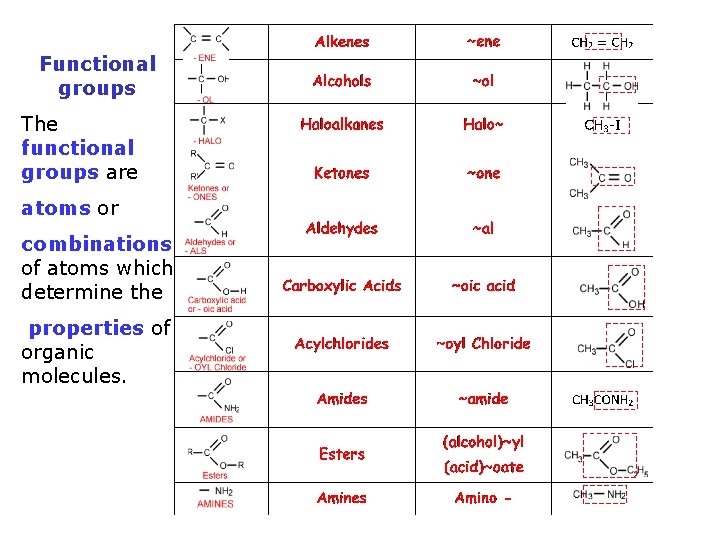
Functional groups The functional groups are atoms or














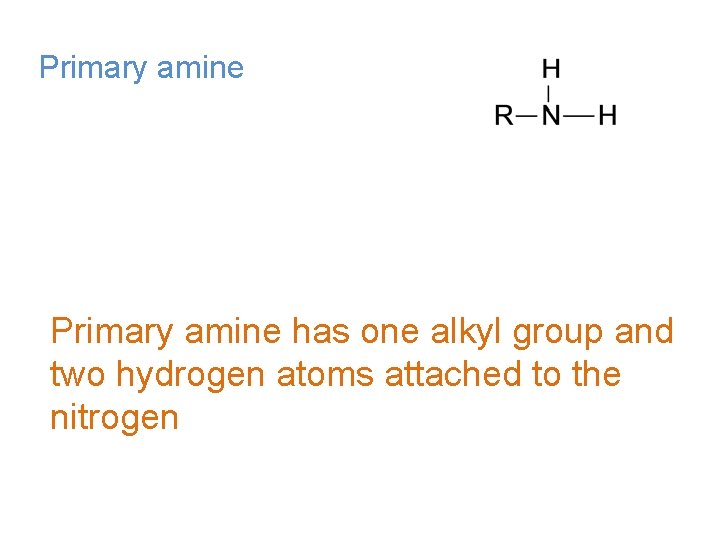
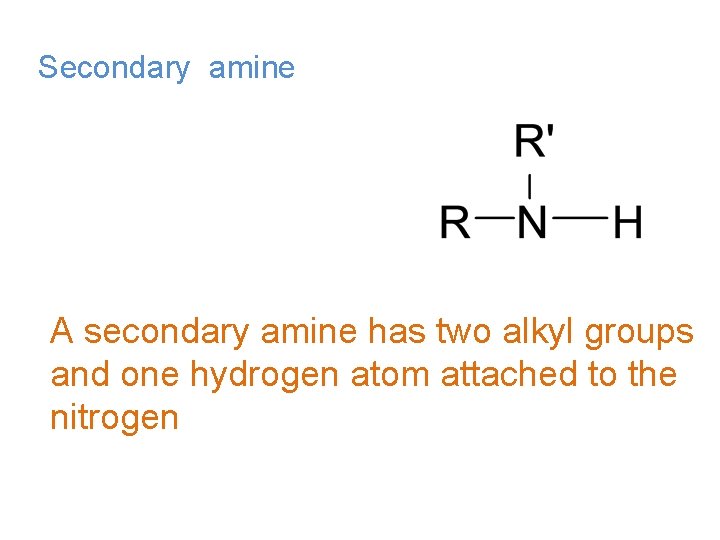
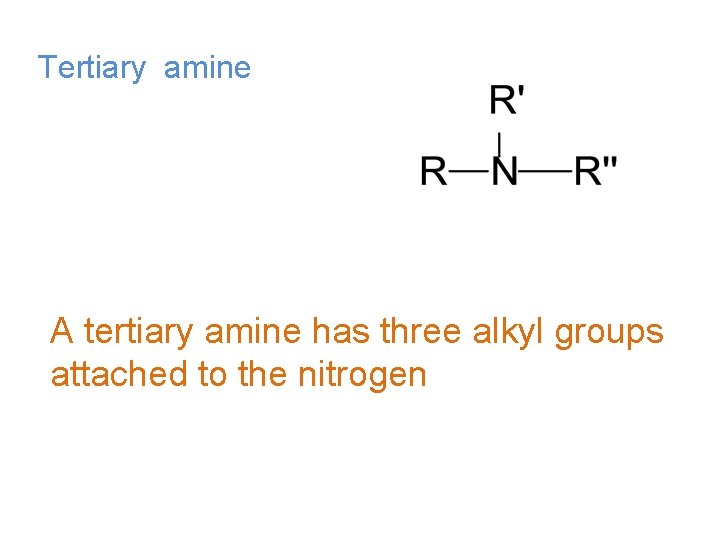


- Slides: 46

Functional groups The functional groups are atoms or combinations of atoms which determine the properties of organic molecules.

