Lecture 12 Alcohols Thiols Ethers Aldehydes and Ketones






























- Slides: 48

Lecture 12: Alcohols, Thiols, Ethers, Aldehydes and Ketones Course lecturer : Assis. Prof. Dr. Altijana Hromic-Jahjefendic

�Book chapter: 12 �Pages: 398 -433

Introduction �Functional groups – organic compounds with oxygen and sulfur atoms �Anesthetics- Diethyl ether �Aldehydes as perfumes �Alcohols as beverages (drings) and desinfectants

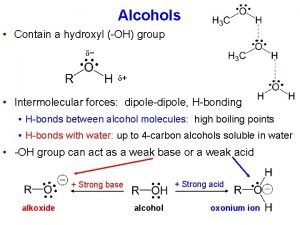
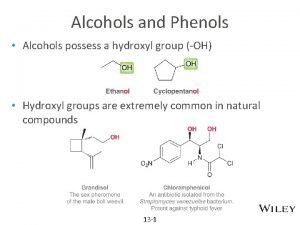
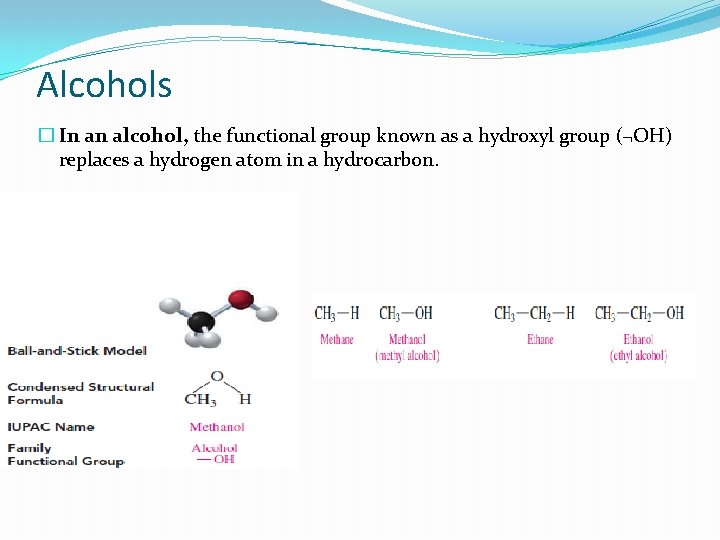
Alcohols � In an alcohol, the functional group known as a hydroxyl group (¬OH) replaces a hydrogen atom in a hydrocarbon.

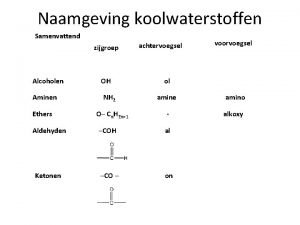
Naming alcohols �Replacing the e of the corresponding alkane with ol �Common name: alkyl group + alcohol �Example: methyl alcohol (methanol) �Cyclic alcohol – naming depends on substituents �The ring is numbered from C 1 which has OH group

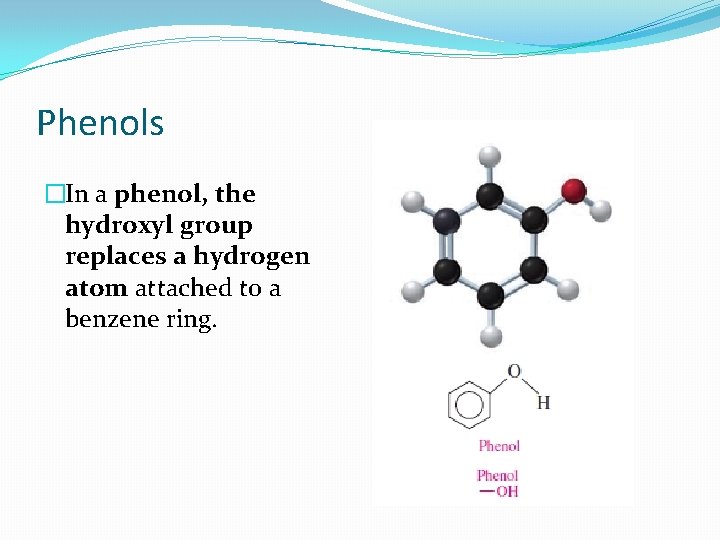
Phenols �In a phenol, the hydroxyl group replaces a hydrogen atom attached to a benzene ring.

