A large portion of the materials on plagiarism








- Slides: 26

• A large portion of the materials on plagiarism on the University of Wisconsin Oshkosh's Writing Center Web site was revealed in February to have been taken verbatim from Purdue University's Web page on plagiarism.

Nomenclature of Ethers, Aldehydes, Ketones, Carboxylic Acids, and Esters 2

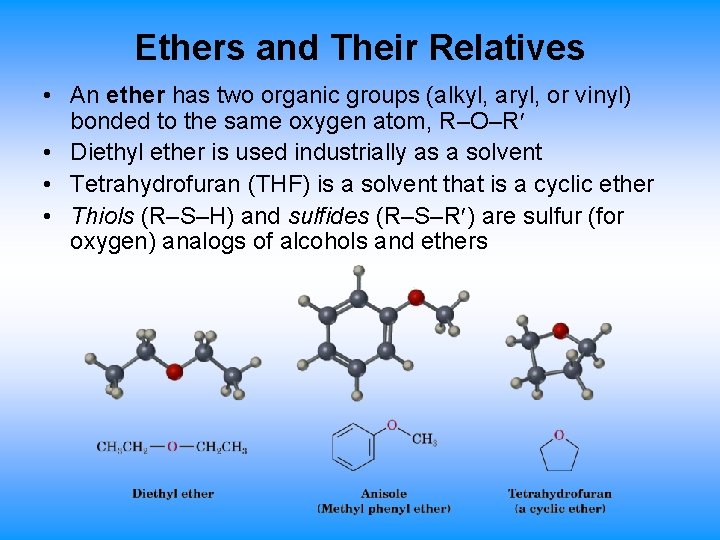
Ethers and Their Relatives • An ether has two organic groups (alkyl, aryl, or vinyl) bonded to the same oxygen atom, R–O–R • Diethyl ether is used industrially as a solvent • Tetrahydrofuran (THF) is a solvent that is a cyclic ether • Thiols (R–S–H) and sulfides (R–S–R ) are sulfur (for oxygen) analogs of alcohols and ethers

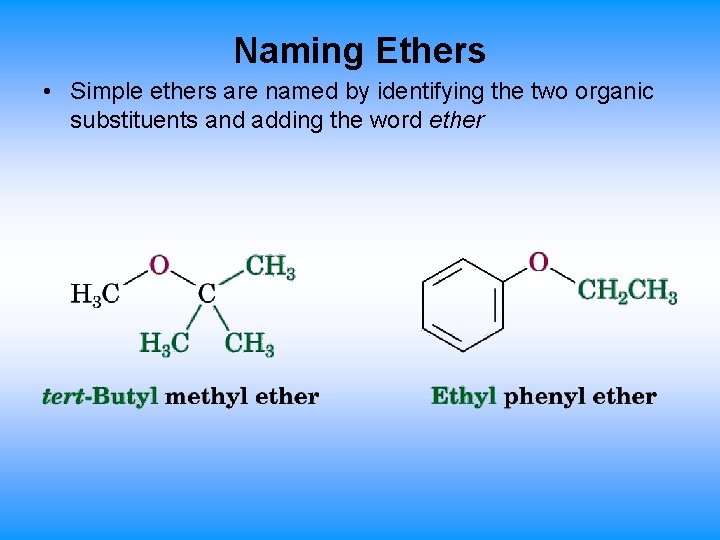
Naming Ethers • Simple ethers are named by identifying the two organic substituents and adding the word ether

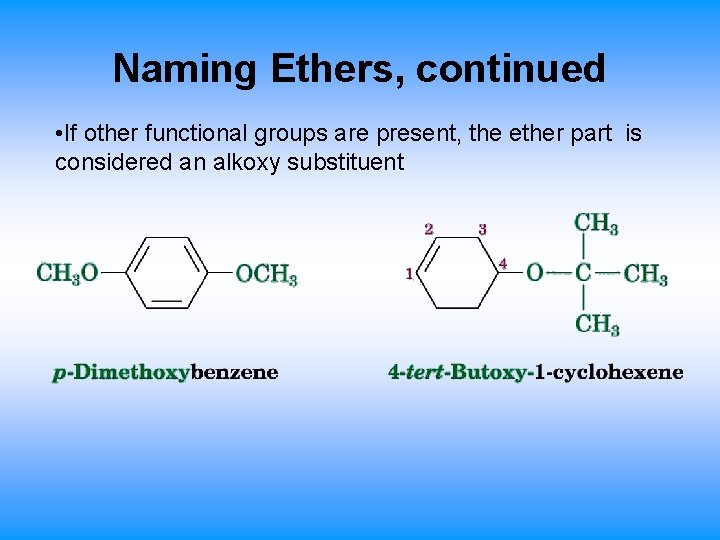
Naming Ethers, continued • If other functional groups are present, the ether part is considered an alkoxy substituent

