Chapter 3 Alcohols Phenols Ethers Thiols Lecture PLUS



- Slides: 15

Chapter 3 Alcohols, Phenols, Ethers, Thiols Lecture. PLUS Timberlake 1

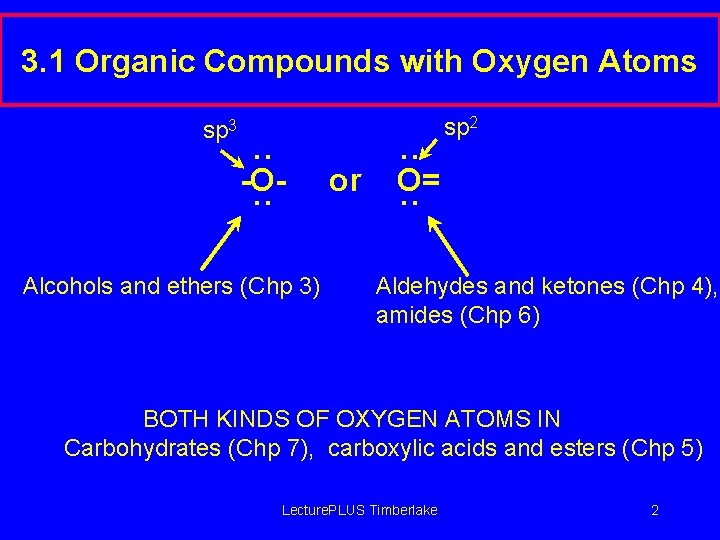
3. 1 Organic Compounds with Oxygen Atoms sp 3 . . -O. . Alcohols and ethers (Chp 3) or . . O=. . sp 2 Aldehydes and ketones (Chp 4), amides (Chp 6) BOTH KINDS OF OXYGEN ATOMS IN Carbohydrates (Chp 7), carboxylic acids and esters (Chp 5) Lecture. PLUS Timberlake 2

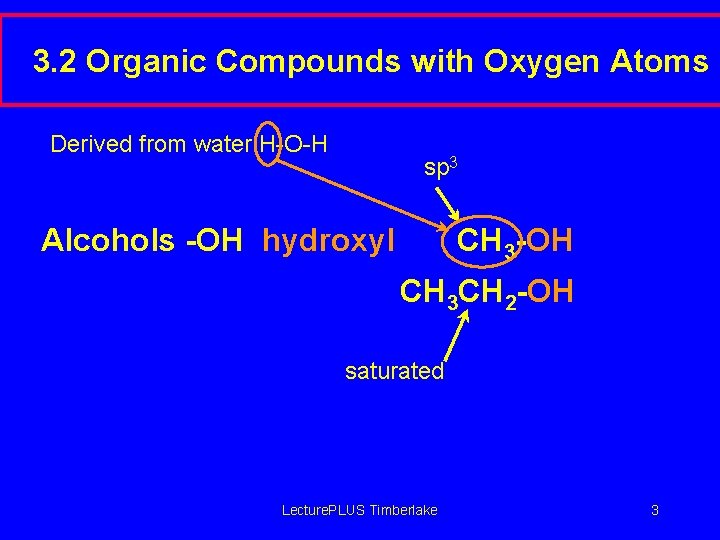
3. 2 Organic Compounds with Oxygen Atoms Derived from water H-O-H sp 3 Alcohols -OH hydroxyl CH 3 -OH CH 3 CH 2 -OH saturated Lecture. PLUS Timberlake 3

