Lecture 5 Properties structures existence and synthesisperparation Dr






- Slides: 25

Lecture: 5 Properties, structures, existence and synthesis/perparation Dr. A. K. M. Shafiqul Islam 01. 08

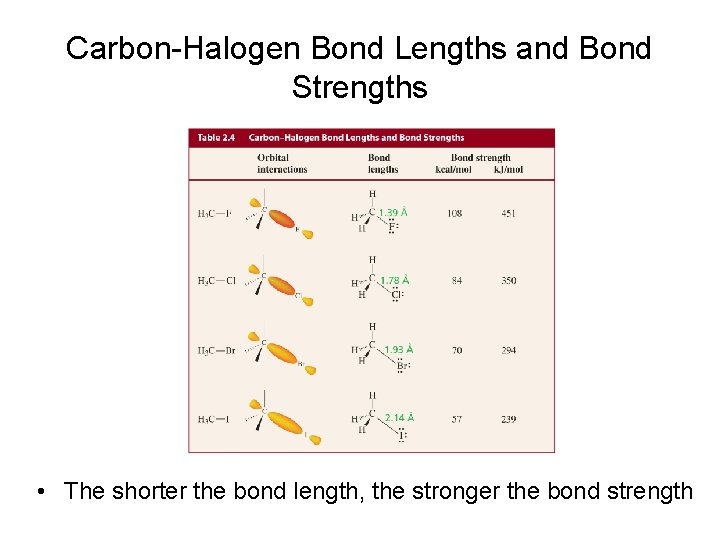
Carbon-Halogen Bond Lengths and Bond Strengths • The shorter the bond length, the stronger the bond strength

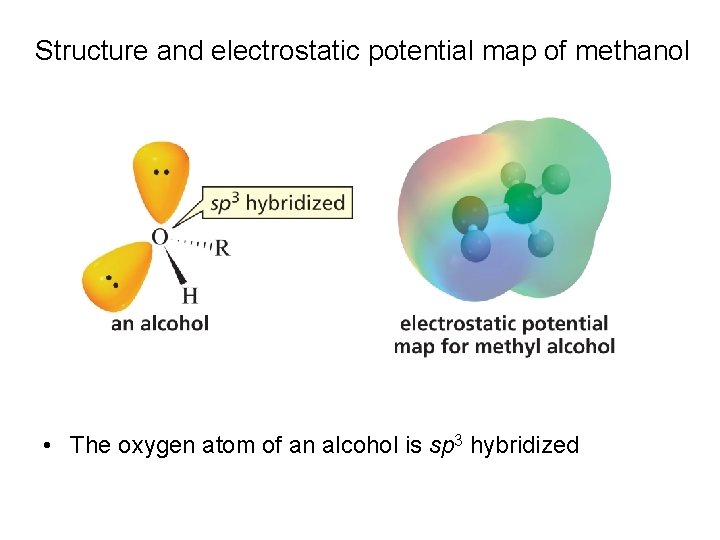
Structure and electrostatic potential map of methanol • The oxygen atom of an alcohol is sp 3 hybridized

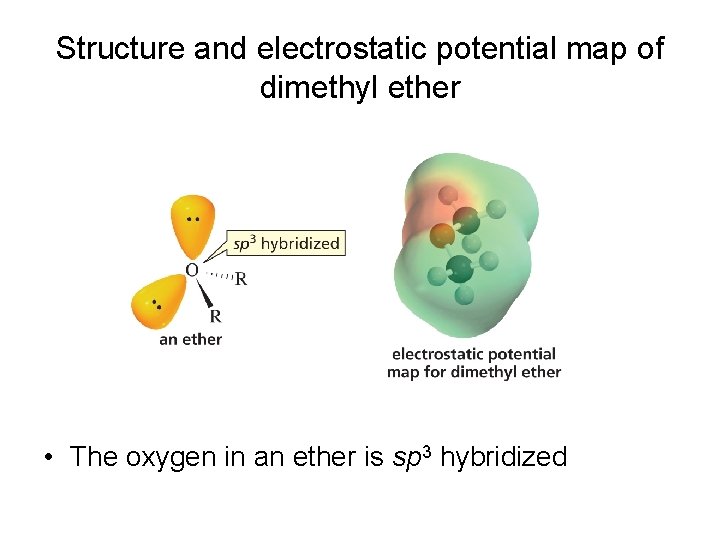
Structure and electrostatic potential map of dimethyl ether • The oxygen in an ether is sp 3 hybridized

