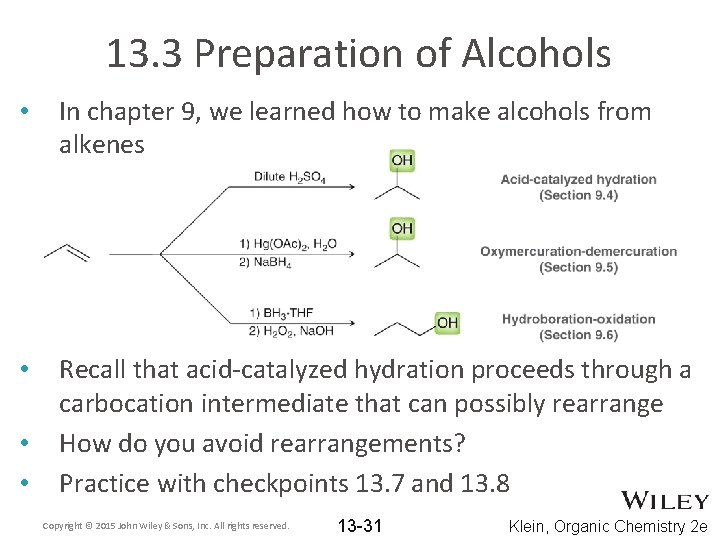
13. 3 Preparation of Alcohols • In chapter 9, we learned how to make alcohols from alkenes • Recall that acid-catalyzed hydration proceeds through a carbocation intermediate that can possibly rearrange How do you avoid rearrangements? Practice with checkpoints 13. 7 and 13. 8 • • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -31 Klein, Organic Chemistry 2 e

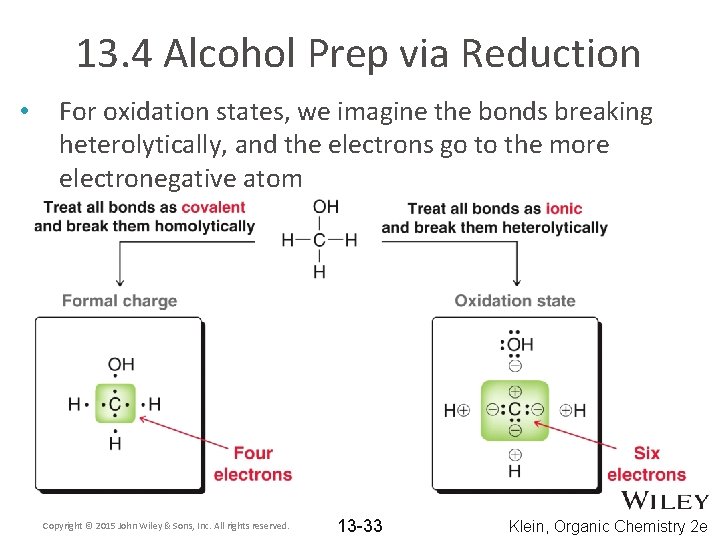
13. 4 Alcohol Prep via Reduction • • A third method to prepare alcohols is by the reduction of a carbonyl. What is a carbonyl? Reductions involve a change in oxidation state Oxidation state are a method of electron bookkeeping Recall how we used formal charge as a method of electron bookkeeping – – Each atom is assigned half of the electrons it is sharing with another atom What is the formal charge on carbon in methanol? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -32 Klein, Organic Chemistry 2 e

13. 4 Alcohol Prep via Reduction • For oxidation states, we imagine the bonds breaking heterolytically, and the electrons go to the more electronegative atom Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -33 Klein, Organic Chemistry 2 e

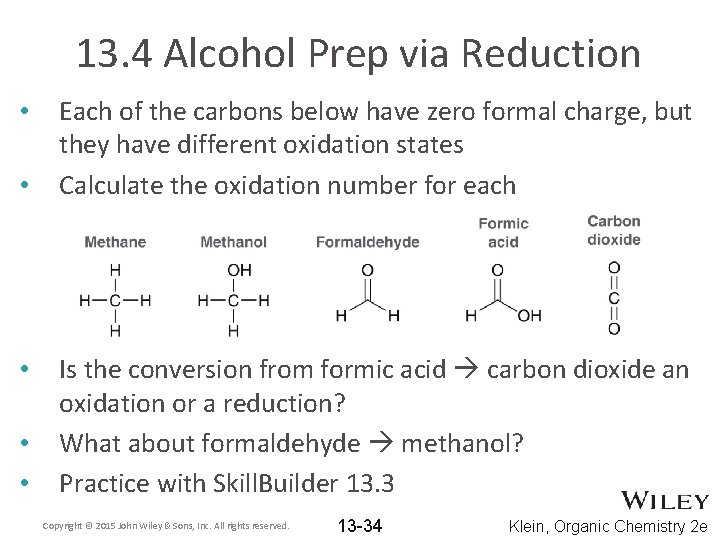
13. 4 Alcohol Prep via Reduction • • • Each of the carbons below have zero formal charge, but they have different oxidation states Calculate the oxidation number for each Is the conversion from formic acid carbon dioxide an oxidation or a reduction? What about formaldehyde methanol? Practice with Skill. Builder 13. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -34 Klein, Organic Chemistry 2 e

13. 4 Alcohol Prep via Reduction • The reduction of a carbonyl requires a reducing agent • • Is the reducing agent oxidized or reduced? If you were to design a reducing agent, what element(s) would be necessary? Would an acid such as HCl be an appropriate reducing agent? WHY or WHY NOT? • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -35 Klein, Organic Chemistry 2 e

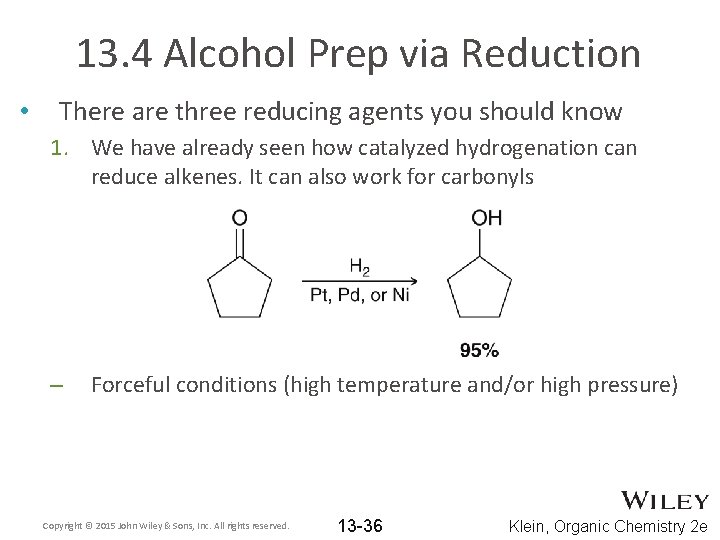
13. 4 Alcohol Prep via Reduction • There are three reducing agents you should know 1. We have already seen how catalyzed hydrogenation can reduce alkenes. It can also work for carbonyls – Forceful conditions (high temperature and/or high pressure) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -36 Klein, Organic Chemistry 2 e

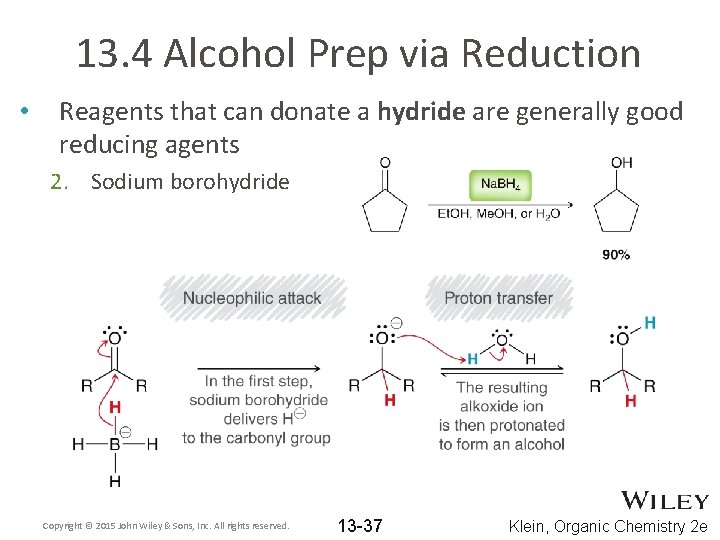
13. 4 Alcohol Prep via Reduction • Reagents that can donate a hydride are generally good reducing agents 2. Sodium borohydride Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -37 Klein, Organic Chemistry 2 e

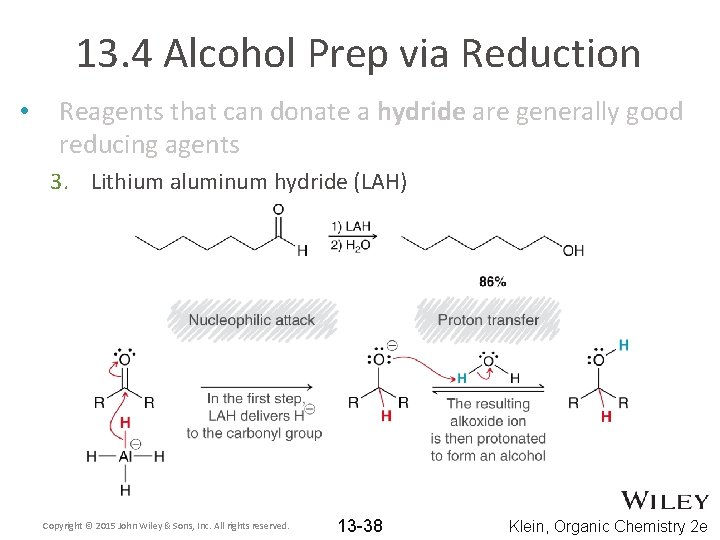
13. 4 Alcohol Prep via Reduction • Reagents that can donate a hydride are generally good reducing agents 3. Lithium aluminum hydride (LAH) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -38 Klein, Organic Chemistry 2 e

