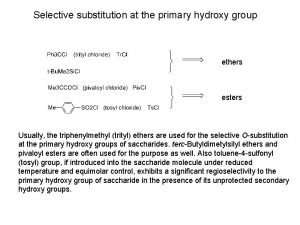
14 1 Introduction to Ethers An ether group





- Slides: 40

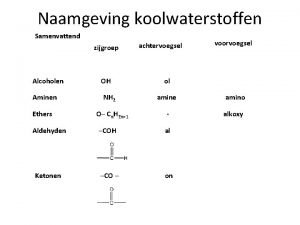
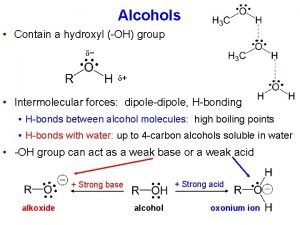
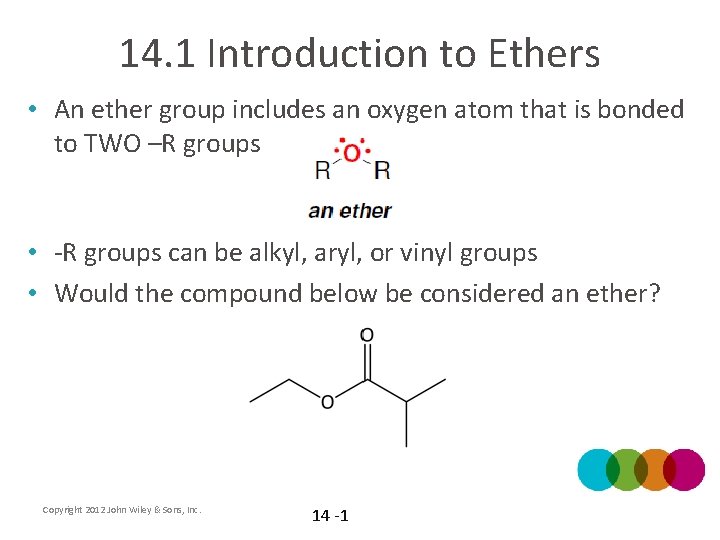
14. 1 Introduction to Ethers • An ether group includes an oxygen atom that is bonded to TWO –R groups • -R groups can be alkyl, aryl, or vinyl groups • Would the compound below be considered an ether? Copyright 2012 John Wiley & Sons, Inc. 14 -1

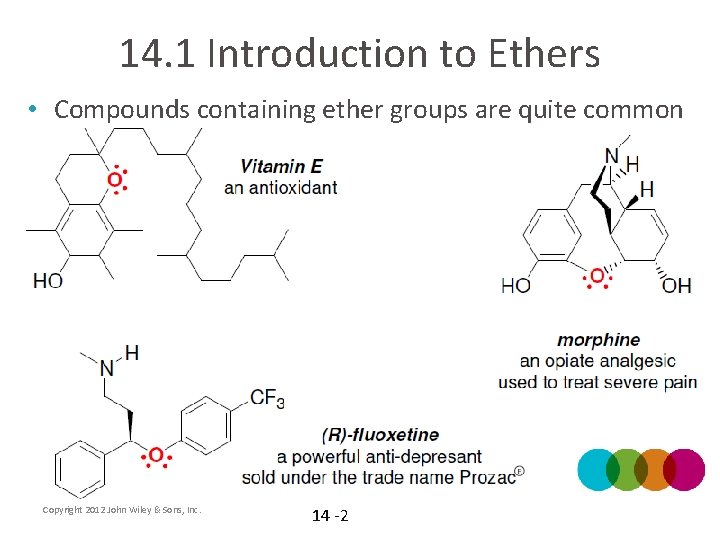
14. 1 Introduction to Ethers • Compounds containing ether groups are quite common Copyright 2012 John Wiley & Sons, Inc. 14 -2

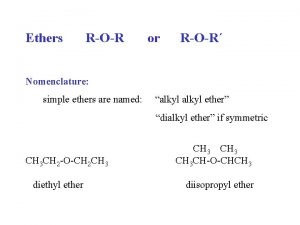
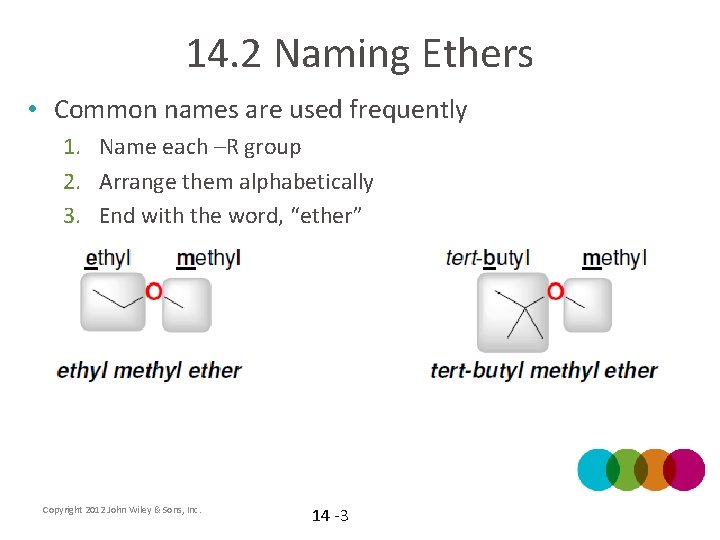
14. 2 Naming Ethers • Common names are used frequently 1. Name each –R group 2. Arrange them alphabetically 3. End with the word, “ether” Copyright 2012 John Wiley & Sons, Inc. 14 -3

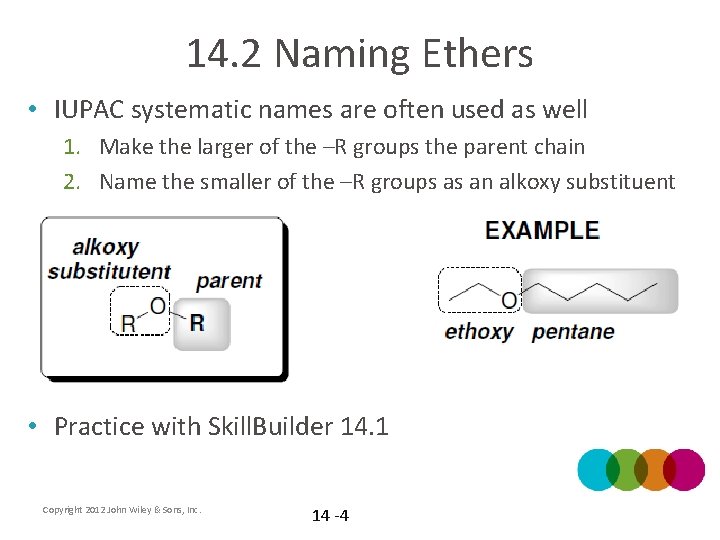
14. 2 Naming Ethers • IUPAC systematic names are often used as well 1. Make the larger of the –R groups the parent chain 2. Name the smaller of the –R groups as an alkoxy substituent • Practice with Skill. Builder 14. 1 Copyright 2012 John Wiley & Sons, Inc. 14 -4