Naming phenols �Benzene ring bonded to a hydroxyl group �If there is a second substituent -the benzene ring is numbered starting from C 1 (carbon that has OH group)

Important alcohols �Methanol (methyl alcohol) – the simplest �Found in many solvents and paint removers �If ingested – oxidized to formaldehyde (headache, blindness and death) �Ethanol (ethyl alcohol) – fermentation of grains, sugars and starches �As solvent for perfumes, some medicines (tincture of iodine, propolis) �“Gasohol” – mixture of ethanol and gasoline (production of alternative fuels)

Important phenols �Essential oils of plants �Eugenol – in cloves �Vanillin – vanilla bean �Thymol – thyme and mint �Bispherol A (BPA) – making polycarbonate (manufacturing beverage bottles; also baby bottles) �Washing with certain detergents at high temperatures – disrupts polymer, small amounts of BPA leach from the bottle �Estrogen mimic – harmful effects �Banned – “BPA free”

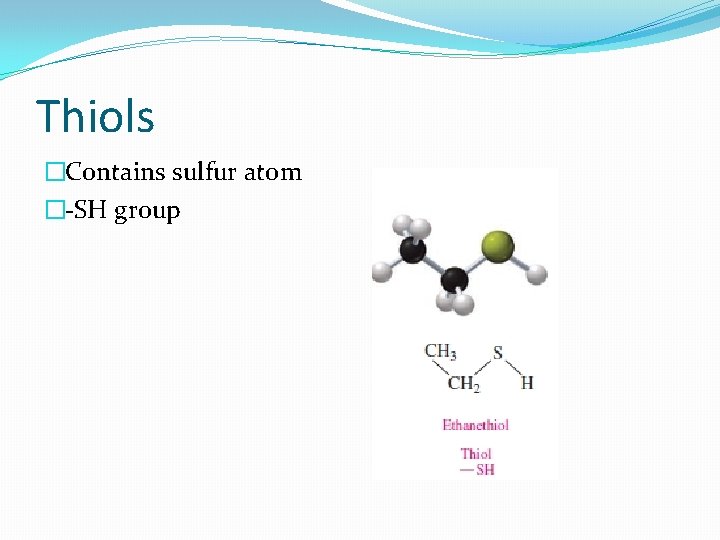
Thiols �Contains sulfur atom �-SH group

Naming thiols �Thiol to the alkane name of the longest carbon chain �Numbering the carbon chain from the end nearer the –SH group �Properties: �Strong, disagreeable odor �Used to detect natural gas leaks (ethanethiol) �Methanethiol – odor of cheddar cheese, onions and garlic

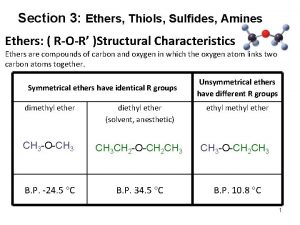
Ether �The functional group consists of an oxygen atom �Attached to 2 C atoms (-O-)

Naming ethers �Most of them have common names �Each alkyl or aromatic group attached to the oxygen atom is written in alphabetical order + ether q. Example: dimethylether (dimethyl group + ether)

Chemistry Link to Health �Ethers as Anesthetics �Anesthesia – loss of sensation and consciousness �Diethyl ether was most widely used for more than a 100 years �Very volatile and highly flammable �Since 1950 s Forane (isofurane), Ethrane and Penthrane were developed – not as flammable �Addition of halogens reduces the flammability �Some of them highly toxic to liver and kidneys replaced

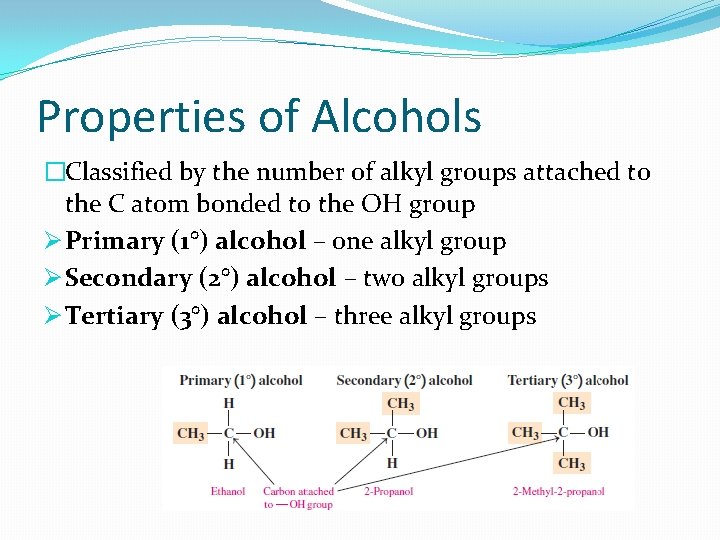
Properties of Alcohols �Classified by the number of alkyl groups attached to the C atom bonded to the OH group Ø Primary (1°) alcohol – one alkyl group Ø Secondary (2°) alcohol – two alkyl groups Ø Tertiary (3°) alcohol – three alkyl groups

