Ethers Sulfides Epoxides Variety of ethers ROR Aprotic




- Slides: 26

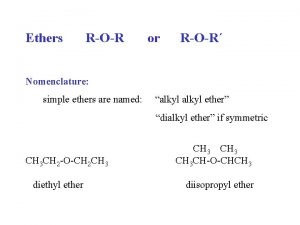
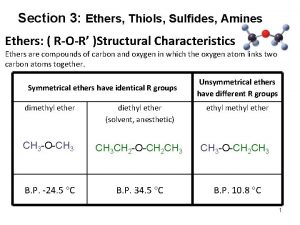
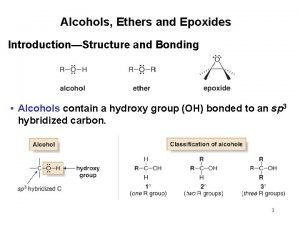
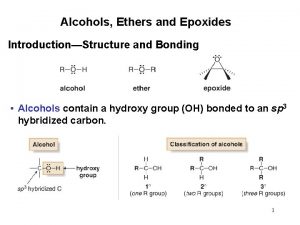
Ethers, Sulfides, Epoxides

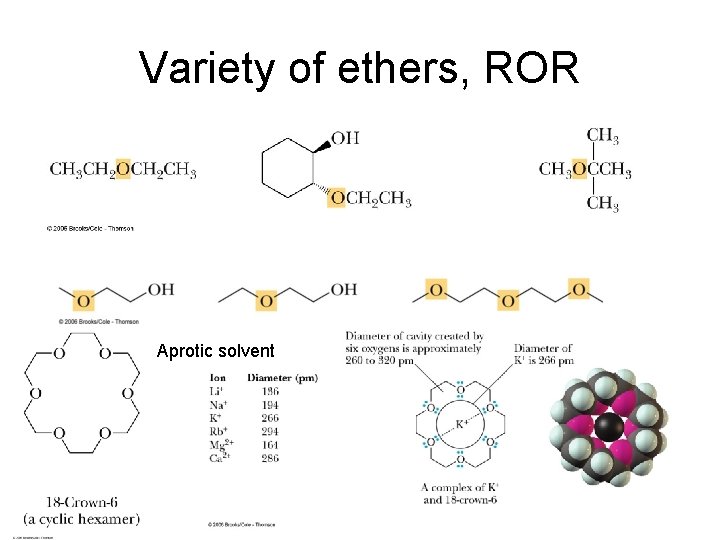
Variety of ethers, ROR Aprotic solvent

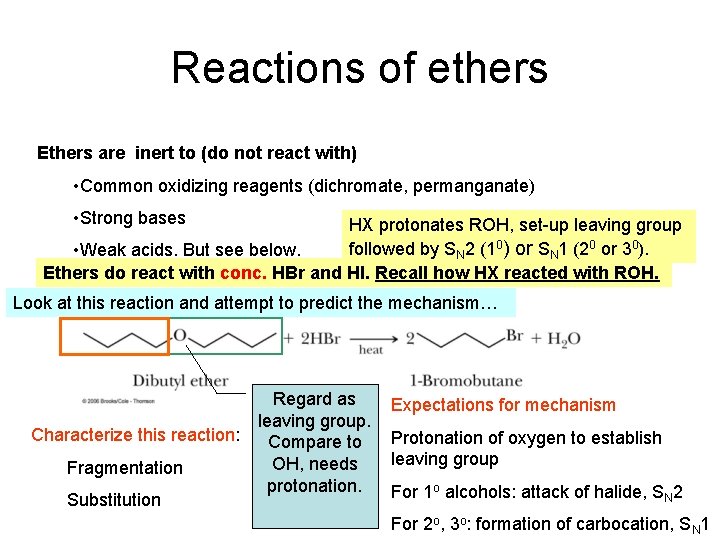
Reactions of ethers Ethers are inert to (do not react with) • Common oxidizing reagents (dichromate, permanganate) • Strong bases HX protonates ROH, set-up leaving group followed by SN 2 (10) or SN 1 (20 or 30). • Weak acids. But see below. Ethers do react with conc. HBr and HI. Recall how HX reacted with ROH. Look at this reaction and attempt to predict the mechanism… Characterize this reaction: Fragmentation Substitution Regard as leaving group. Compare to OH, needs protonation. Expectations for mechanism Protonation of oxygen to establish leaving group For 1 o alcohols: attack of halide, SN 2 For 2 o, 3 o: formation of carbocation, SN 1