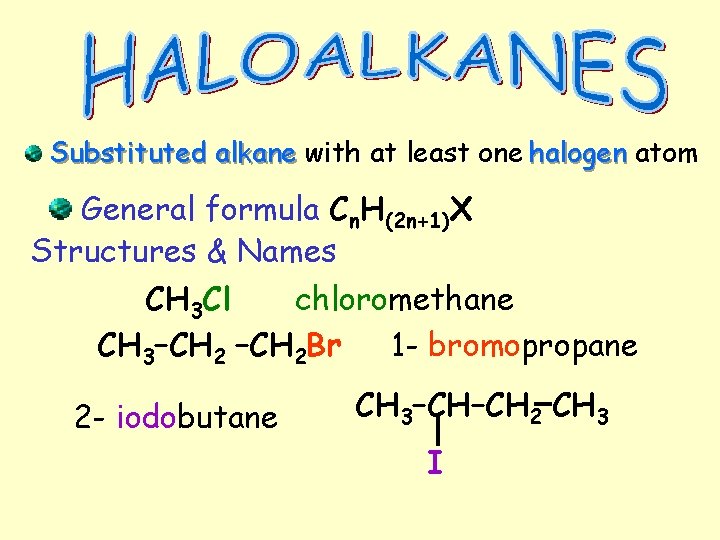
Substituted alkane with at least one halogen atom General formula Cn. H(2 n+1)X Structures & Names CH 3 Cl chloromethane CH 3–CH 2 Br 1 - bromopropane 2 - iodobutane CH 3–CH–CH 2 CH 3 I

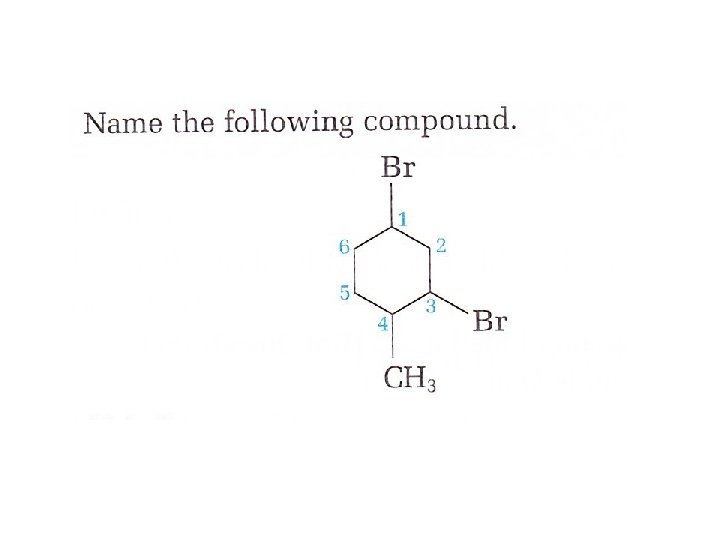
Alkyl Halides An alkyl halide (also known as a haloalkane) is an alkane in which one or more hydrogen atoms have been replaced with halogen atoms, such as F, Cl, Br, or I The functional group is R - X, where X represents a halogen atom. • Similar in structure , polarity, and reactivity to alcohols • To name first name the parent hydrocarbon • Then use the prefix, fluoro, chloro, bromo. or iodo • Use a position number to indicate the halogen



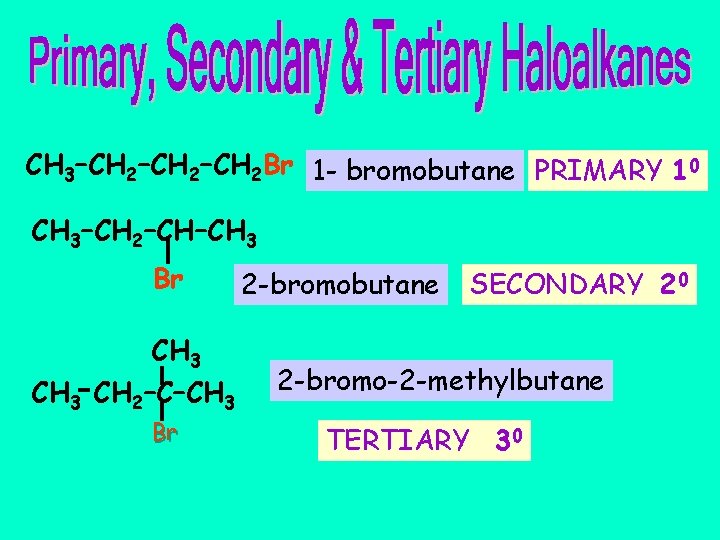
CH 3–CH 2–CH 2 Br 1 - bromobutane PRIMARY 10 CH 3–CH 2–CH–CH 3 Br CH 3 CH 2–C–CH 3 Br 2 -bromobutane SECONDARY 20 2 -bromo-2 -methylbutane TERTIARY 30

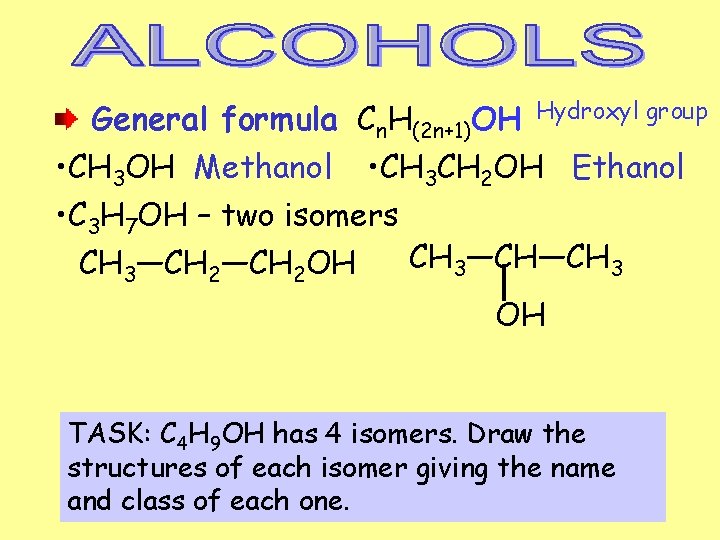
General formula Cn. H(2 n+1)OH Hydroxyl group • CH 3 OH Methanol • CH 3 CH 2 OH Ethanol • C 3 H 7 OH – two isomers CH 3—CH—CH 3—CH 2 OH OH TASK: C 4 H 9 OH has 4 isomers. Draw the structures of each isomer giving the name and class of each one.