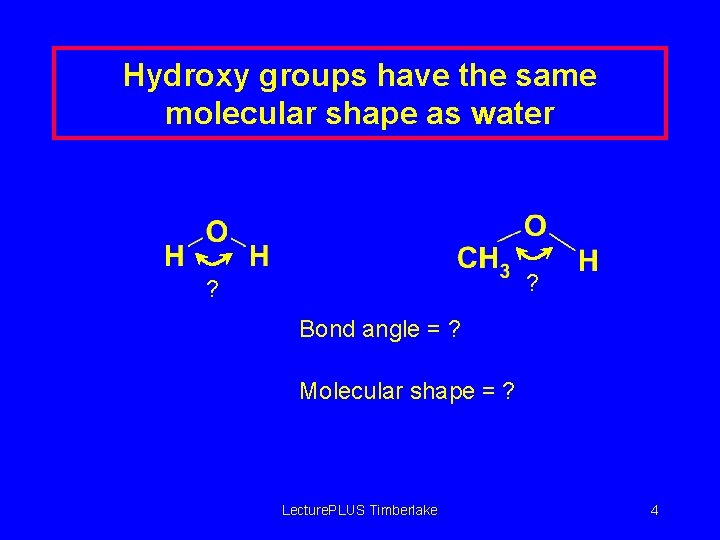
Hydroxy groups have the same molecular shape as water ? ? Bond angle = ? Molecular shape = ? Lecture. PLUS Timberlake 4

3. 3 Naming Alcohols n A carbon compound that contain -OH (hydroxyl) group n In IUPAC name, the -e in alkane name is replaced with -ol. CH 4 methane CH 3 OH methanol (methyl alcohol) CH 3 ethane CH 3 CH 2 OH ethanol Lecture. PLUS Timberlake (ethyl alcohol) 5

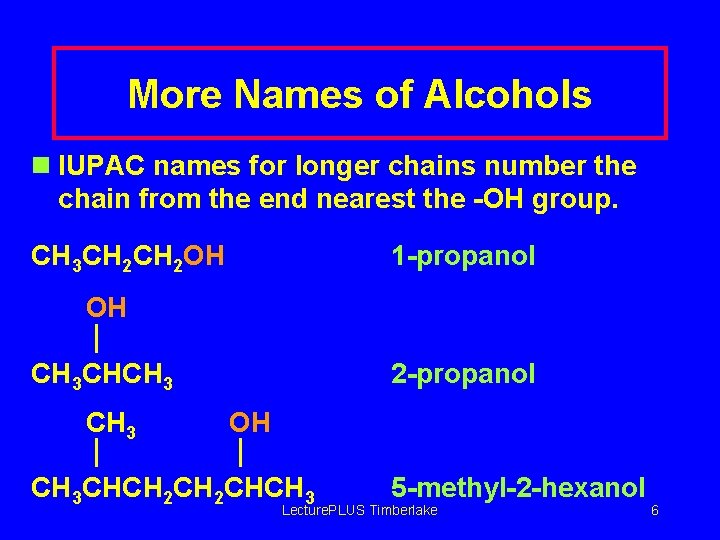
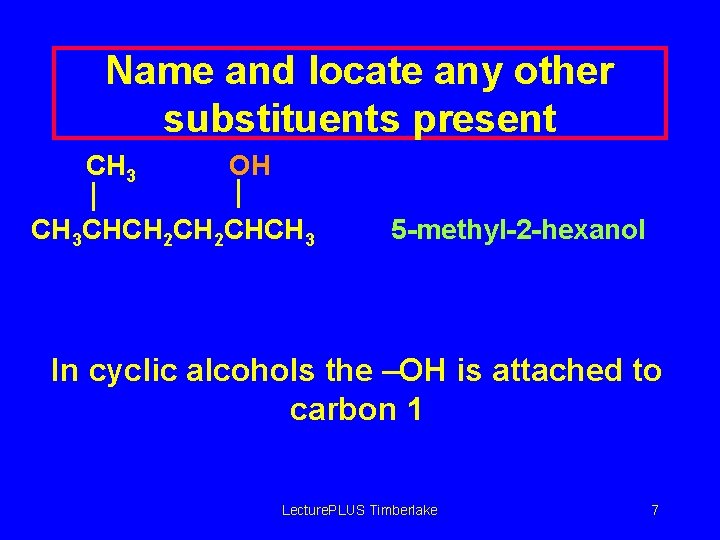
More Names of Alcohols n IUPAC names for longer chains number the chain from the end nearest the -OH group. CH 3 CH 2 OH 1 -propanol OH CH 3 CHCH 3 2 -propanol OH CH 3 CHCH 2 CHCH 3 5 -methyl-2 -hexanol Lecture. PLUS Timberlake 6