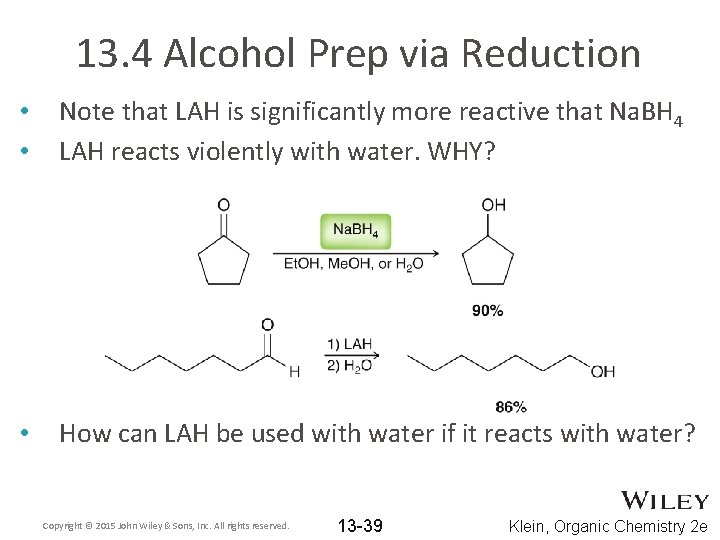
13. 4 Alcohol Prep via Reduction • • Note that LAH is significantly more reactive that Na. BH 4 LAH reacts violently with water. WHY? • How can LAH be used with water if it reacts with water? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -39 Klein, Organic Chemistry 2 e

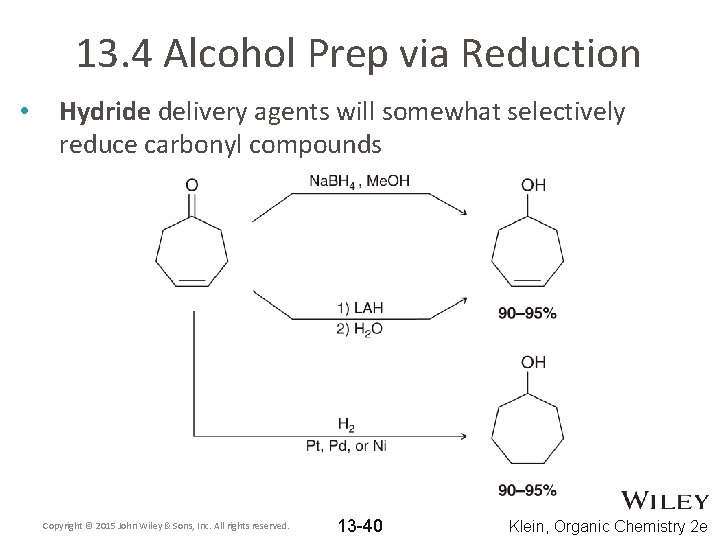
13. 4 Alcohol Prep via Reduction • Hydride delivery agents will somewhat selectively reduce carbonyl compounds Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -40 Klein, Organic Chemistry 2 e

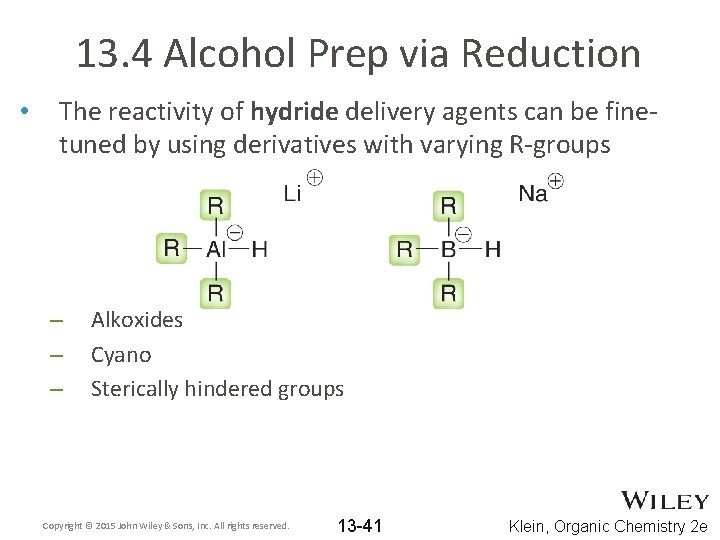
13. 4 Alcohol Prep via Reduction • The reactivity of hydride delivery agents can be finetuned by using derivatives with varying R-groups – – – Alkoxides Cyano Sterically hindered groups Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -41 Klein, Organic Chemistry 2 e

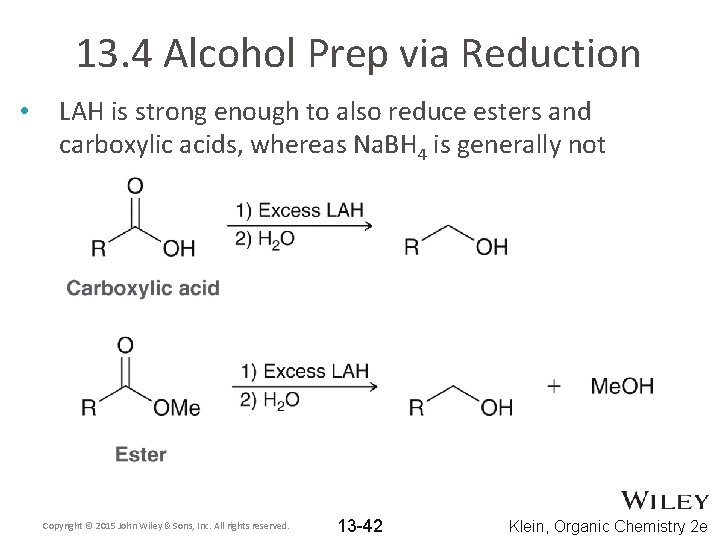
13. 4 Alcohol Prep via Reduction • LAH is strong enough to also reduce esters and carboxylic acids, whereas Na. BH 4 is generally not Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -42 Klein, Organic Chemistry 2 e

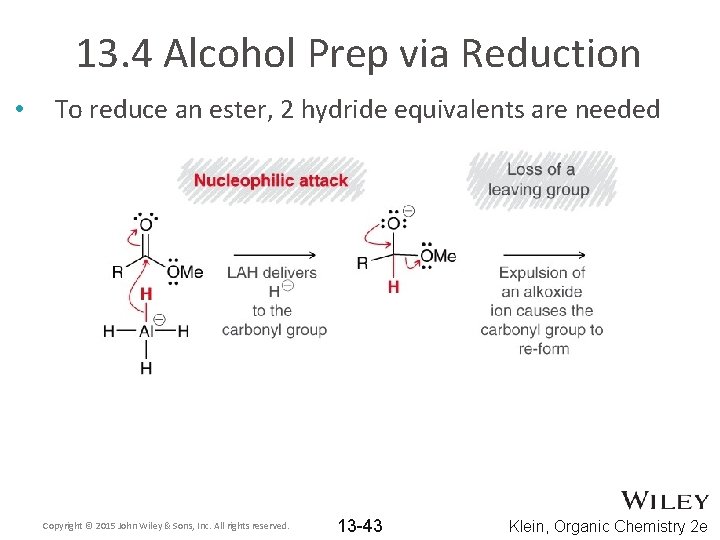
13. 4 Alcohol Prep via Reduction • To reduce an ester, 2 hydride equivalents are needed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -43 Klein, Organic Chemistry 2 e

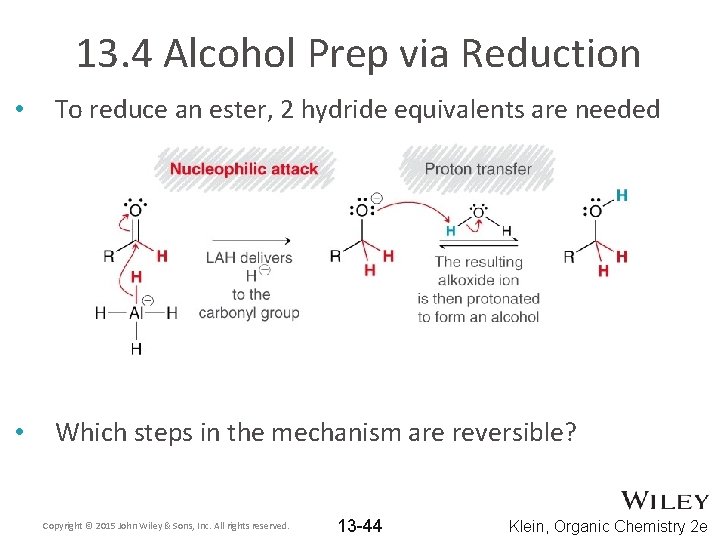
13. 4 Alcohol Prep via Reduction • To reduce an ester, 2 hydride equivalents are needed • Which steps in the mechanism are reversible? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -44 Klein, Organic Chemistry 2 e

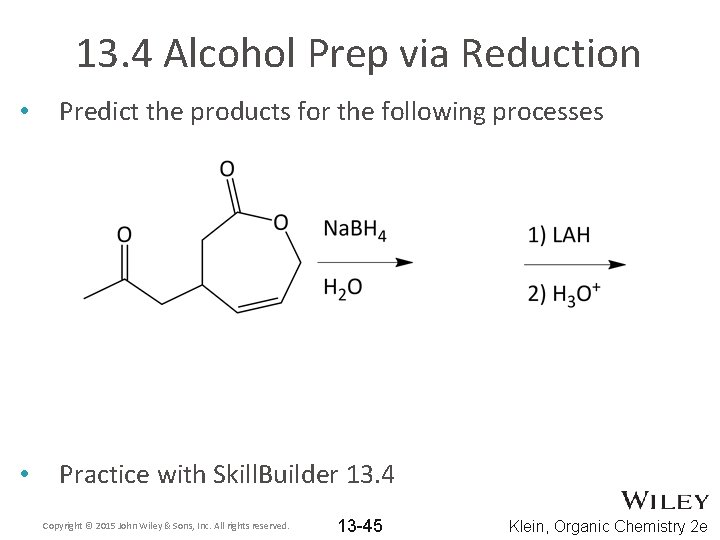
13. 4 Alcohol Prep via Reduction • Predict the products for the following processes • Practice with Skill. Builder 13. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -45 Klein, Organic Chemistry 2 e