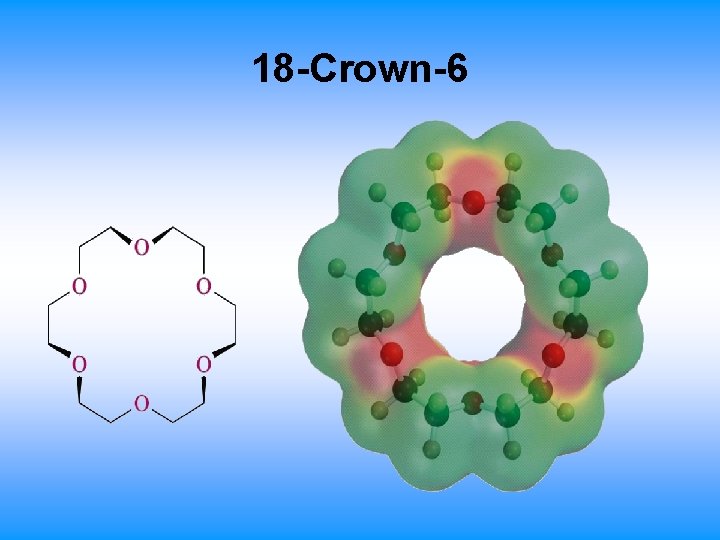
18. 9 Crown Ethers • Large rings consisting repeating (-OCH 2 -) or similar units • Named as x-crown-y – x is the total number of atoms in the ring – y is the number of oxygen atoms – 18 -crown-6 ether: 18 -membered ring containing 6 oxygens atoms • Central cavity is electronegative and attracts cations

18 -Crown-6

Uses of Crown Ethers • Complexes between crown ethers and ionic salts are soluble in nonpolar organic solvents • Creates reagents that are free of water that have useful properties • Inorganic salts dissolve in organic solvents leaving the anion unassociated, enhancing reactivity

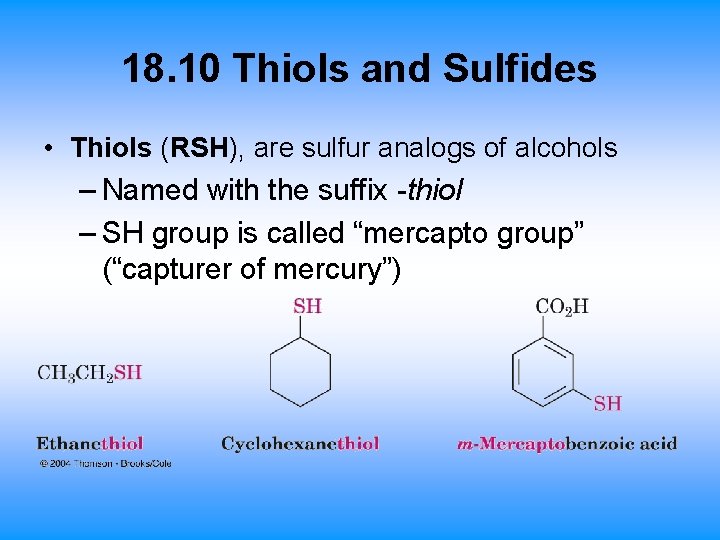
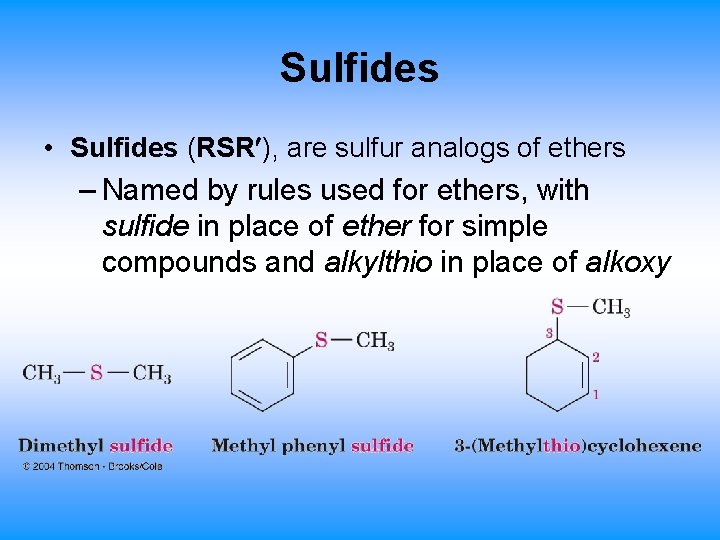
18. 10 Thiols and Sulfides • Thiols (RSH), are sulfur analogs of alcohols – Named with the suffix -thiol – SH group is called “mercapto group” (“capturer of mercury”)