Solubility of Alcohols in water �Polar –OH group �Can form hydrogen bonds with the H and O atoms of water �Makes alcohols more soluble in water �Alcohols with one-three C atoms makes them more miscible in water – any amount of alcohol is completely soluble in water �Solubility decreases as the number of C atoms increases


Chemistry link to health: Hand sanitizers and Ethanol �Hand sanitizers – alternative to washing hands �Kills most bacteria and viruses that spread colds and flu �Use ethanol as active ingredient �Safe for most adults; supervision when used by children �The ethanol amount is 60 % (v/v) but can go up to 85 % �Importnat to rub the hands until they are completely dry – flammable �Keep them in storage away from heat sources

�Some hand sanitizers are ethanol-free �Active ingredient – triclosan �Aromathic, ether and phenol functional groups �Banning it – when mixed with tap water for disposal accumulates in the environment �Promotes the growth of antibiotic-resistant bacteria

Solubility of Phenols �Slightly soluble in water �Weak acids �Very corrosive and highly irritating to the skin �Causes severe burns �Ingestion can be fatal �In the past was used to disinfect wounds to prevent post-surgical infections �Replaced by other disinfectants and later by antibiotics

Summary �Alcohols and naming �Phenols and naming �Ethers and naming �Thiols and naming �Properties of alcohols �Hand sanitizers �Solubility of phenols

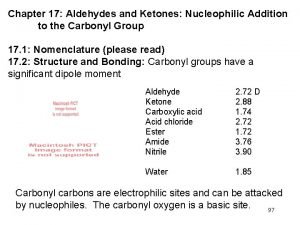
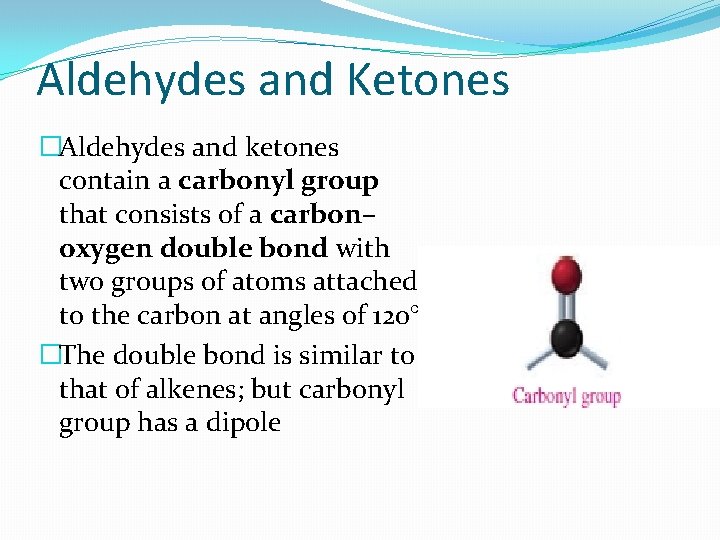
Aldehydes and Ketones �Aldehydes and ketones contain a carbonyl group that consists of a carbon– oxygen double bond with two groups of atoms attached to the carbon at angles of 120°. �The double bond is similar to that of alkenes; but carbonyl group has a dipole