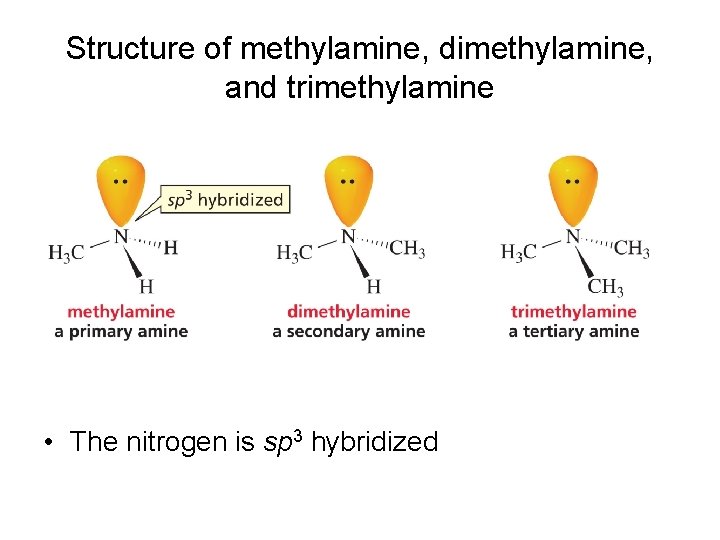
Structure of methylamine, dimethylamine, and trimethylamine • The nitrogen is sp 3 hybridized

The physical properties of Alkanes, Alkyl Halides, Alcohol, Esters, and Amines • The boiling point of a compound is the temperature at which the liquid form become gas • In order for a compound to vaporize, the force that held the individual molecules close to each other in the liquid must be overcome • This means that the boiling point of a compound depends on the strength of the attractive forces between the individual molecules • If the molecules are held together by strong force, a lot of energy will be needed to pull the molecules away from each other and the compound will have a high boiling point

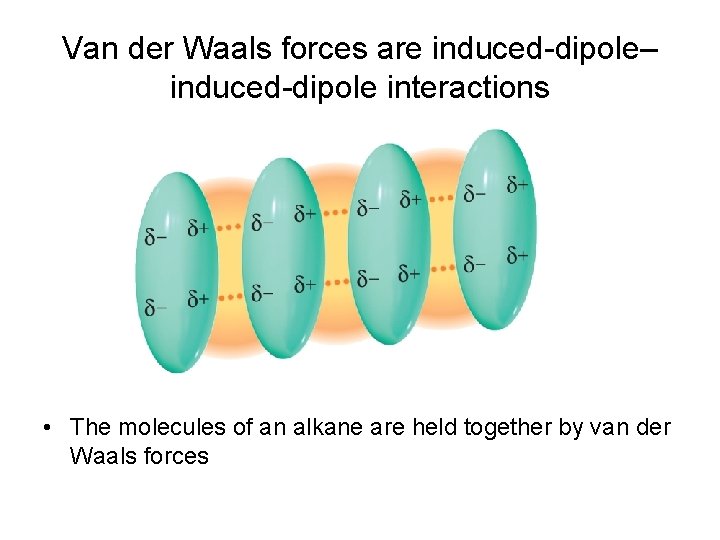
Van der Waals forces are induced-dipole– induced-dipole interactions • The molecules of an alkane are held together by van der Waals forces