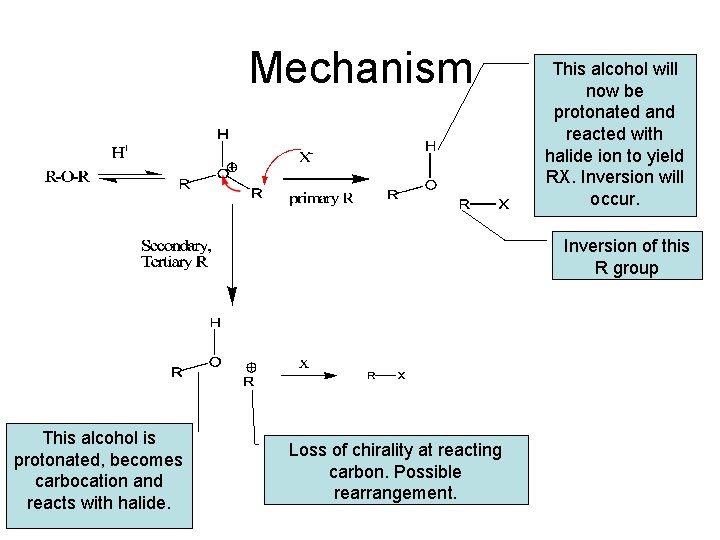
Mechanism This alcohol will now be protonated and reacted with halide ion to yield RX. Inversion will occur. Inversion of this R group This alcohol is protonated, becomes carbocation and reacts with halide. Loss of chirality at reacting carbon. Possible rearrangement.

Properties of ethers Aprotic Solvent, cannot supply the H in Hbonding, no ether to ether hydrogen bonding Ethers are polar and have boiling points close to the alkanes. propane (bp: -42) dimethyl ether (-24) ethanol (78)

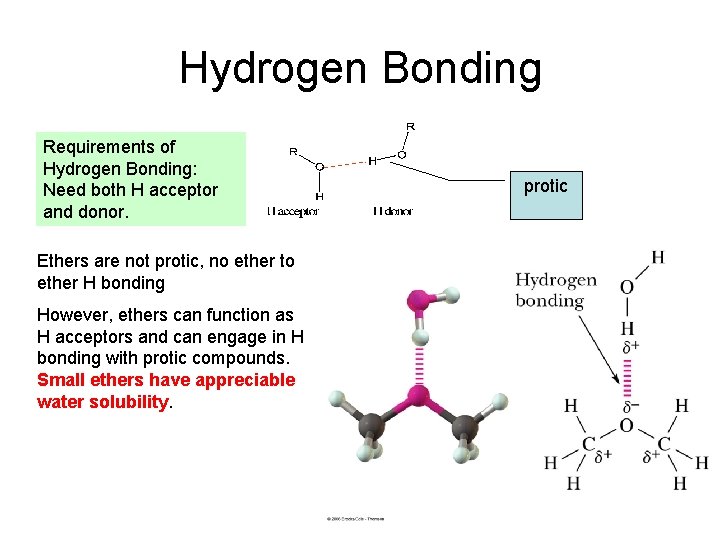
Hydrogen Bonding Requirements of Hydrogen Bonding: Need both H acceptor and donor. Ethers are not protic, no ether to ether H bonding However, ethers can function as H acceptors and can engage in H bonding with protic compounds. Small ethers have appreciable water solubility. protic

Synthesis of ethers Williamson ether synthesis RO- + R’X ROR’ nucleophile electrophile Characteristics • RO-, an alkoxide ion, is both a strong nucleophile (unless bulky and hindered) and a strong base. Both SN 2 (desired) and E 2 (undesired side product) can occur. • Choose nucleophile and electrophile carefully. Maximize SN 2 and minimize E 2 reaction by choosing the R’X to have least substituted carbon undergoing substitution (electrophile). Methyl best, then primary, secondary marginal, tertiary never (get E 2 instead). • Stereochemistry: the reacting carbon in R’, the electrophile which undergoes substitution, experiences inversion. The alkoxide undergoes no change of configuration.

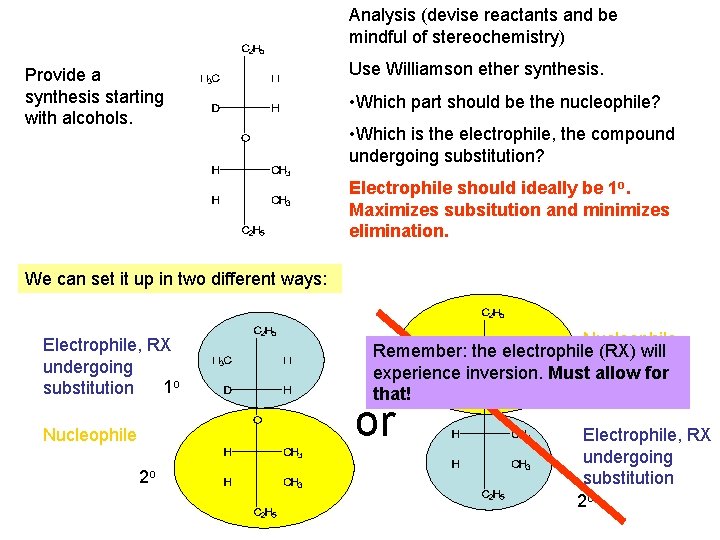
Analysis (devise reactants and be mindful of stereochemistry) Provide a synthesis starting with alcohols. Use Williamson ether synthesis. • Which part should be the nucleophile? • Which is the electrophile, the compound undergoing substitution? Electrophile should ideally be 1 o. Maximizes subsitution and minimizes elimination. We can set it up in two different ways: Electrophile, RX undergoing 1 o substitution Nucleophile 2 o Nucleophile Remember: the electrophile (RX) will experience inversion. Must 1 oallow for that! or Electrophile, RX undergoing substitution 2 o

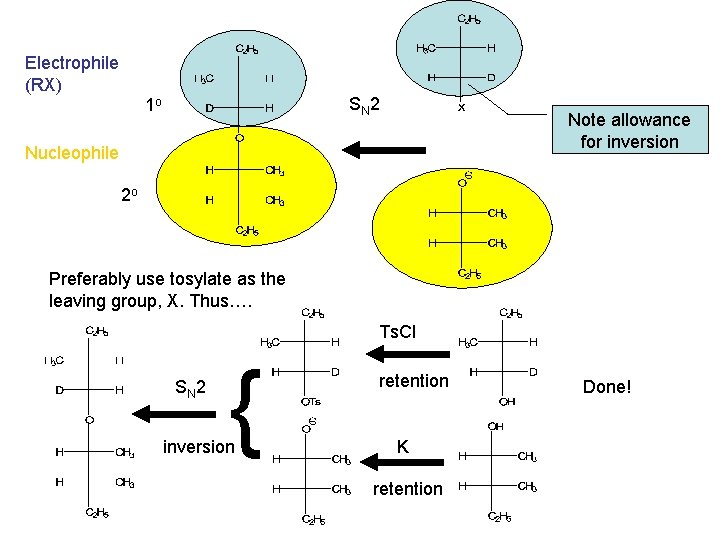
Electrophile (RX) SN 2 1 o Note allowance for inversion Nucleophile 2 o Preferably use tosylate as the leaving group, X. Thus…. Ts. Cl SN 2 { inversion retention K retention Done!

Acid catalyzed dehydration of alcohols to yield ethers. Key ideas: • Acid will protonate alcohol, setting up good leaving group. • A second alcohol molecule can act as a nucleophile. The nucleophile (ROH) is weak but the leaving group (ROH) is good. Mechanism is totally as expected: • Protonation of alcohol (setting up good leaving group) • For 2 o and 3 o ionization to yield a carbocation with alkene formation as side product. Attack of nucleophile (second alcohol molecule) on carbocation. • For 1 o attack of nucleophile (second alcohol molecule) on the protonated alcohol.

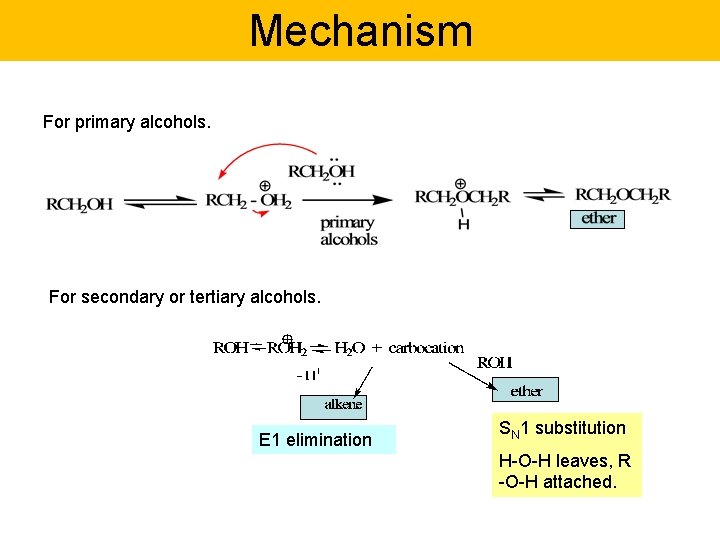
Mechanism For primary alcohols. For secondary or tertiary alcohols. E 1 elimination SN 1 substitution H-O-H leaves, R -O-H attached.