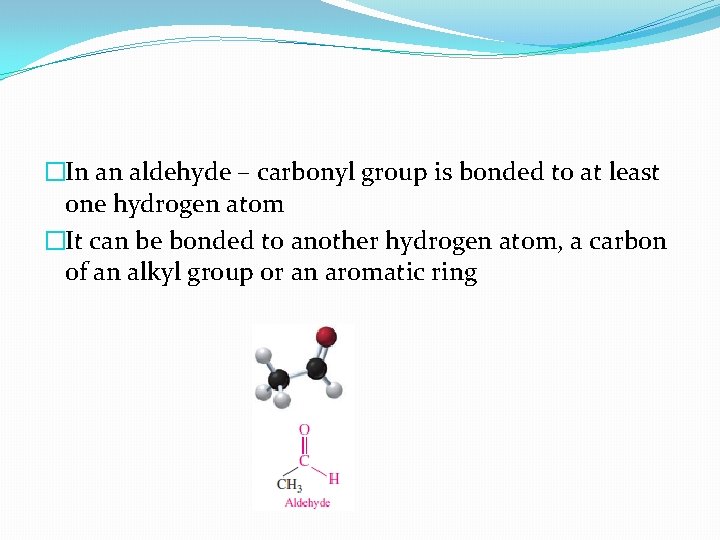
�In an aldehyde – carbonyl group is bonded to at least one hydrogen atom �It can be bonded to another hydrogen atom, a carbon of an alkyl group or an aromatic ring

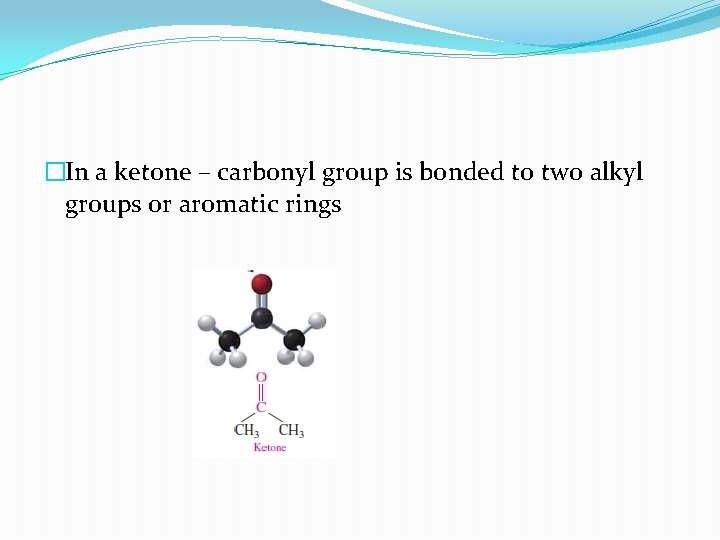
�In a ketone – carbonyl group is bonded to two alkyl groups or aromatic rings

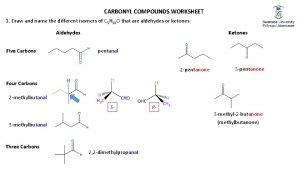
Naming aldehydes �Replacing e of the corresponding alkane with al �No number is needed for the aldehyde group – appears at the end of the chain � 1 -4 C atoms – common names which ends in aldehyde

Naming ketons �Common anmes for unbranched ketones are still in use �The alkyl groups bonded to the carbonyl group are named as substituents �Listed alphabetically followed by ketone �In the IUPAC system – replacing the e in the corresponding alkane name with one q. Example: propanon (propan + one)

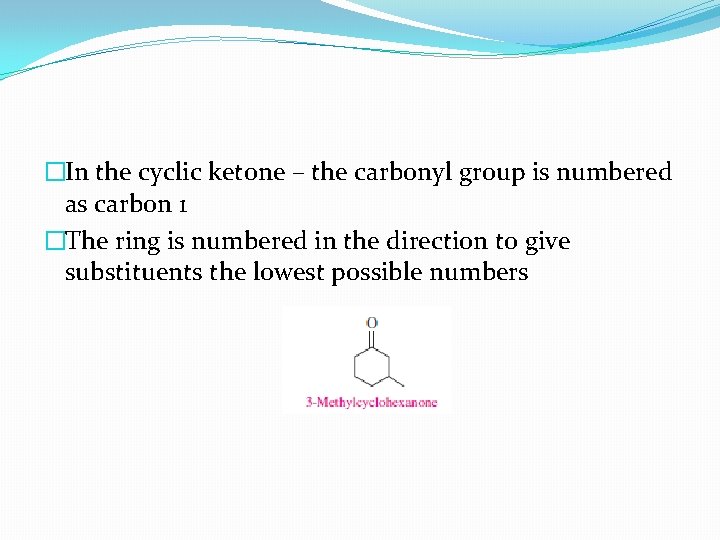
�In the cyclic ketone – the carbonyl group is numbered as carbon 1 �The ring is numbered in the direction to give substituents the lowest possible numbers