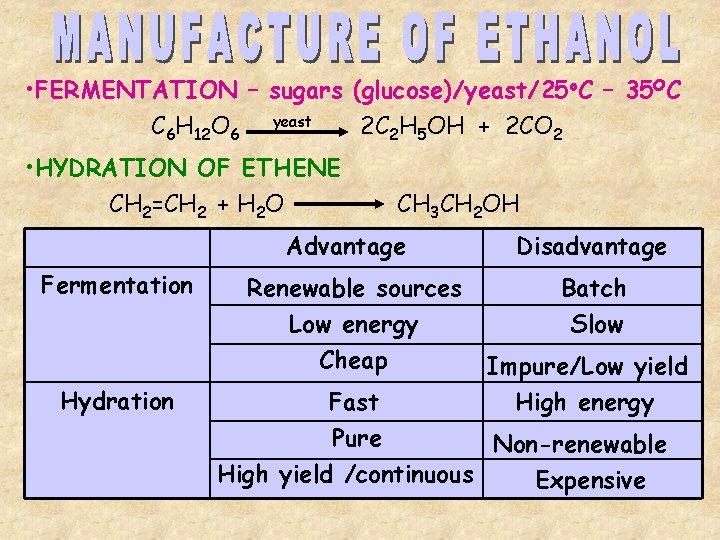
• FERMENTATION – sugars (glucose)/yeast/25 o. C – 35 OC C 6 H 12 O 6 yeast 2 C 2 H 5 OH + 2 CO 2 • HYDRATION OF ETHENE CH 2=CH 2 + H 2 O CH 3 CH 2 OH Advantage Fermentation Hydration Renewable sources Low energy Cheap Disadvantage Batch Slow Impure/Low yield High energy Fast Pure Non-renewable High yield /continuous Expensive

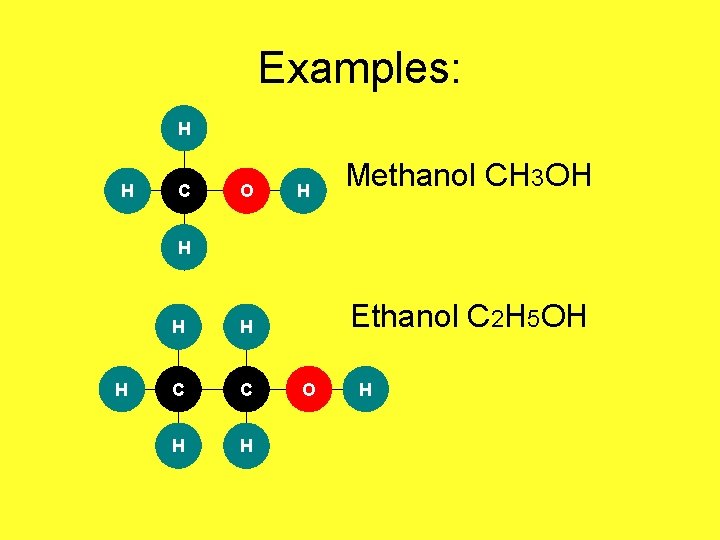
Examples: H H C O H Methanol CH 3 OH H H C C H H Ethanol C 2 H 5 OH O H

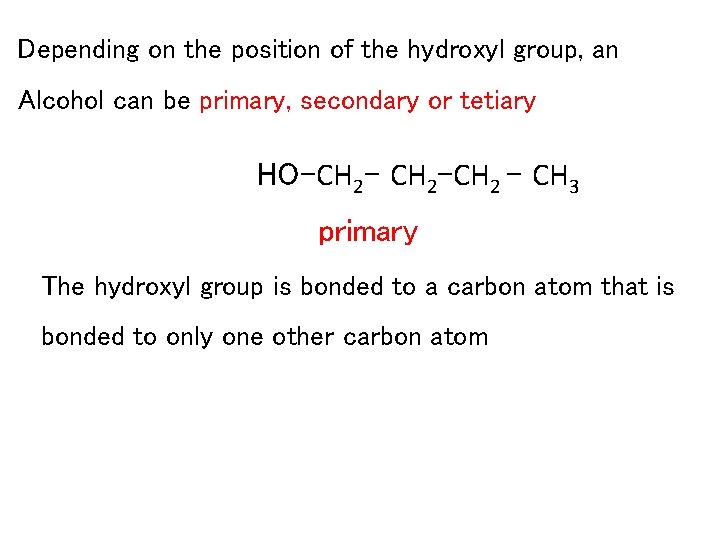
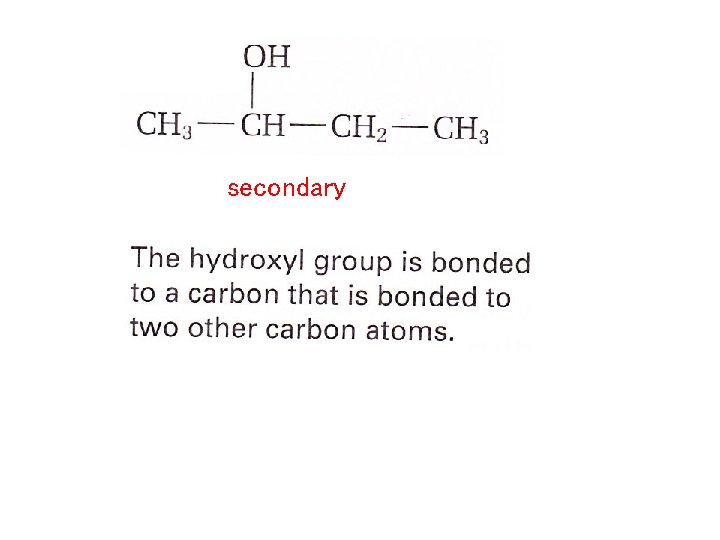
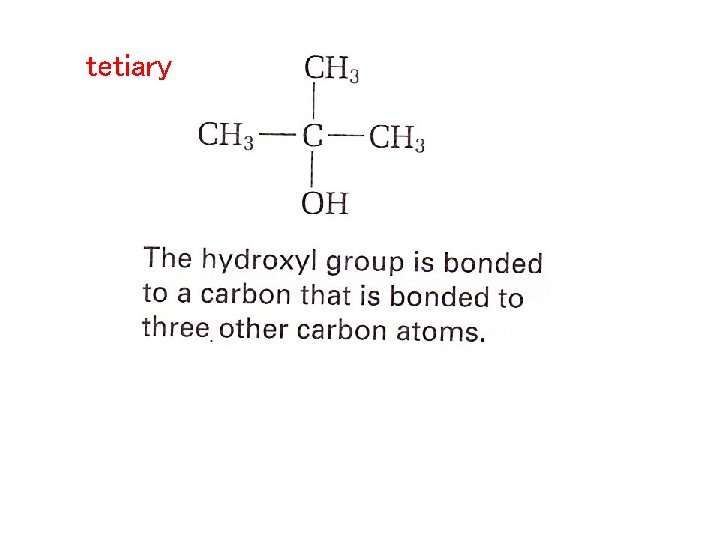
Depending on the position of the hydroxyl group, an Alcohol can be primary, secondary or tetiary HO-CH 2 -CH 2 – CH 3 primary The hydroxyl group is bonded to a carbon atom that is bonded to only one other carbon atom