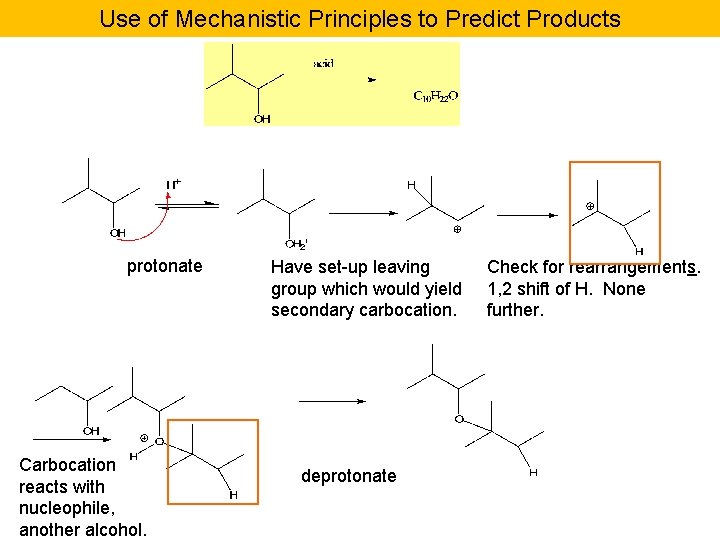
Use of Mechanistic Principles to Predict Products protonate Carbocation reacts with nucleophile, another alcohol. Have set-up leaving group which would yield secondary carbocation. deprotonate Check for rearrangements. 1, 2 shift of H. None further.

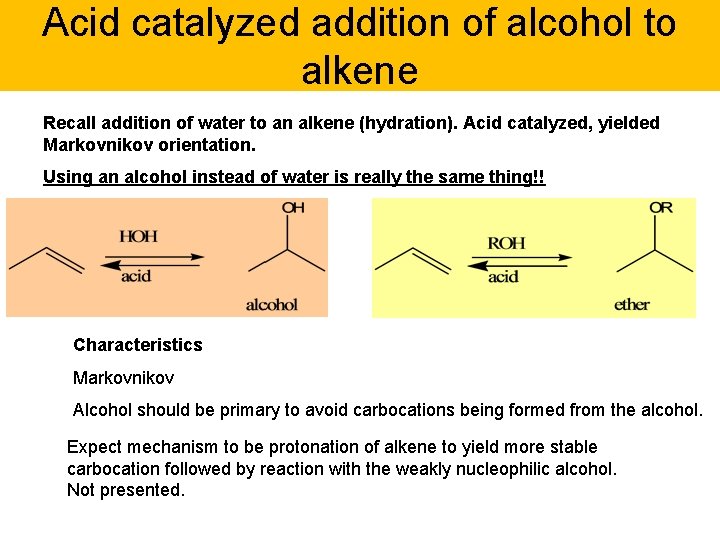
Acid catalyzed addition of alcohol to alkene Recall addition of water to an alkene (hydration). Acid catalyzed, yielded Markovnikov orientation. Using an alcohol instead of water is really the same thing!! Characteristics Markovnikov Alcohol should be primary to avoid carbocations being formed from the alcohol. Expect mechanism to be protonation of alkene to yield more stable carbocation followed by reaction with the weakly nucleophilic alcohol. Not presented.

Important Synthetic Technique: protecting groups. Using Silyl ethers to Protect Alcohols Protecting groups are used to temporarily deactivate a functional group while reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily Silyl ether. Does render the OH inert. not react with non Sequence of Steps: aqueous acid and bases or moderate aq. acids and 1. Protect: bases. 2. Do work: 3. Deprotect: THF

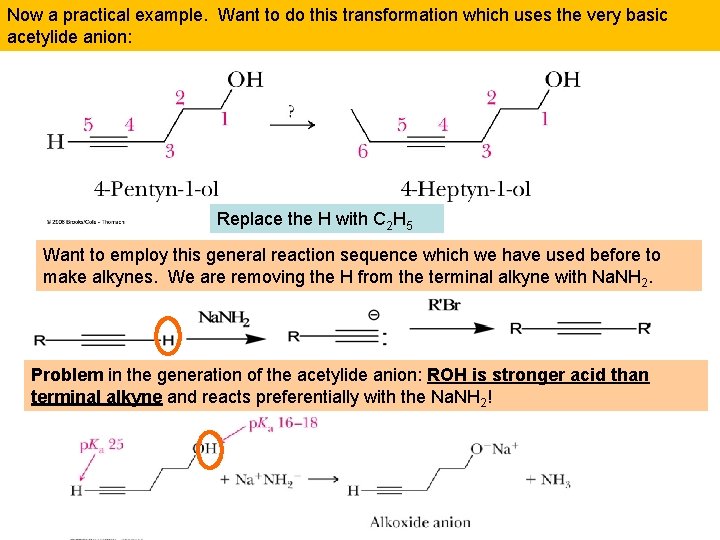
Now a practical example. Want to do this transformation which uses the very basic acetylide anion: Replace the H with C 2 H 5 Want to employ this general reaction sequence which we have used before to make alkynes. We are removing the H from the terminal alkyne with Na. NH 2. Problem in the generation of the acetylide anion: ROH is stronger acid than terminal alkyne and reacts preferentially with the Na. NH 2!