Properties of Aldehydes and Ketones in Water �Both contain polar carbonyl group �Partially negative oxygen atom and partially positive C atom �Electronegative oxygen forms hydrogen bondswith water molecules – both compounds with 1 -4 C atoms are very soluble � 5 and more C atoms – not soluble; longer hydrocarbon chains (nonpolar) diminish the solubility effect of the polar carbonyl group


Chemistry Link to Health: Some important aldehydes and ketones �Formaldehyde – colorless gas, pungent odor �An aqueous solution – formalin (germicide and to preserve biological specimens) �In industry – reactant in the synthesis of polymers (used to make fabrics, insulation materials, carpeting, pressed wood products etc. ) �Exposure to the gas – irritation of eyes, nose and upper respiratory tract, skin rashes, headache, dizziness

�Acetone – colorless liquid with mild odor �Wide use in cleaning fluids, paint, nail polish removers �Very flammable �In the body- produced in uncontrolled diabetes, fasting and high-protein diets �Muscone – used to make musk perfumes �Naturally occuring aromatic aldehydes – used to flavor food and as fragnances in perfumes �Benzaldehyde – almonds

Reactions of Alcohols, Thiols, Aledydes and Ketones �Alcohols – combustion in the presence of oxygen �Example: flamming dessert; prepared by pouring liquor on fruit or ice cream and lighten it

Dehydration of Alcohols �Lose water when they are heated with an acid as catalyst �A double bond forms between the same two C atoms – produces alkenes

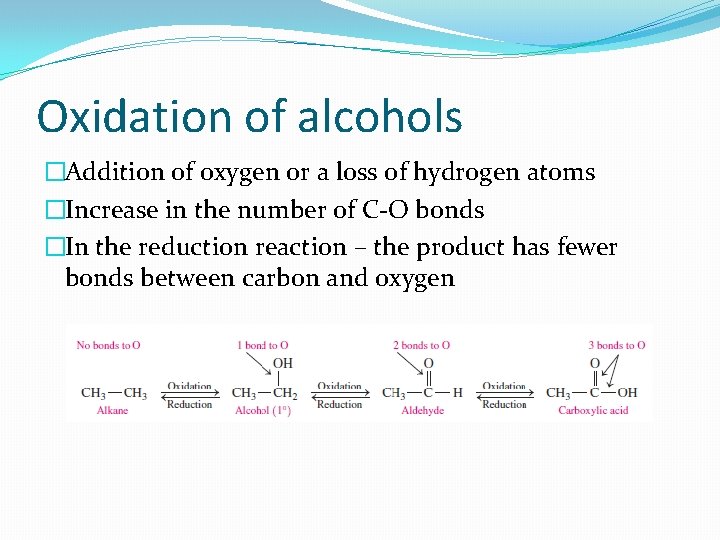
Oxidation of alcohols �Addition of oxygen or a loss of hydrogen atoms �Increase in the number of C-O bonds �In the reduction reaction – the product has fewer bonds between carbon and oxygen

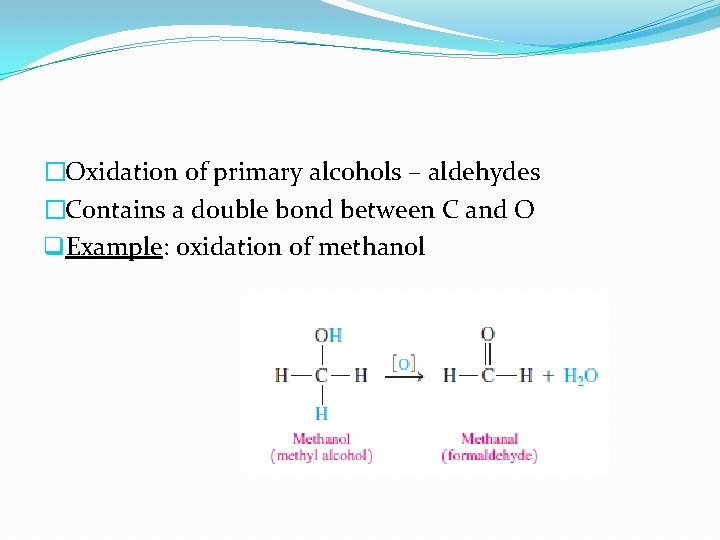
�Oxidation of primary alcohols – aldehydes �Contains a double bond between C and O q. Example: oxidation of methanol

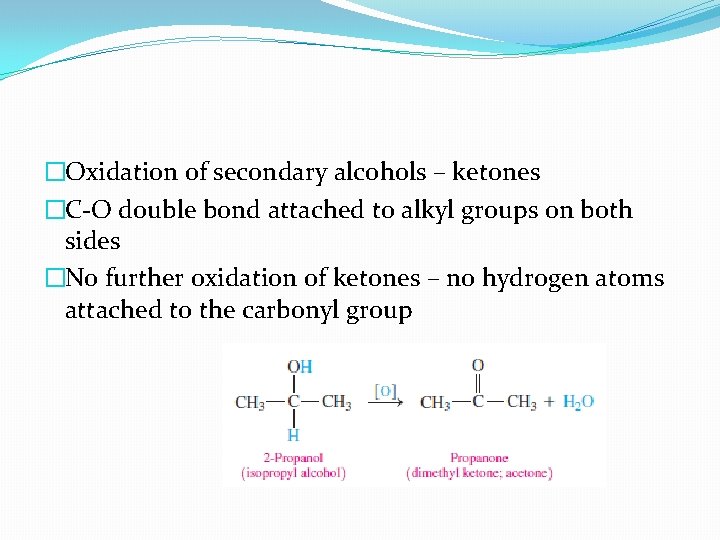
�Oxidation of secondary alcohols – ketones �C-O double bond attached to alkyl groups on both sides �No further oxidation of ketones – no hydrogen atoms attached to the carbonyl group

�Tertiary alcohols do not oxidize! �There is no hydrogen atom on the carbon bonded to the –OH group �C-C bonds are too strong to oxidize

Chemistry Link to Health: Methanol Poisoning �Methanol (methyl alcohol) – highly toxic �Present in products such as winshield washer fluid, paint strippers �Rapidly absorbed in the gastrointestinal tract �In the liver – oxidized to formaldehyde and formic acid �Causes severe abdominal pain and blurred vision �Blindness – intermediate products destroy the retina �Formic acid – lowers blood p. H so severly; 30 m. L of Me. OH can lead to coma and death

�Treatment: Ø Sodium bicarbonate – neutralize the formic acid in the blood Ø Ethanol – intravenously �Enzymes in the liver pick up ethanol molecules to oxidize it instead of methanol �Gives time for the methanol to be eliminated via the lungs �Without formation of its dangerous oxidation products

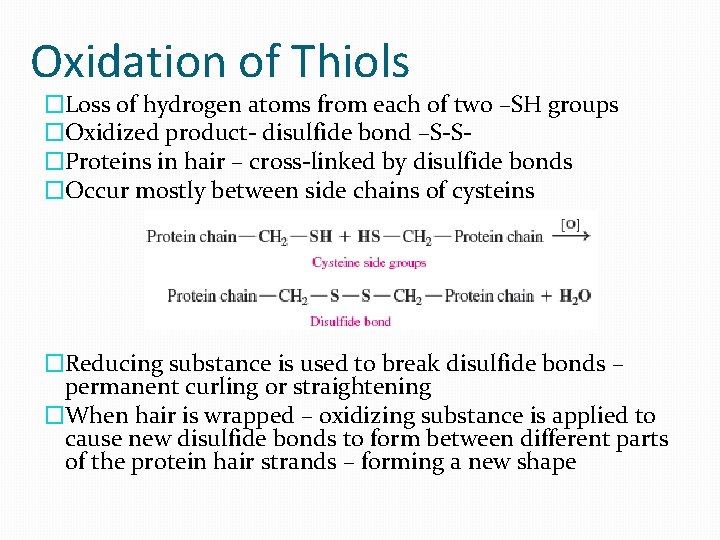
Oxidation of Thiols �Loss of hydrogen atoms from each of two –SH groups �Oxidized product- disulfide bond –S-S�Proteins in hair – cross-linked by disulfide bonds �Occur mostly between side chains of cysteins �Reducing substance is used to break disulfide bonds – permanent curling or straightening �When hair is wrapped – oxidizing substance is applied to cause new disulfide bonds to form between different parts of the protein hair strands – forming a new shape

Oxidation of aldehydes �Adding O to aldehydes – to form a carboxylic acid �Has a carboxyl functional group �This step occurs readily – often difficult to isolate the aldehyde product during the oxidation reaction �In contrast, ketones do not undergo further oxidation

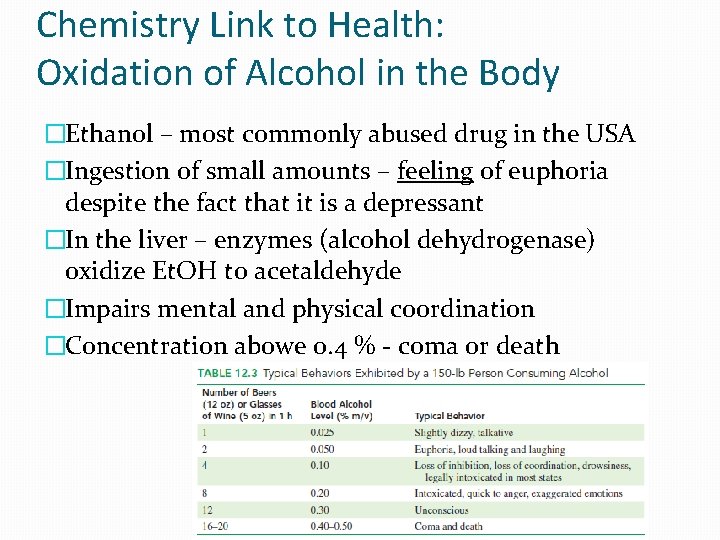
Chemistry Link to Health: Oxidation of Alcohol in the Body �Ethanol – most commonly abused drug in the USA �Ingestion of small amounts – feeling of euphoria despite the fact that it is a depressant �In the liver – enzymes (alcohol dehydrogenase) oxidize Et. OH to acetaldehyde �Impairs mental and physical coordination �Concentration abowe 0. 4 % - coma or death

�The acetaldehyde – further oxidized to acetic acid �Converted to CO 2 and water in the citric acid cycle �Enzymes in the liver – can break down ethanol �However, aldehyde and carboxylic acid intermediates can cause considerable damage within the cells of the liver �Rate of Et. OH metabolism varies between drinkers and nondrinkers �Some effects of alcohol metabolism include an increase in liver lipids, gastritis, alcoholic hepatitis and psychological disturbances

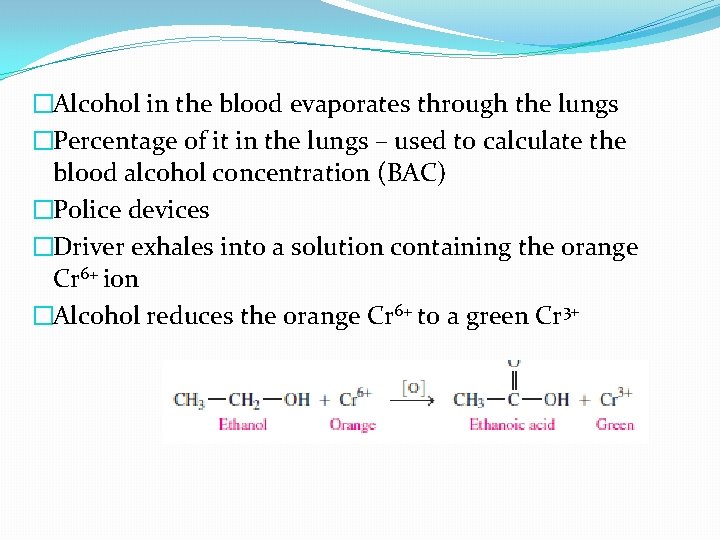
�Alcohol in the blood evaporates through the lungs �Percentage of it in the lungs – used to calculate the blood alcohol concentration (BAC) �Police devices �Driver exhales into a solution containing the orange Cr 6+ ion �Alcohol reduces the orange Cr 6+ to a green Cr 3+

�Treatment: �Antabuse (disulfiram) �Prevents oxidation of acetaldehyde to acetic acid �Result: acetaldehyde accumulates in the blood �Causes nausea, sweating, headache, dizziness and respiratory difficulties �Because of side effects – the person is less likely to use alcohol

Tollens’ Test �Uses solution of Ag+ (Ag. NO 3) and ammonia �Oxidizes aldehydes but not ketones �The silver ion is reduced and forms a “silver mirror” �Comertially, similar process is used to make mirrors by applying a solution of Ag. NO 3 and ammonia on a glass (spray gun)

