Chapter 13 Alcohols Phenols and Thiols 13 4



![Oxidation of Primary (1 ) Alcohols When a primary alcohol is oxidized [O], § Oxidation of Primary (1 ) Alcohols When a primary alcohol is oxidized [O], §](https://slidetodoc.com/presentation_image_h/f82887e4f633cc803169b7f7267e4941/image-10.jpg)
![Oxidation of Secondary (2 ) Alcohols When a secondary alcohol is oxidized [O], § Oxidation of Secondary (2 ) Alcohols When a secondary alcohol is oxidized [O], §](https://slidetodoc.com/presentation_image_h/f82887e4f633cc803169b7f7267e4941/image-11.jpg)
- Slides: 20

Chapter 13 Alcohols, Phenols, and Thiols 13. 4 Reactions of Alcohols and Thiols General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

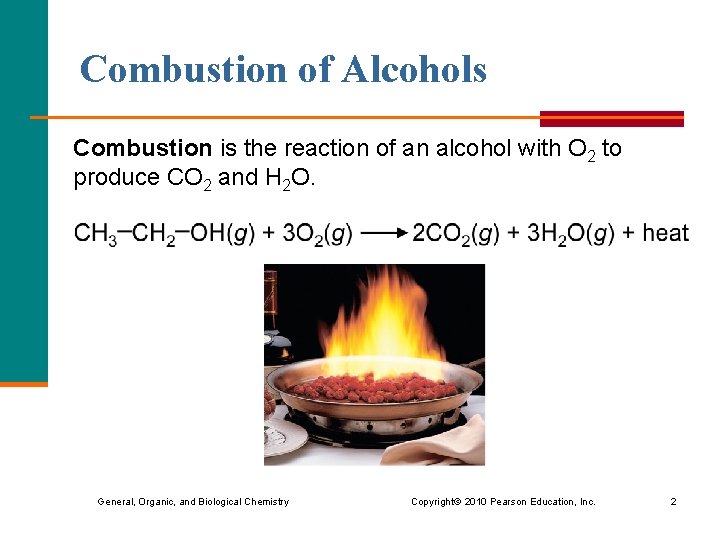
Combustion of Alcohols Combustion is the reaction of an alcohol with O 2 to produce CO 2 and H 2 O. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

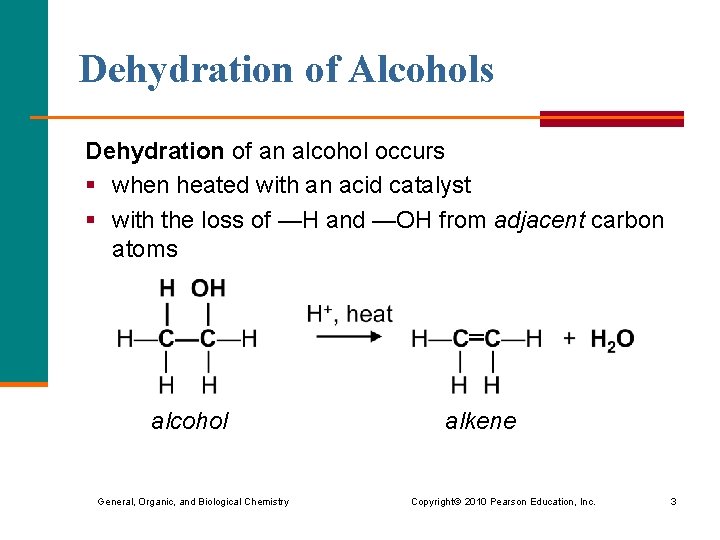
Dehydration of Alcohols Dehydration of an alcohol occurs § when heated with an acid catalyst § with the loss of —H and —OH from adjacent carbon atoms alcohol alkene General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

Saytzeff’s Rule According to Saytzeff’s rule, the dehydration of a secondary alcohol favors the product in which § hydrogen is removed from the adjacent carbon atom in the chain with the smaller number of H atoms General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