secondary

tetiary

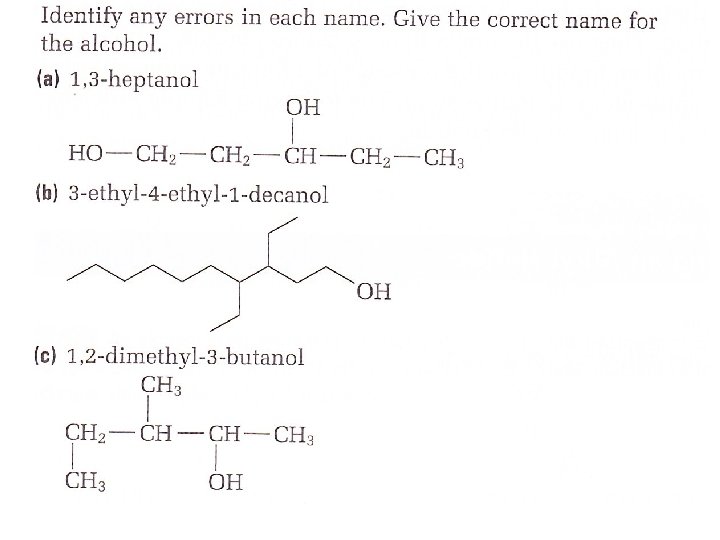
How to name an Alcohol Step 1 Locate the longest chain that contains an — OH group attached to one of the carbon atoms. Name the parent alkane Step 2 Replace the — e ending of the name of the parent alkane with — ol

Step 3 Add a position number before the root name to indicate the location of the — group OH (Remember to number the main chain of the hydrocarbon so that the hydroxyl group has the lowest possible position number Step 4 Name and number any other branches on the main chain. Add the names of these branches to the prefix If the functional group occurs multiple times, place a prefix (di, tri, tetra etc)

Step 5 Put the name together: prefix + root + suffix

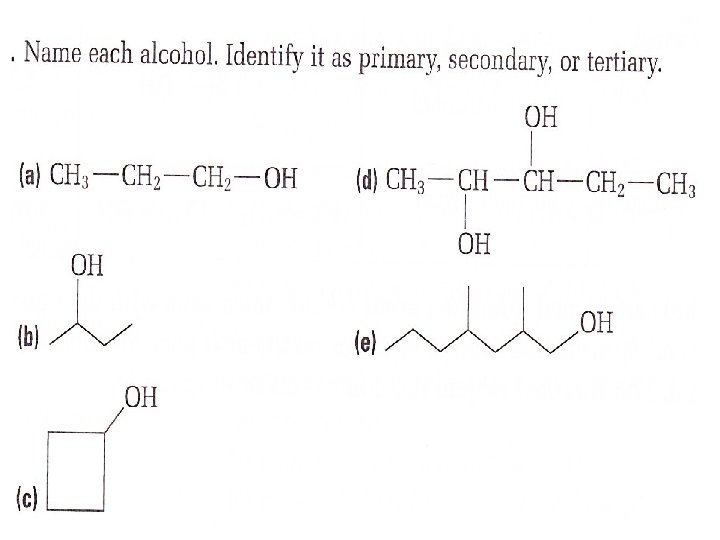
Try this: Name the following alcohol. Identify it as a primary, secondary or tertiary alcohol




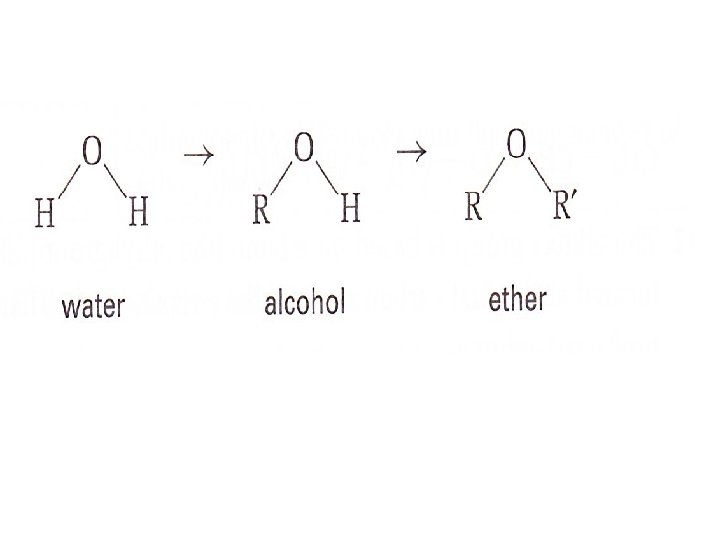
Ethers Suppose that you remove the H atom from the - OH group of an alcohol. This would leave space for another alkyl group to attach to the oxygen atom. CH 3 CH 2 -OH → CH 3 CH 2 -O – CH 3 The compound you have just made is called an ether

An ether is an organic compound that has two alkyl groups joined by an oxygen atom. The general formula of an ether is R – O – R You can think of alcohols and ethers as derivatives of the water molecule