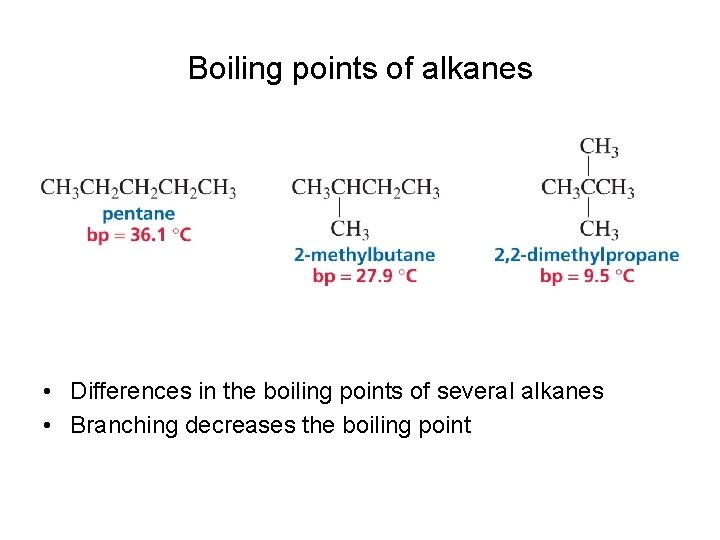
Boiling points of alkanes • Differences in the boiling points of several alkanes • Branching decreases the boiling point

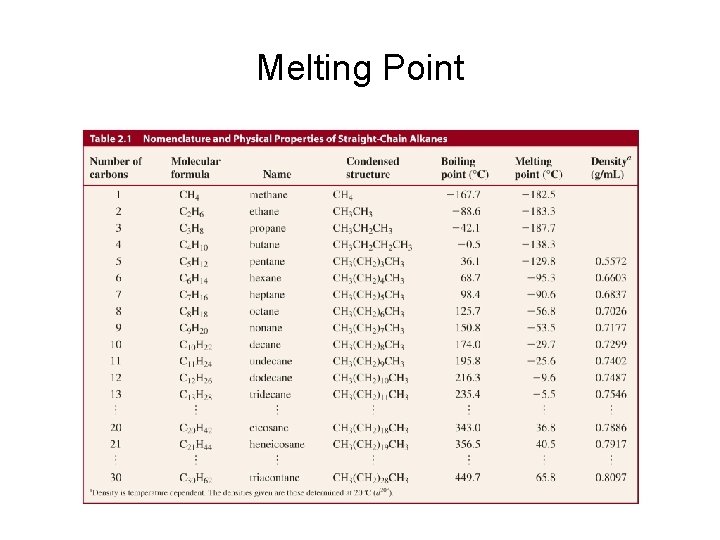
Boiling Point • The boiling points of the compounds in any homologous series increase as their molecular weights increase • Because of the increase in van der Waals forces

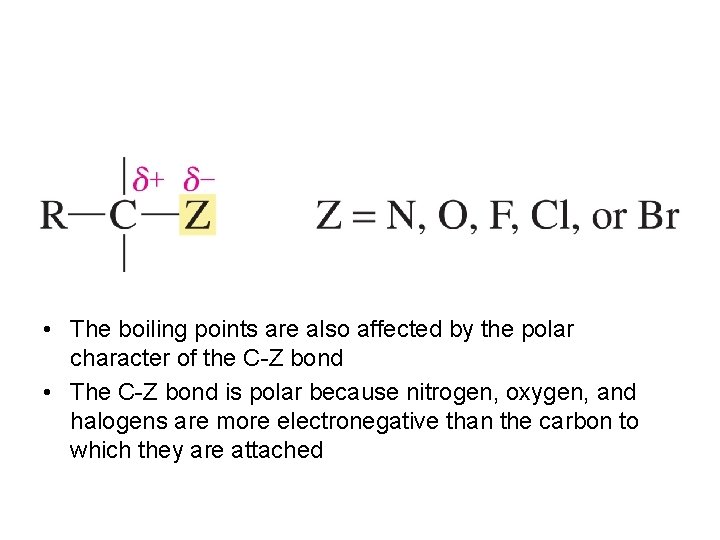
• The boiling points are also affected by the polar character of the C-Z bond • The C-Z bond is polar because nitrogen, oxygen, and halogens are more electronegative than the carbon to which they are attached