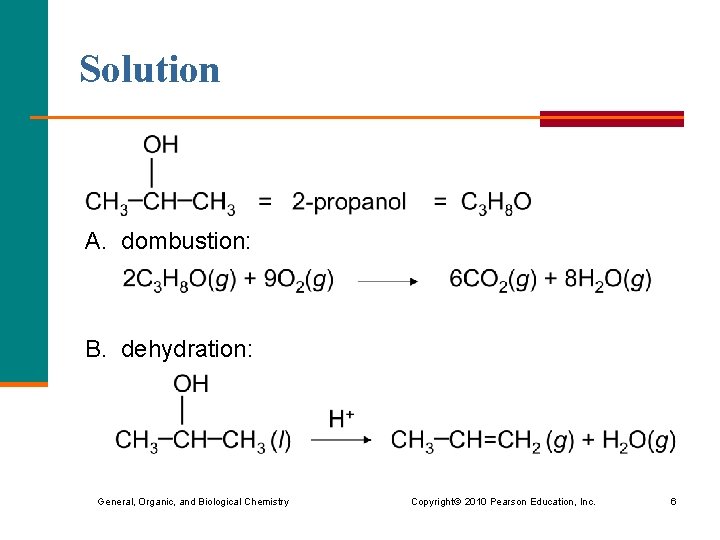
Learning Check Write the equations for the reactions when 2 -propanol undergoes: A. combustion B. dehydration General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

Solution A. dombustion: B. dehydration: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

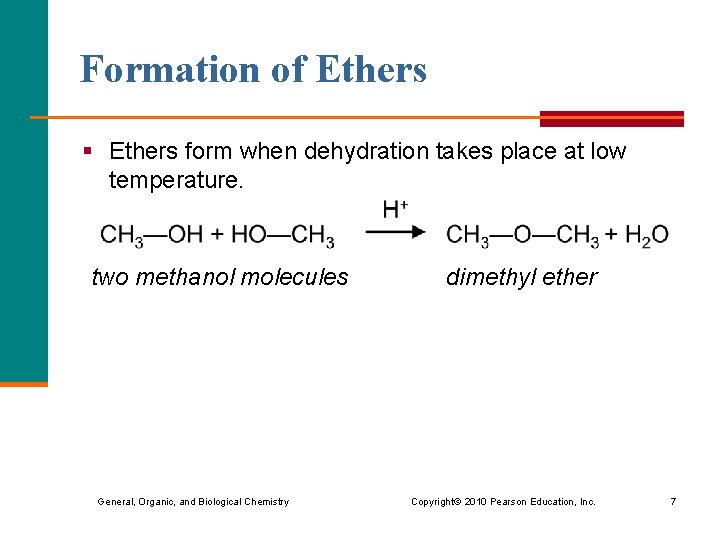
Formation of Ethers § Ethers form when dehydration takes place at low temperature. two methanol molecules dimethyl ether General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

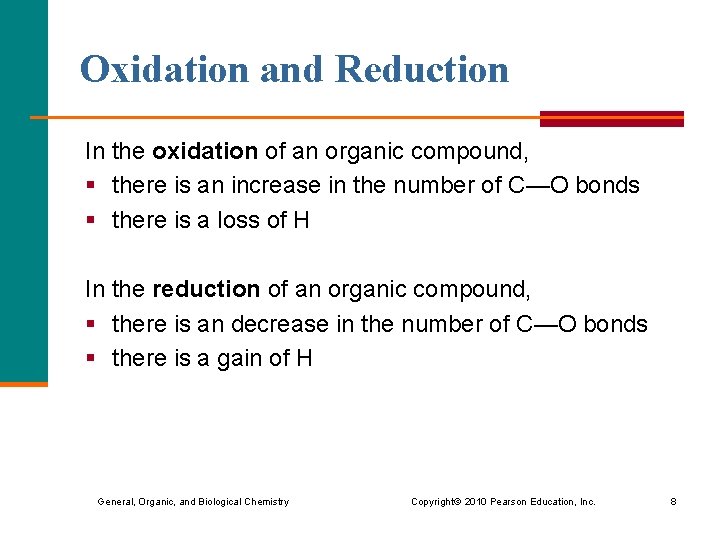
Oxidation and Reduction In the oxidation of an organic compound, § there is an increase in the number of C—O bonds § there is a loss of H In the reduction of an organic compound, § there is an decrease in the number of C—O bonds § there is a gain of H General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