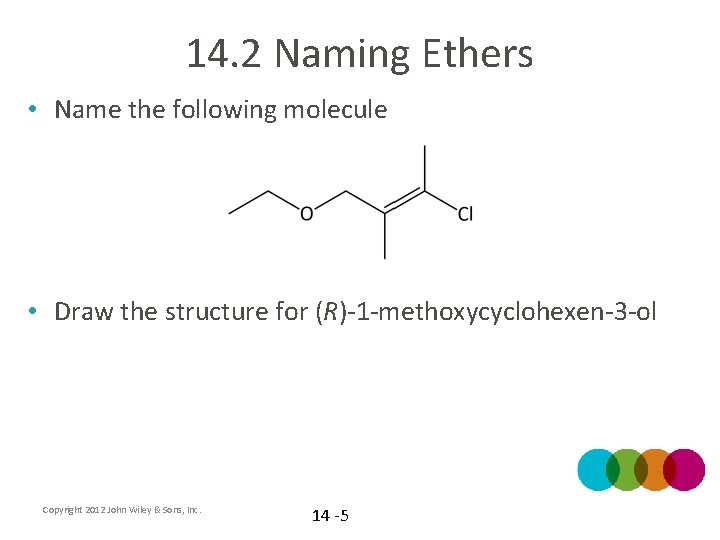
14. 2 Naming Ethers • Name the following molecule • Draw the structure for (R)-1 -methoxycyclohexen-3 -ol Copyright 2012 John Wiley & Sons, Inc. 14 -5

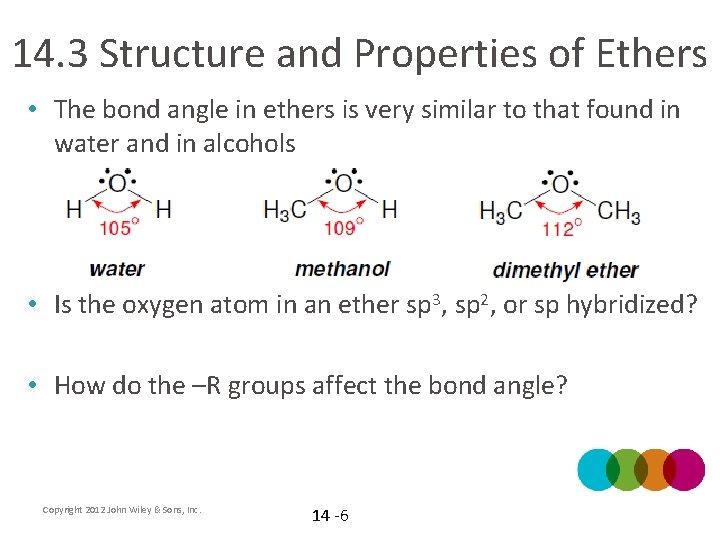
14. 3 Structure and Properties of Ethers • The bond angle in ethers is very similar to that found in water and in alcohols • Is the oxygen atom in an ether sp 3, sp 2, or sp hybridized? • How do the –R groups affect the bond angle? Copyright 2012 John Wiley & Sons, Inc. 14 -6

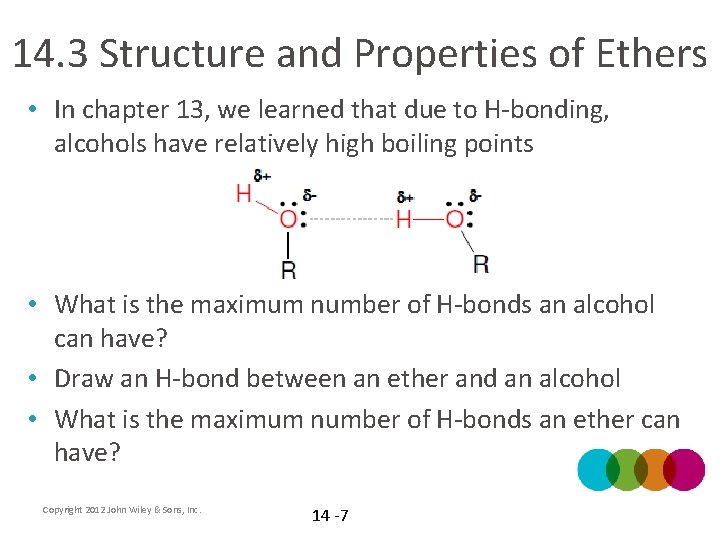
14. 3 Structure and Properties of Ethers • In chapter 13, we learned that due to H-bonding, alcohols have relatively high boiling points • What is the maximum number of H-bonds an alcohol can have? • Draw an H-bond between an ether and an alcohol • What is the maximum number of H-bonds an ether can have? Copyright 2012 John Wiley & Sons, Inc. 14 -7

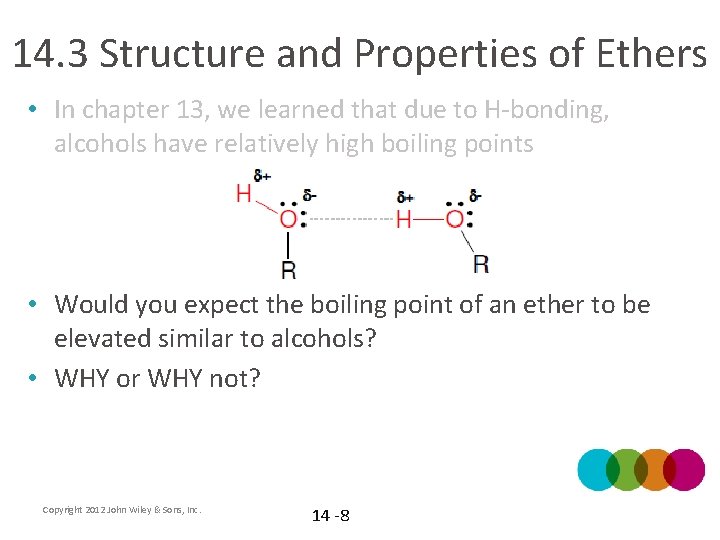
14. 3 Structure and Properties of Ethers • In chapter 13, we learned that due to H-bonding, alcohols have relatively high boiling points • Would you expect the boiling point of an ether to be elevated similar to alcohols? • WHY or WHY not? Copyright 2012 John Wiley & Sons, Inc. 14 -8

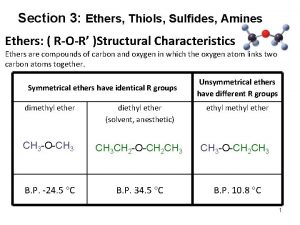
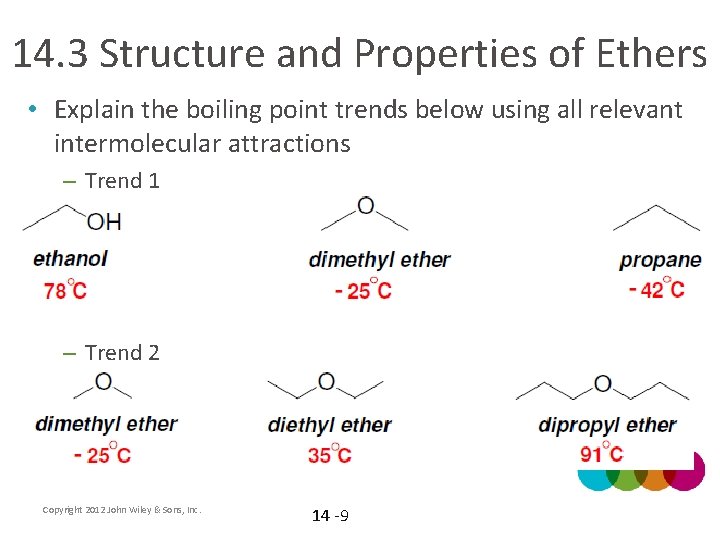
14. 3 Structure and Properties of Ethers • Explain the boiling point trends below using all relevant intermolecular attractions – Trend 1 – Trend 2 Copyright 2012 John Wiley & Sons, Inc. 14 -9