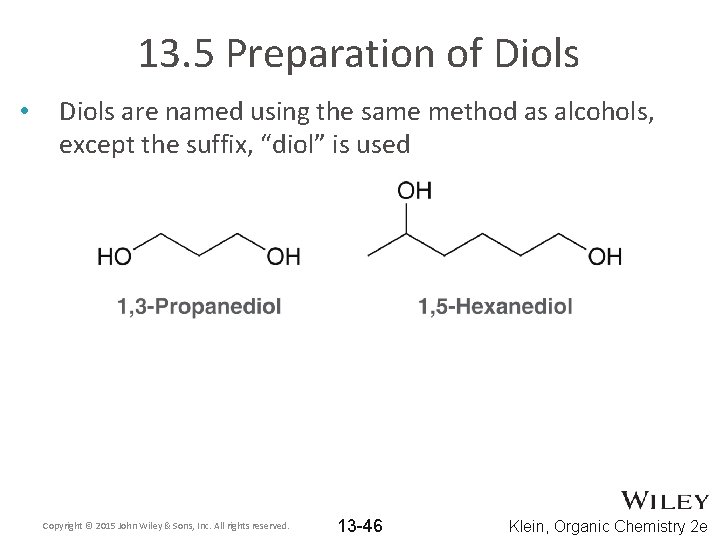
13. 5 Preparation of Diols • Diols are named using the same method as alcohols, except the suffix, “diol” is used Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -46 Klein, Organic Chemistry 2 e

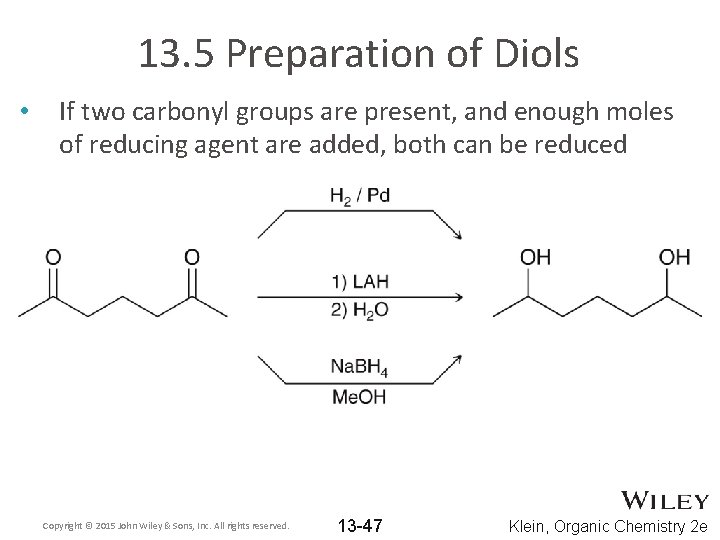
13. 5 Preparation of Diols • If two carbonyl groups are present, and enough moles of reducing agent are added, both can be reduced Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -47 Klein, Organic Chemistry 2 e

13. 5 Preparation of Diols • Recall the methods we discussed in chapter 9 to convert an alkene into a diol Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -48 Klein, Organic Chemistry 2 e

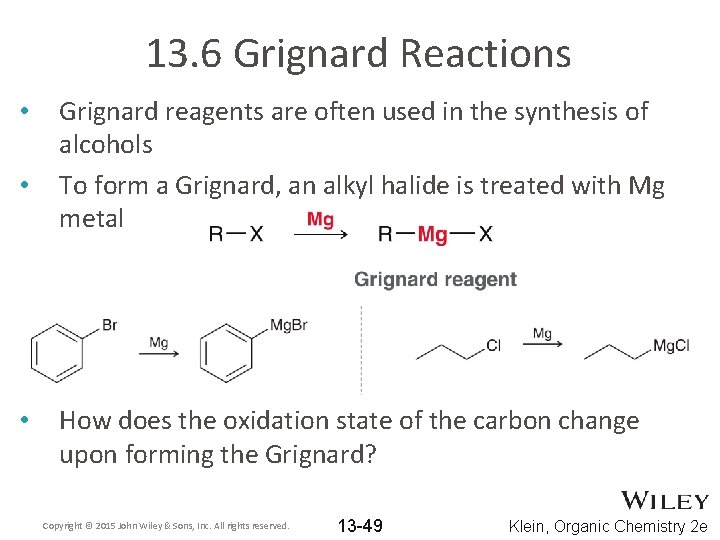
13. 6 Grignard Reactions • • • Grignard reagents are often used in the synthesis of alcohols To form a Grignard, an alkyl halide is treated with Mg metal How does the oxidation state of the carbon change upon forming the Grignard? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -49 Klein, Organic Chemistry 2 e

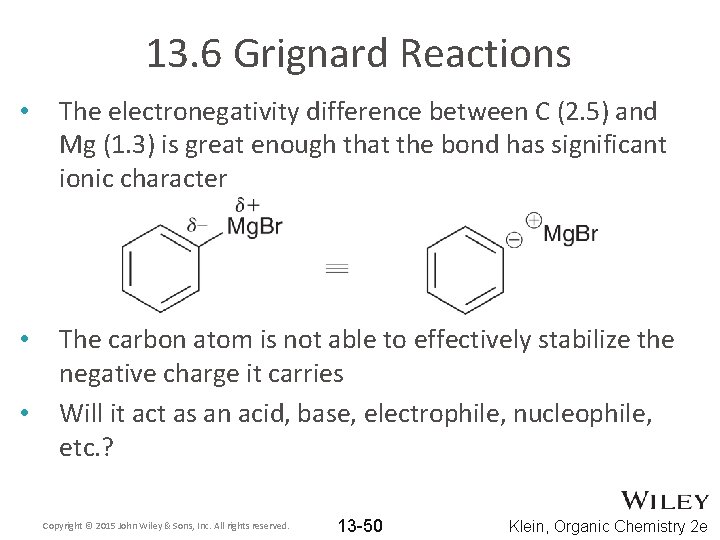
13. 6 Grignard Reactions • The electronegativity difference between C (2. 5) and Mg (1. 3) is great enough that the bond has significant ionic character • The carbon atom is not able to effectively stabilize the negative charge it carries Will it act as an acid, base, electrophile, nucleophile, etc. ? • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -50 Klein, Organic Chemistry 2 e

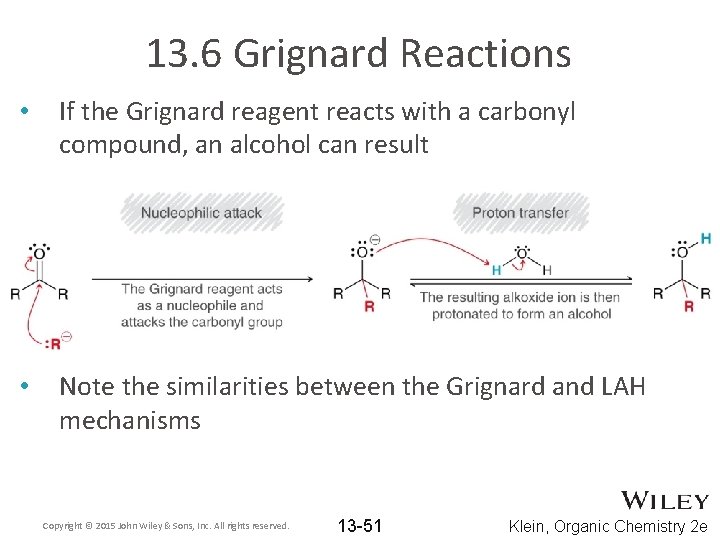
13. 6 Grignard Reactions • If the Grignard reagent reacts with a carbonyl compound, an alcohol can result • Note the similarities between the Grignard and LAH mechanisms Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -51 Klein, Organic Chemistry 2 e

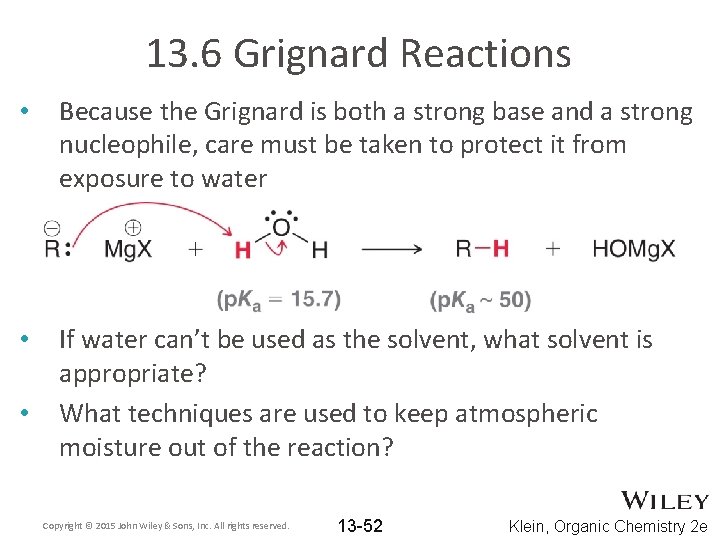
13. 6 Grignard Reactions • Because the Grignard is both a strong base and a strong nucleophile, care must be taken to protect it from exposure to water • If water can’t be used as the solvent, what solvent is appropriate? What techniques are used to keep atmospheric moisture out of the reaction? • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -52 Klein, Organic Chemistry 2 e

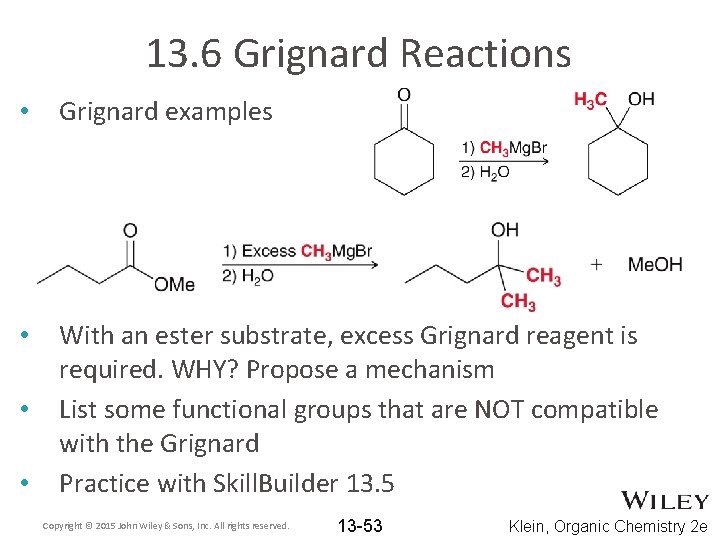
13. 6 Grignard Reactions • Grignard examples • With an ester substrate, excess Grignard reagent is required. WHY? Propose a mechanism List some functional groups that are NOT compatible with the Grignard Practice with Skill. Builder 13. 5 • • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -53 Klein, Organic Chemistry 2 e