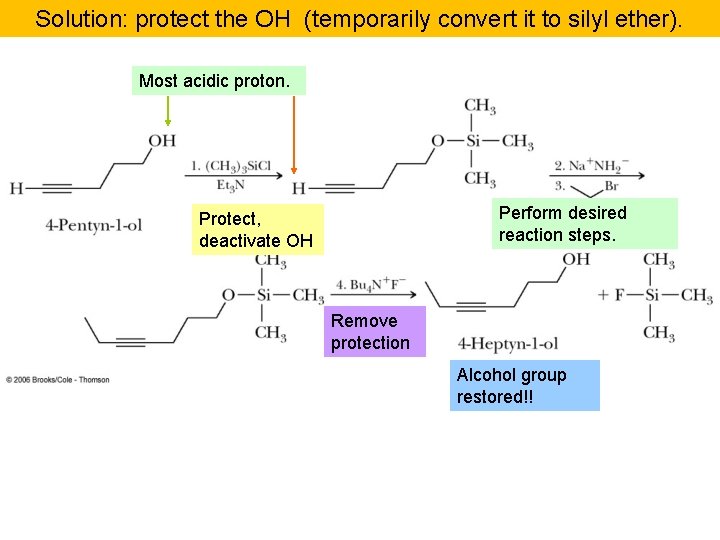
Solution: protect the OH (temporarily convert it to silyl ether). Most acidic proton. Perform desired reaction steps. Protect, deactivate OH Remove protection Alcohol group restored!!

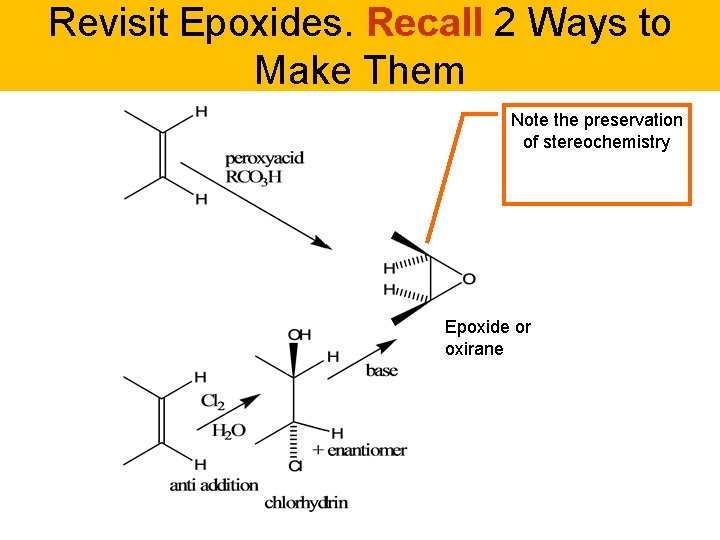
Revisit Epoxides. Recall 2 Ways to Make Them Note the preservation of stereochemistry Epoxide or oxirane

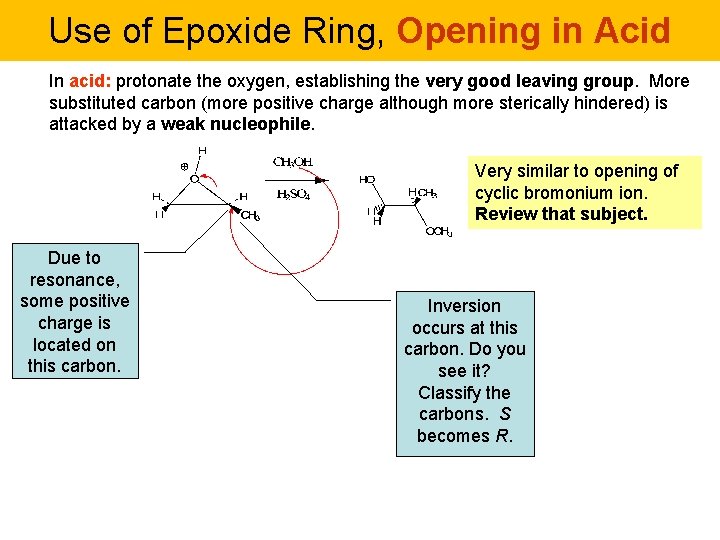
Use of Epoxide Ring, Opening in Acid In acid: protonate the oxygen, establishing the very good leaving group. More substituted carbon (more positive charge although more sterically hindered) is attacked by a weak nucleophile. Very similar to opening of cyclic bromonium ion. Review that subject. Due to resonance, some positive charge is located on this carbon. Inversion occurs at this carbon. Do you see it? Classify the carbons. S becomes R.

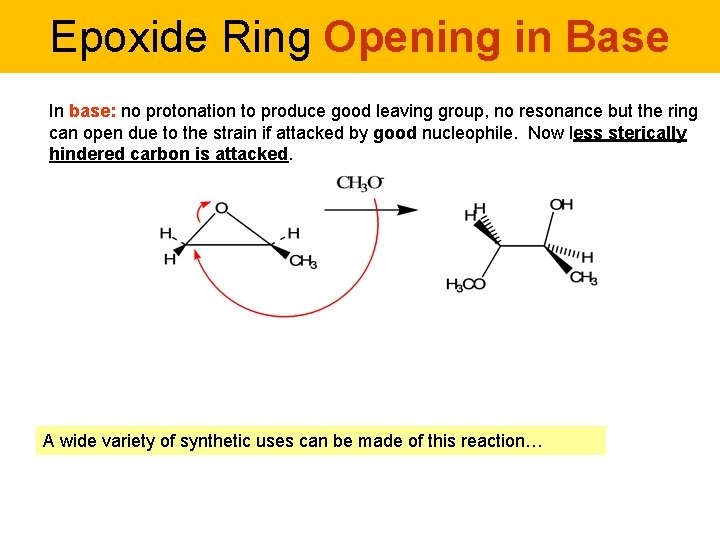
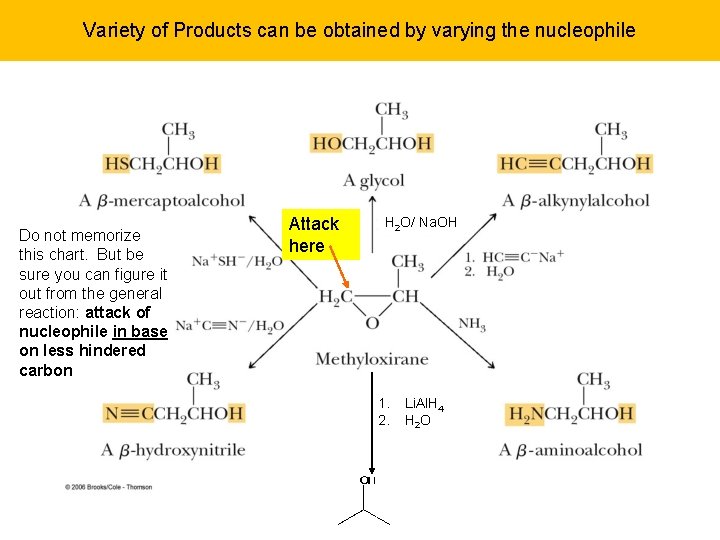
Epoxide Ring Opening in Base In base: no protonation to produce good leaving group, no resonance but the ring can open due to the strain if attacked by good nucleophile. Now less sterically hindered carbon is attacked. A wide variety of synthetic uses can be made of this reaction…

Variety of Products can be obtained by varying the nucleophile Do not memorize this chart. But be sure you can figure it out from the general reaction: attack of nucleophile in base on less hindered carbon Attack here H 2 O/ Na. OH 1. 2. Li. Al. H 4 H 2 O

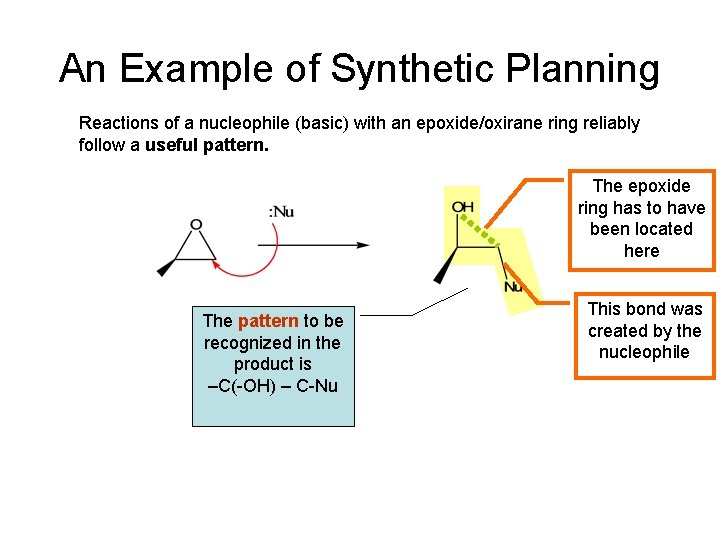
An Example of Synthetic Planning Reactions of a nucleophile (basic) with an epoxide/oxirane ring reliably follow a useful pattern. The epoxide ring has to have been located here The pattern to be recognized in the product is –C(-OH) – C-Nu This bond was created by the nucleophile

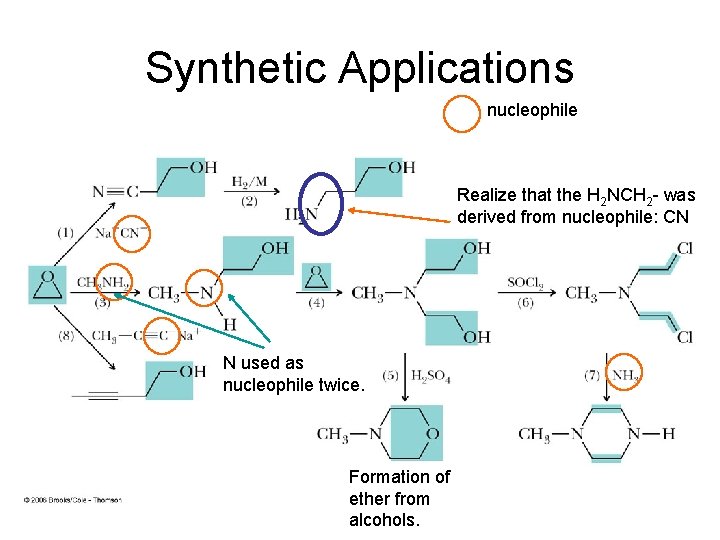
Synthetic Applications nucleophile Realize that the H 2 NCH 2 - was derived from nucleophile: CN N used as nucleophile twice. Formation of ether from alcohols.

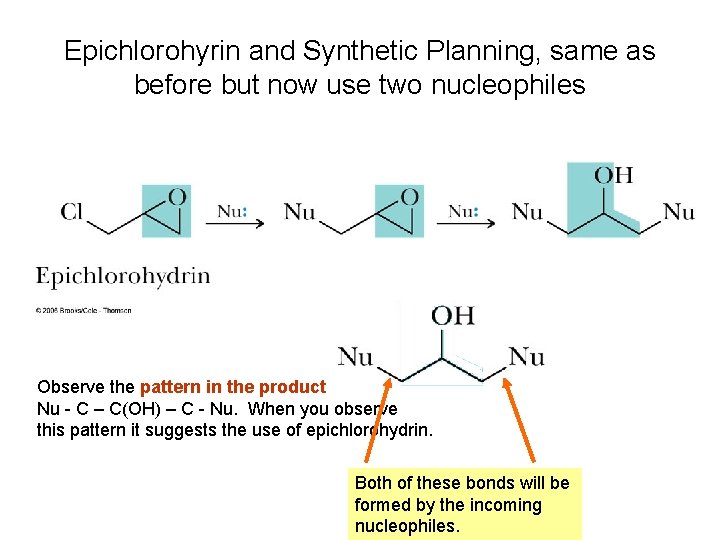
Epichlorohyrin and Synthetic Planning, same as before but now use two nucleophiles Observe the pattern in the product Nu - C – C(OH) – C - Nu. When you observe this pattern it suggests the use of epichlorohydrin. Both of these bonds will be formed by the incoming nucleophiles.

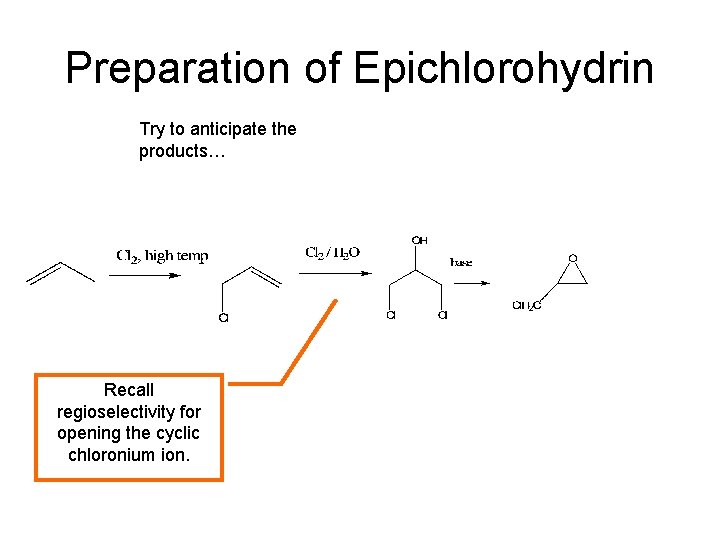
Preparation of Epichlorohydrin Try to anticipate the products… Recall regioselectivity for opening the cyclic chloronium ion.

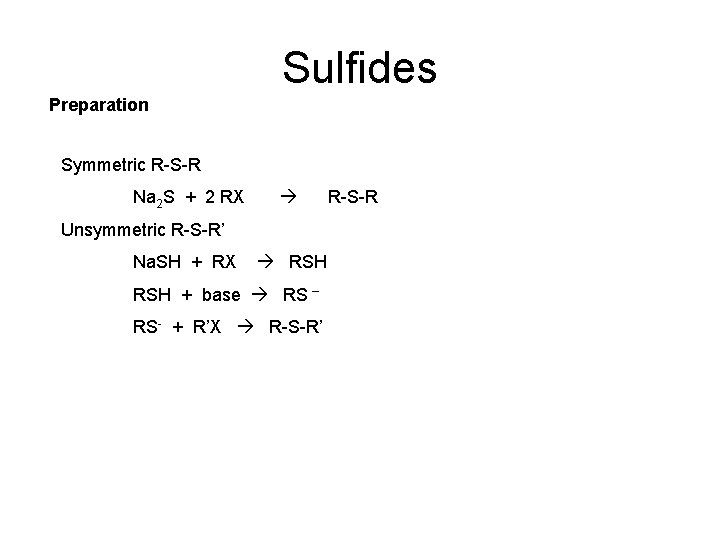
Sulfides Preparation Symmetric R-S-R Na 2 S + 2 RX Unsymmetric R-S-R’ Na. SH + RX RSH + base RS – RS- + R’X R-S-R’ R-S-R

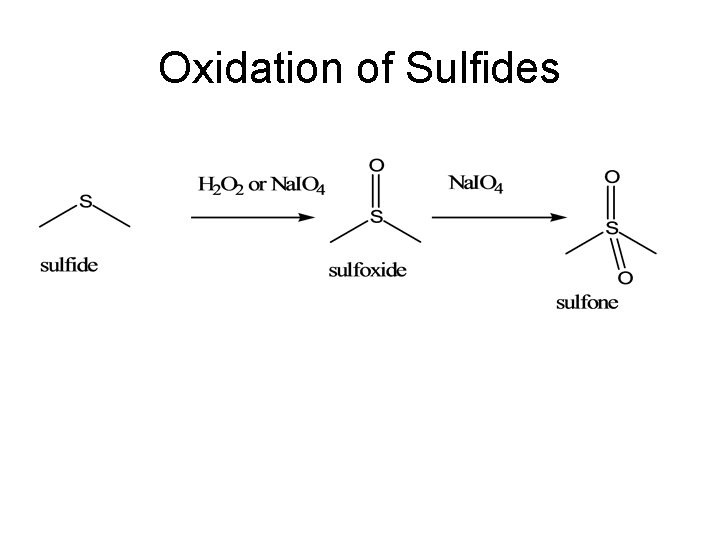
Oxidation of Sulfides
 2 methylpropyl
2 methylpropyl Praktik makabra
Praktik makabra Ror + hx
Ror + hx When else vhdl
When else vhdl Compiled languages
Compiled languages Ror 8086
Ror 8086 Qin shi huang ror
Qin shi huang ror Ror model
Ror model Classification of instruction set of 8086
Classification of instruction set of 8086 The ether group
The ether group Organic chemistry william h brown
Organic chemistry william h brown Ester naming
Ester naming Ethers boiling point
Ethers boiling point Why are ethers relatively inert compounds
Why are ethers relatively inert compounds Ether naming
Ether naming Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Ethers naming
Ethers naming Nomenclature of ethers
Nomenclature of ethers Acidic cleavage of ethers
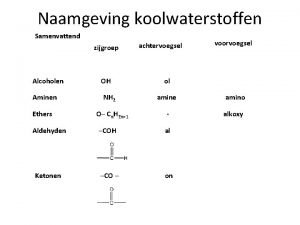
Acidic cleavage of ethers Ethers naamgeving
Ethers naamgeving Direct water pollution
Direct water pollution Process volume and variety
Process volume and variety Interesting sentence openers
Interesting sentence openers Hypocotyl necrosis in french bean is due to
Hypocotyl necrosis in french bean is due to Starry night harmony unity and variety
Starry night harmony unity and variety Variety of life on earth
Variety of life on earth Dr anbari
Dr anbari