14. 3 Structure and Properties of Ethers • Ethers are often used by organic chemists as solvents – Relatively low boiling points allow them to be evaporated after the reaction is complete – Their dipole moment allows them to stabilize charged or partially charged transition states. HOW? – They are NOT protic. WHY is that an advantage for a solvent in many reactions? Copyright 2012 John Wiley & Sons, Inc. 14 -10

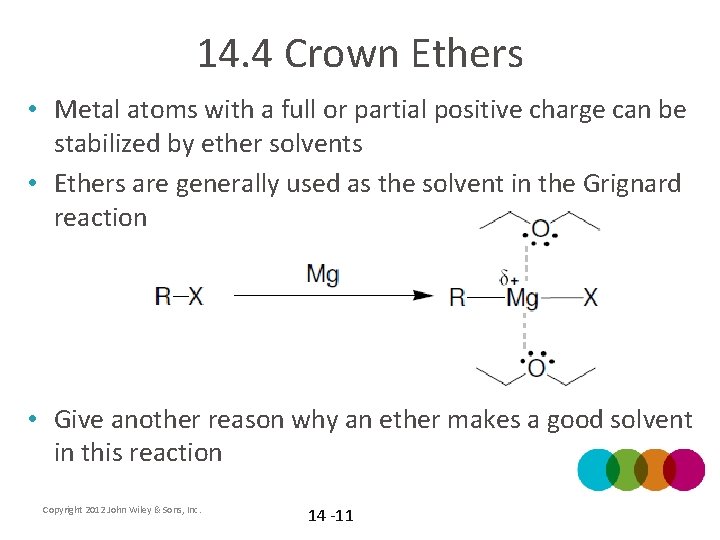
14. 4 Crown Ethers • Metal atoms with a full or partial positive charge can be stabilized by ether solvents • Ethers are generally used as the solvent in the Grignard reaction • Give another reason why an ether makes a good solvent in this reaction Copyright 2012 John Wiley & Sons, Inc. 14 -11

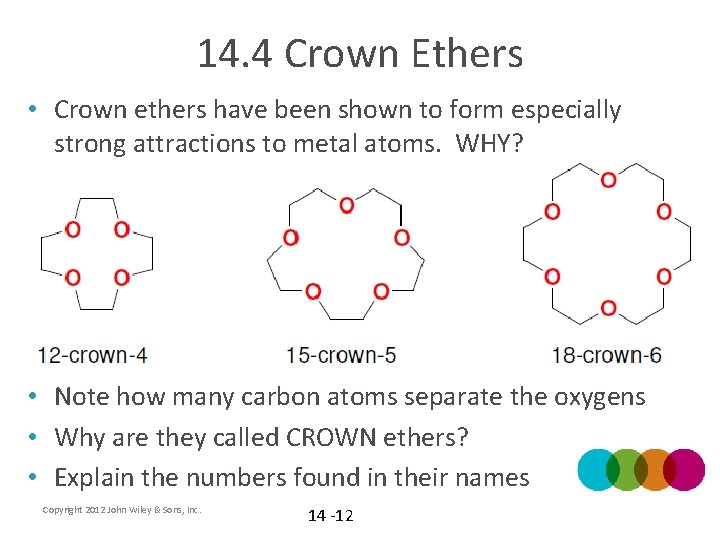
14. 4 Crown Ethers • Crown ethers have been shown to form especially strong attractions to metal atoms. WHY? • Note how many carbon atoms separate the oxygens • Why are they called CROWN ethers? • Explain the numbers found in their names Copyright 2012 John Wiley & Sons, Inc. 14 -12

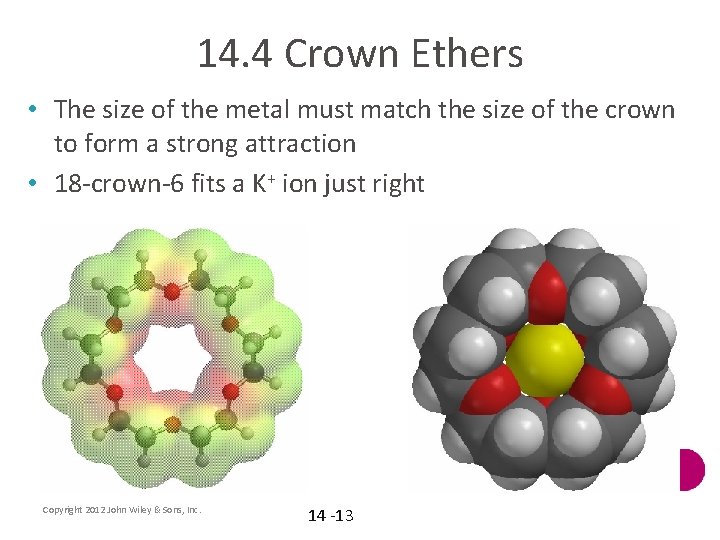
14. 4 Crown Ethers • The size of the metal must match the size of the crown to form a strong attraction • 18 -crown-6 fits a K+ ion just right Copyright 2012 John Wiley & Sons, Inc. 14 -13

14. 4 Crown Ethers • Normally metal ions are not soluble in low polarity solvents. WHY? • The crown ether – metal complex should dissolve nicely in low polarity solvents. WHY? • Imagine how a crown ether could be used to aid reactions between ion (especially anions) and low polarity organic substrates Copyright 2012 John Wiley & Sons, Inc. 14 -14

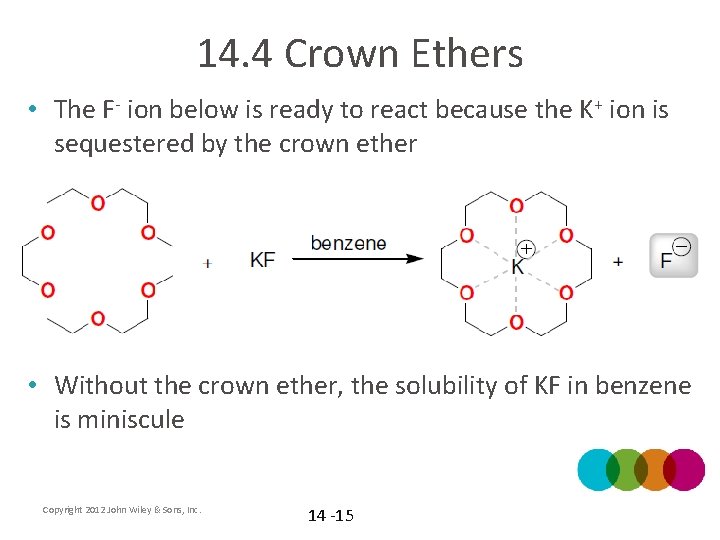
14. 4 Crown Ethers • The F- ion below is ready to react because the K+ ion is sequestered by the crown ether • Without the crown ether, the solubility of KF in benzene is miniscule Copyright 2012 John Wiley & Sons, Inc. 14 -15