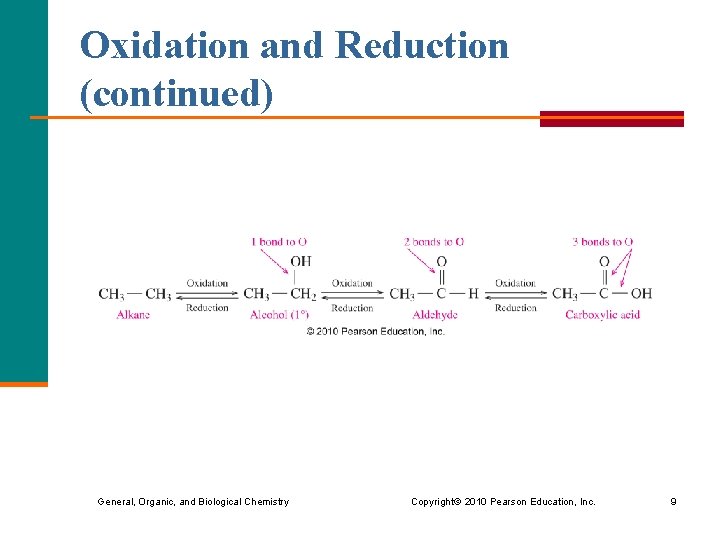
Oxidation and Reduction (continued) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9
![Oxidation of Primary 1 Alcohols When a primary alcohol is oxidized O Oxidation of Primary (1 ) Alcohols When a primary alcohol is oxidized [O], §](https://slidetodoc.com/presentation_image_h/f82887e4f633cc803169b7f7267e4941/image-10.jpg)
Oxidation of Primary (1 ) Alcohols When a primary alcohol is oxidized [O], § one H is removed from the –OH § another –H is removed from the carbon bonded to –OH § an aldehyde is produced Ethanol (ethyl alcohol) Ethanal (acetaldehyde) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10
![Oxidation of Secondary 2 Alcohols When a secondary alcohol is oxidized O Oxidation of Secondary (2 ) Alcohols When a secondary alcohol is oxidized [O], §](https://slidetodoc.com/presentation_image_h/f82887e4f633cc803169b7f7267e4941/image-11.jpg)
Oxidation of Secondary (2 ) Alcohols When a secondary alcohol is oxidized [O], § one H is removed from the –OH § another –H is removed from the carbon bonded to the –OH § a ketone is produced 2 -Propanol (isopropyl alcohol) 2 -Propanone(dimethyl ketone, “acetone”) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

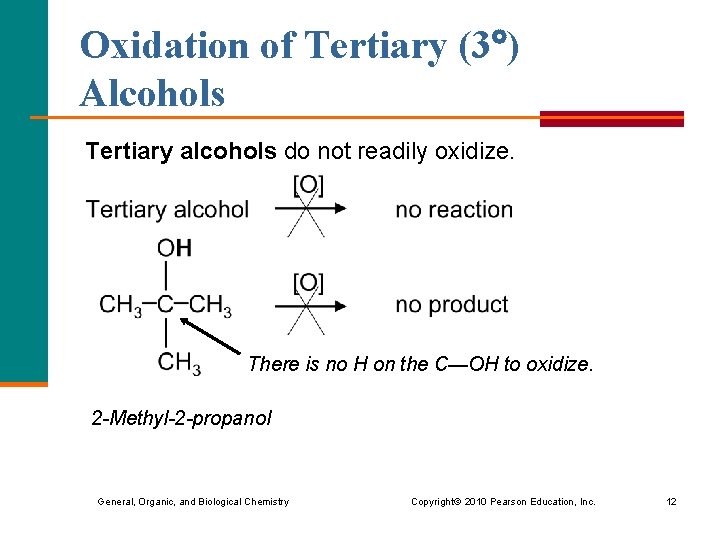
Oxidation of Tertiary (3 ) Alcohols Tertiary alcohols do not readily oxidize. There is no H on the C—OH to oxidize. 2 -Methyl-2 -propanol General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