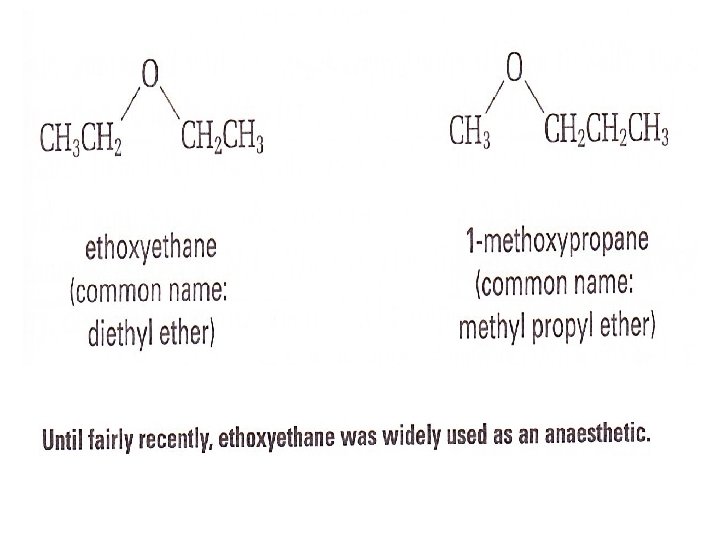
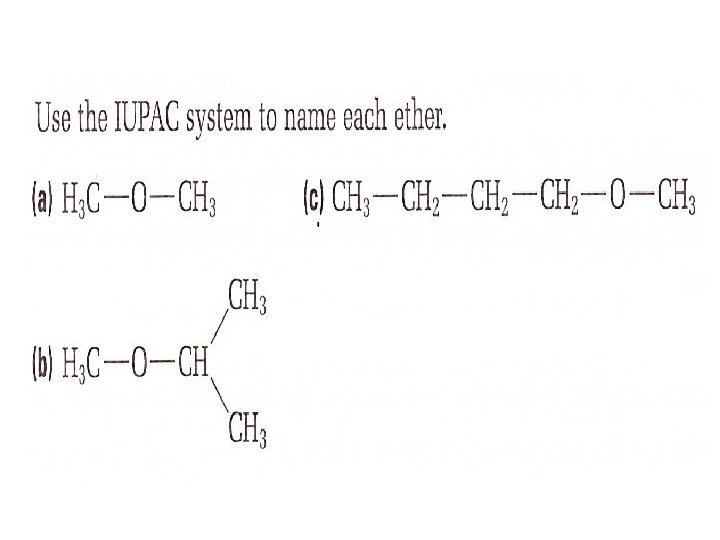
How to name an Ether • Choose the longest alkyl group as the parent alkane. Give it an alkane name • Treat the second alkyl group (shorter), along with the oxygen atom as an alkoxy group attached to the parent alkane • Name it by replacing the —yl ending of the corresponding alkyl group’s name with — oxy • give it a position number • put the prefix and suffix together: • alkoxy group + parent name



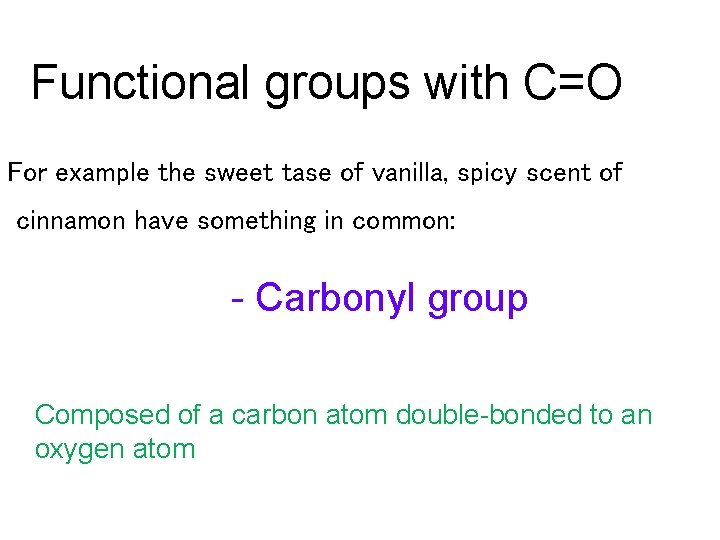
Functional groups with C=O For example the sweet tase of vanilla, spicy scent of cinnamon have something in common: - Carbonyl group Composed of a carbon atom double-bonded to an oxygen atom

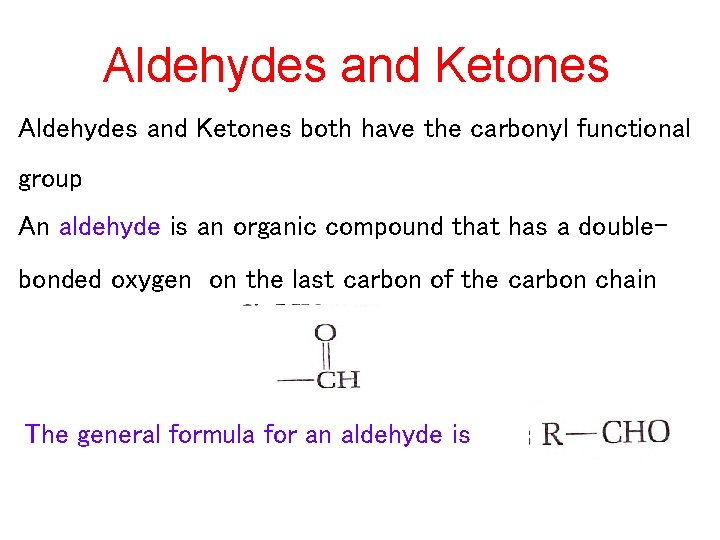
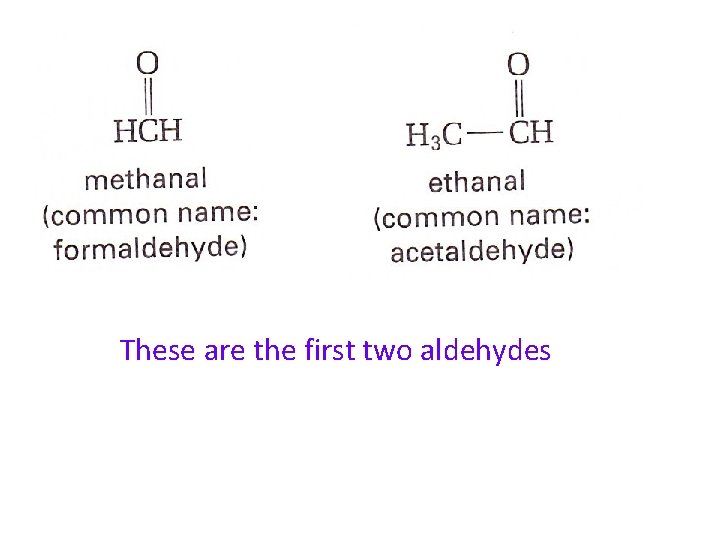
Aldehydes and Ketones both have the carbonyl functional group An aldehyde is an organic compound that has a doublebonded oxygen on the last carbon of the carbon chain The general formula for an aldehyde is