Sulfides • Sulfides (RSR ), are sulfur analogs of ethers – Named by rules used for ethers, with sulfide in place of ether for simple compounds and alkylthio in place of alkoxy

Aldehydes and Ketones • Aldehydes and ketones are characterized by the carbonyl functional group (C=O) • The compounds occur widely in nature as intermediates in metabolism and biosynthesis • They are also common as chemicals, as solvents, monomers, adhesives, agrichemicals and pharmaceuticals

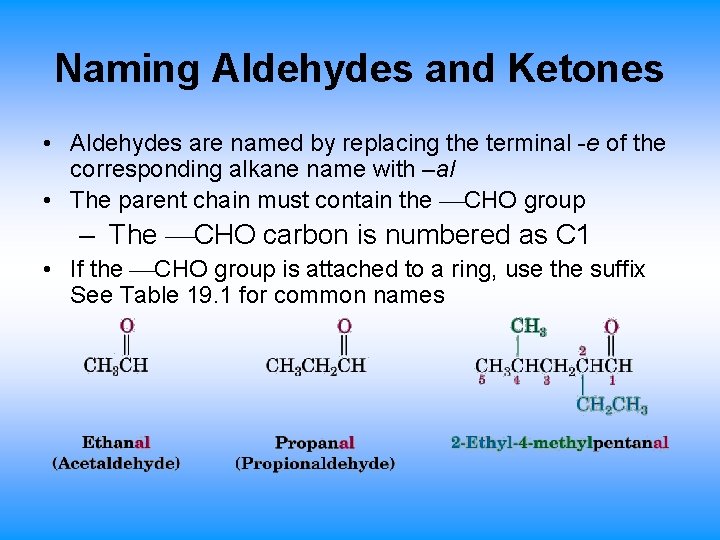
Naming Aldehydes and Ketones • Aldehydes are named by replacing the terminal -e of the corresponding alkane name with –al • The parent chain must contain the CHO group – The CHO carbon is numbered as C 1 • If the CHO group is attached to a ring, use the suffix See Table 19. 1 for common names

Common Name of Some Simple Aldehydes Formula Common Name Systematic Name HCHO Formaldehye Methanal CH 3 CHO Acetaldehyde Ethanal Propionaldehyde Propanal CH 3 CH 2 CHO Butyraldehyde Butanal CH 3 CH 2 CH 2 CHO Valeraldehyde Pentanal Acrolein Propenal Benzaldehyde Benzenecarbaldehyde CH 3 CH 2 CHO H 2 C=CHCHO Ph. CHO

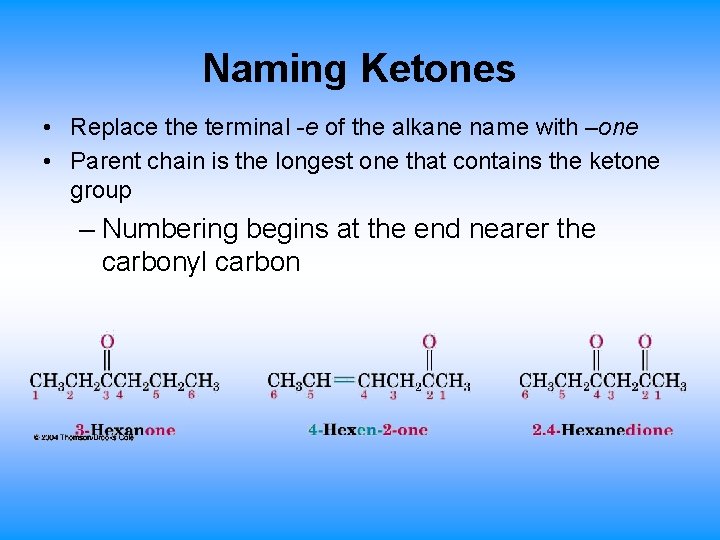
Naming Ketones • Replace the terminal -e of the alkane name with –one • Parent chain is the longest one that contains the ketone group – Numbering begins at the end nearer the carbonyl carbon