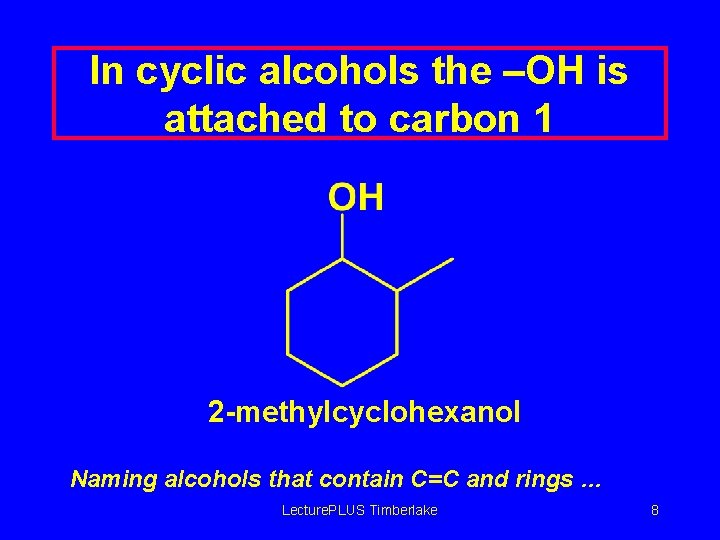
Name and locate any other substituents present CH 3 OH CH 3 CHCH 2 CHCH 3 5 -methyl-2 -hexanol In cyclic alcohols the –OH is attached to carbon 1 Lecture. PLUS Timberlake 7

In cyclic alcohols the –OH is attached to carbon 1 2 -methylcyclohexanol Naming alcohols that contain C=C and rings … Lecture. PLUS Timberlake 8

Alcohols with more than one hydroxyl antifreeze HO-CH 2 -OH 1, 2 -ethanediol (ethylene glycol) OH glycerol HO-CH 2 -CH-CH 2 OH glycerin 1, 2, 3 -propanetriol IUPAC name? Lecture. PLUS Timberlake 9

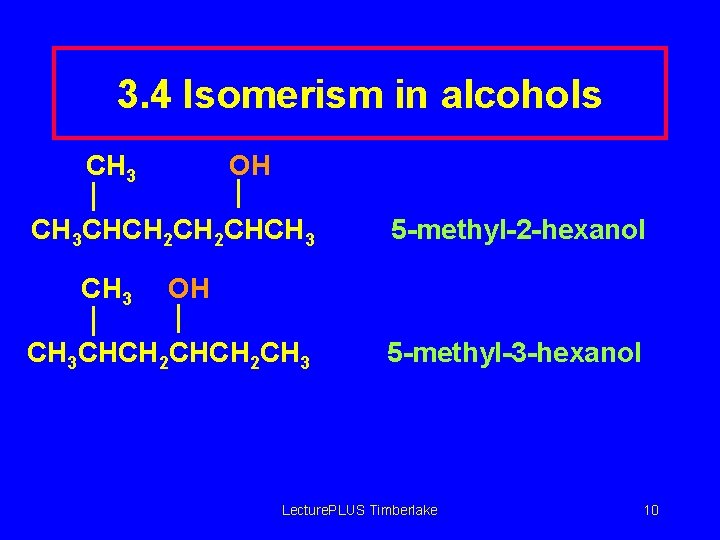
3. 4 Isomerism in alcohols CH 3 OH CH 3 CHCH 2 CHCH 3 5 -methyl-2 -hexanol OH CH 3 CHCH 2 CH 3 5 -methyl-3 -hexanol Lecture. PLUS Timberlake 10