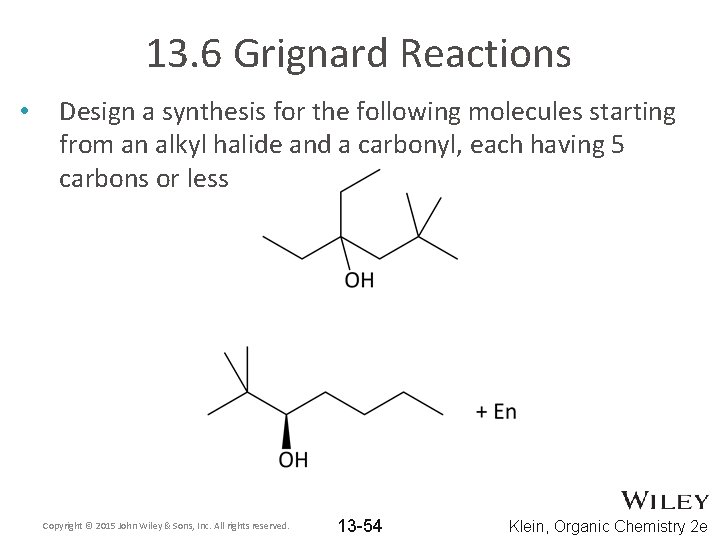
13. 6 Grignard Reactions • Design a synthesis for the following molecules starting from an alkyl halide and a carbonyl, each having 5 carbons or less Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -54 Klein, Organic Chemistry 2 e

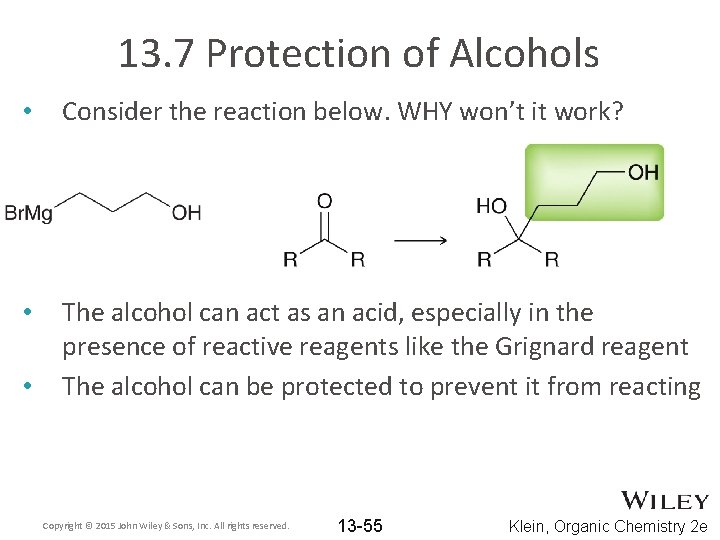
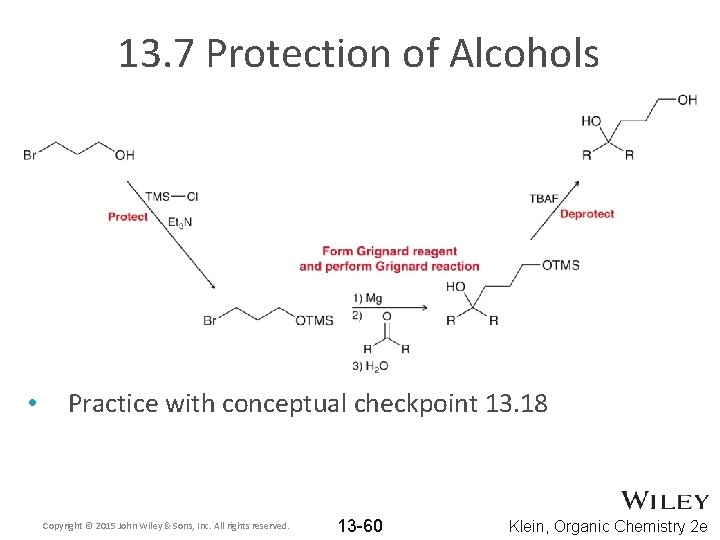
13. 7 Protection of Alcohols • Consider the reaction below. WHY won’t it work? • The alcohol can act as an acid, especially in the presence of reactive reagents like the Grignard reagent The alcohol can be protected to prevent it from reacting • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -55 Klein, Organic Chemistry 2 e

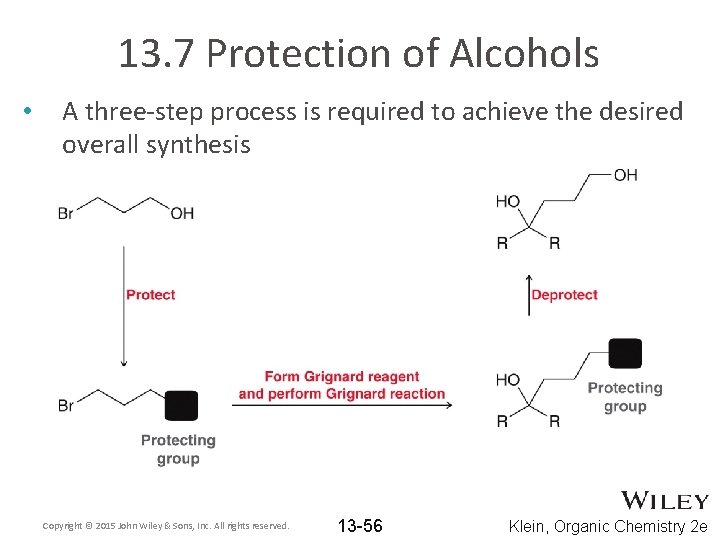
13. 7 Protection of Alcohols • A three-step process is required to achieve the desired overall synthesis Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -56 Klein, Organic Chemistry 2 e

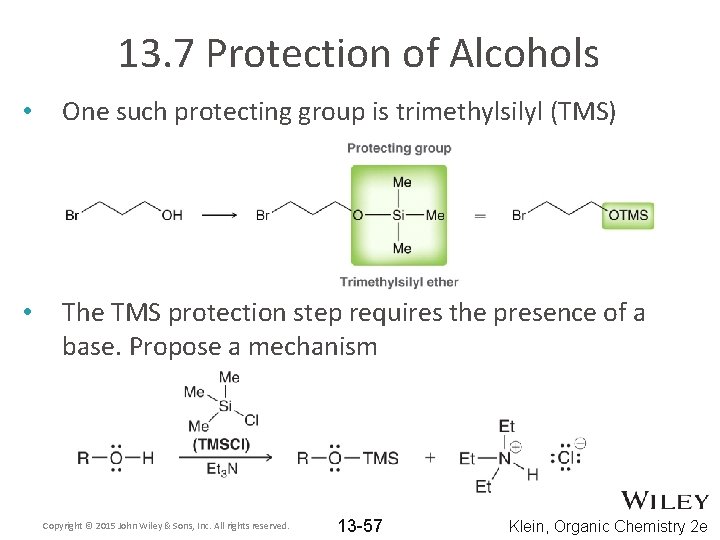
13. 7 Protection of Alcohols • One such protecting group is trimethylsilyl (TMS) • The TMS protection step requires the presence of a base. Propose a mechanism Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -57 Klein, Organic Chemistry 2 e

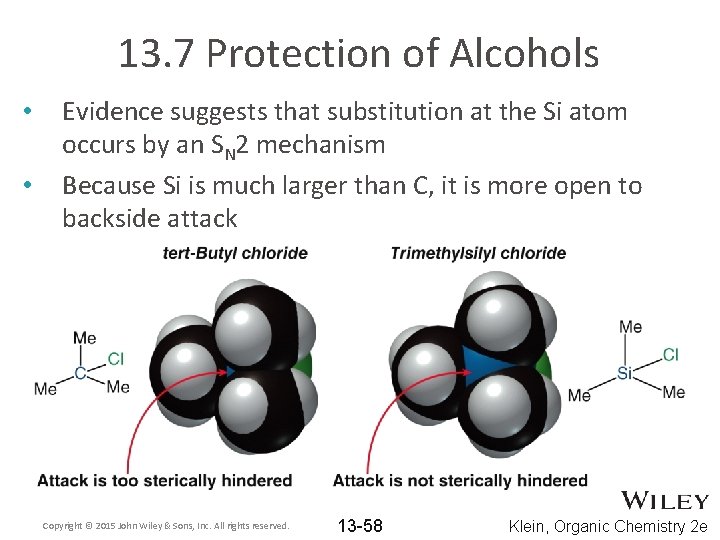
13. 7 Protection of Alcohols • • Evidence suggests that substitution at the Si atom occurs by an SN 2 mechanism Because Si is much larger than C, it is more open to backside attack Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -58 Klein, Organic Chemistry 2 e

13. 7 Protection of Alcohols • • The TMS group can later be removed with H 3 O+ or FTBAF is often used to supply fluoride ions Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -59 Klein, Organic Chemistry 2 e

13. 7 Protection of Alcohols • Practice with conceptual checkpoint 13. 18 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -60 Klein, Organic Chemistry 2 e

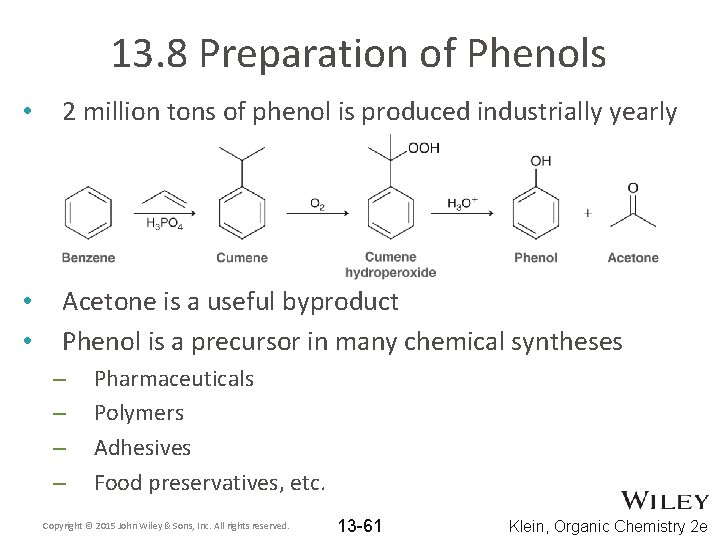
13. 8 Preparation of Phenols • 2 million tons of phenol is produced industrially yearly • • Acetone is a useful byproduct Phenol is a precursor in many chemical syntheses – – Pharmaceuticals Polymers Adhesives Food preservatives, etc. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -61 Klein, Organic Chemistry 2 e

