Ethers Epoxides Ether Nomenclature Compounds that contain two









- Slides: 17

Ethers & Epoxides

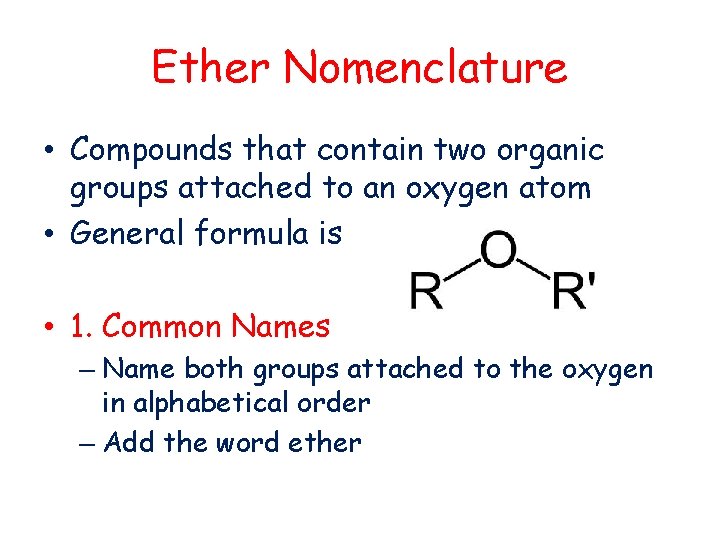
Ether Nomenclature • Compounds that contain two organic groups attached to an oxygen atom • General formula is • 1. Common Names – Name both groups attached to the oxygen in alphabetical order – Add the word ether

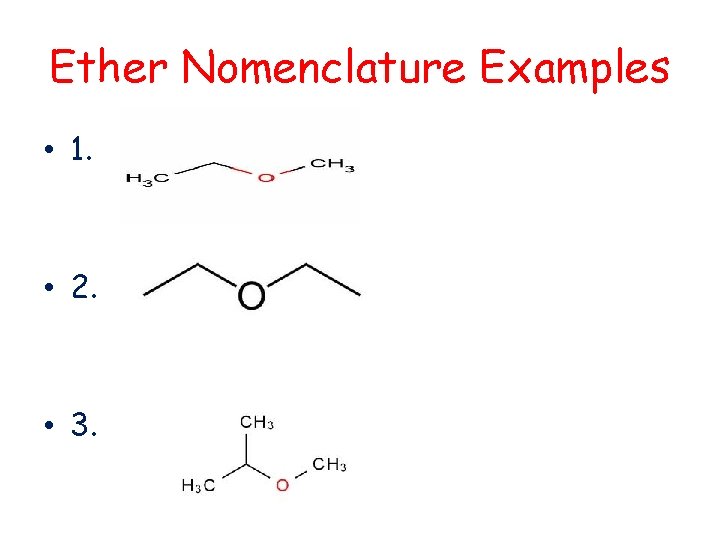
Ether Nomenclature Examples • 1. • 2. • 3.


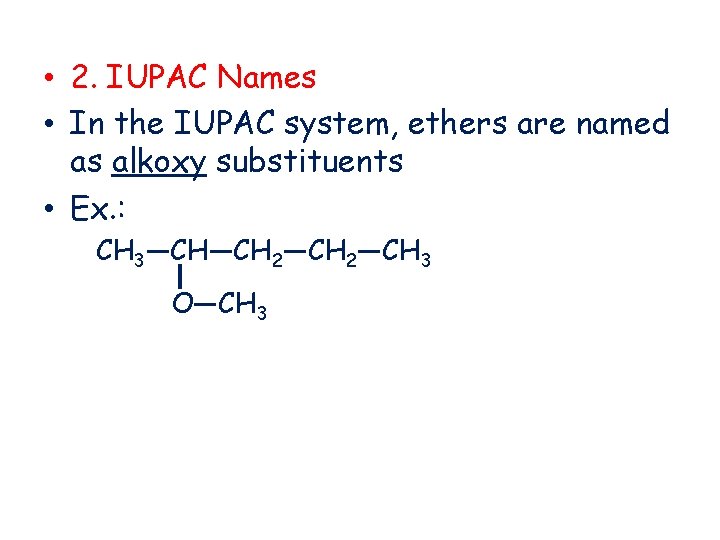
• 2. IUPAC Names • In the IUPAC system, ethers are named as alkoxy substituents • Ex. : CH 3—CH—CH 2—CH 3 O—CH 3

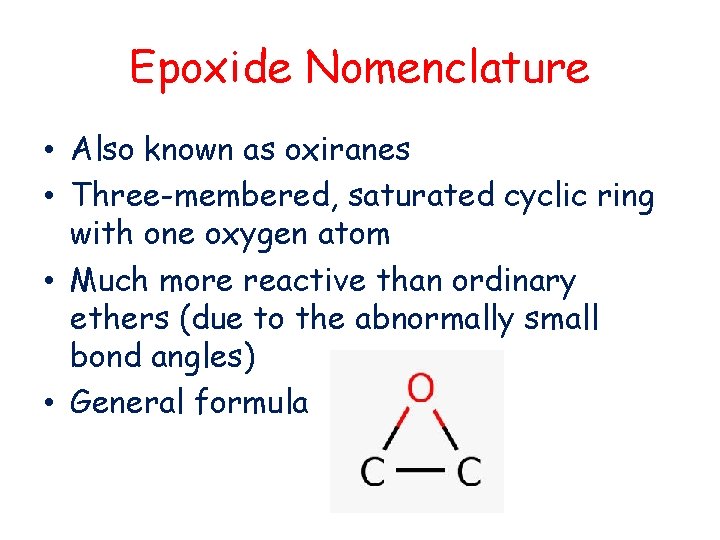
Epoxide Nomenclature • Also known as oxiranes • Three-membered, saturated cyclic ring with one oxygen atom • Much more reactive than ordinary ethers (due to the abnormally small bond angles) • General formula