These are the first two aldehydes

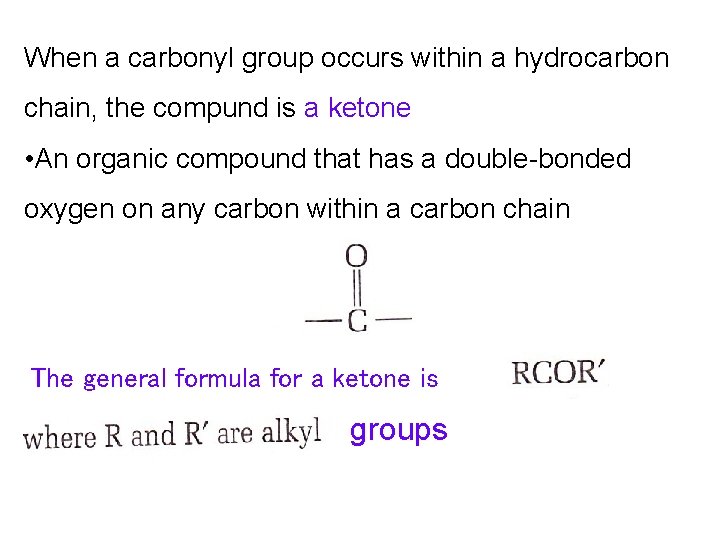
When a carbonyl group occurs within a hydrocarbon chain, the compund is a ketone • An organic compound that has a double-bonded oxygen on any carbon within a carbon chain The general formula for a ketone is groups

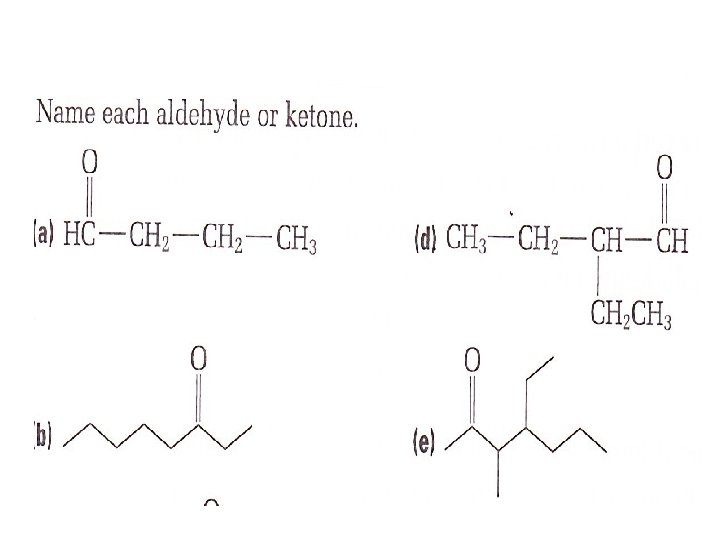
How to name an Aldehyde • Name the parent alkane always give the carbon atom of the carbonyl group position number 1 • replace the —e at the end of the name of the parent alkane with — al. • the carbonyl group is always given position number 1. therefore you do not need to include a position number for it.



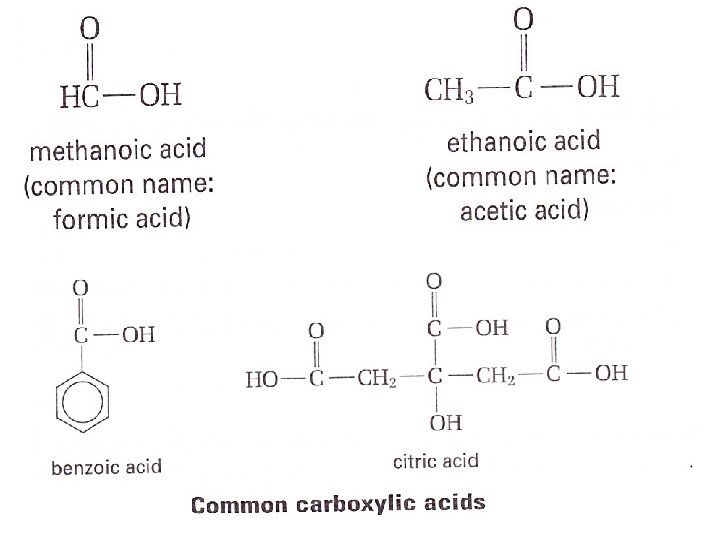
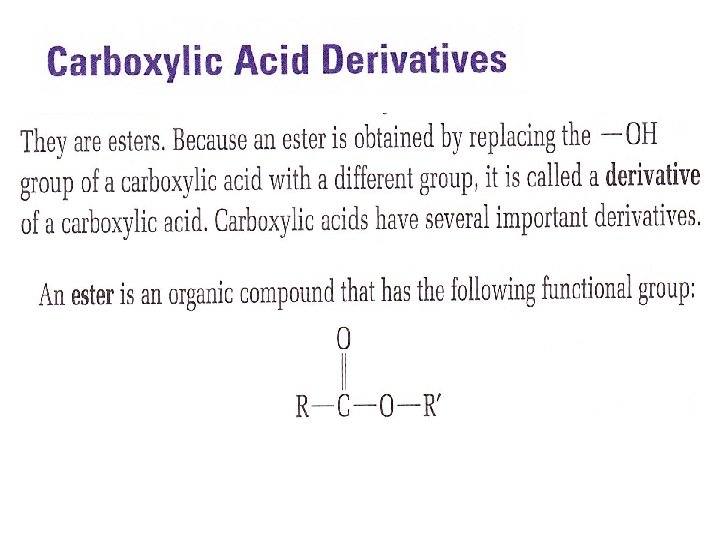
Carboxylic Acids You are familiar with one You sprinkle it over your french fries or salad Vinegar 5% solution of Acetic Acid in water The IUPAC name for acetic acid, CH 3 COOH, is ethanoic acid Functional group