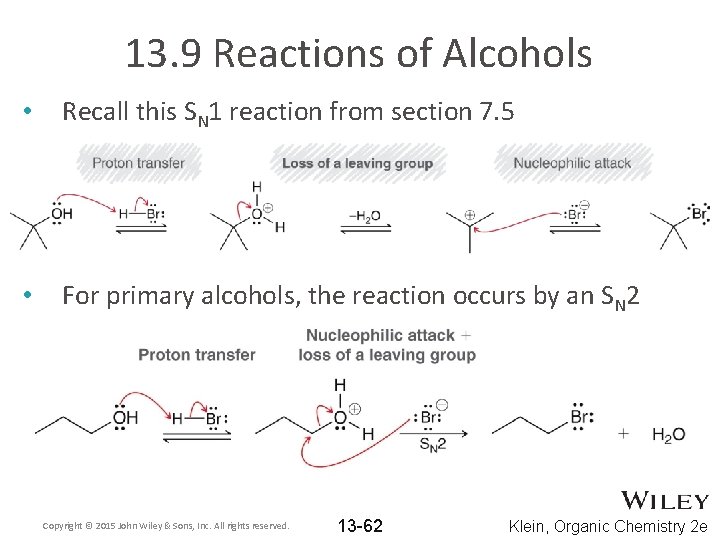
13. 9 Reactions of Alcohols • Recall this SN 1 reaction from section 7. 5 • For primary alcohols, the reaction occurs by an SN 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -62 Klein, Organic Chemistry 2 e

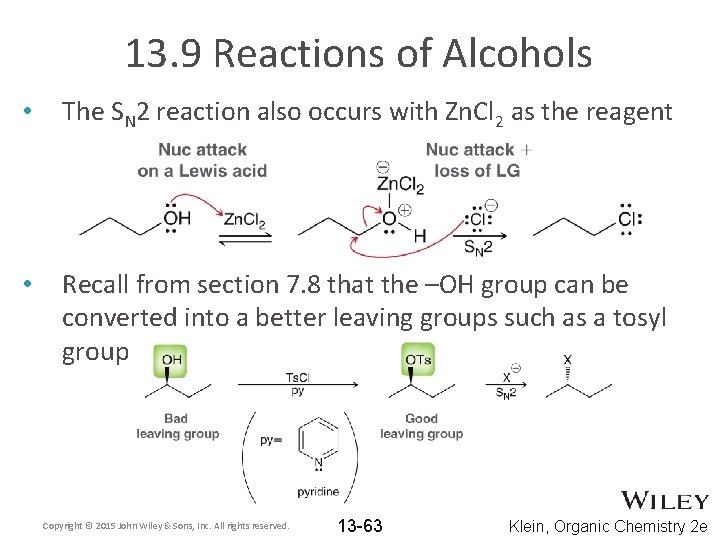
13. 9 Reactions of Alcohols • The SN 2 reaction also occurs with Zn. Cl 2 as the reagent • Recall from section 7. 8 that the –OH group can be converted into a better leaving groups such as a tosyl group Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -63 Klein, Organic Chemistry 2 e

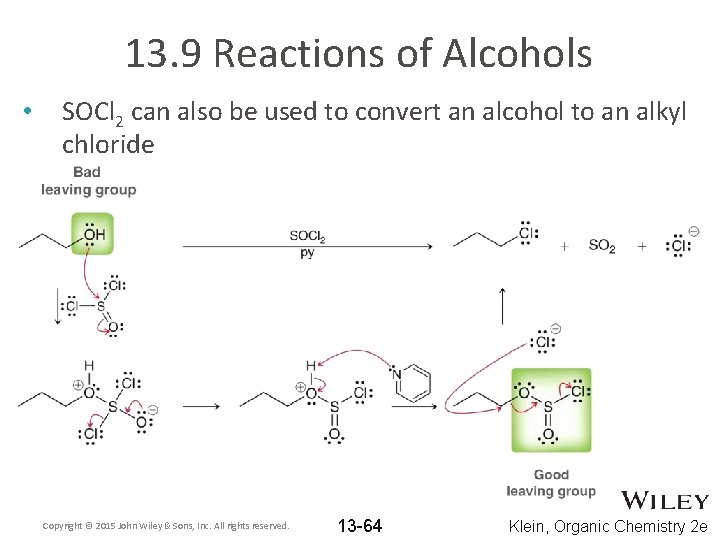
13. 9 Reactions of Alcohols • SOCl 2 can also be used to convert an alcohol to an alkyl chloride Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -64 Klein, Organic Chemistry 2 e

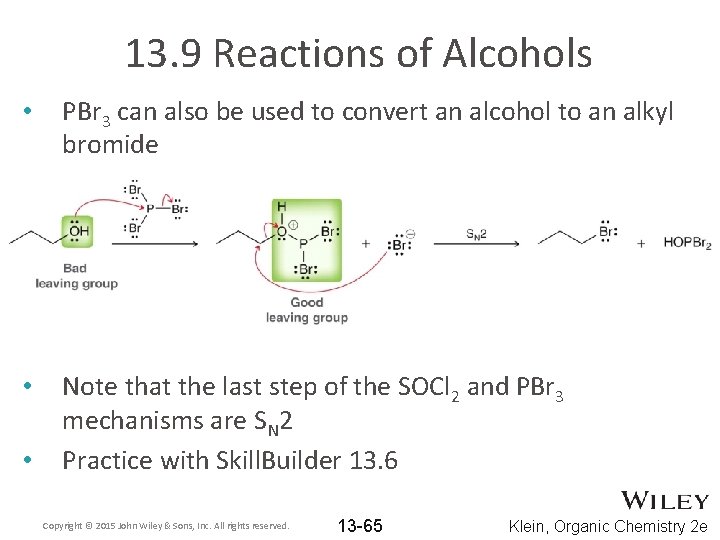
13. 9 Reactions of Alcohols • PBr 3 can also be used to convert an alcohol to an alkyl bromide • Note that the last step of the SOCl 2 and PBr 3 mechanisms are SN 2 Practice with Skill. Builder 13. 6 • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -65 Klein, Organic Chemistry 2 e

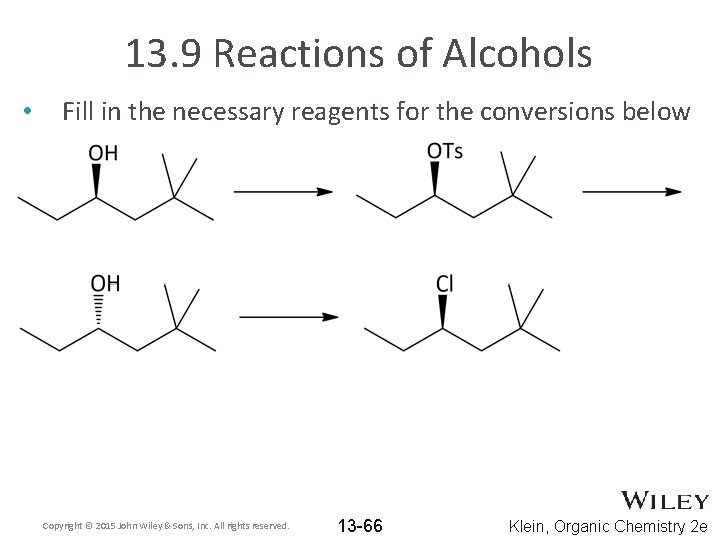
13. 9 Reactions of Alcohols • Fill in the necessary reagents for the conversions below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -66 Klein, Organic Chemistry 2 e

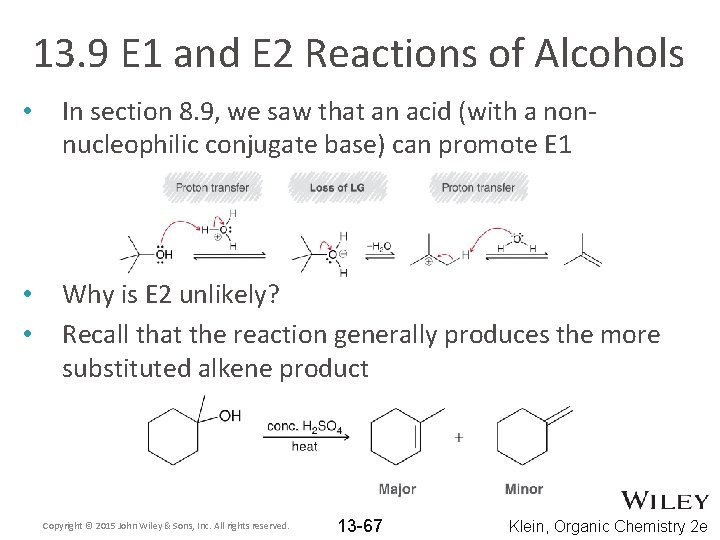
13. 9 E 1 and E 2 Reactions of Alcohols • In section 8. 9, we saw that an acid (with a nonnucleophilic conjugate base) can promote E 1 • • Why is E 2 unlikely? Recall that the reaction generally produces the more substituted alkene product Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -67 Klein, Organic Chemistry 2 e

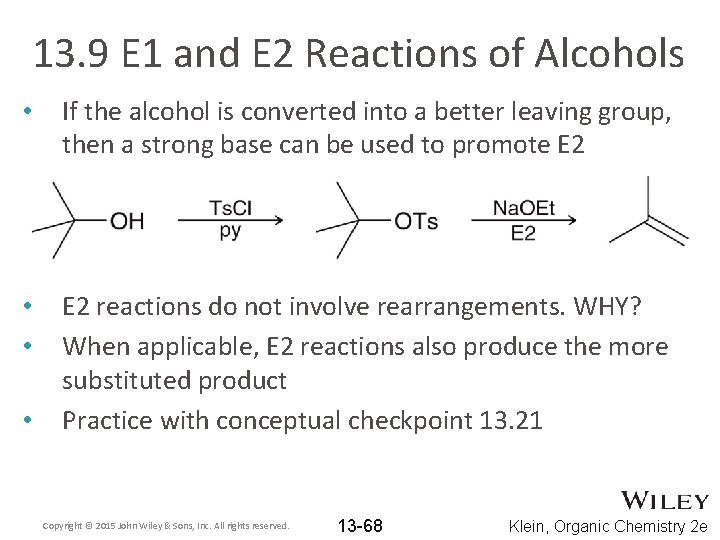
13. 9 E 1 and E 2 Reactions of Alcohols • If the alcohol is converted into a better leaving group, then a strong base can be used to promote E 2 • • E 2 reactions do not involve rearrangements. WHY? When applicable, E 2 reactions also produce the more substituted product Practice with conceptual checkpoint 13. 21 • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -68 Klein, Organic Chemistry 2 e