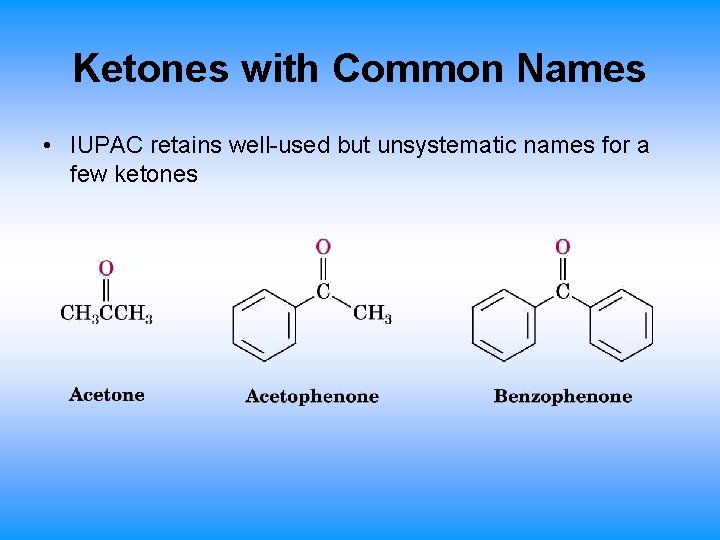
Ketones with Common Names • IUPAC retains well-used but unsystematic names for a few ketones

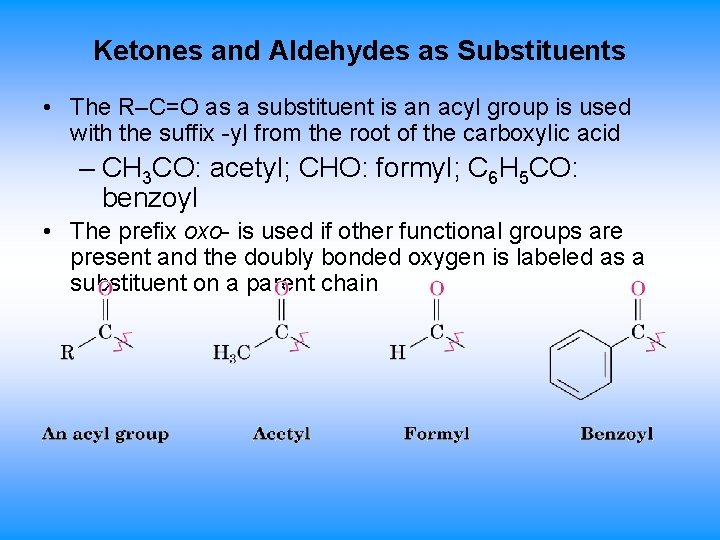
Ketones and Aldehydes as Substituents • The R–C=O as a substituent is an acyl group is used with the suffix -yl from the root of the carboxylic acid – CH 3 CO: acetyl; CHO: formyl; C 6 H 5 CO: benzoyl • The prefix oxo- is used if other functional groups are present and the doubly bonded oxygen is labeled as a substituent on a parent chain

The Importance of Carboxylic Acids (RCO 2 H or RCOOH) • Starting materials for acyl derivatives (esters, amides, and acid chlorides) • Abundant in nature from oxidation of aldehydes and alcohols in metabolism – Acetic acid, CH 3 CO 2 H, - vinegar – Butanoic acid, CH 3 CH 2 CO 2 H (rancid butter) – Long-chain aliphatic acids from the breakdown of fats

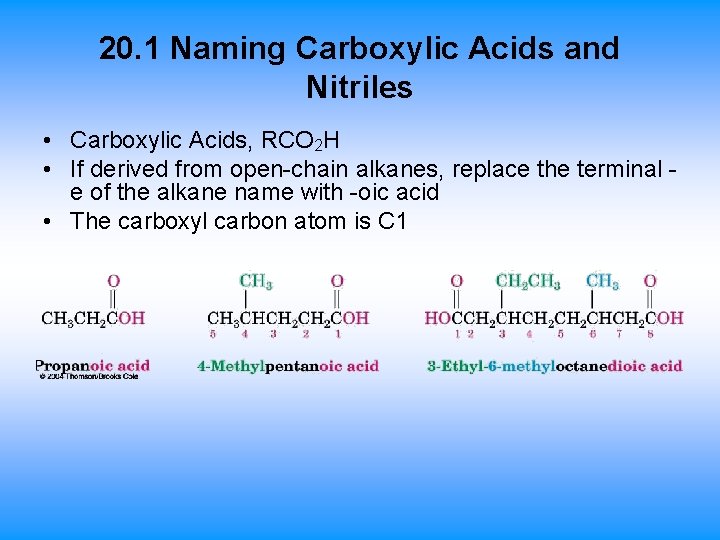
20. 1 Naming Carboxylic Acids and Nitriles • Carboxylic Acids, RCO 2 H • If derived from open-chain alkanes, replace the terminal e of the alkane name with -oic acid • The carboxyl carbon atom is C 1