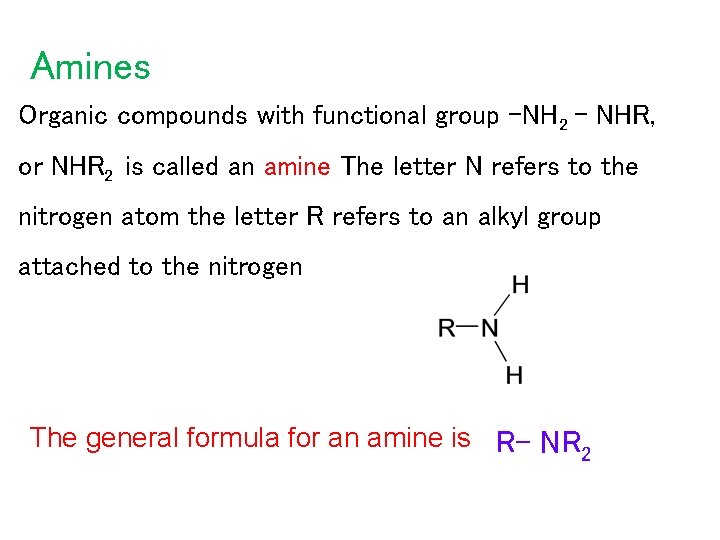
Amines Organic compounds with functional group -NH 2 – NHR, or NHR 2 is called an amine The letter N refers to the nitrogen atom the letter R refers to an alkyl group attached to the nitrogen The general formula for an amine is R– NR 2
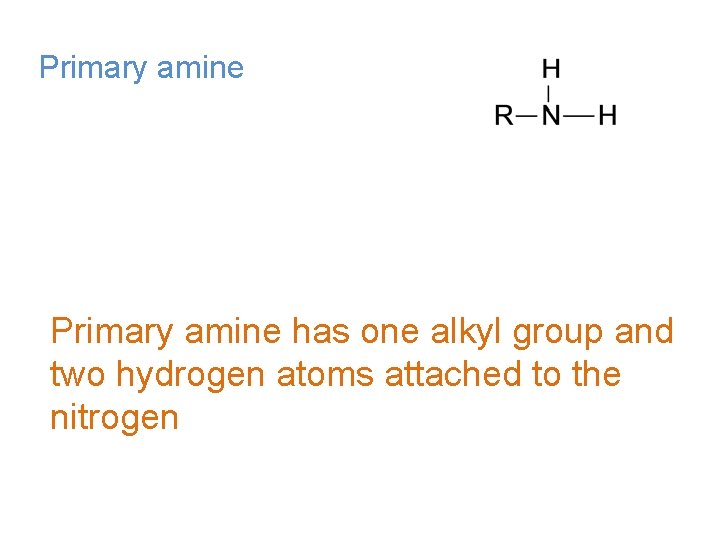
Primary amine has one alkyl group and two hydrogen atoms attached to the nitrogen
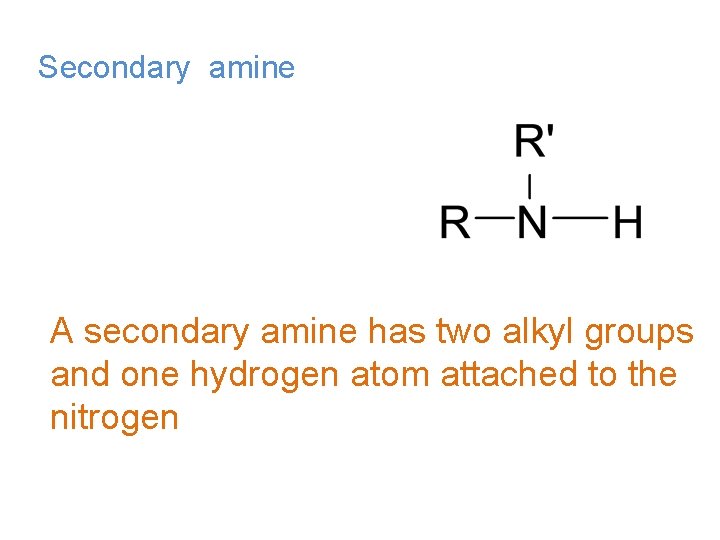
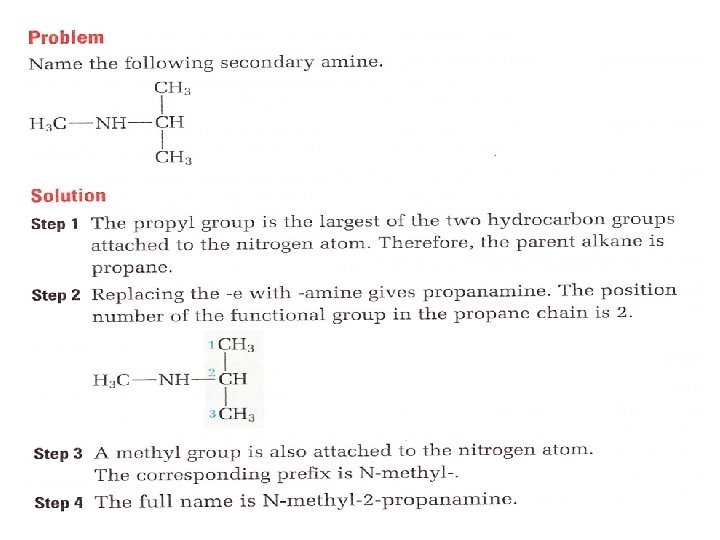
Secondary amine A secondary amine has two alkyl groups and one hydrogen atom attached to the nitrogen
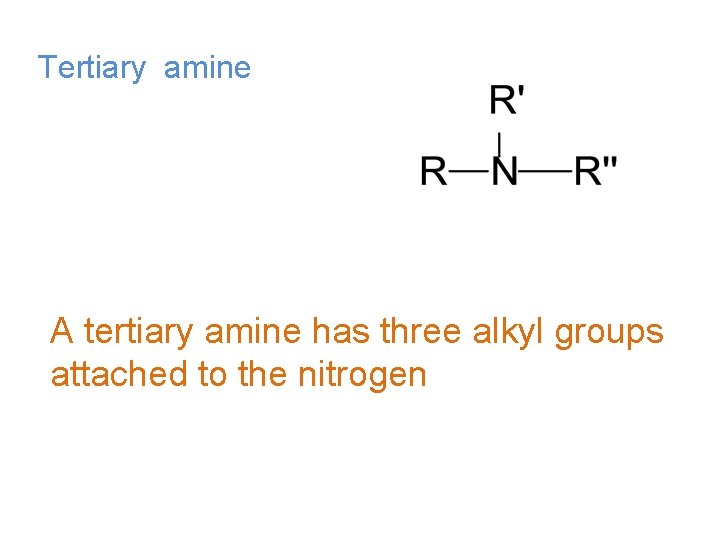
Tertiary amine A tertiary amine has three alkyl groups attached to the nitrogen

How to name an Amine • Identify the largest hydrocarbon group attached to the nitrogen as the parent alkane • Replace the — e at the end of the name with — amine. Include the position number to show the position of the functional group • Name other alkyl group(s) attached to the nitrogen atom. Instead of position numbers, use the letter — N to locate the group(s) • if two identical alkyl groups are attached to the nitrogen atom, use N, N — • this is the prefix • Prefix + Root + Suffix



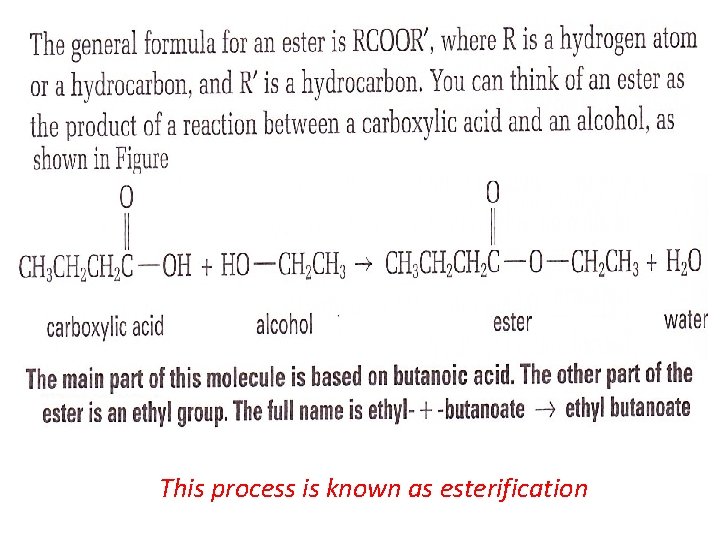
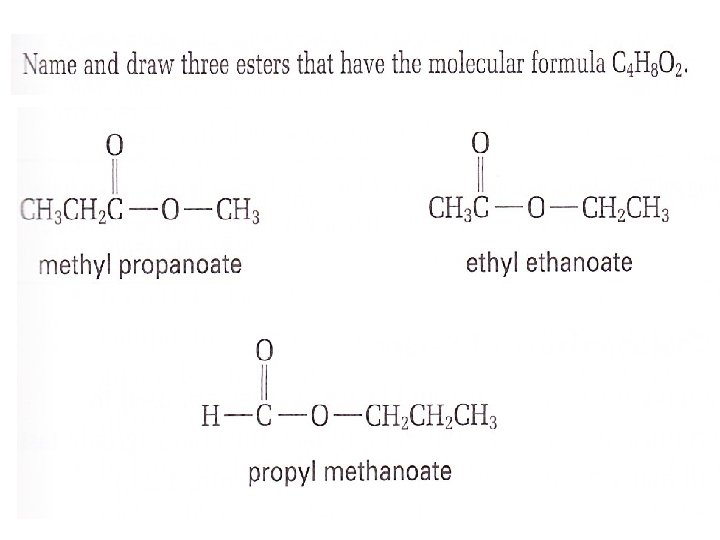
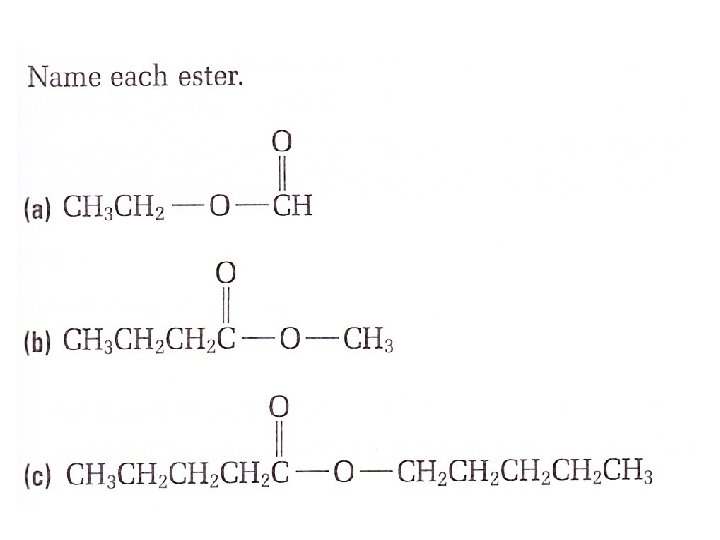
This process is known as esterification