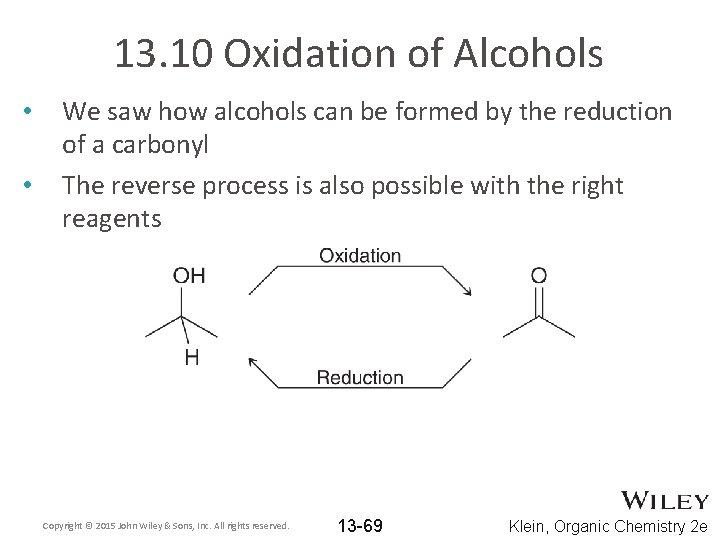
13. 10 Oxidation of Alcohols • • We saw how alcohols can be formed by the reduction of a carbonyl The reverse process is also possible with the right reagents Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -69 Klein, Organic Chemistry 2 e

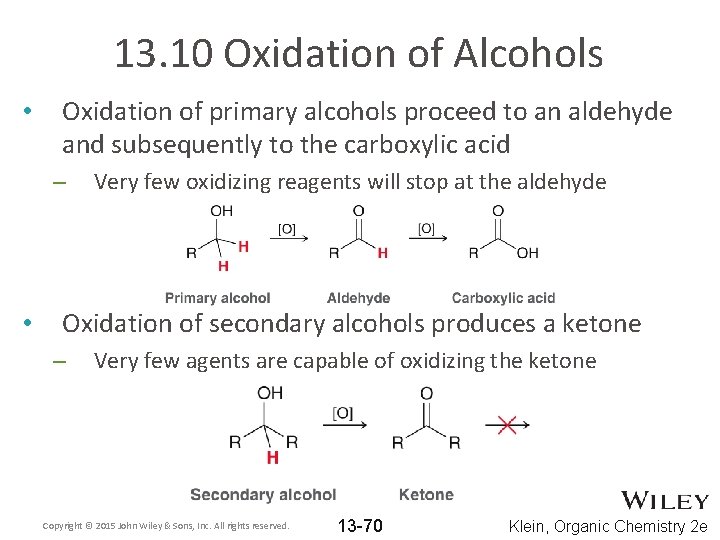
13. 10 Oxidation of Alcohols • Oxidation of primary alcohols proceed to an aldehyde and subsequently to the carboxylic acid – • Very few oxidizing reagents will stop at the aldehyde Oxidation of secondary alcohols produces a ketone – Very few agents are capable of oxidizing the ketone Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -70 Klein, Organic Chemistry 2 e

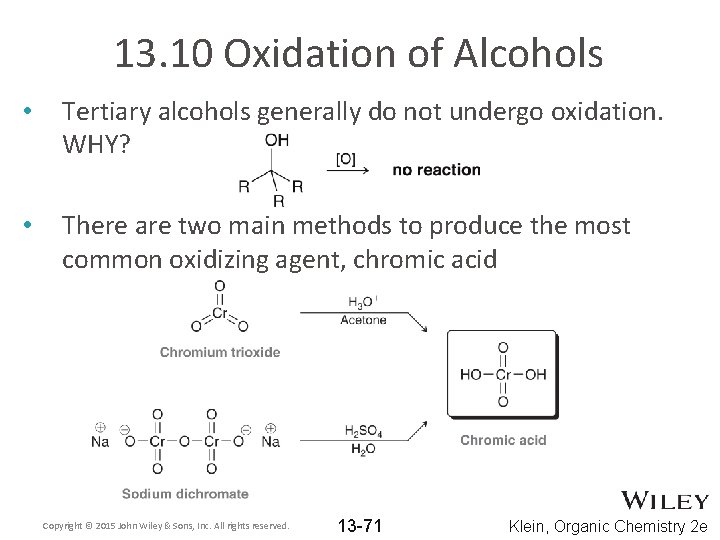
13. 10 Oxidation of Alcohols • Tertiary alcohols generally do not undergo oxidation. WHY? • There are two main methods to produce the most common oxidizing agent, chromic acid Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -71 Klein, Organic Chemistry 2 e

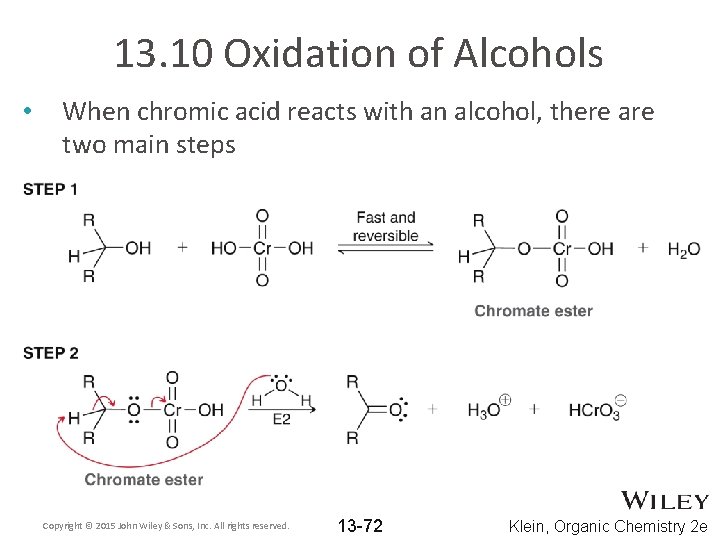
13. 10 Oxidation of Alcohols • When chromic acid reacts with an alcohol, there are two main steps Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -72 Klein, Organic Chemistry 2 e

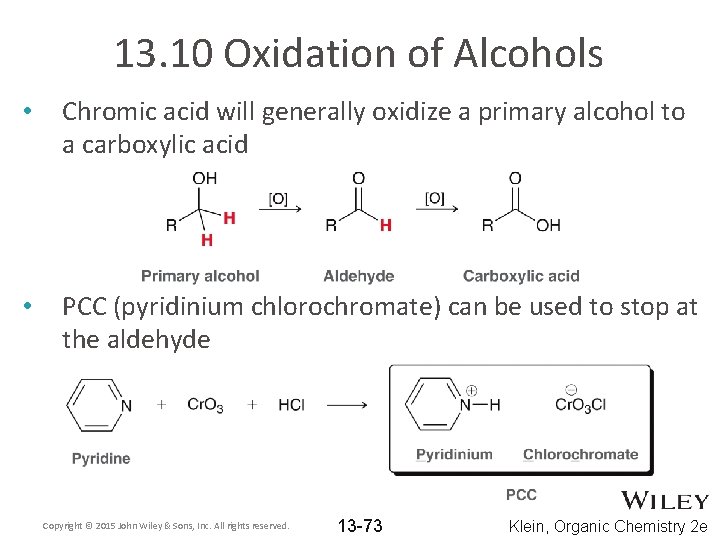
13. 10 Oxidation of Alcohols • Chromic acid will generally oxidize a primary alcohol to a carboxylic acid • PCC (pyridinium chlorochromate) can be used to stop at the aldehyde Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -73 Klein, Organic Chemistry 2 e

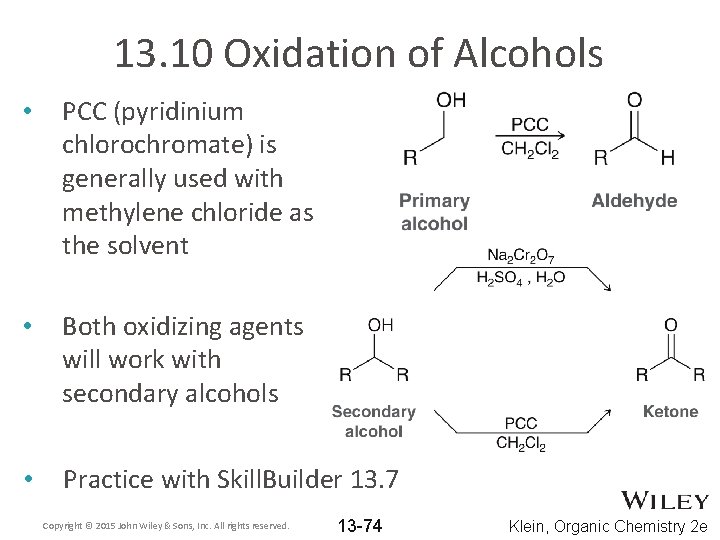
13. 10 Oxidation of Alcohols • PCC (pyridinium chlorochromate) is generally used with methylene chloride as the solvent • Both oxidizing agents will work with secondary alcohols • Practice with Skill. Builder 13. 7 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -74 Klein, Organic Chemistry 2 e

13. 10 Oxidation of Alcohols • Predict the product for the following reaction Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -75 Klein, Organic Chemistry 2 e