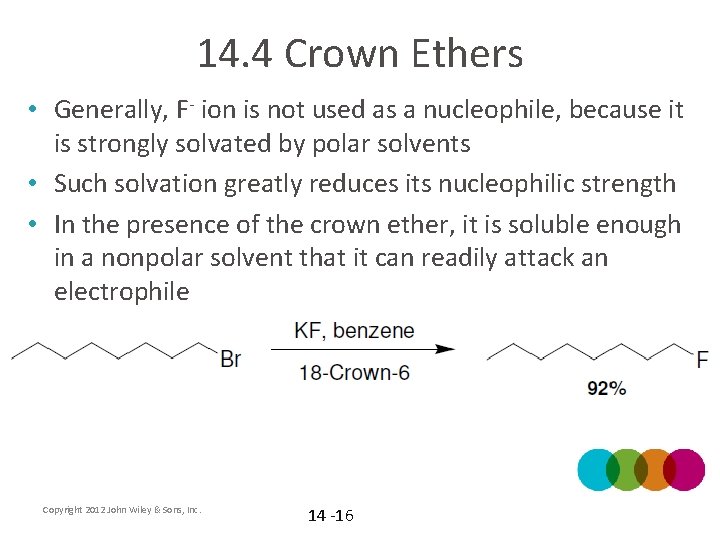
14. 4 Crown Ethers • Generally, F- ion is not used as a nucleophile, because it is strongly solvated by polar solvents • Such solvation greatly reduces its nucleophilic strength • In the presence of the crown ether, it is soluble enough in a nonpolar solvent that it can readily attack an electrophile Copyright 2012 John Wiley & Sons, Inc. 14 -16

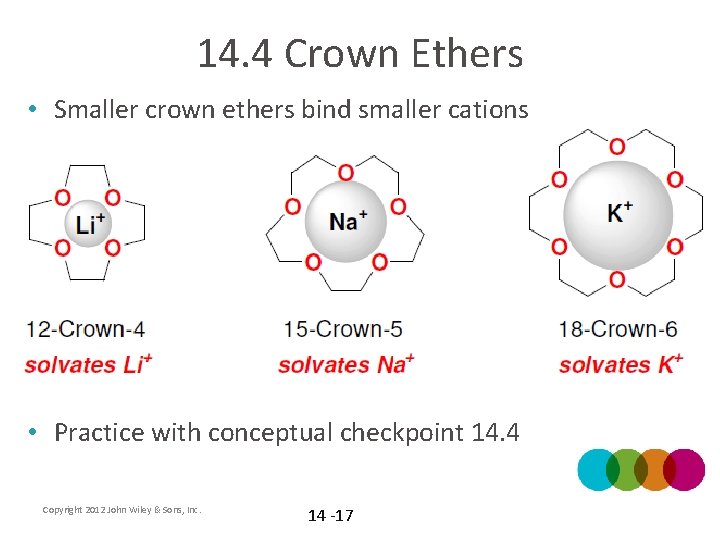
14. 4 Crown Ethers • Smaller crown ethers bind smaller cations • Practice with conceptual checkpoint 14. 4 Copyright 2012 John Wiley & Sons, Inc. 14 -17

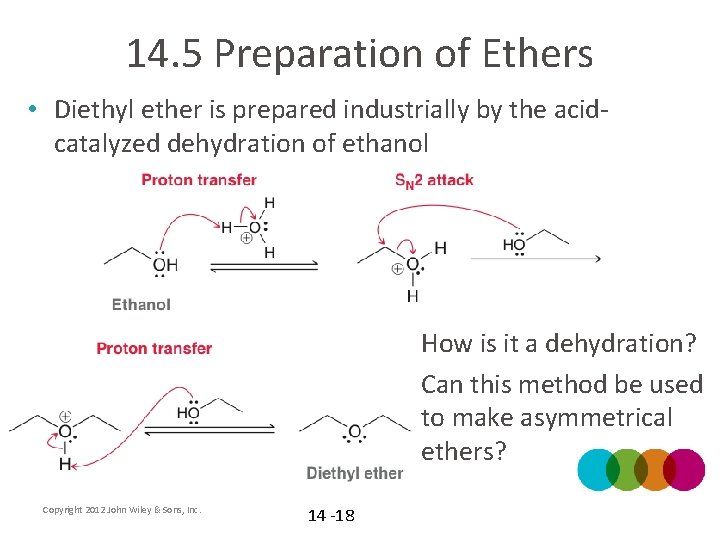
14. 5 Preparation of Ethers • Diethyl ether is prepared industrially by the acidcatalyzed dehydration of ethanol • How is it a dehydration? • Can this method be used to make asymmetrical ethers? Copyright 2012 John Wiley & Sons, Inc. 14 -18

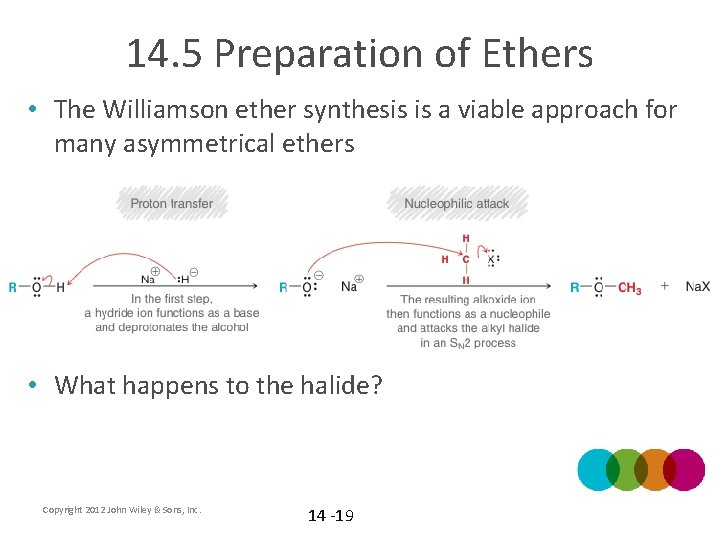
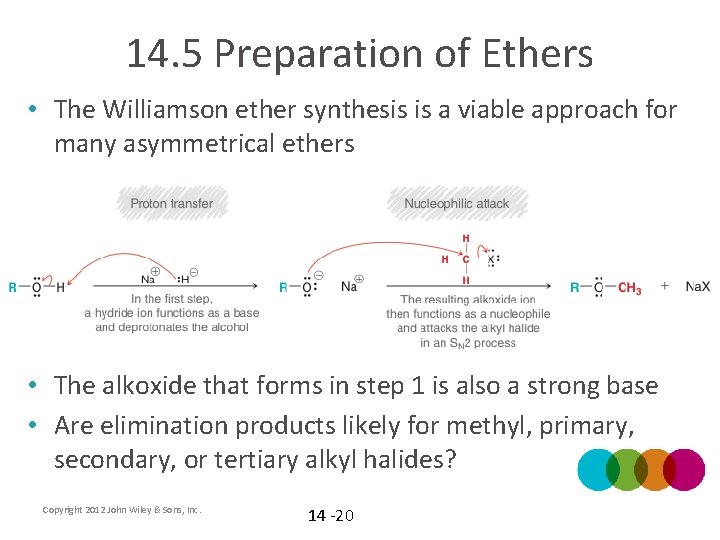
14. 5 Preparation of Ethers • The Williamson ether synthesis is a viable approach for many asymmetrical ethers • What happens to the halide? Copyright 2012 John Wiley & Sons, Inc. 14 -19

14. 5 Preparation of Ethers • The Williamson ether synthesis is a viable approach for many asymmetrical ethers • The alkoxide that forms in step 1 is also a strong base • Are elimination products likely for methyl, primary, secondary, or tertiary alkyl halides? Copyright 2012 John Wiley & Sons, Inc. 14 -20

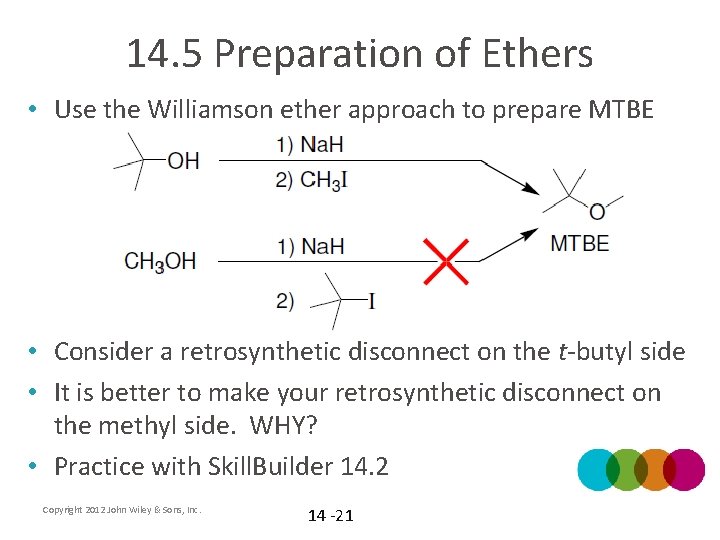
14. 5 Preparation of Ethers • Use the Williamson ether approach to prepare MTBE • Consider a retrosynthetic disconnect on the t-butyl side • It is better to make your retrosynthetic disconnect on the methyl side. WHY? • Practice with Skill. Builder 14. 2 Copyright 2012 John Wiley & Sons, Inc. 14 -21

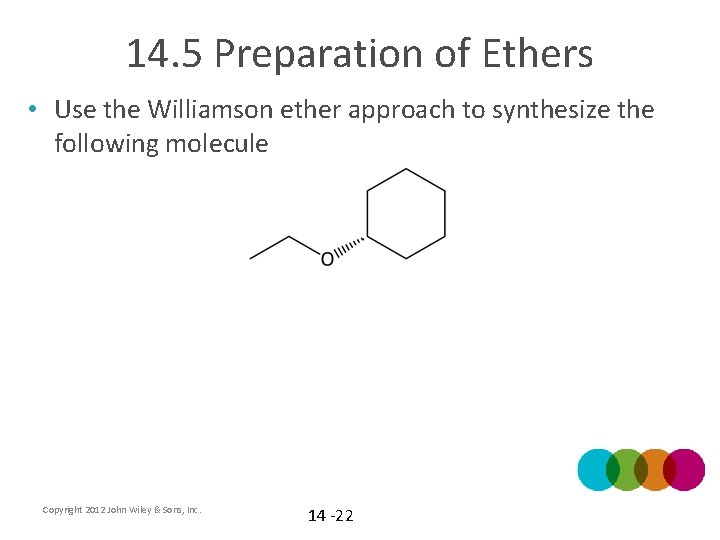
14. 5 Preparation of Ethers • Use the Williamson ether approach to synthesize the following molecule Copyright 2012 John Wiley & Sons, Inc. 14 -22

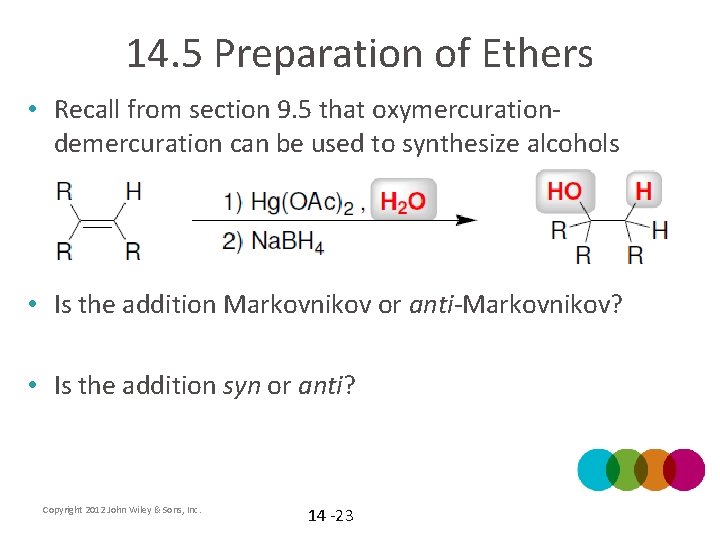
14. 5 Preparation of Ethers • Recall from section 9. 5 that oxymercurationdemercuration can be used to synthesize alcohols • Is the addition Markovnikov or anti-Markovnikov? • Is the addition syn or anti? Copyright 2012 John Wiley & Sons, Inc. 14 -23

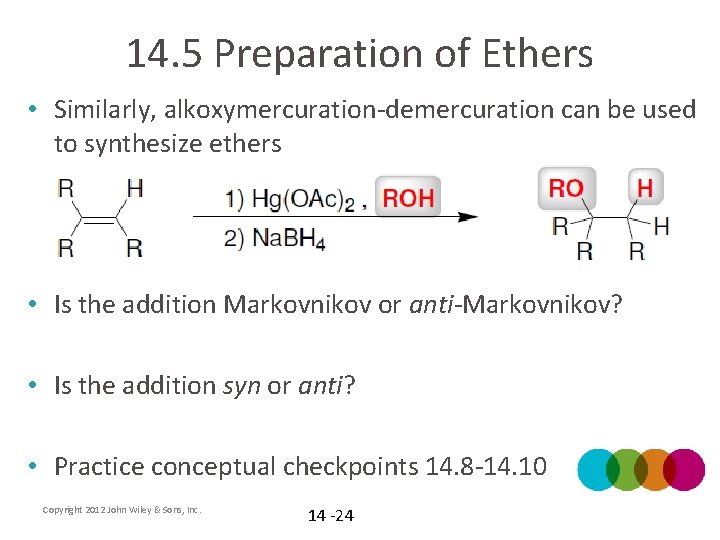
14. 5 Preparation of Ethers • Similarly, alkoxymercuration-demercuration can be used to synthesize ethers • Is the addition Markovnikov or anti-Markovnikov? • Is the addition syn or anti? • Practice conceptual checkpoints 14. 8 -14. 10 Copyright 2012 John Wiley & Sons, Inc. 14 -24

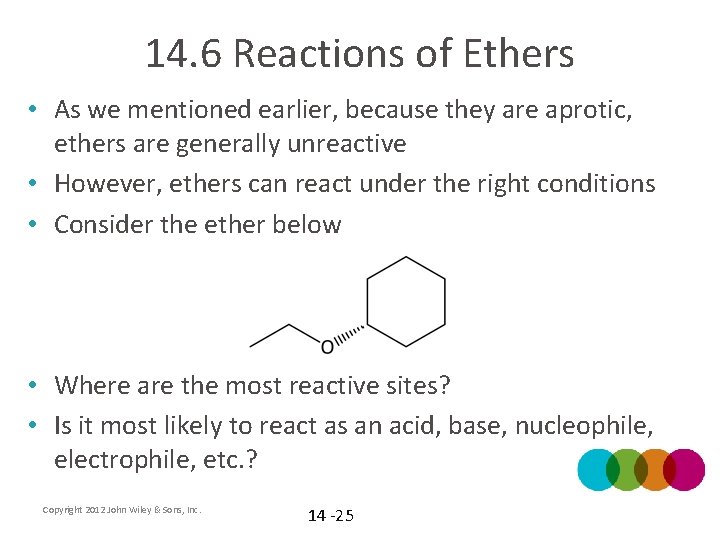
14. 6 Reactions of Ethers • As we mentioned earlier, because they are aprotic, ethers are generally unreactive • However, ethers can react under the right conditions • Consider the ether below • Where are the most reactive sites? • Is it most likely to react as an acid, base, nucleophile, electrophile, etc. ? Copyright 2012 John Wiley & Sons, Inc. 14 -25