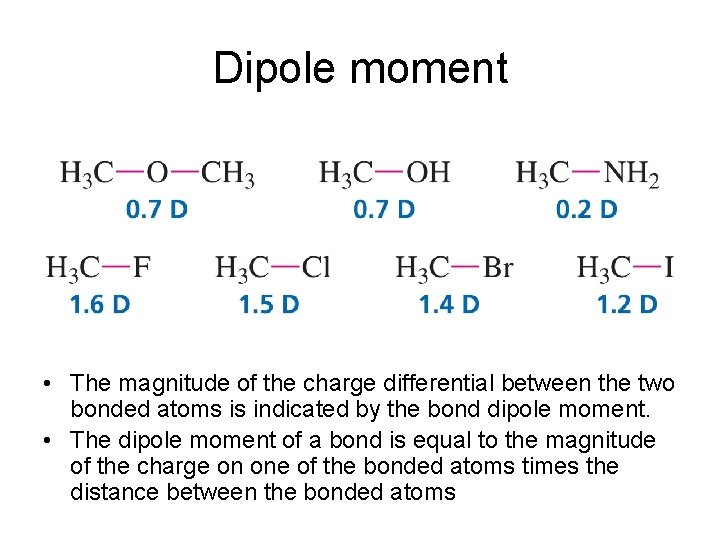
Dipole moment • The magnitude of the charge differential between the two bonded atoms is indicated by the bond dipole moment. • The dipole moment of a bond is equal to the magnitude of the charge on one of the bonded atoms times the distance between the bonded atoms

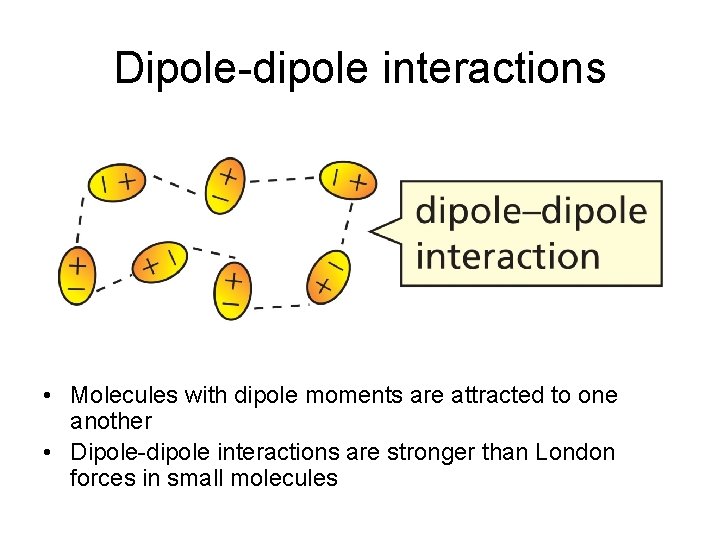
Dipole-dipole interactions • Molecules with dipole moments are attracted to one another • Dipole-dipole interactions are stronger than London forces in small molecules

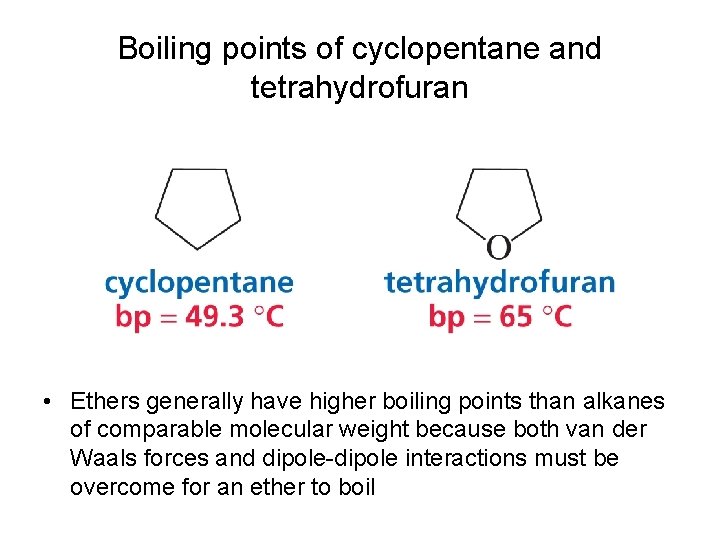
Boiling points of cyclopentane and tetrahydrofuran • Ethers generally have higher boiling points than alkanes of comparable molecular weight because both van der Waals forces and dipole-dipole interactions must be overcome for an ether to boil