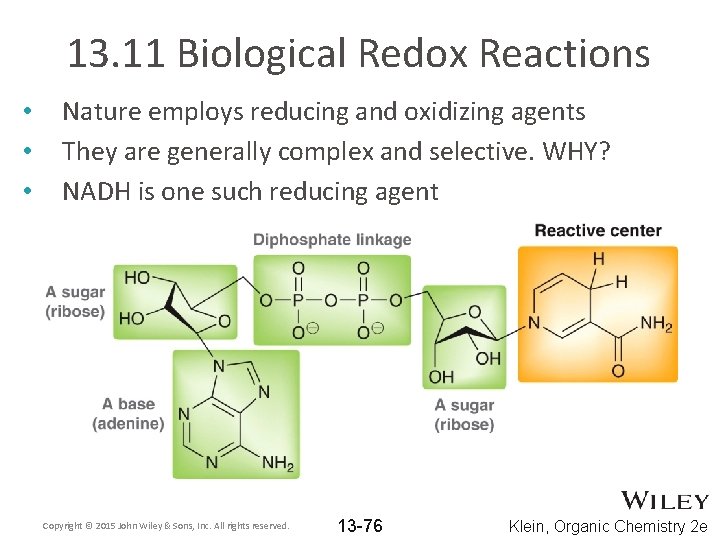
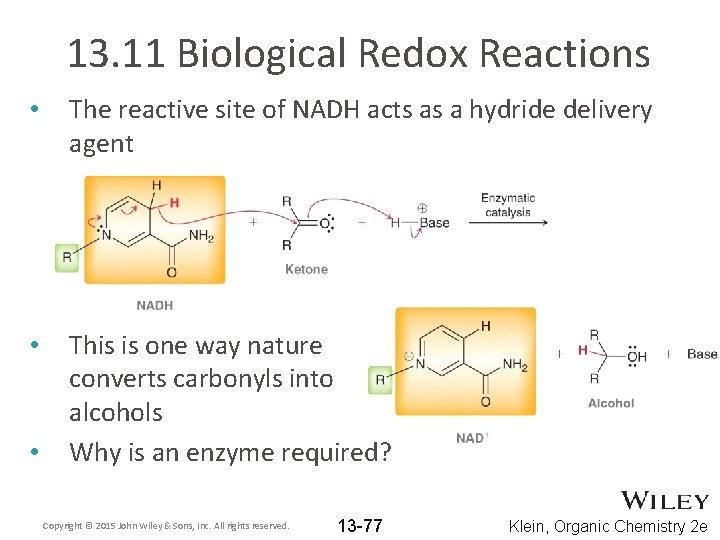
13. 11 Biological Redox Reactions • • • Nature employs reducing and oxidizing agents They are generally complex and selective. WHY? NADH is one such reducing agent Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -76 Klein, Organic Chemistry 2 e

13. 11 Biological Redox Reactions • The reactive site of NADH acts as a hydride delivery agent • This is one way nature converts carbonyls into alcohols Why is an enzyme required? • Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -77 Klein, Organic Chemistry 2 e

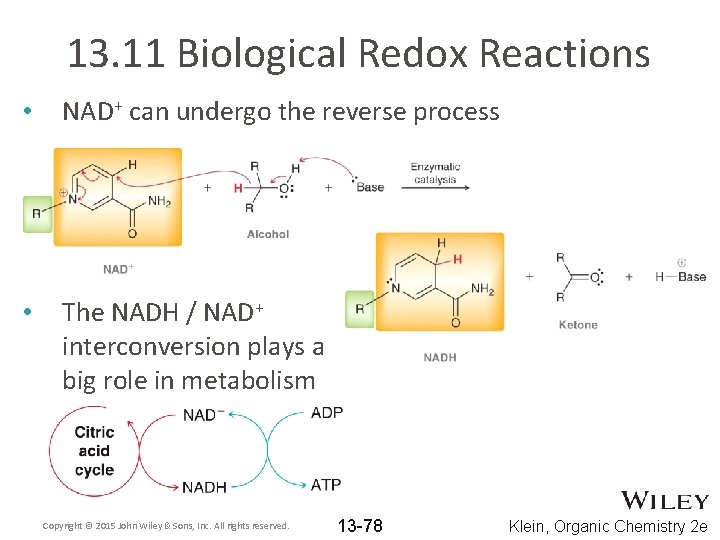
13. 11 Biological Redox Reactions • NAD+ can undergo the reverse process • The NADH / NAD+ interconversion plays a big role in metabolism Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -78 Klein, Organic Chemistry 2 e

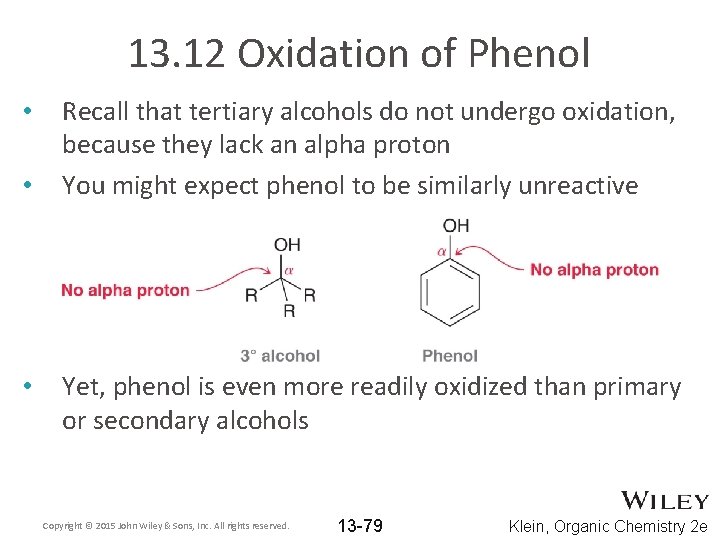
13. 12 Oxidation of Phenol • • • Recall that tertiary alcohols do not undergo oxidation, because they lack an alpha proton You might expect phenol to be similarly unreactive Yet, phenol is even more readily oxidized than primary or secondary alcohols Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -79 Klein, Organic Chemistry 2 e

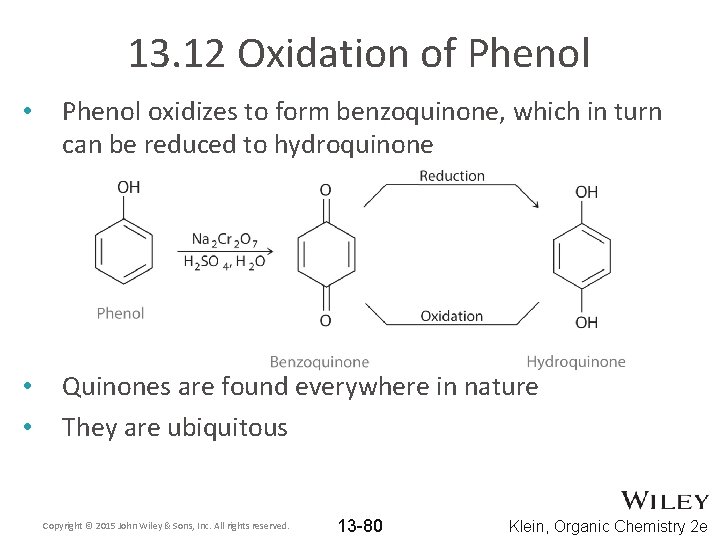
13. 12 Oxidation of Phenol • Phenol oxidizes to form benzoquinone, which in turn can be reduced to hydroquinone • • Quinones are found everywhere in nature They are ubiquitous Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -80 Klein, Organic Chemistry 2 e

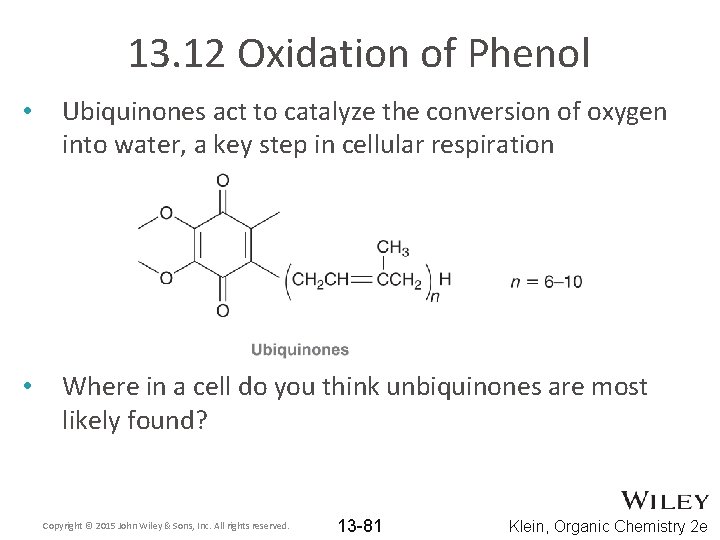
13. 12 Oxidation of Phenol • Ubiquinones act to catalyze the conversion of oxygen into water, a key step in cellular respiration • Where in a cell do you think unbiquinones are most likely found? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -81 Klein, Organic Chemistry 2 e

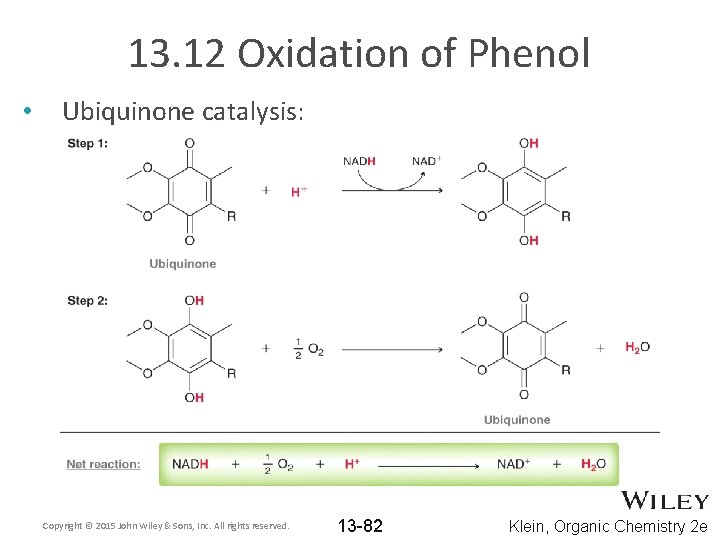
13. 12 Oxidation of Phenol • Ubiquinone catalysis: Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -82 Klein, Organic Chemistry 2 e