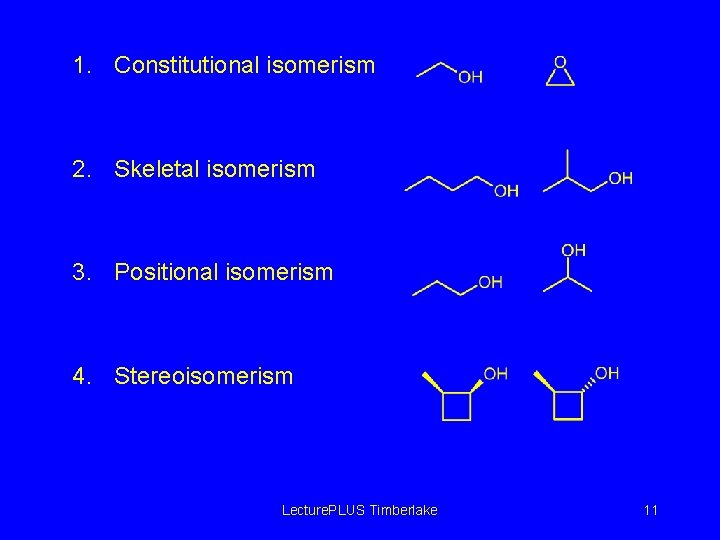
1. Constitutional isomerism 2. Skeletal isomerism 3. Positional isomerism 4. Stereoisomerism Lecture. PLUS Timberlake 11

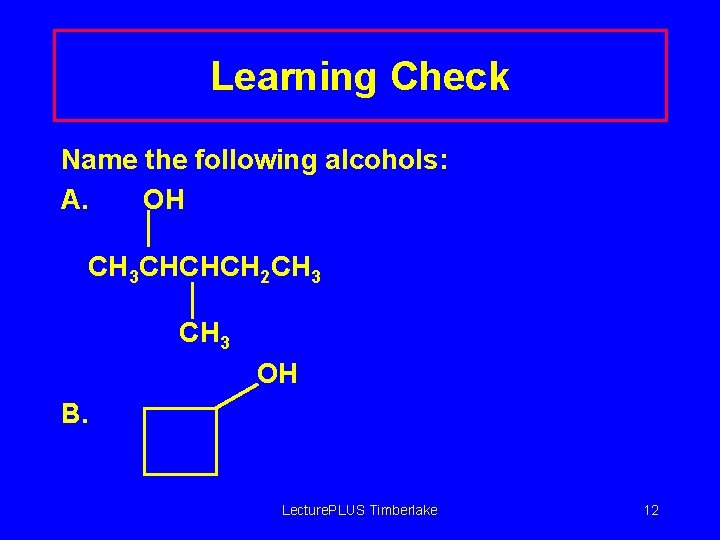
Learning Check Name the following alcohols: A. OH CH 3 CHCHCH 2 CH 3 OH B. Lecture. PLUS Timberlake 12

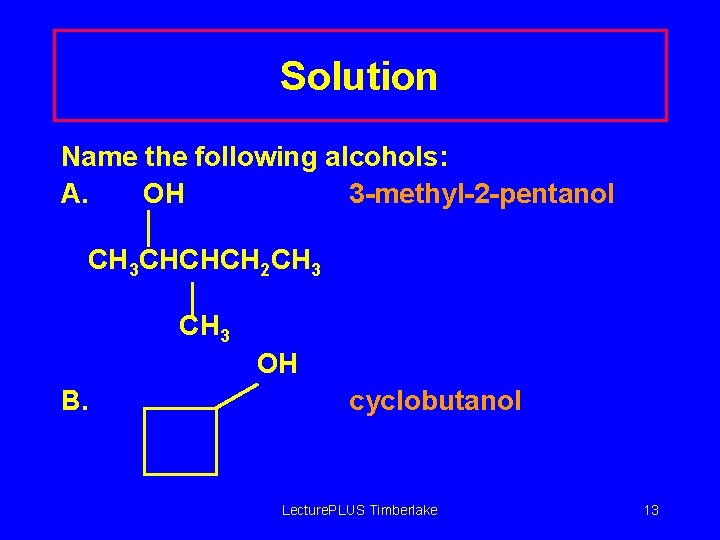
Solution Name the following alcohols: A. OH 3 -methyl-2 -pentanol CH 3 CHCHCH 2 CH 3 OH B. cyclobutanol Lecture. PLUS Timberlake 13