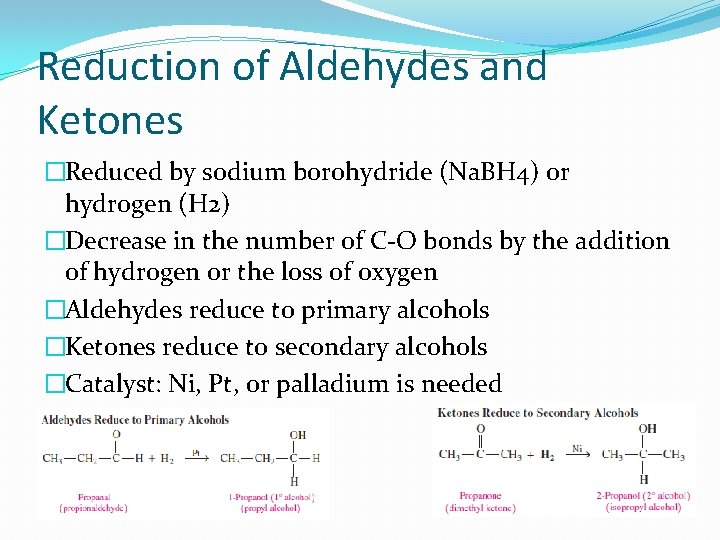
Reduction of Aldehydes and Ketones �Reduced by sodium borohydride (Na. BH 4) or hydrogen (H 2) �Decrease in the number of C-O bonds by the addition of hydrogen or the loss of oxygen �Aldehydes reduce to primary alcohols �Ketones reduce to secondary alcohols �Catalyst: Ni, Pt, or palladium is needed

Summary �Aldehydes and naming �Ketones and naming �Properties in water �Aldehydes and ketones in everyday life �Reactions of alcohols, thiols, aldehydes and ketones �Methanol poisoning �Oxidation of alcohol in the body �Tollens Test �Reduction of aldehydes and ketones
 Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Aldehydes and ketones structure
Aldehydes and ketones structure Polyhydroxy aldehydes and ketones
Polyhydroxy aldehydes and ketones Aldehydes and ketones
Aldehydes and ketones Uses of alkanals and alkanones
Uses of alkanals and alkanones Aldehyde skeletal structure
Aldehyde skeletal structure Carbonyl group aldehyde and ketone
Carbonyl group aldehyde and ketone Aldehyde ending
Aldehyde ending Chemsheets
Chemsheets Chemical properties of ketones
Chemical properties of ketones Reactivity of ketones
Reactivity of ketones Reactions of aldehydes and ketones summary
Reactions of aldehydes and ketones summary Carbonyl compounds
Carbonyl compounds Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Cyanohydrin formation
Cyanohydrin formation Nomenclature of ether
Nomenclature of ether Sulfhydryl group
Sulfhydryl group Acidity of thiols
Acidity of thiols Ioc revelation thiols
Ioc revelation thiols Ethers naamgeving
Ethers naamgeving How to name esters
How to name esters Ether naming
Ether naming Ethers naming
Ethers naming Preparation of ether
Preparation of ether Ethers boiling point
Ethers boiling point David klein organic chemistry
David klein organic chemistry Cis-2 3-dimethyloxirane
Cis-2 3-dimethyloxirane Why are ethers relatively inert compounds
Why are ethers relatively inert compounds Acidic cleavage of ethers
Acidic cleavage of ethers Primary alcohol oxidation
Primary alcohol oxidation 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Bilateral cataract ketones and raised liver enzymes
Bilateral cataract ketones and raised liver enzymes Aldehyde and ketones
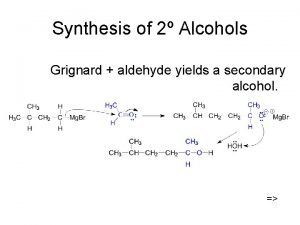
Aldehyde and ketones Synthesis of secondary alcohol
Synthesis of secondary alcohol Cppp cholesterol
Cppp cholesterol High boiling point alcohols
High boiling point alcohols Bleach and ethanol reaction
Bleach and ethanol reaction Lucas test reagent
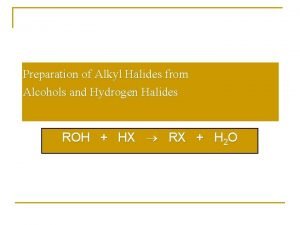
Lucas test reagent Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Acidity of alcohols
Acidity of alcohols Acidity of alcohols
Acidity of alcohols Erythritol production process
Erythritol production process Alcohols nomenclature
Alcohols nomenclature Alcohols nomenclature
Alcohols nomenclature Butanal isomers
Butanal isomers Alcohol and hbr
Alcohol and hbr Can test for alcohols
Can test for alcohols Site:slidetodoc.com
Site:slidetodoc.com Aldehyde + ag2o
Aldehyde + ag2o