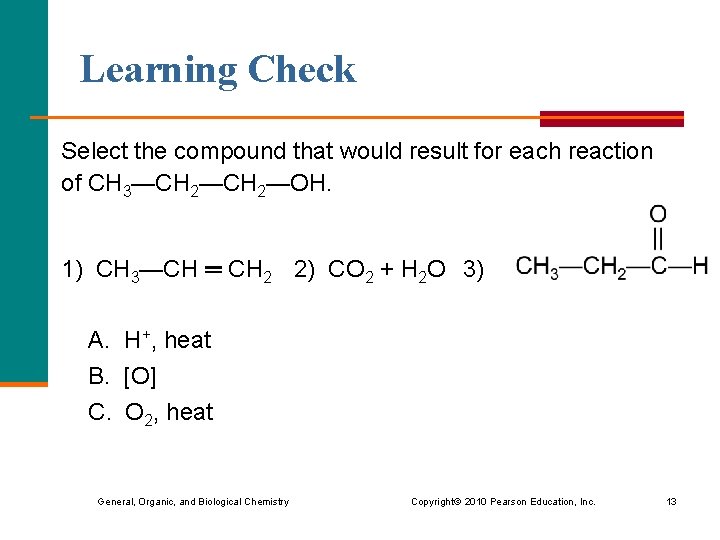
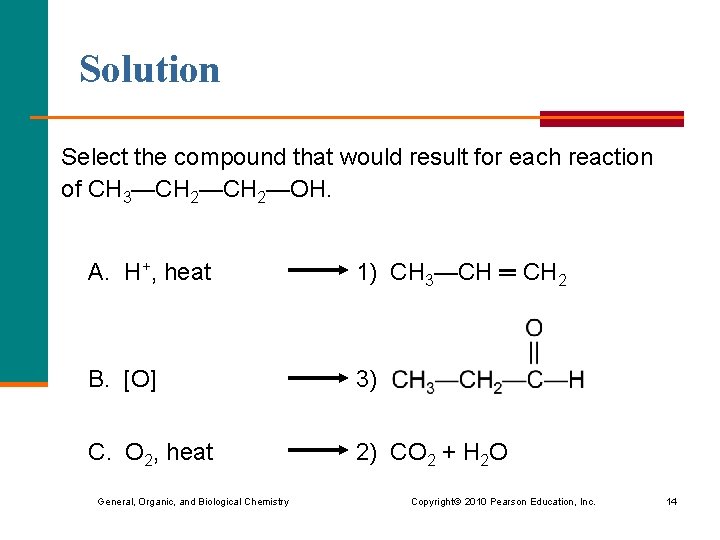
Learning Check Select the compound that would result for each reaction of CH 3—CH 2—OH. 1) CH 3—CH ═ CH 2 2) CO 2 + H 2 O 3) A. H+, heat B. [O] C. O 2, heat General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13

Solution Select the compound that would result for each reaction of CH 3—CH 2—OH. A. H+, heat 1) CH 3—CH ═ CH 2 B. [O] 3) C. O 2, heat 2) CO 2 + H 2 O General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14

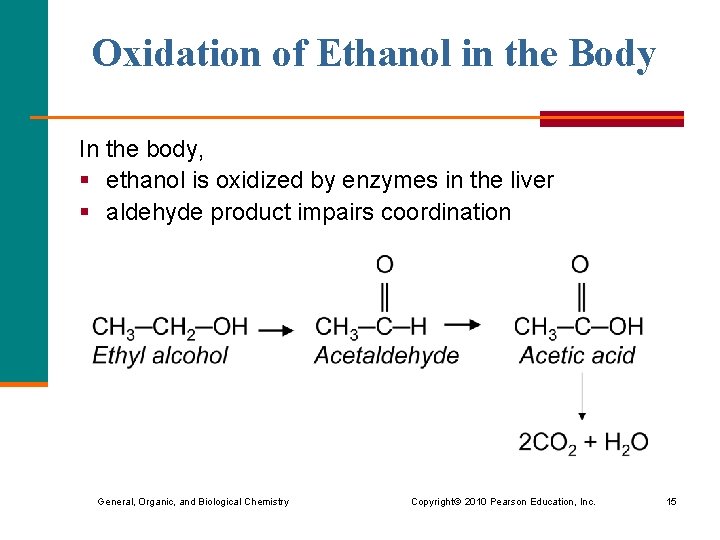
Oxidation of Ethanol in the Body In the body, § ethanol is oxidized by enzymes in the liver § aldehyde product impairs coordination General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

Ethanol CH 3─CH 2─OH Ethanol § acts as a depressant § kills or disables more people than any other drug § is metabolized at a rate of 12– 15 mg/d. L per hour by a social drinker § is metabolized at a rate of 30 mg/d. L per hour by an alcoholic General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16

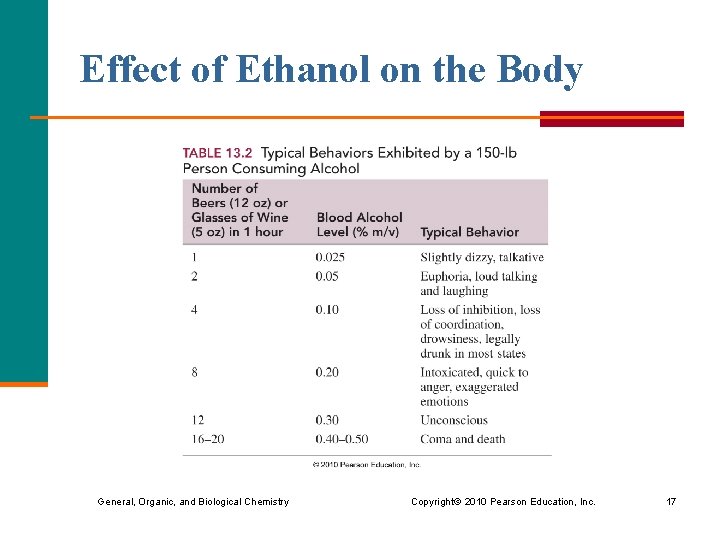
Effect of Ethanol on the Body General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