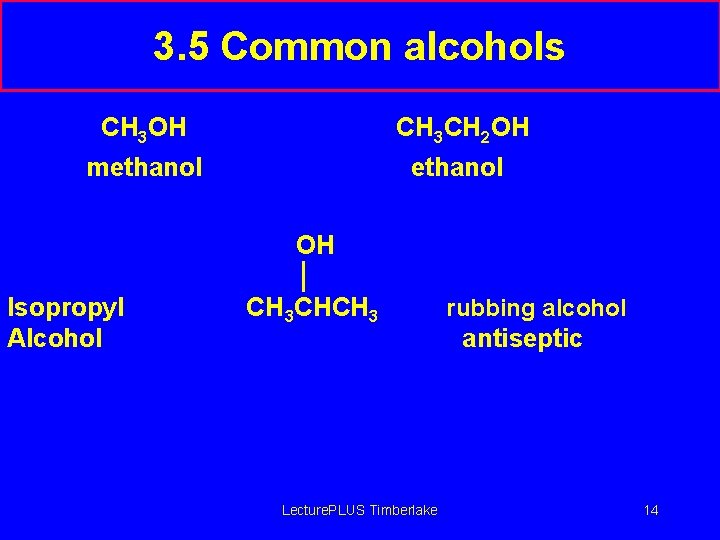
3. 5 Common alcohols CH 3 OH CH 3 CH 2 OH methanol OH Isopropyl Alcohol CH 3 CHCH 3 Lecture. PLUS Timberlake rubbing alcohol antiseptic 14

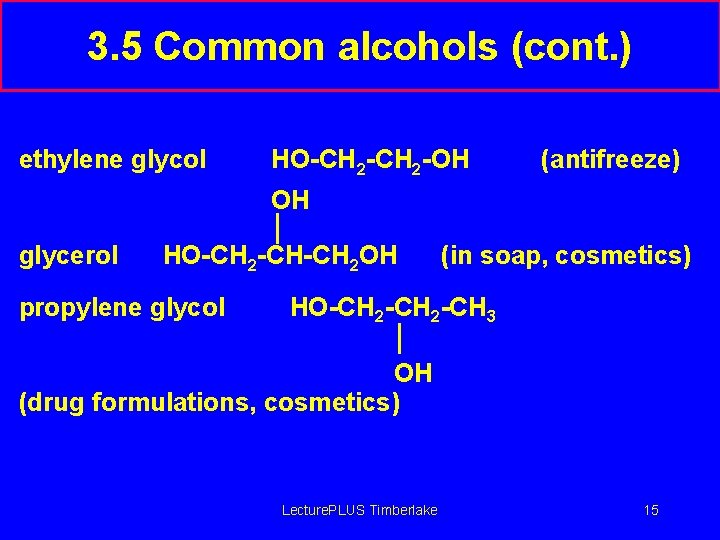
3. 5 Common alcohols (cont. ) ethylene glycol HO-CH 2 -OH (antifreeze) OH glycerol HO-CH 2 -CH-CH 2 OH propylene glycol (in soap, cosmetics) HO-CH 2 -CH 3 OH (drug formulations, cosmetics) Lecture. PLUS Timberlake 15