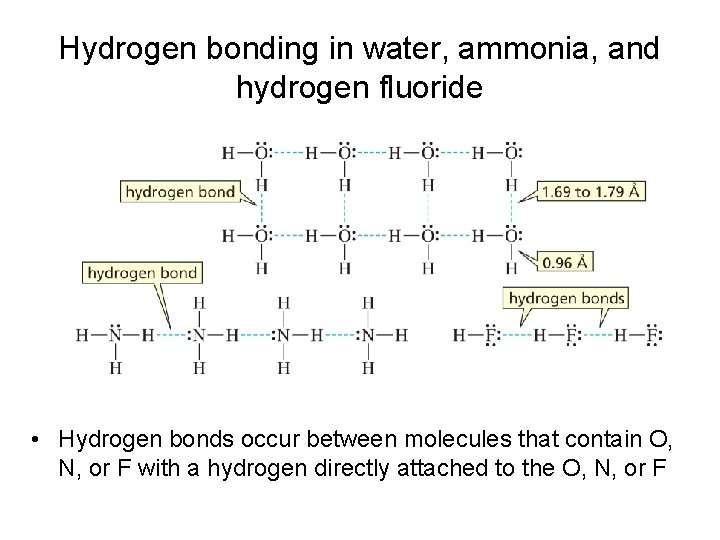
Hydrogen bonding in water, ammonia, and hydrogen fluoride • Hydrogen bonds occur between molecules that contain O, N, or F with a hydrogen directly attached to the O, N, or F

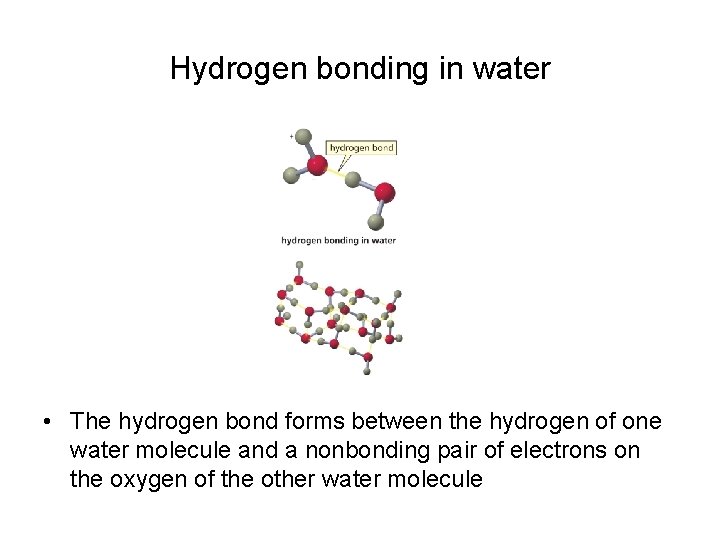
Hydrogen bonding in water • The hydrogen bond forms between the hydrogen of one water molecule and a nonbonding pair of electrons on the oxygen of the other water molecule

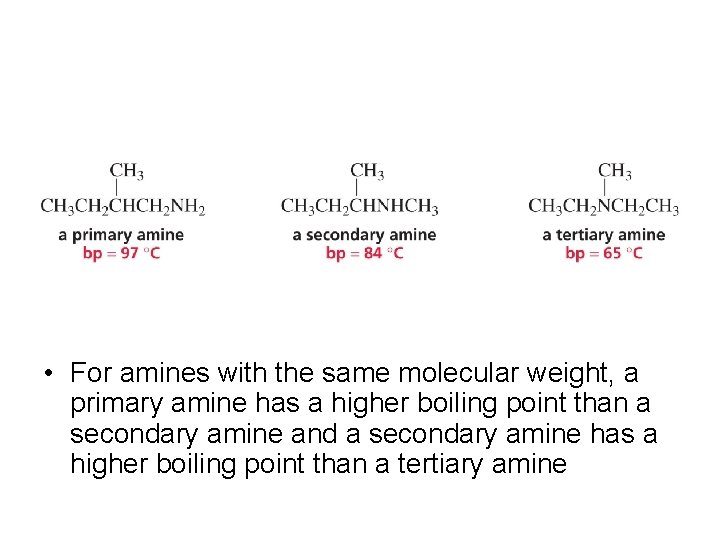
• For amines with the same molecular weight, a primary amine has a higher boiling point than a secondary amine and a secondary amine has a higher boiling point than a tertiary amine