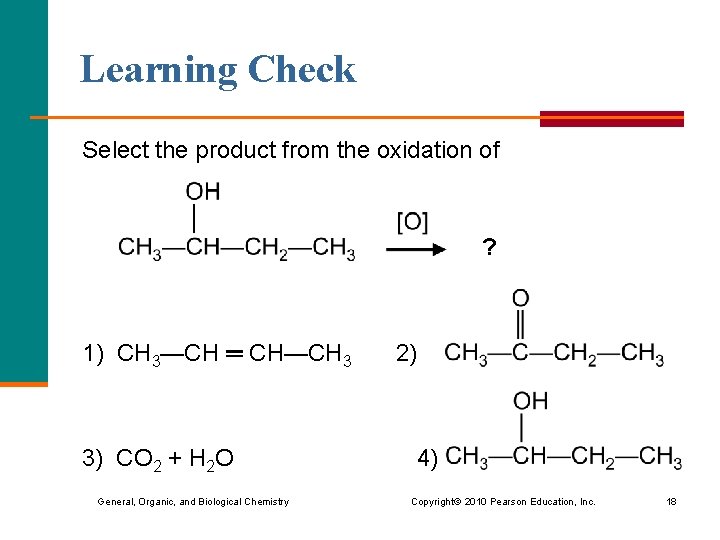
Learning Check Select the product from the oxidation of ? 1) CH 3—CH ═ CH—CH 3 2) 3) CO 2 + H 2 O 4) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

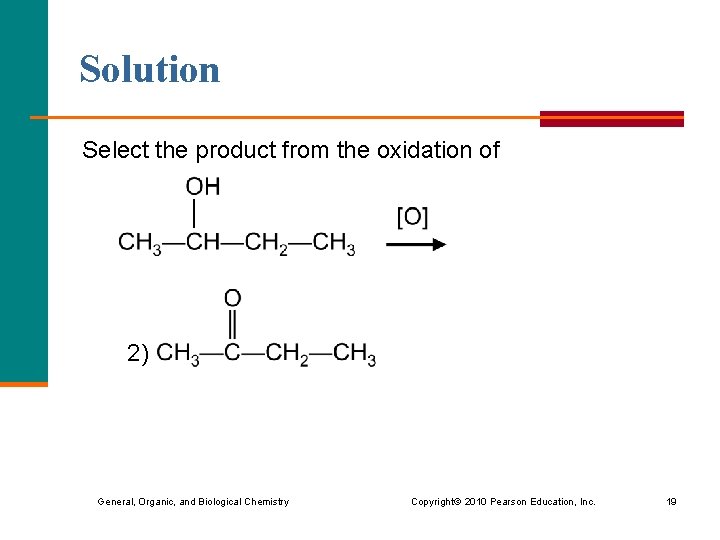
Solution Select the product from the oxidation of 2) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

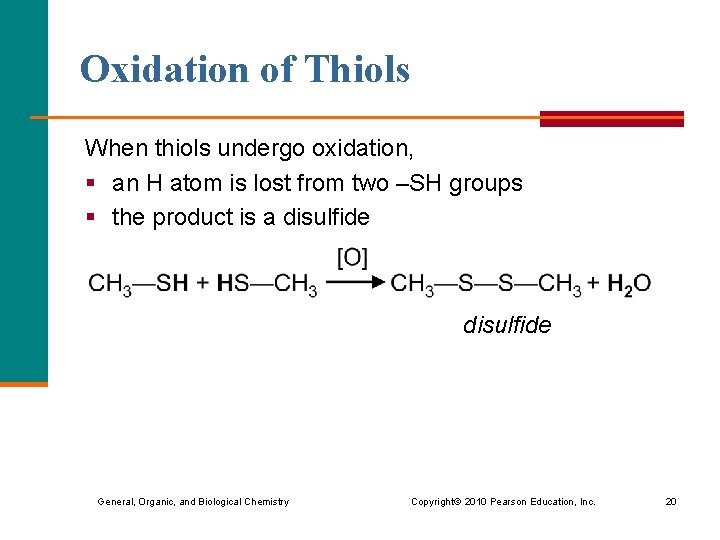
Oxidation of Thiols When thiols undergo oxidation, § an H atom is lost from two –SH groups § the product is a disulfide General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20