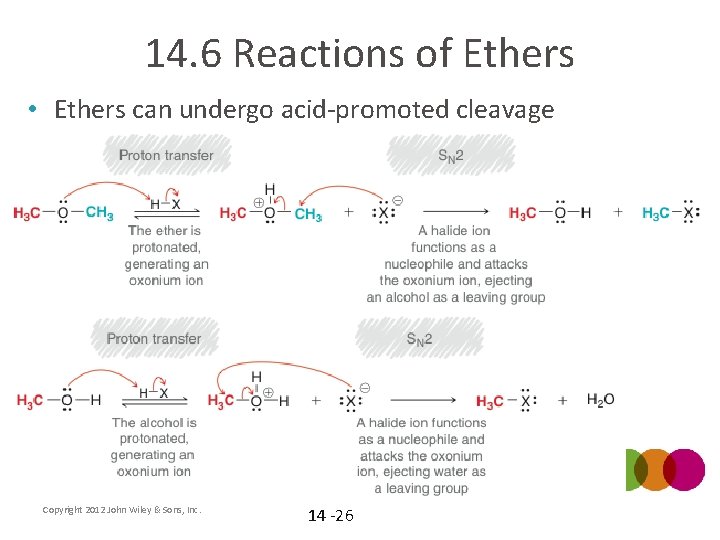
14. 6 Reactions of Ethers • Ethers can undergo acid-promoted cleavage Copyright 2012 John Wiley & Sons, Inc. 14 -26

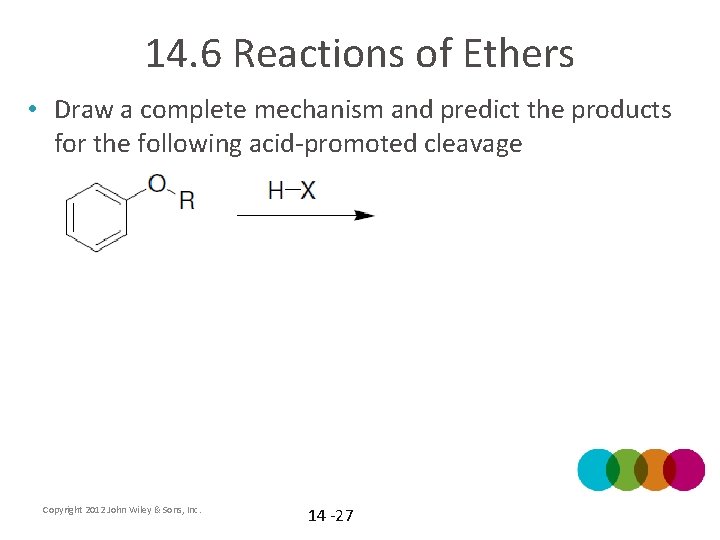
14. 6 Reactions of Ethers • Draw a complete mechanism and predict the products for the following acid-promoted cleavage Copyright 2012 John Wiley & Sons, Inc. 14 -27

14. 6 Reactions of Ethers • To promote cleavage, HI and HBr are generally effective • HCl is less effective, and HF does not cause significant cleavage • Explain the trend above considering the relative strength of the halide nucleophiles • Why is the cleavage considered acid-promoted rather than acid-catalyzed? • Practice with conceptual checkpoint 14. 11 Copyright 2012 John Wiley & Sons, Inc. 14 -28

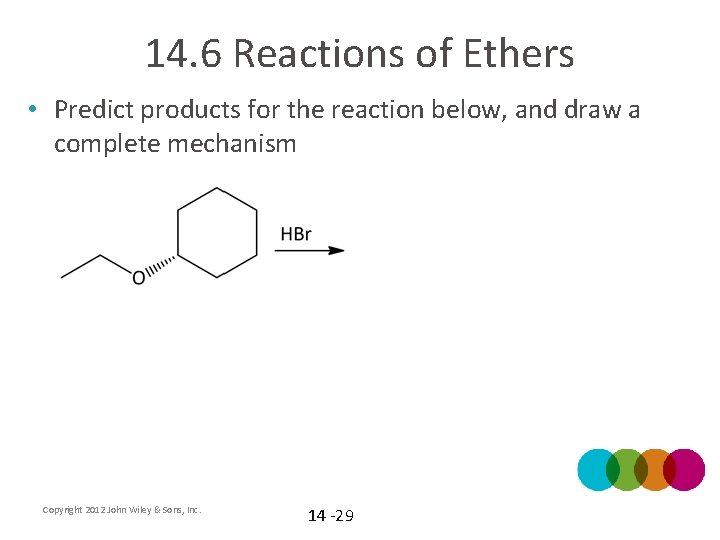
14. 6 Reactions of Ethers • Predict products for the reaction below, and draw a complete mechanism Copyright 2012 John Wiley & Sons, Inc. 14 -29

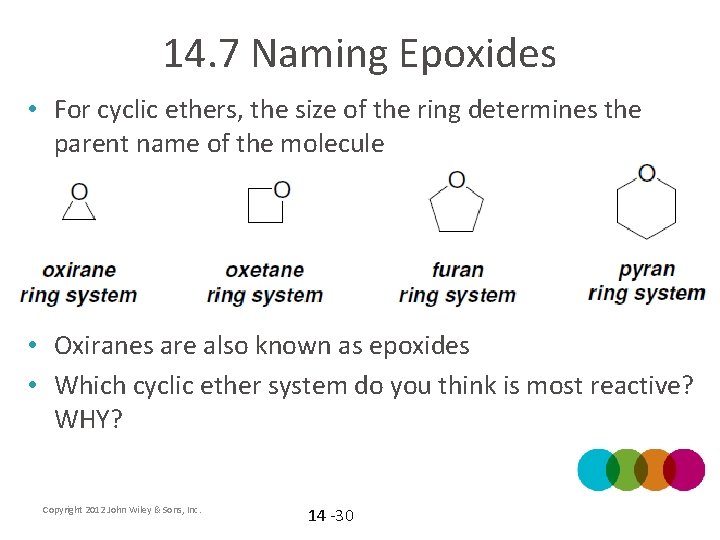
14. 7 Naming Epoxides • For cyclic ethers, the size of the ring determines the parent name of the molecule • Oxiranes are also known as epoxides • Which cyclic ether system do you think is most reactive? WHY? Copyright 2012 John Wiley & Sons, Inc. 14 -30