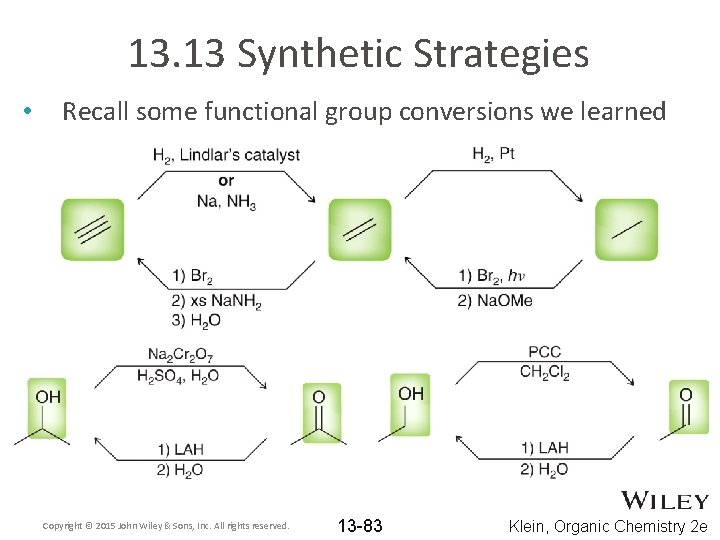
13. 13 Synthetic Strategies • Recall some functional group conversions we learned Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -83 Klein, Organic Chemistry 2 e

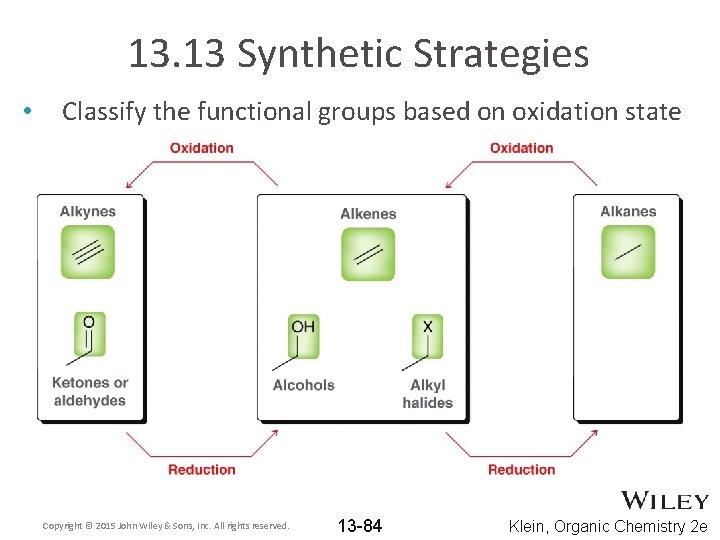
13. 13 Synthetic Strategies • Classify the functional groups based on oxidation state Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -84 Klein, Organic Chemistry 2 e

13. 13 Synthetic Strategies Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -85 Klein, Organic Chemistry 2 e

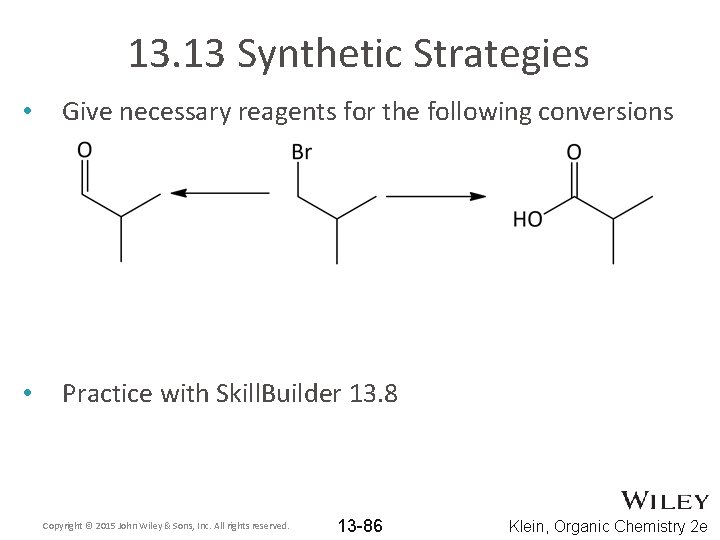
13. 13 Synthetic Strategies • Give necessary reagents for the following conversions • Practice with Skill. Builder 13. 8 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -86 Klein, Organic Chemistry 2 e

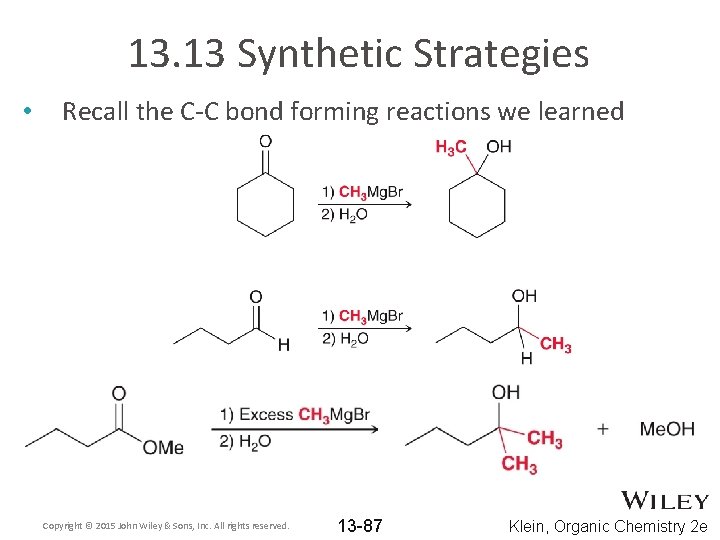
13. 13 Synthetic Strategies • Recall the C-C bond forming reactions we learned Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -87 Klein, Organic Chemistry 2 e

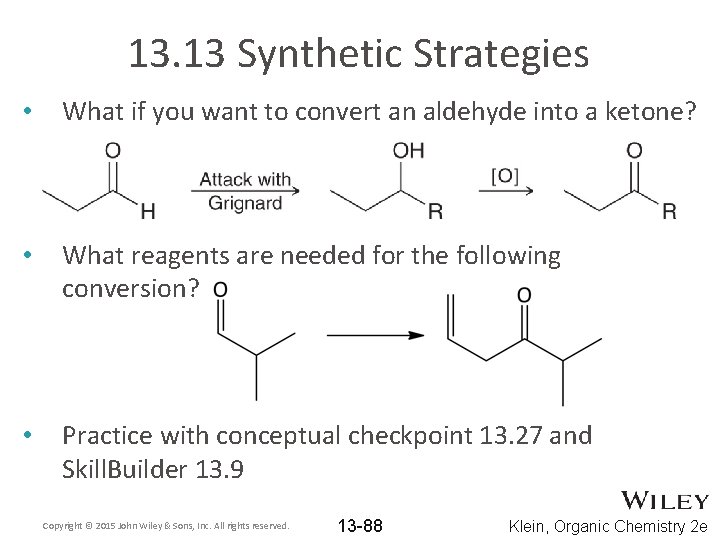
13. 13 Synthetic Strategies • What if you want to convert an aldehyde into a ketone? • What reagents are needed for the following conversion? • Practice with conceptual checkpoint 13. 27 and Skill. Builder 13. 9 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -88 Klein, Organic Chemistry 2 e

Additional Practice Problems • Name the following molecule • Draw (1 R, 2 R)-1 -(3, 3 -dimethylbutyl)-3, 5 -cyclohexadien 1, 2 -diol Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -89 Klein, Organic Chemistry 2 e

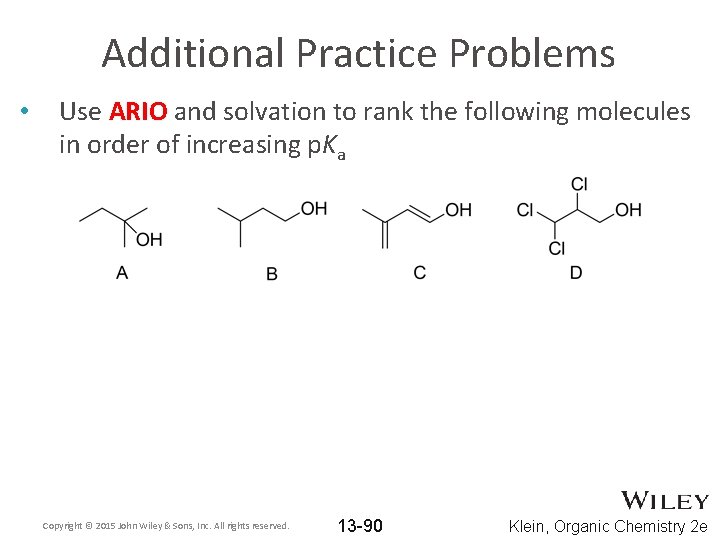
Additional Practice Problems • Use ARIO and solvation to rank the following molecules in order of increasing p. Ka Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -90 Klein, Organic Chemistry 2 e

Additional Practice Problems • Predict the products for the following processes Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -91 Klein, Organic Chemistry 2 e

Additional Practice Problems • Design a synthesis for the following molecule starting from an alkyl halide and a carbonyl, each having 5 carbons or less Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -92 Klein, Organic Chemistry 2 e

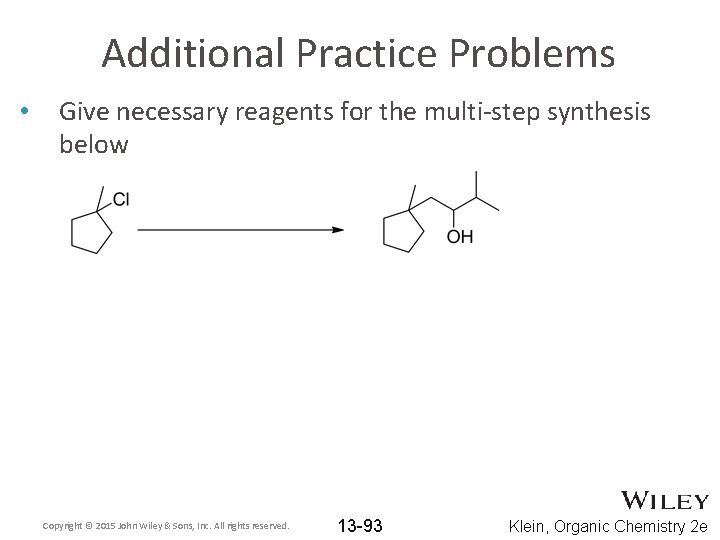
Additional Practice Problems • Give necessary reagents for the multi-step synthesis below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 13 -93 Klein, Organic Chemistry 2 e