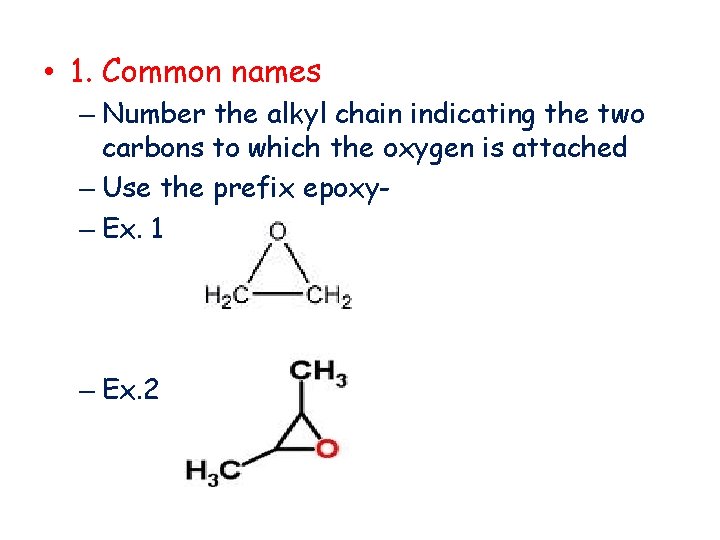
• 1. Common names – Number the alkyl chain indicating the two carbons to which the oxygen is attached – Use the prefix epoxy– Ex. 1 – Ex. 2.

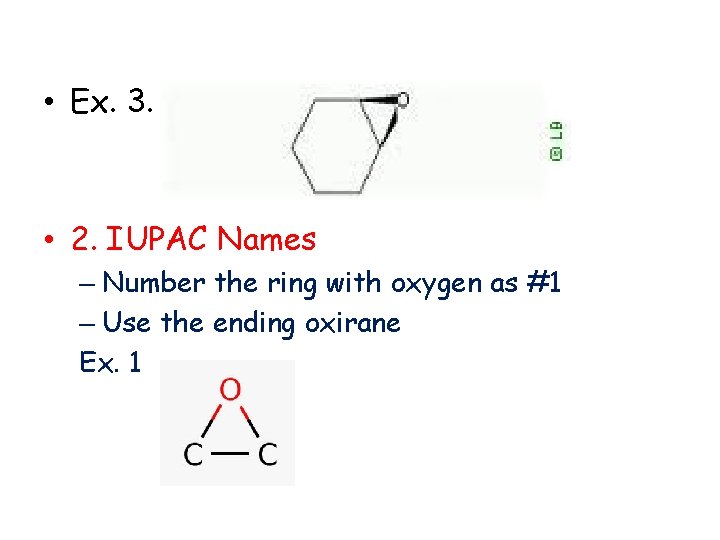
• Ex. 3. • 2. IUPAC Names – Number the ring with oxygen as #1 – Use the ending oxirane Ex. 1


Reactions of Ethers • Ethers are relatively stable, especially to dilute acids or alkalis & many oxidizing & reducing agents • Do not react with sodium (this property distinguishes them from alcohols) • Are weak bases which dissolve in concentrated sulfuric acid (this property distinguishes them from saturated hydrocarbons) • React with oxygen in the air to form dangerous explosive peroxides

Preparation of Ethers • • 1. Intermolecular Dehydration of Alcohols Used to make symmetrical ethers Reagent is H 2 SO 4 at 140°C Ex. Dehydrate two moles of ethanol • Ex. Dehydrate tertbutanol

• 2. Williamson Synthesis • Reaction of an alkyl halide with an alcohol (or an alkoxide ion) • Can be used to make either symmetrical or unsymmetrical ethers • The alkyl halide must be primary or secondary • Ex. React methanol with ethyl chloride.

• Alkyl sulfates, in the presence of Na. OH, are used to prepare ethers of phenols • Ex. React phenol with dimethylsulfate

Preparation of Epoxides • 1. Oxidation of Ethylene • Uses silver as the catalyst at 250°C • Ex: • 2. Intramolecular Williamson Synthesis • Uses hypochloric acid (HCl. O) and hydroxide • Ex:

Reactions of Epoxides • the three membered ring is highly strained and opens easily • 1. Acid Catalyzed Addition – A. Hydrolysis of epoxyethane • Uses dilute acid; used to make trans diols • Ex. ; Hydrolyze 1, 2 -epoxycyclohexane with dilute acid – B. Treatment with concentrated acid Ex. React oxirane with concentrated HCl.

– C. In alcoholic solutions – Ex. React oxirane with methanol – D. with ethylene glycol – Ex. React oxirane with ethylene glycol

• 2. Base Catalyzed Addition • Uses ammonia • 3. Reaction with a Grignard Reagent • Extends the chain of the Grignard reagent by two carbons • Product is a primary alcohol • Ex. React oxirane with phenylmagnesium bromide