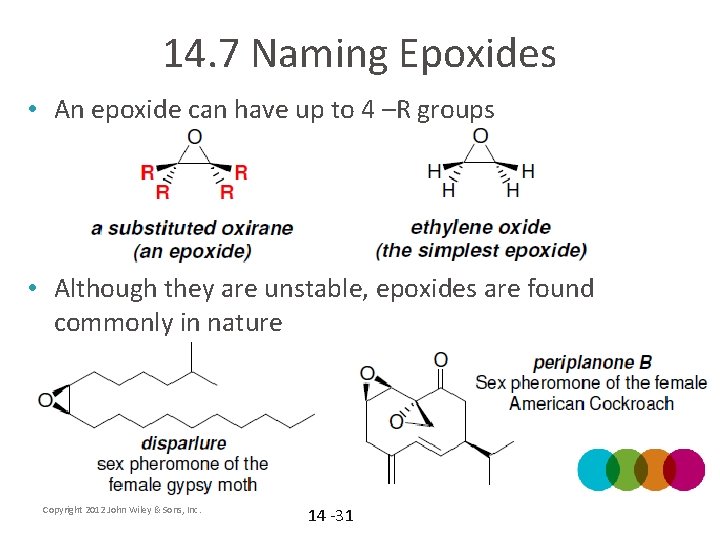
14. 7 Naming Epoxides • An epoxide can have up to 4 –R groups • Although they are unstable, epoxides are found commonly in nature Copyright 2012 John Wiley & Sons, Inc. 14 -31

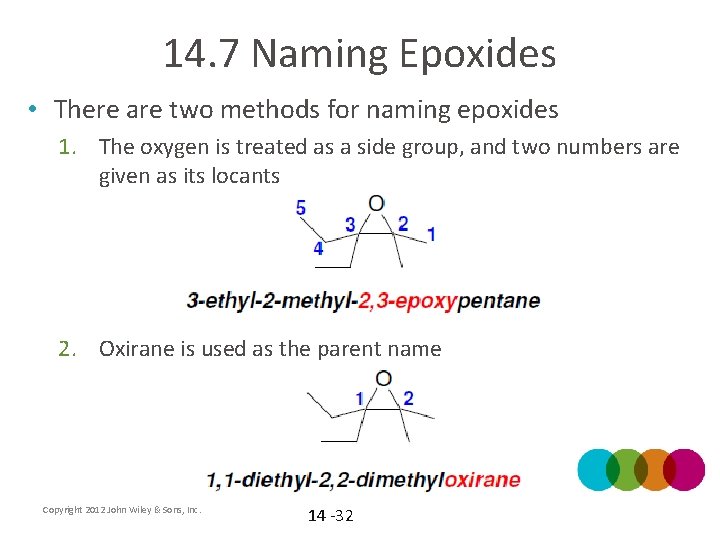
14. 7 Naming Epoxides • There are two methods for naming epoxides 1. The oxygen is treated as a side group, and two numbers are given as its locants 2. Oxirane is used as the parent name Copyright 2012 John Wiley & Sons, Inc. 14 -32

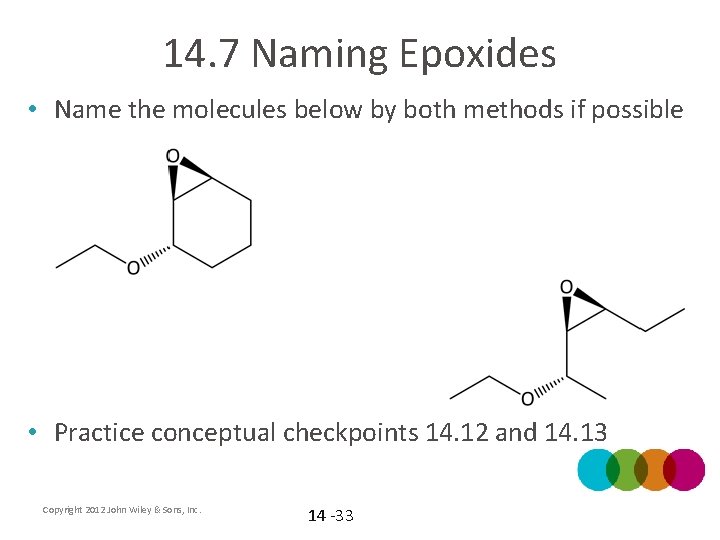
14. 7 Naming Epoxides • Name the molecules below by both methods if possible • Practice conceptual checkpoints 14. 12 and 14. 13 Copyright 2012 John Wiley & Sons, Inc. 14 -33

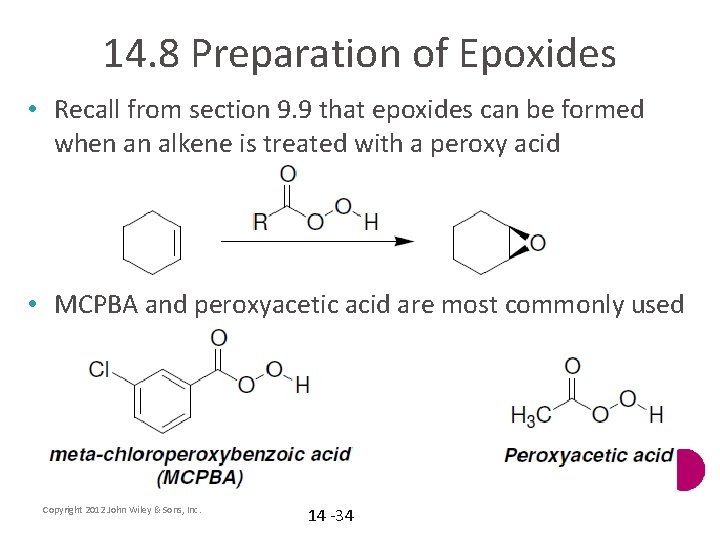
14. 8 Preparation of Epoxides • Recall from section 9. 9 that epoxides can be formed when an alkene is treated with a peroxy acid • MCPBA and peroxyacetic acid are most commonly used Copyright 2012 John Wiley & Sons, Inc. 14 -34

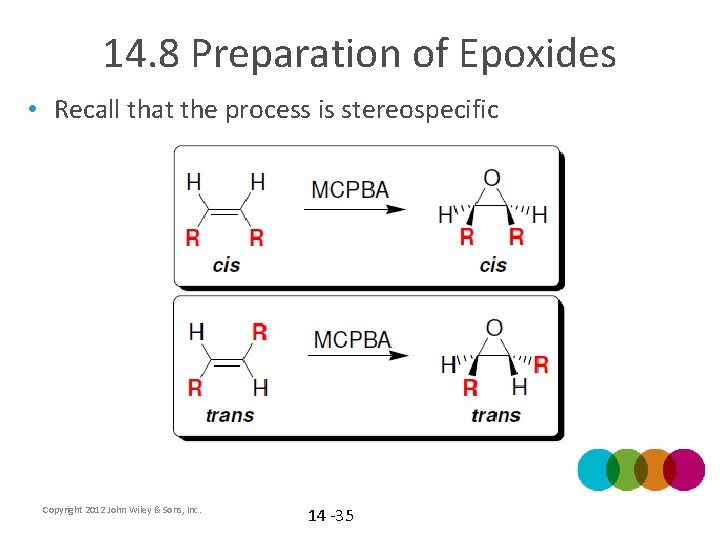
14. 8 Preparation of Epoxides • Recall that the process is stereospecific Copyright 2012 John Wiley & Sons, Inc. 14 -35

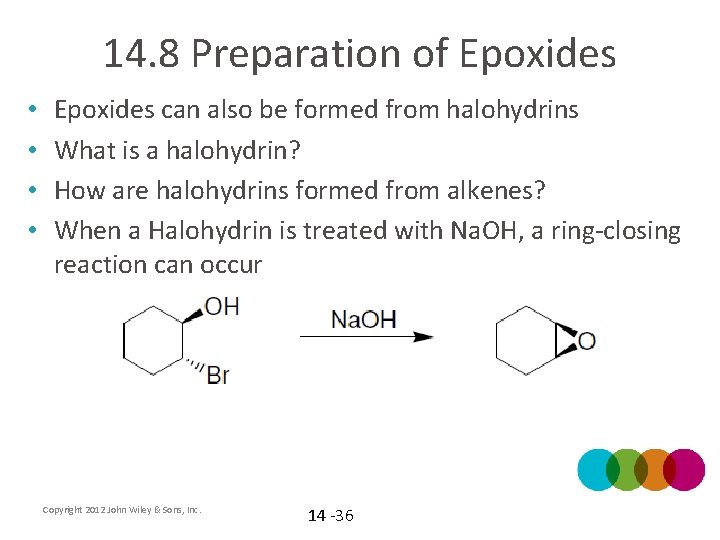
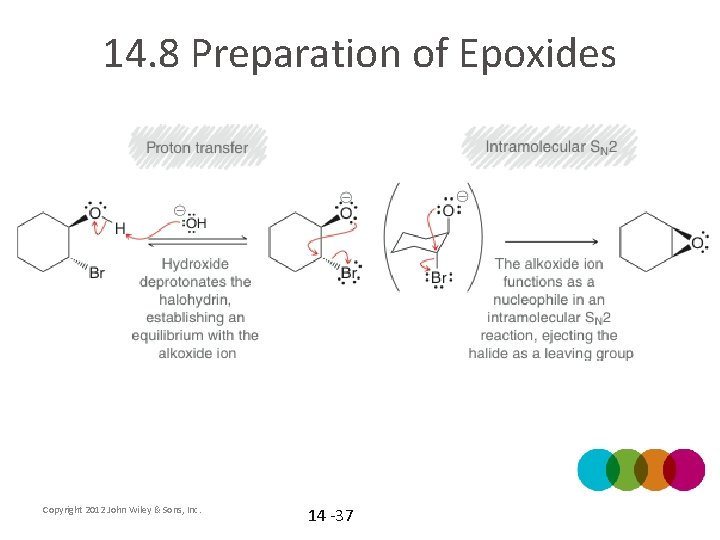
14. 8 Preparation of Epoxides • • Epoxides can also be formed from halohydrins What is a halohydrin? How are halohydrins formed from alkenes? When a Halohydrin is treated with Na. OH, a ring-closing reaction can occur Copyright 2012 John Wiley & Sons, Inc. 14 -36

14. 8 Preparation of Epoxides Copyright 2012 John Wiley & Sons, Inc. 14 -37

Study Guide for Sections 14. 1 -14. 8 DAY 8, Terms to know: Sections 14. 1 -14. 8 ethers, crown ether, dehydration, Williamson ether synthesis, alkoxymercurationdemercuration, acid-promoted, Oxiranes, epoxides, MCPBA, halohydrin DAY 8, Specific outcomes and skills that may be tested on exam 2: Sections 14. 1 -14. 8 • Be able to use both common naming and IUPAC naming systems to name an ether • Given either a common or IUPAC name, be able to draw the structure for an ether • Be able to identify intermolecular attractions that ethers undergo and how that affects their physical properties such as BP, solubility, etc. • Be able to give three reasons why ethers often make good solvents for many organic reactions • Be able to explain how crown ethers work to improve reactions involving salts • Be able to predict products and give a complete mechanism for the formation of an ether by dehydration of two moles of an alcohol • Given products and reactant, be able to choose appropriate reagents for a Williamson ether synthesis • Given a specific alcohol and specific reagents, be able to predict the products of a Williamson ether reaction and draw a complete mechanism • Be able to design a Williamson ether synthesis in a way that gives the best possible yield avoiding elimination side products. • Given products and reactant, be able to choose appropriate reagents for an ether synthesis via alkoxymercuration-demercuration • Given a specific alcohol and specific reagents, be able to predict the products of an alkoxymercuration -demercuration including regio and stereo outcomes • Given a specific ether and specific reagents, be able to predict the products of an acid-promoted ether cleavage and draw a complete mechanism • Be able to name simple epoxides or draw the structure from a given name

Extra Practice Problems for Sections 14. 1 -14. 8 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 14. 1 14. 2 14. 3 14. 5 14. 7 14. 40 14. 8 14. 9 14. 11 14. 12 14. 13

Prep for Day 9 Must Watch videos: https: //www. youtube. com/watch? v=Kf. Tosr. Ms 5 W 0 (epoxide formation with a peracid like MCPBA and acid catalyzed epoxide opening, Khan) https: //www. youtube. com/watch? v=J 6 wh-1 t_F 50 (epoxides from halohydrins, Khan) https: //www. youtube. com/watch? v=r. Wfs 1 hygua. M (opening an epoxide with a nucleophile, Khan) https: //www. youtube. com/watch? v=r. LPKsbo. OH 3 U (naming thiols, Leah) https: //www. youtube. com/watch? v=THg 43 u 7 Ac. DU (formation of sulfides and disulfides, Clutch Prep) https: //www. youtube. com/watch? v=-Dyoxm_MKXs (oxidation of sulfides, Clutch Prep) Other helpful videos: https: //www. youtube. com/watch? v=u-Ko_Tc. JLns (epoxide formation, Leah) http: //ocw. uci. edu/lectures/chem_51 b_lec_05_organic_chemistry_alcohols_ethers_and_expodites_part_4. html (alcohols, UC-Irvine) start 28 minutes in. https: //www. youtube. com/watch? v=N 1 AFVCJu. UIs (naming compounds with sulfur, Clutch Prep) https: //www. youtube. com/watch? v=MC 8 Mc 6 t. Sj. YI (formation of sulfides, Khan) Read Sections 14. 8 -14. 12
 Nhr functional group
Nhr functional group How to make ether from alcohol
How to make ether from alcohol Butaanzuur
Butaanzuur Naming ethers
Naming ethers Ether cleavage
Ether cleavage Alcohols nomenclature
Alcohols nomenclature Ethers boiling point
Ethers boiling point David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Cis-2 3-dimethyloxirane
Cis-2 3-dimethyloxirane Greenyar
Greenyar Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Acidic cleavage of ethers
Acidic cleavage of ethers Ir spec
Ir spec Petroleum ether composition
Petroleum ether composition 4-heptanone
4-heptanone Intermolecular forces formaldehyde
Intermolecular forces formaldehyde R-o-r name in chemistry
R-o-r name in chemistry Hydrolysis of ether gives
Hydrolysis of ether gives Difference between alcohol and ether
Difference between alcohol and ether Alcohol protonation
Alcohol protonation Methyl propyl ether
Methyl propyl ether Aldehydes scary
Aldehydes scary Williamson synthesis
Williamson synthesis Dry ether example
Dry ether example Dipropyl ether structure
Dipropyl ether structure Ether 2:12
Ether 2:12 Sedimentation technique for stool concentration
Sedimentation technique for stool concentration Preanesthetic medication
Preanesthetic medication Ether oxygen
Ether oxygen Petroleum ether nfpa
Petroleum ether nfpa Ether 12 4
Ether 12 4 Ether
Ether Etx 203 ax
Etx 203 ax Giardia lamblia
Giardia lamblia Nomenclature of ether
Nomenclature of ether Diphenyl ether
Diphenyl ether What is the most important physical property of alcohol?
What is the most important physical property of alcohol? Feve polymer
Feve polymer Propoxycyclopentane
Propoxycyclopentane Cognex ether inspect
Cognex ether inspect Group polarization
Group polarization