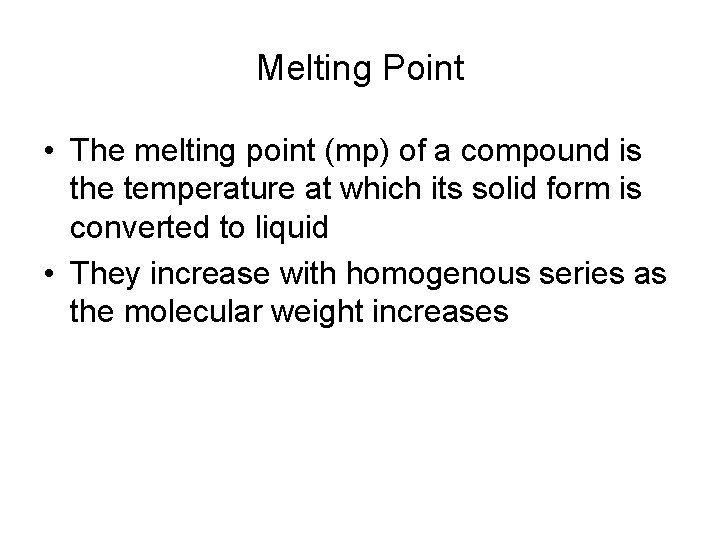
Melting Point • The melting point (mp) of a compound is the temperature at which its solid form is converted to liquid • They increase with homogenous series as the molecular weight increases

Melting Point

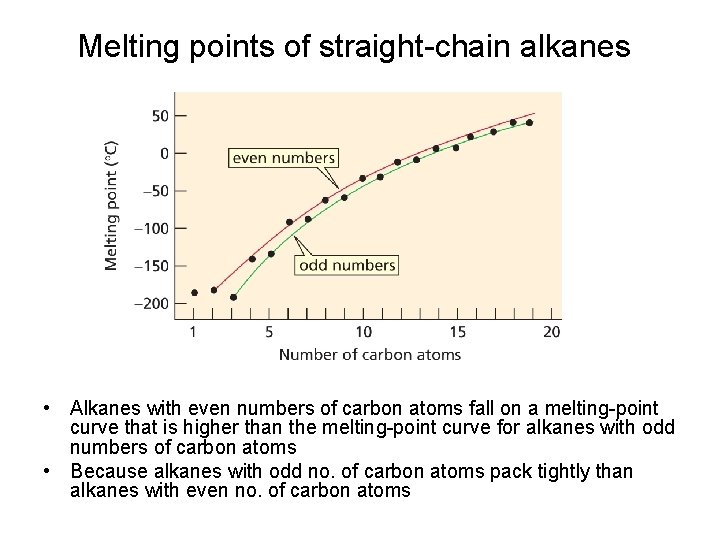
Melting points of straight-chain alkanes • Alkanes with even numbers of carbon atoms fall on a melting-point curve that is higher than the melting-point curve for alkanes with odd numbers of carbon atoms • Because alkanes with odd no. of carbon atoms pack tightly than alkanes with even no. of carbon atoms

• Alkanes with odd numbers of carbon atoms have weaker intermolecular attractions and corresponding lower melting points. • Alkanes with an odd number of carbons pack less tightly than alkanes with an even number of carbon atoms.

Solubility • The general rule that govern solubility is “like dissolves like” • Polar compounds dissolve in polar solvent, and nonpolar compound dissolve in nonpolar solvent.