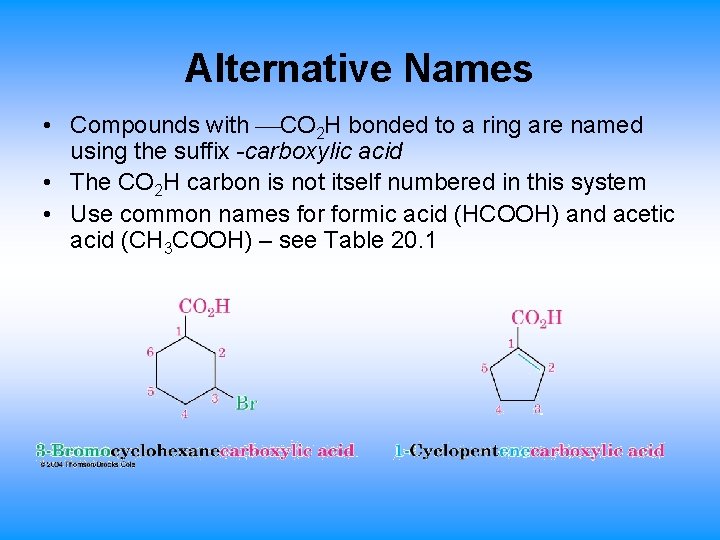
Alternative Names • Compounds with CO 2 H bonded to a ring are named using the suffix -carboxylic acid • The CO 2 H carbon is not itself numbered in this system • Use common names formic acid (HCOOH) and acetic acid (CH 3 COOH) – see Table 20. 1

Common Names – common names are based on natural origin rather than structure O HCOH Systematic Name Common Name methanoic acid formic acid ethanoic acid acetic acid octadecanoic acid stearic acid O CH 3 COH O CH 3(CH 2)16 COH

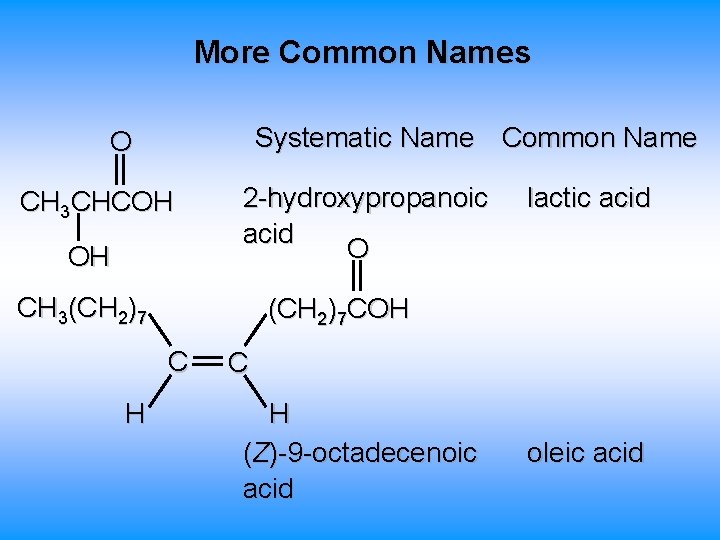
More Common Names Systematic Name Common Name O CH 3 CHCOH OH 2 -hydroxypropanoic acid O CH 3(CH 2)7 COH C H lactic acid C H (Z)-9 -octadecenoic acid oleic acid