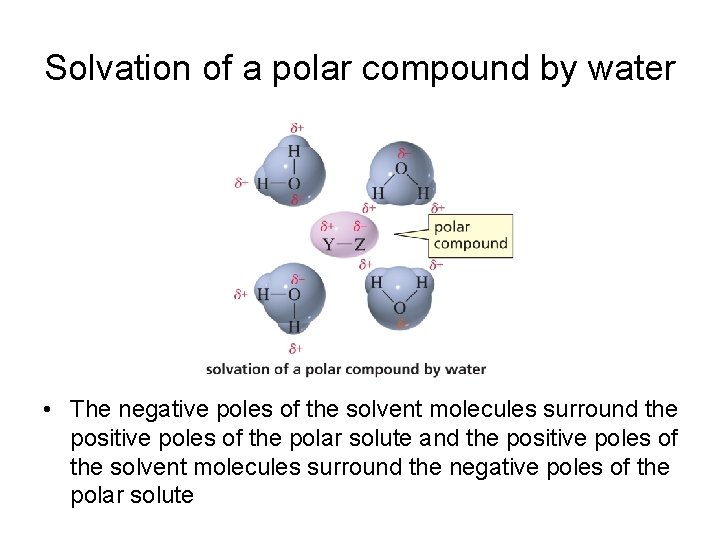
Solvation of a polar compound by water • The negative poles of the solvent molecules surround the positive poles of the polar solute and the positive poles of the solvent molecules surround the negative poles of the polar solute

Solubility of Ether in Water

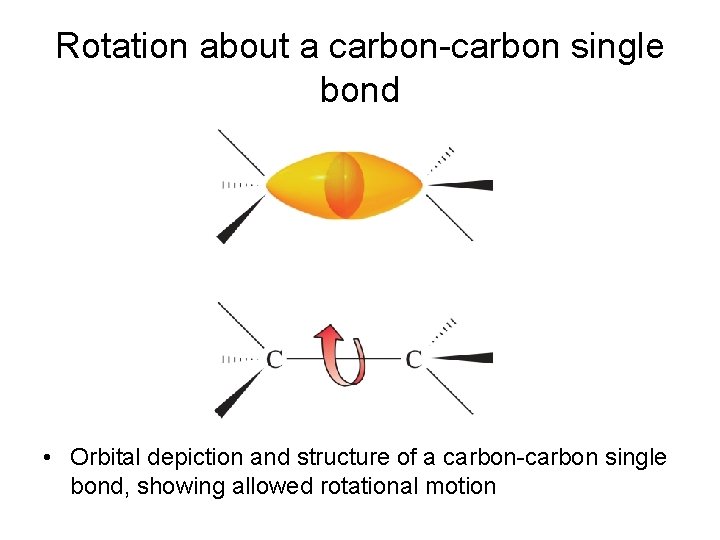
Rotation about a carbon-carbon single bond • Orbital depiction and structure of a carbon-carbon single bond, showing allowed rotational motion

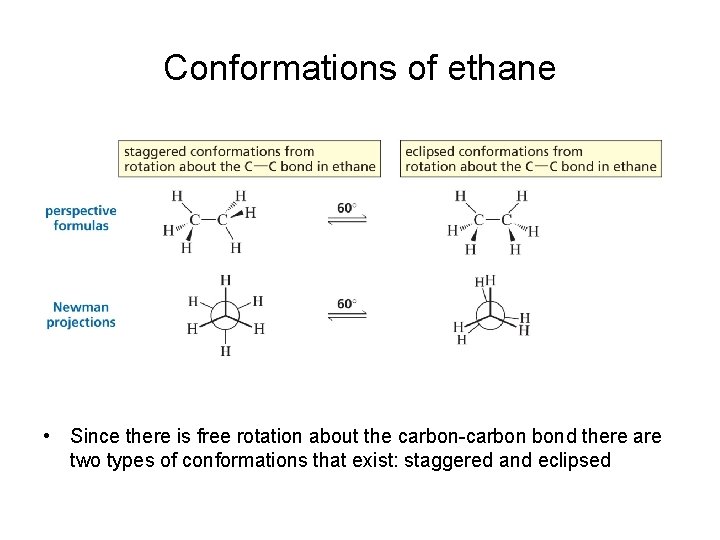
Conformations of ethane • Since there is free rotation about the carbon-carbon bond there are two types of conformations that exist: staggered and eclipsed