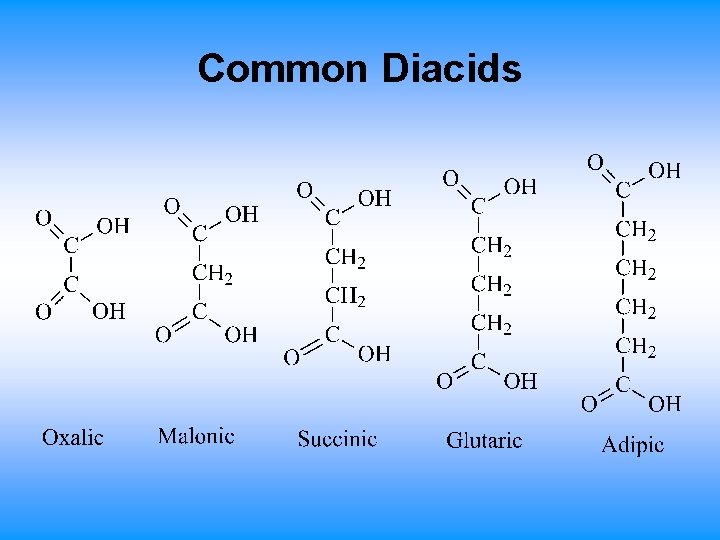
Common Diacids

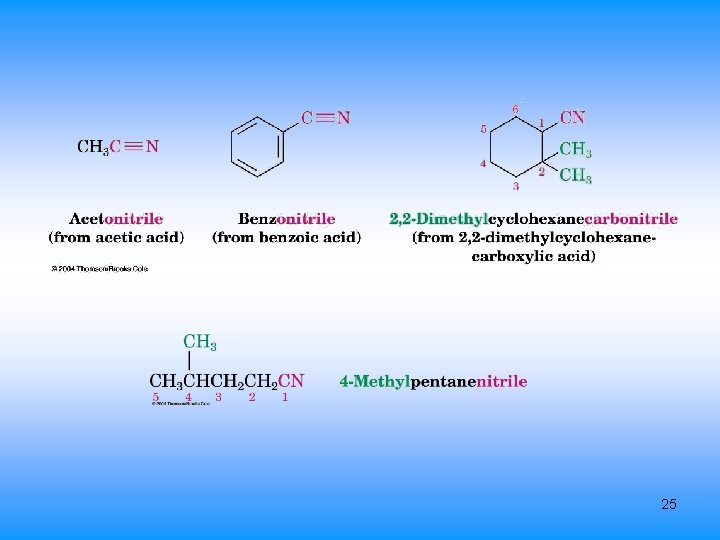
Nitriles, RC N • Closely related to carboxylic acids named by adding nitrile as a suffix to the alkane name, with the nitrile carbon numbered C 1 • Complex nitriles are named as derivatives of carboxylic acids. – Replace -ic acid or -oic acid ending with onitrile 24

25

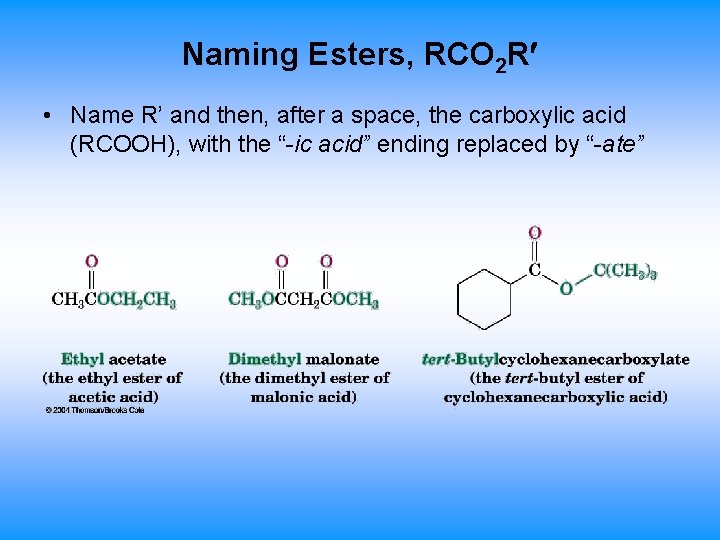
Naming Esters, RCO 2 R • Name R’ and then, after a space, the carboxylic acid (RCOOH), with the “-ic acid” ending replaced by “-ate”
 Natural man made
Natural man made Differentiate adopting materials and adapting materials
Differentiate adopting materials and adapting materials Cant stop the feeling trolls go noodle
Cant stop the feeling trolls go noodle Direct materials budget with multiple materials
Direct materials budget with multiple materials Useful and harmful at home
Useful and harmful at home Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Frameset trong html5
Frameset trong html5 Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tư thế worm breton là gì
Tư thế worm breton là gì đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính thế năng
Công thức tính thế năng Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan