Chapter 16 Ethers Epoxides and Sulfides Copyright The










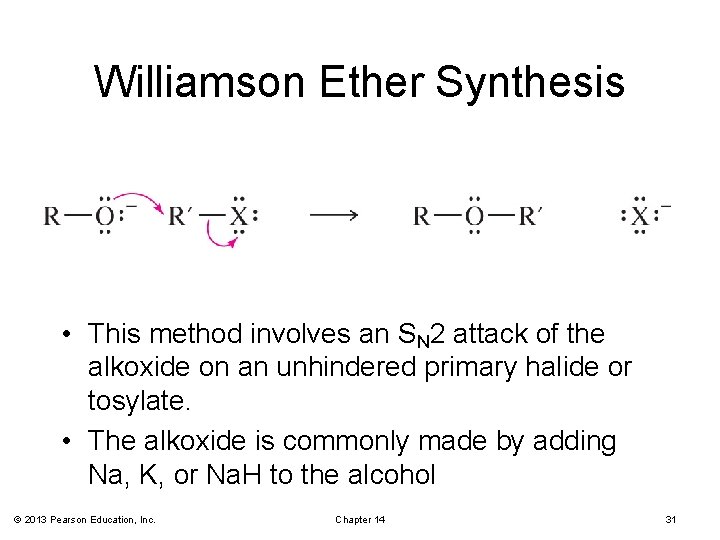







- Slides: 71

Chapter 16 Ethers, Epoxides, and Sulfides Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Ethers • Formula is R—O—R¢ where R and R¢ are alkyl or aryl. • Symmetrical or unsymmetrical © 2013 Pearson Education, Inc. Chapter 14 2

Nomenclature of Ethers, Epoxides, and Sulfides

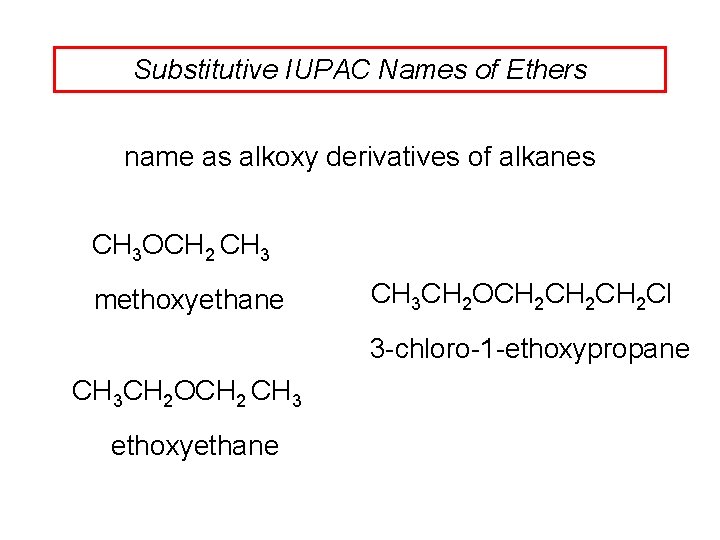
Substitutive IUPAC Names of Ethers name as alkoxy derivatives of alkanes CH 3 OCH 2 CH 3 methoxyethane CH 3 CH 2 OCH 2 CH 2 Cl 3 -chloro-1 -ethoxypropane CH 3 CH 2 OCH 2 CH 3 ethoxyethane

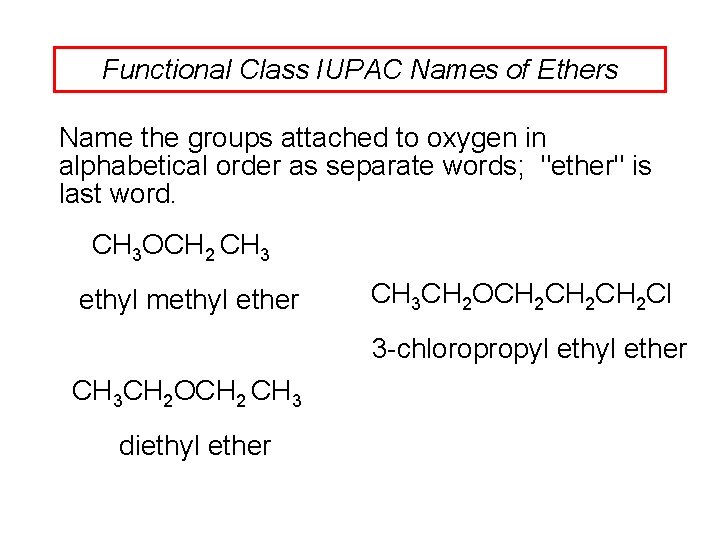
Functional Class IUPAC Names of Ethers Name the groups attached to oxygen in alphabetical order as separate words; "ether" is last word. CH 3 OCH 2 CH 3 ethyl methyl ether CH 3 CH 2 OCH 2 CH 2 Cl 3 -chloropropyl ether CH 3 CH 2 OCH 2 CH 3 diethyl ether

Structure and Bonding in Ethers and Epoxides bent geometry at oxygen analogous to water and alcohols

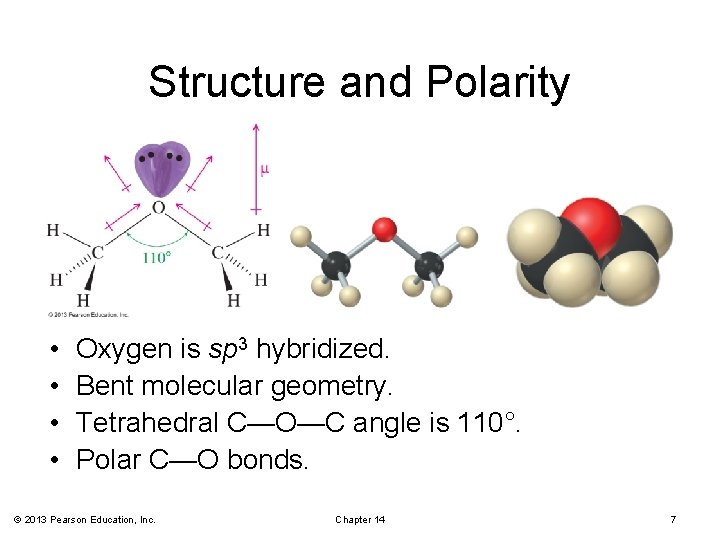
Structure and Polarity • • Oxygen is sp 3 hybridized. Bent molecular geometry. Tetrahedral C—O—C angle is 110°. Polar C—O bonds. © 2013 Pearson Education, Inc. Chapter 14 7

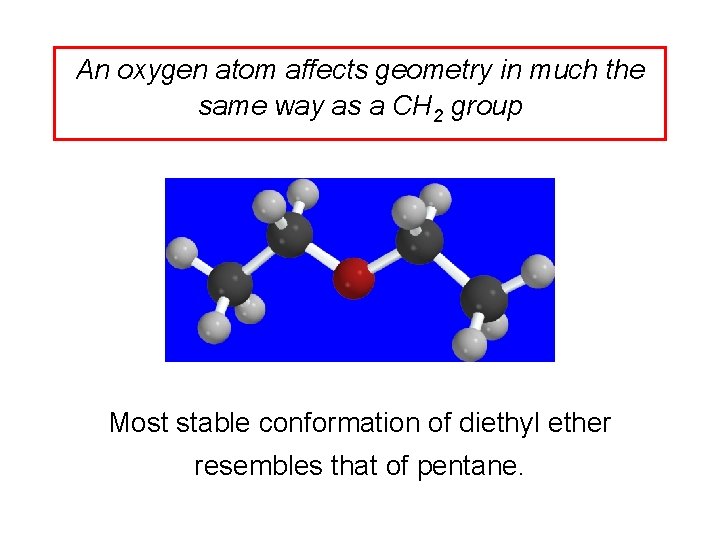
Bond angles at oxygen are sensitive to steric effects O O H H 105° 108. 5° O O CH 3 112° H CH 3 C(CH 3)3 C 132°

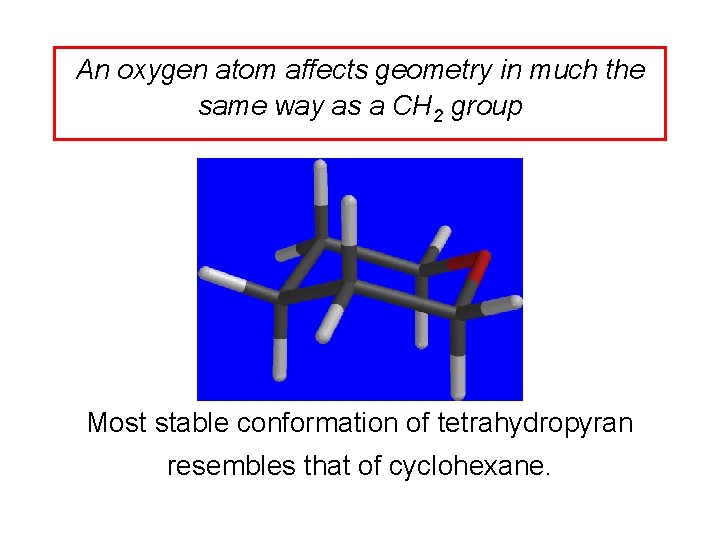
An oxygen atom affects geometry in much the same way as a CH 2 group Most stable conformation of diethyl ether resembles that of pentane.

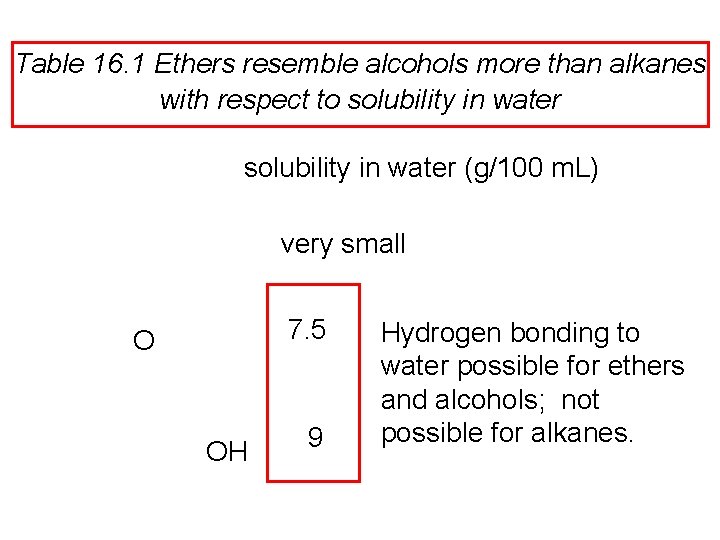
An oxygen atom affects geometry in much the same way as a CH 2 group Most stable conformation of tetrahydropyran resembles that of cyclohexane.

Physical Properties of Ethers

Table 16. 1 Ethers resemble alkanes more than alcohols with respect to boiling point 36°C O 35°C 117°C OH Intermolecular hydrogen bonding possible in alcohols; not possible in alkanes or ethers.

Table 16. 1 Ethers resemble alcohols more than alkanes with respect to solubility in water (g/100 m. L) very small 7. 5 O OH 9 Hydrogen bonding to water possible for ethers and alcohols; not possible for alkanes.

Hydrogen Bond Acceptor • Ethers cannot hydrogen bond with other ether molecules, so they have a lower boiling point than alcohols. • Ether molecules can hydrogen bond with water and alcohol molecules. • They are hydrogen bond acceptors. © 2013 Pearson Education, Inc. Chapter 14 14

Ethers as Solvents • Ethers are widely used as solvents because § they can dissolve nonpolar and polar substances. § they are unreactive toward strong bases. Ethers are relatively unreactive. Their low level of reactivity is one reason why ethers are often used as solvents in chemical reactions. © 2013 Pearson Education, Inc. Chapter 14 15

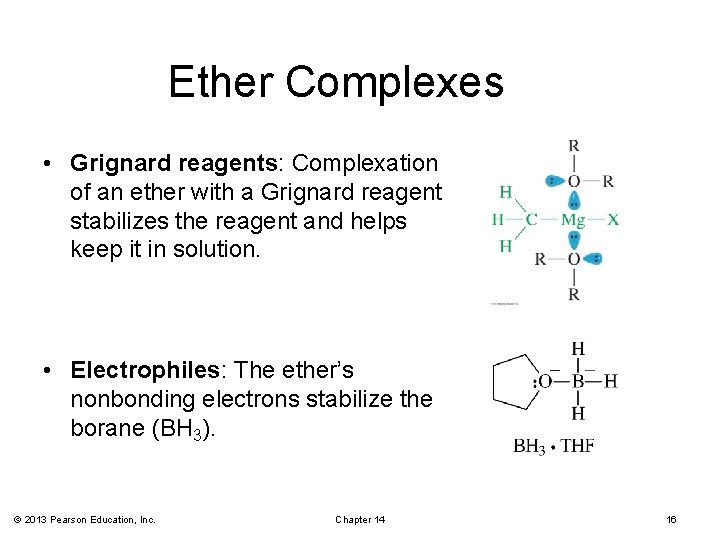
Ether Complexes • Grignard reagents: Complexation of an ether with a Grignard reagent stabilizes the reagent and helps keep it in solution. • Electrophiles: The ether’s nonbonding electrons stabilize the borane (BH 3). © 2013 Pearson Education, Inc. Chapter 14 16

Crown Ethers Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

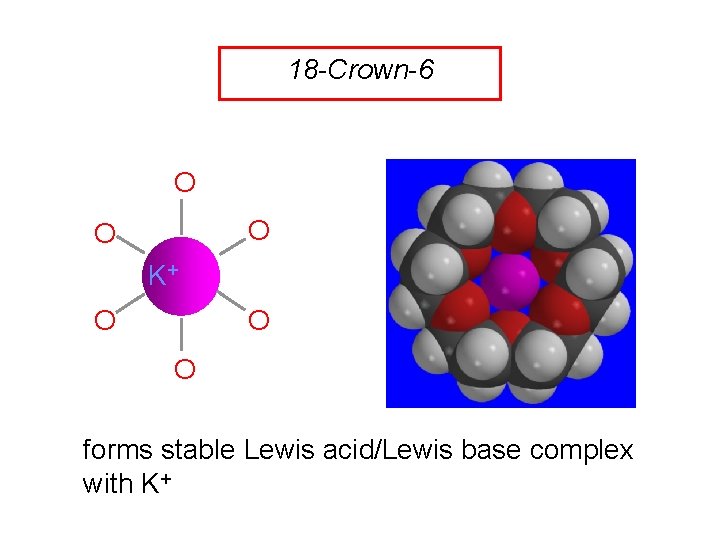
Crown Ethers structure cyclic polyethers derived from repeating —OCH 2— units properties form stable complexes with metal ions applications synthetic reactions involving anions

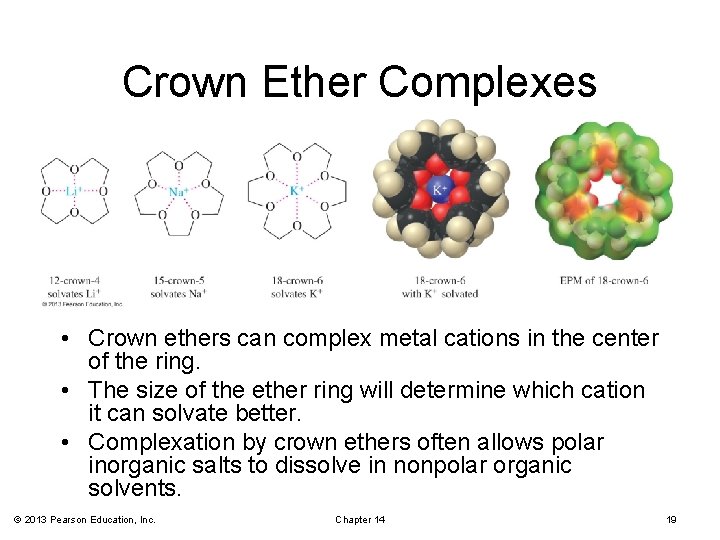
Crown Ether Complexes • Crown ethers can complex metal cations in the center of the ring. • The size of the ether ring will determine which cation it can solvate better. • Complexation by crown ethers often allows polar inorganic salts to dissolve in nonpolar organic solvents. © 2013 Pearson Education, Inc. Chapter 14 19

18 -Crown-6 O O O K+ O O O forms stable Lewis acid/Lewis base complex with K+

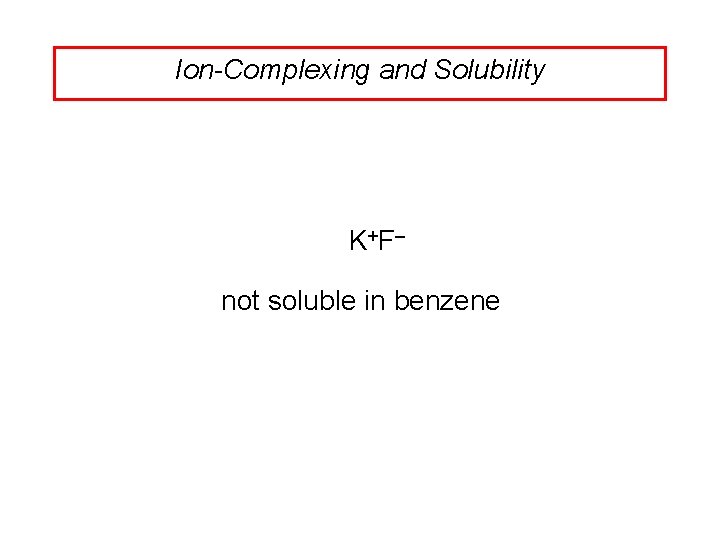
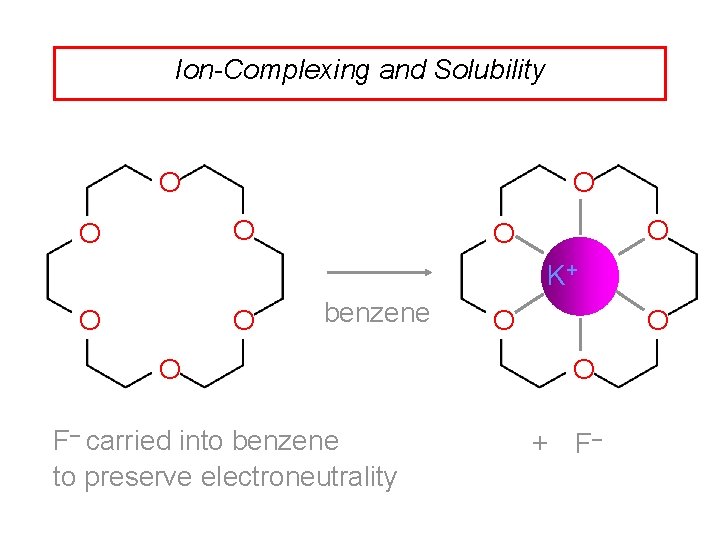
Ion-Complexing and Solubility K +F– not soluble in benzene

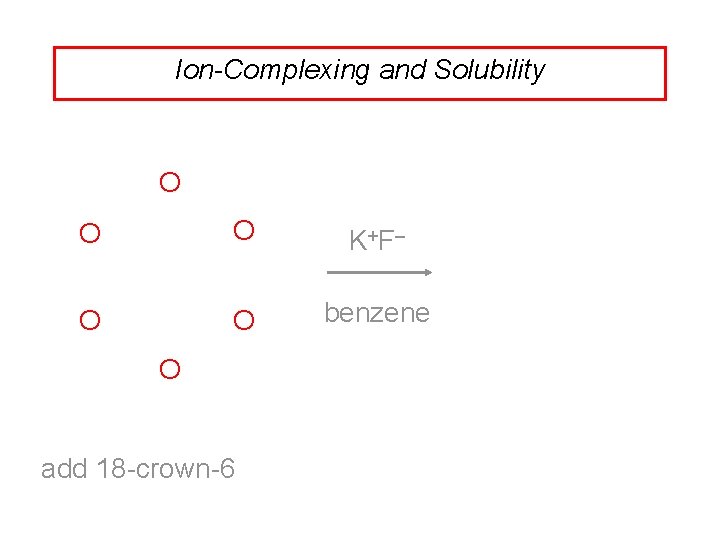
Ion-Complexing and Solubility O O O K +F– O O benzene O add 18 -crown-6

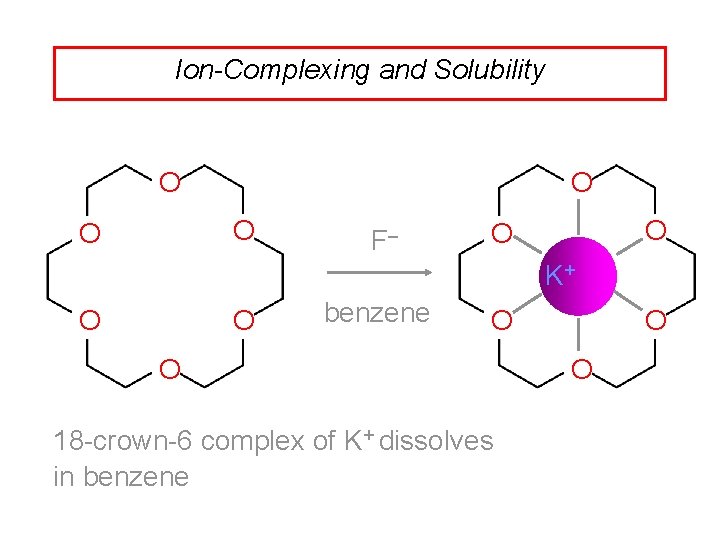
Ion-Complexing and Solubility O O F– O O K+ O O benzene O O 18 -crown-6 complex of K+ dissolves in benzene O O

Ion-Complexing and Solubility O O O K+ O O benzene O F– carried into benzene to preserve electroneutrality O O O + F–

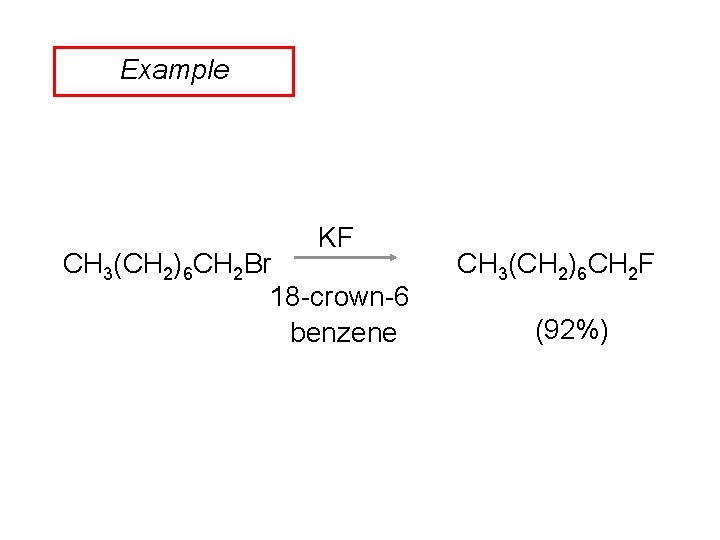
Application to organic synthesis Complexation of K+ by 18 -crown-6 solubilizes potassium salts in benzene. Anion of salt is in a relatively unsolvated state in benzene (sometimes referred to as a "naked anion"). Unsolvated anion is very reactive. Only catalytic quantities of 18 -crown-6 are needed.

Example KF CH 3(CH 2)6 CH 2 Br 18 -crown-6 benzene CH 3(CH 2)6 CH 2 F (92%)

Preparation of Ethers

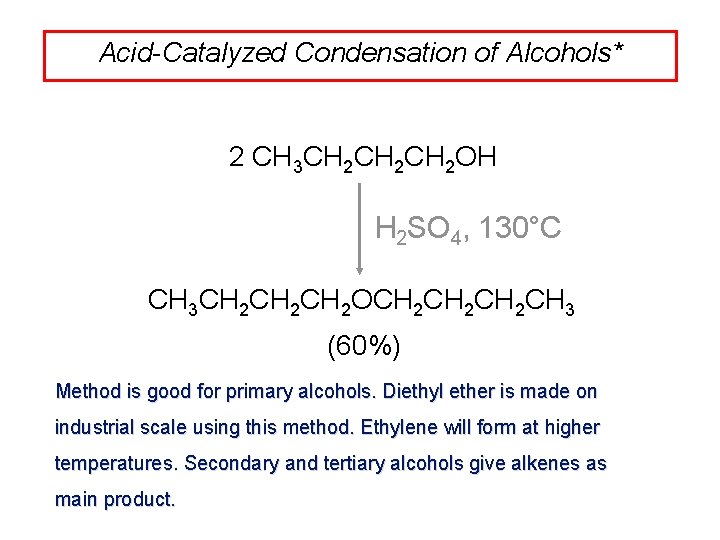
Acid-Catalyzed Condensation of Alcohols* 2 CH 3 CH 2 CH 2 OH H 2 SO 4, 130°C CH 3 CH 2 CH 2 OCH 2 CH 2 CH 3 (60%) Method is good for primary alcohols. Diethyl ether is made on industrial scale using this method. Ethylene will form at higher temperatures. Secondary and tertiary alcohols give alkenes as main product.

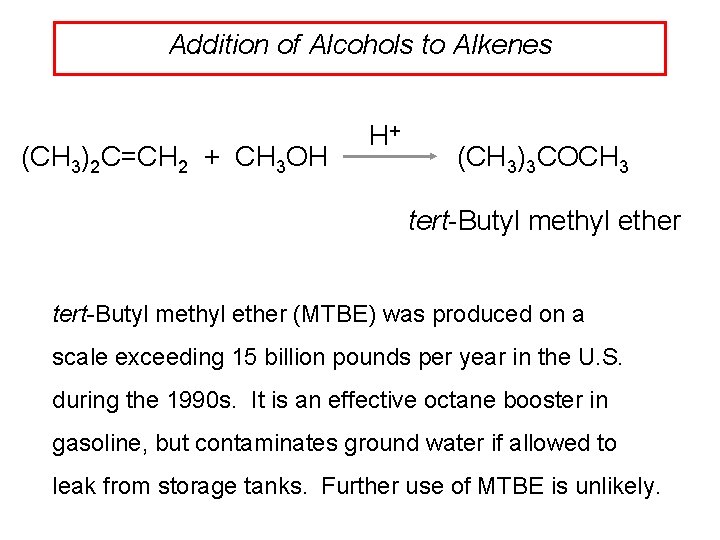
Addition of Alcohols to Alkenes (CH 3)2 C=CH 2 + CH 3 OH H+ (CH 3)3 COCH 3 tert-Butyl methyl ether (MTBE) was produced on a scale exceeding 15 billion pounds per year in the U. S. during the 1990 s. It is an effective octane booster in gasoline, but contaminates ground water if allowed to leak from storage tanks. Further use of MTBE is unlikely.

The Williamson Ether Synthesis
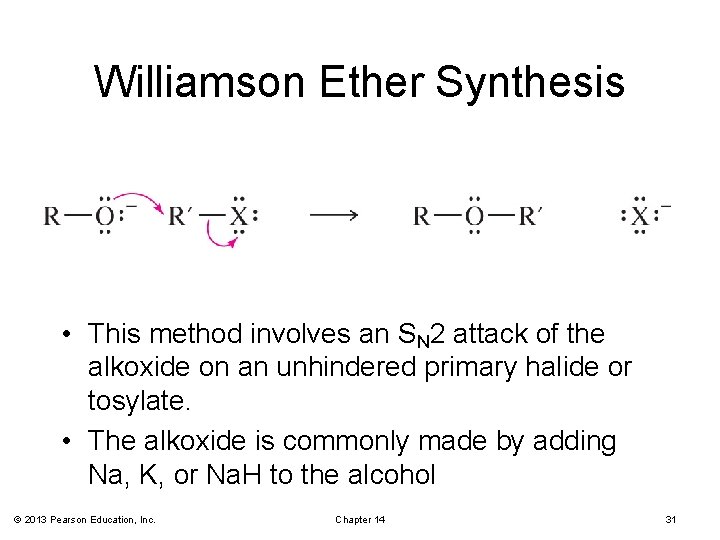
Williamson Ether Synthesis • This method involves an SN 2 attack of the alkoxide on an unhindered primary halide or tosylate. • The alkoxide is commonly made by adding Na, K, or Na. H to the alcohol © 2013 Pearson Education, Inc. Chapter 14 31

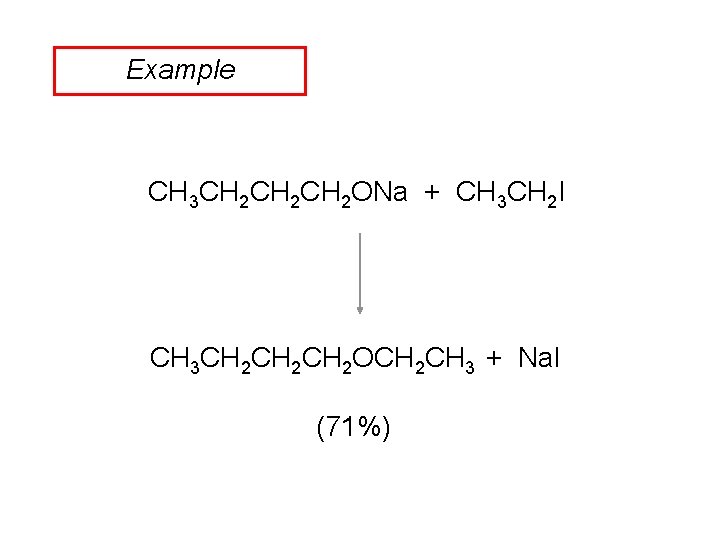
Example CH 3 CH 2 CH 2 ONa + CH 3 CH 2 I CH 3 CH 2 CH 2 OCH 2 CH 3 + Na. I (71%)

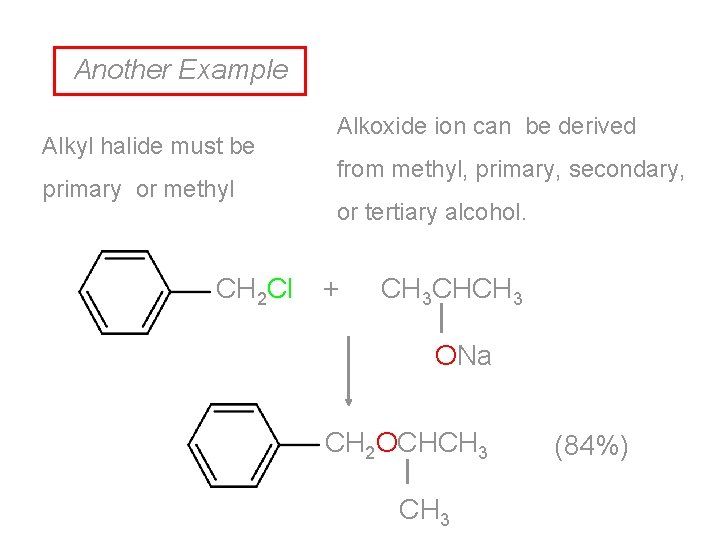
Another Example Alkyl halide must be primary or methyl CH 2 Cl Alkoxide ion can be derived from methyl, primary, secondary, or tertiary alcohol. + CH 3 CHCH 3 ONa CH 2 OCHCH 3 (84%)

Origin of Reactants CH 3 CHCH 3 CH 2 OH OH HCl CH 2 Cl Na + CH 3 CHCH 3 ONa CH 2 OCHCH 3 (84%)

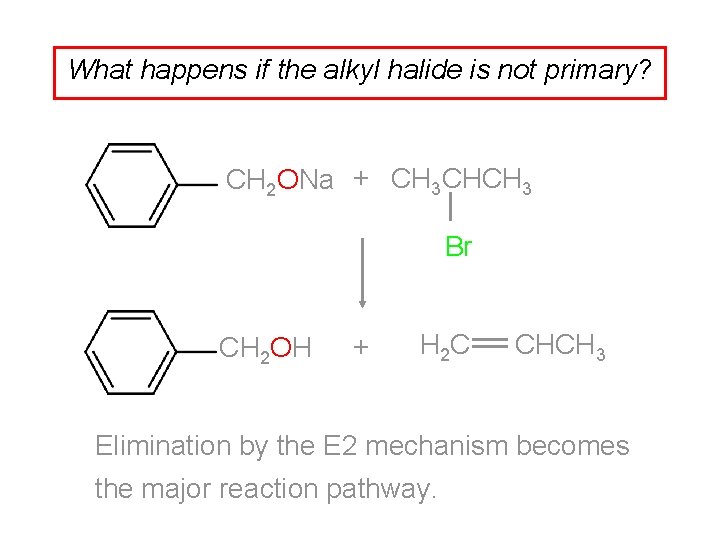
What happens if the alkyl halide is not primary? CH 2 ONa + CH 3 CHCH 3 Br CH 2 OH + H 2 C CHCH 3 Elimination by the E 2 mechanism becomes the major reaction pathway.

Reactions of Ethers:

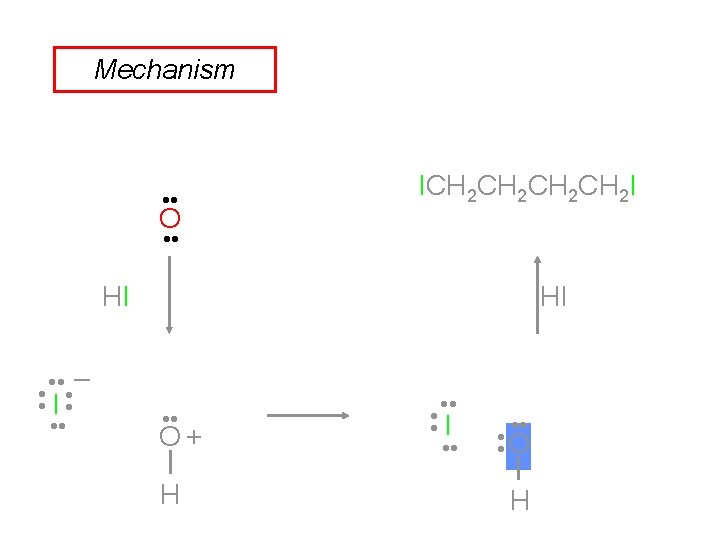
Acid-Catalyzed Cleavage of Ethers can be cleaved by heating with concentrated HBr and HI. Reactivity: HI > HBr

Example CH 3 CHCH 2 CH 3 HBr OCH 3 heat CH 3 CHCH 2 CH 3 Br (81%) + CH 3 Br

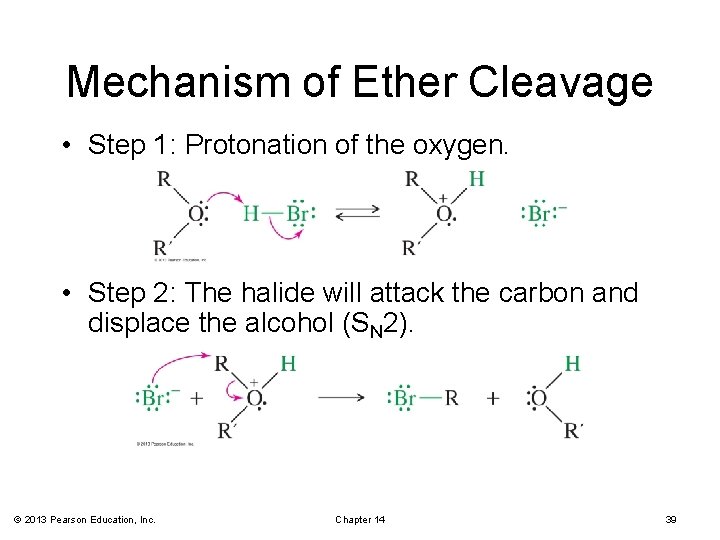
Mechanism of Ether Cleavage • Step 1: Protonation of the oxygen. • Step 2: The halide will attack the carbon and displace the alcohol (SN 2). © 2013 Pearson Education, Inc. Chapter 14 39

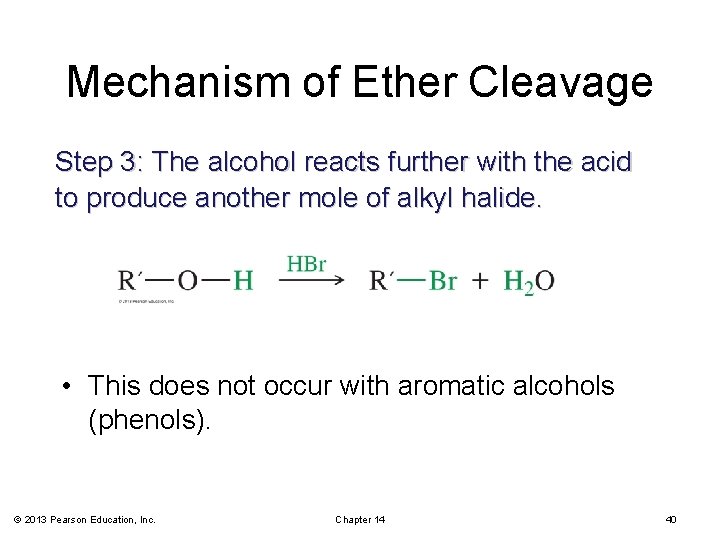
Mechanism of Ether Cleavage Step 3: The alcohol reacts further with the acid to produce another mole of alkyl halide. • This does not occur with aromatic alcohols (phenols). © 2013 Pearson Education, Inc. Chapter 14 40

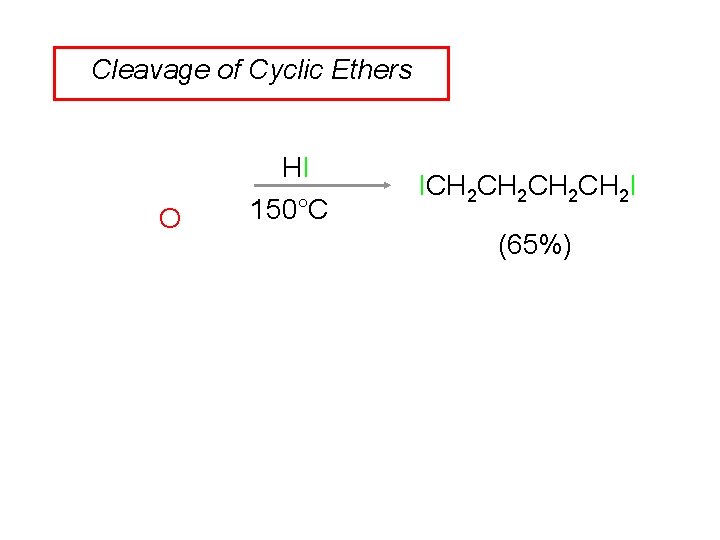
Cleavage of Cyclic Ethers O HI 150°C ICH 2 CH 2 I (65%)


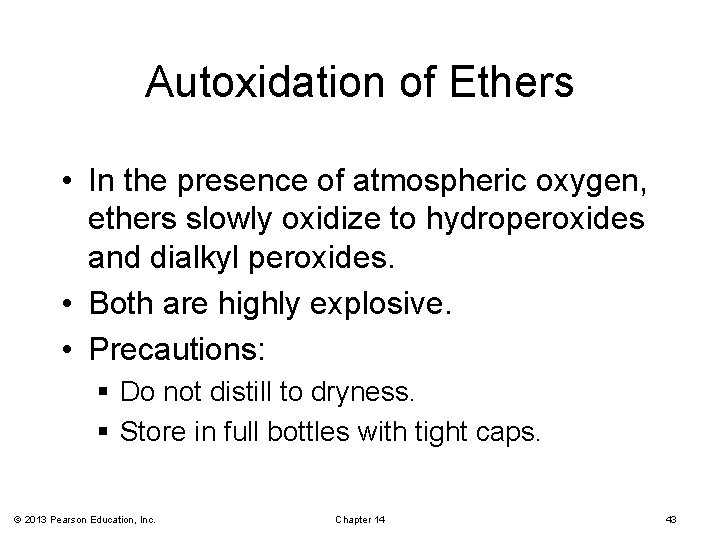
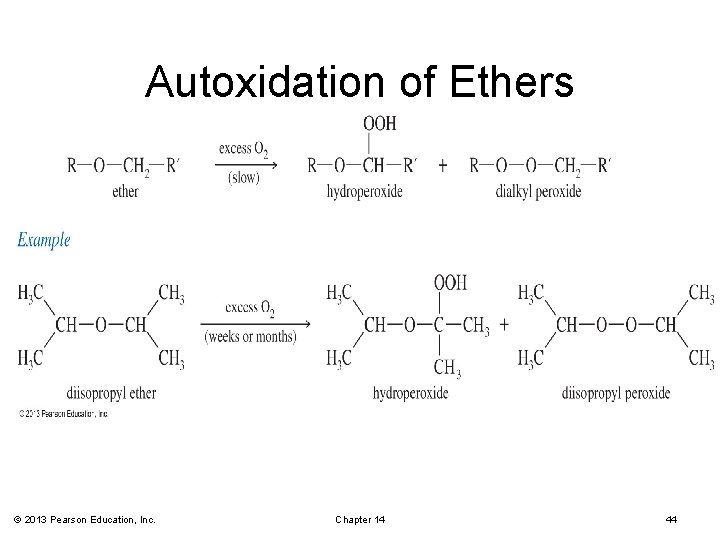
Autoxidation of Ethers • In the presence of atmospheric oxygen, ethers slowly oxidize to hydroperoxides and dialkyl peroxides. • Both are highly explosive. • Precautions: § Do not distill to dryness. § Store in full bottles with tight caps. © 2013 Pearson Education, Inc. Chapter 14 43

Autoxidation of Ethers © 2013 Pearson Education, Inc. Chapter 14 44

Preparation of Epoxides: A Review and a Preview © 2013 Pearson Education, Inc. Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 14 45

Preparation of Epoxides are prepared by two major methods. Both begin with alkenes. Reaction of alkenes with peroxy acids (Section 6. 19) Conversion of alkenes to vicinal halohydrins, followed by treatment with base (Section 16. 10, this chapter) © 2013 Pearson Education, Inc. Chapter 14 46

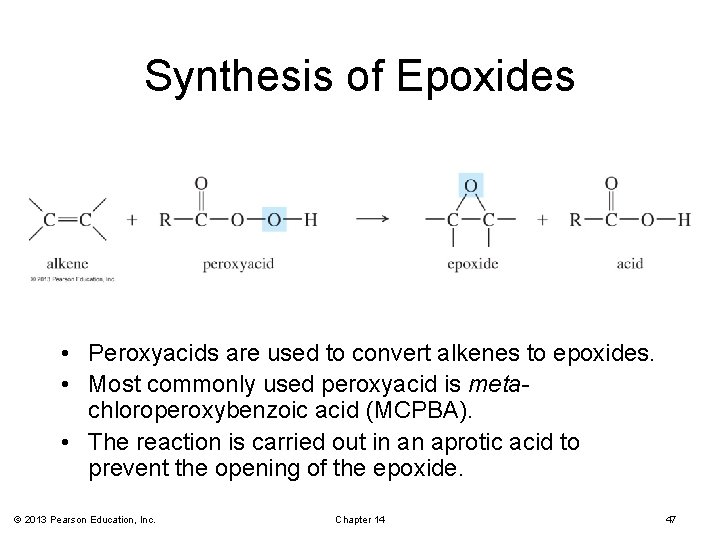
Synthesis of Epoxides • Peroxyacids are used to convert alkenes to epoxides. • Most commonly used peroxyacid is metachloroperoxybenzoic acid (MCPBA). • The reaction is carried out in an aprotic acid to prevent the opening of the epoxide. © 2013 Pearson Education, Inc. Chapter 14 47

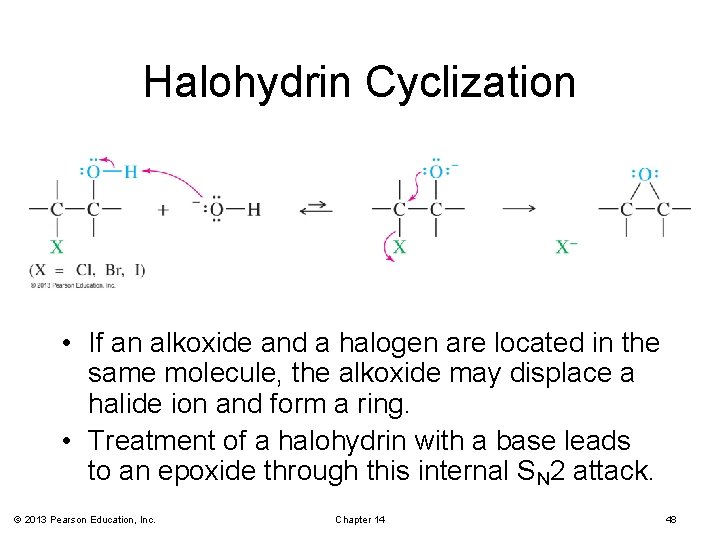
Halohydrin Cyclization • If an alkoxide and a halogen are located in the same molecule, the alkoxide may displace a halide ion and form a ring. • Treatment of a halohydrin with a base leads to an epoxide through this internal SN 2 attack. © 2013 Pearson Education, Inc. Chapter 14 48

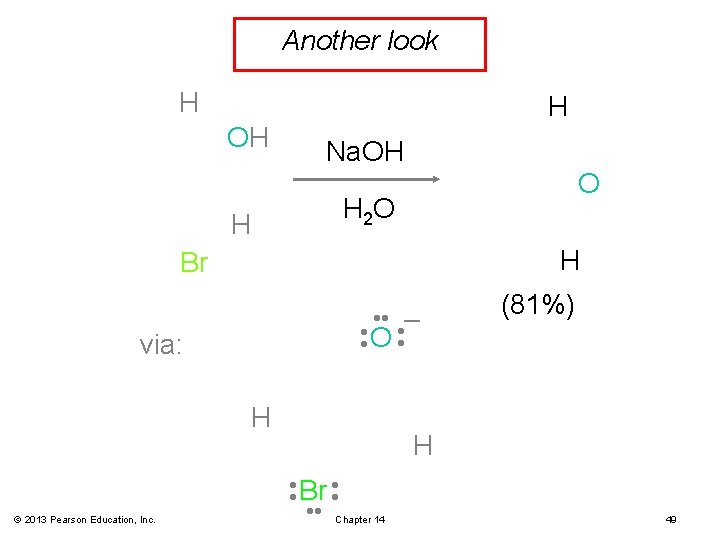
Another look H H OH Na. OH O H 2 O H H Br • • O • • – via: H © 2013 Pearson Education, Inc. (81%) H • • Br • • Chapter 14 49

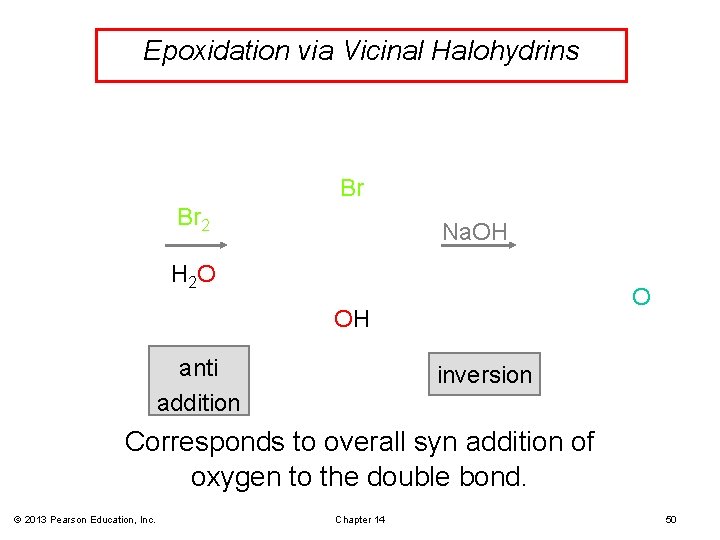
Epoxidation via Vicinal Halohydrins Br Br 2 Na. OH H 2 O O OH anti addition inversion Corresponds to overall syn addition of oxygen to the double bond. © 2013 Pearson Education, Inc. Chapter 14 50

Reactions of Epoxides: A Review and a Preview

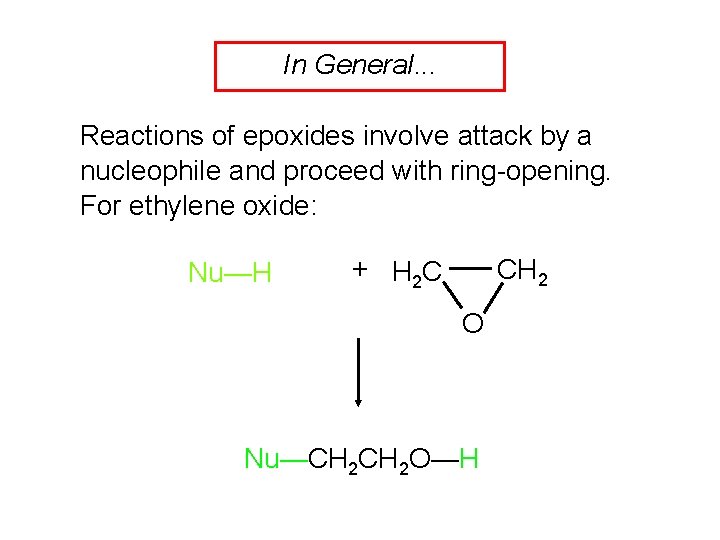
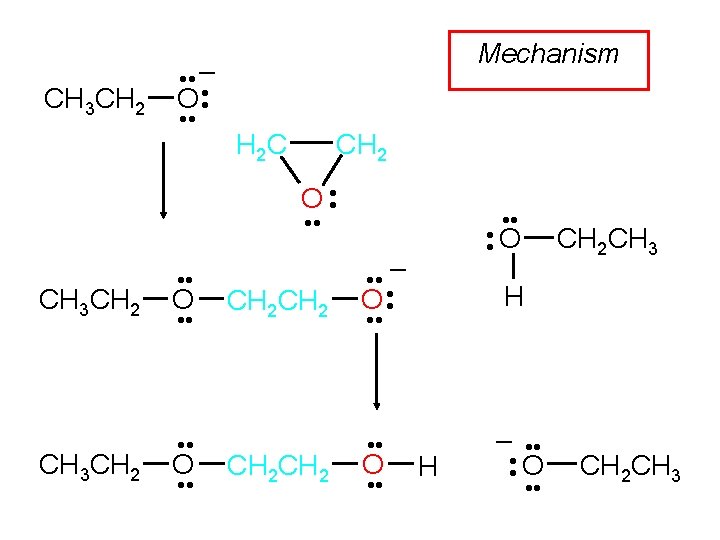
In General. . . Reactions of epoxides involve attack by a nucleophile and proceed with ring-opening. For ethylene oxide: Nu—H + H 2 C CH 2 O Nu—CH 2 O—H

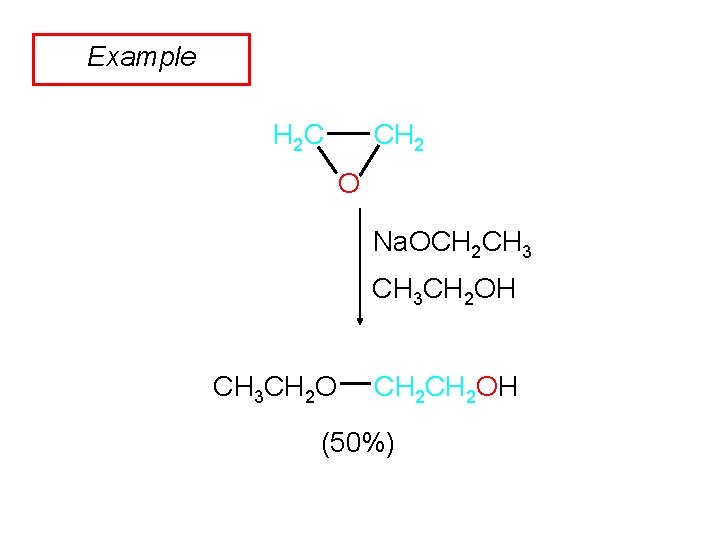
Example CH 2 H 2 C O Na. OCH 2 CH 3 CH 2 OH CH 3 CH 2 OH (50%)


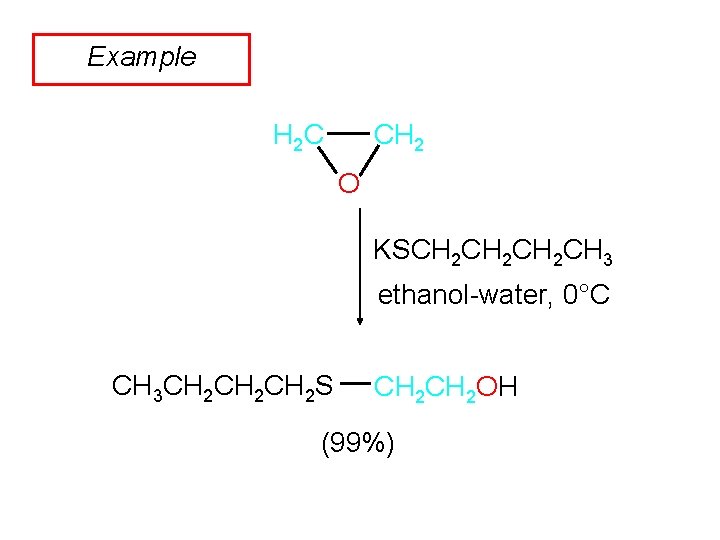
Example CH 2 H 2 C O KSCH 2 CH 2 CH 3 ethanol-water, 0°C CH 3 CH 2 CH 2 S CH 2 OH (99%)

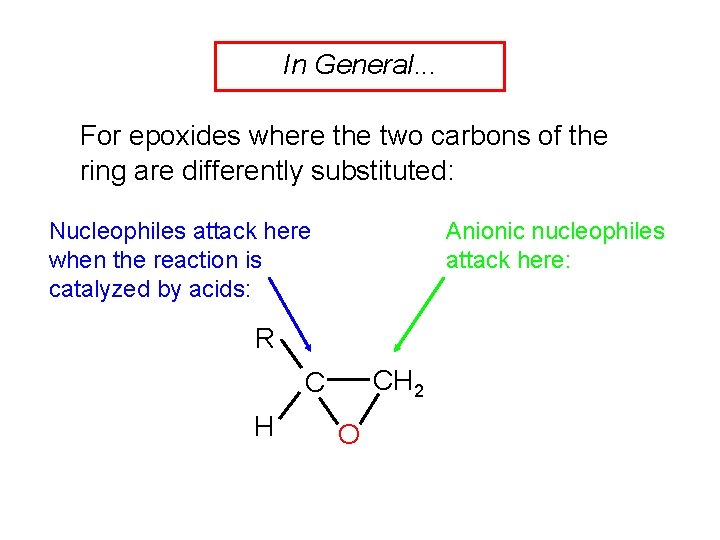
In General. . . For epoxides where the two carbons of the ring are differently substituted: Nucleophiles attack here when the reaction is catalyzed by acids: Anionic nucleophiles attack here: R CH 2 C H O

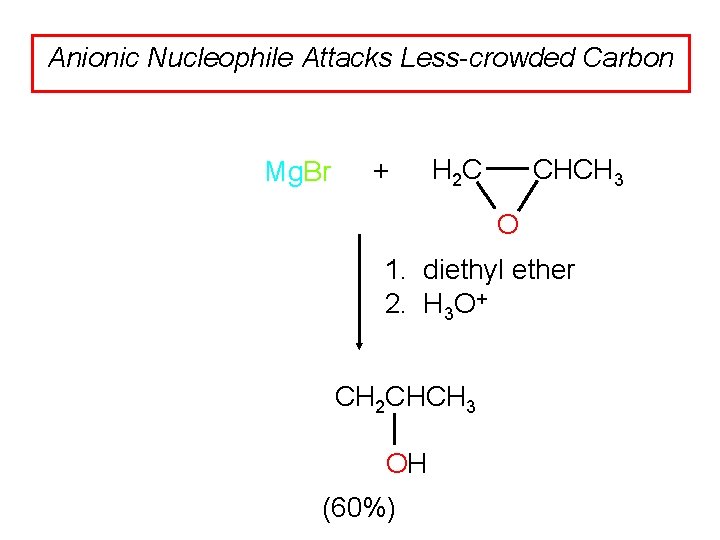
Anionic Nucleophile Attacks Less-crowded Carbon Mg. Br + CHCH 3 H 2 C O 1. diethyl ether 2. H 3 O+ CH 2 CHCH 3 OH (60%)

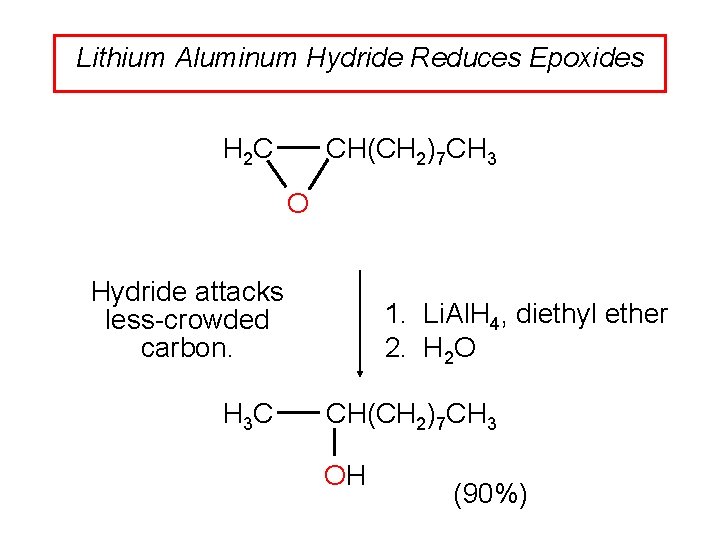
Lithium Aluminum Hydride Reduces Epoxides CH(CH 2)7 CH 3 H 2 C O Hydride attacks less-crowded carbon. H 3 C 1. Li. Al. H 4, diethyl ether 2. H 2 O CH(CH 2)7 CH 3 OH (90%)

Acid-Catalyzed Ring-Opening Reactions of Epoxides

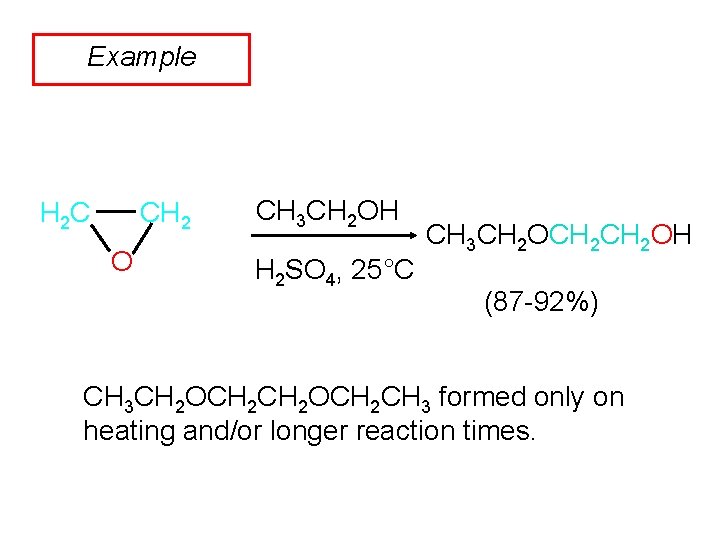
Example CH 2 H 2 C O CH 3 CH 2 OH H 2 SO 4, 25°C CH 3 CH 2 OCH 2 OH (87 -92%) CH 3 CH 2 OCH 2 CH 3 formed only on heating and/or longer reaction times.

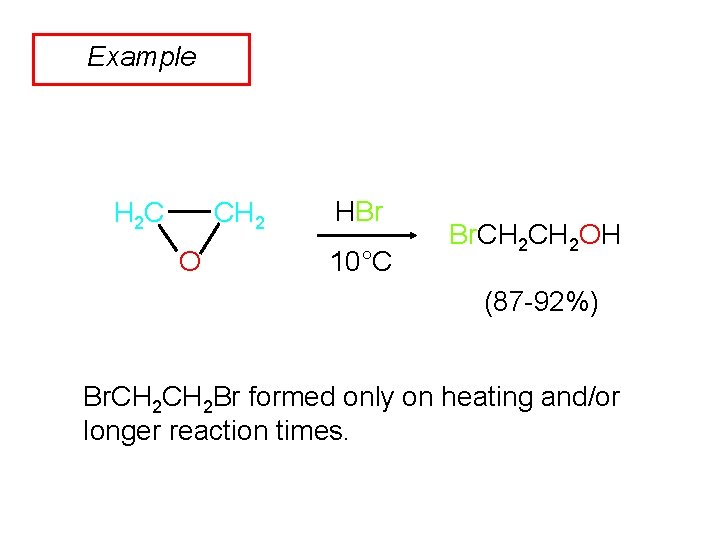
Example CH 2 H 2 C O HBr 10°C Br. CH 2 OH (87 -92%) Br. CH 2 Br formed only on heating and/or longer reaction times.


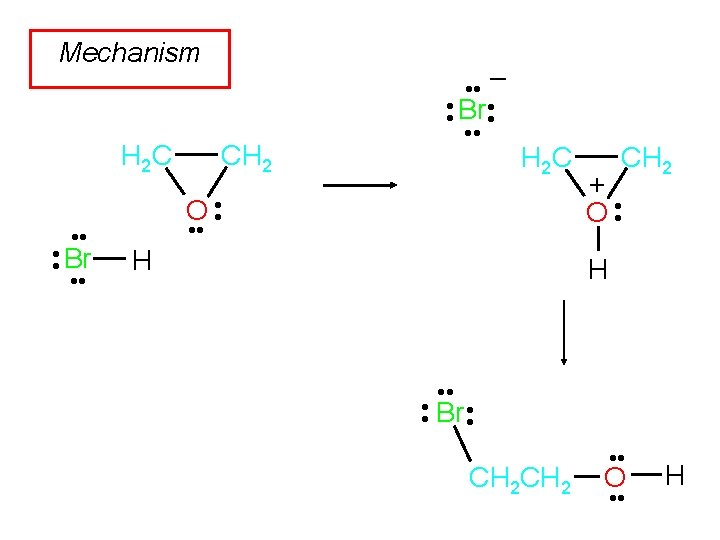
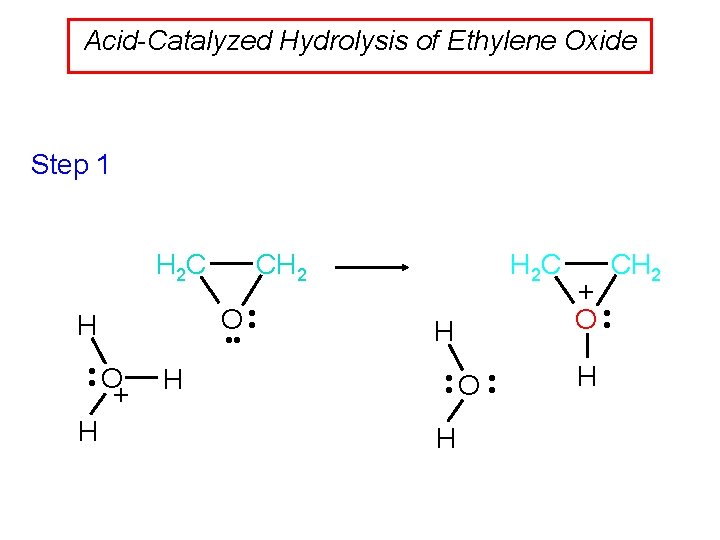
Acid-Catalyzed Hydrolysis of Ethylene Oxide Step 1 CH 2 H 2 C O • • H • • O + H H H 2 C H • • O • • H CH 2 + O • • H

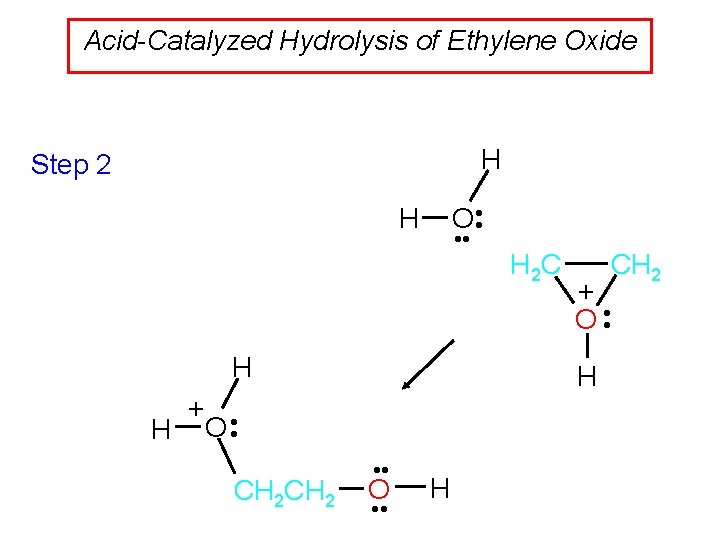
Acid-Catalyzed Hydrolysis of Ethylene Oxide H Step 2 O • • H CH 2 + O • • H + • H O • CH 2 H 2 C • • O • • H

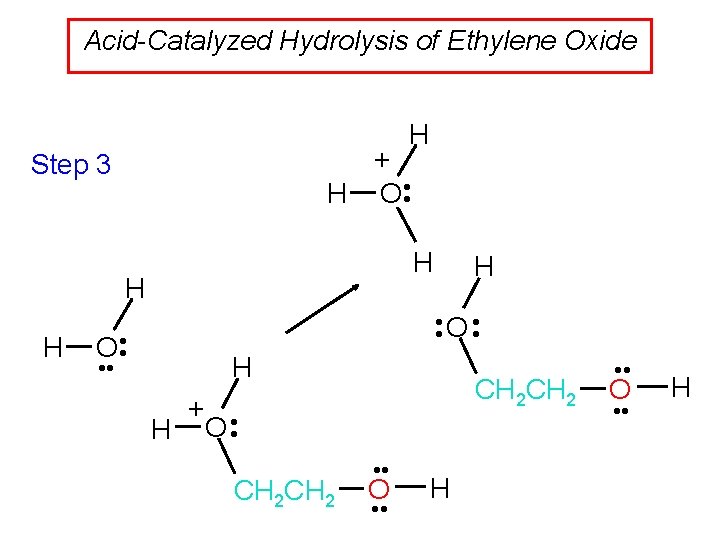
Acid-Catalyzed Hydrolysis of Ethylene Oxide H + H O • • Step 3 H H H O • • H • • O • • H CH 2 + • H O • CH 2 • • O • • H

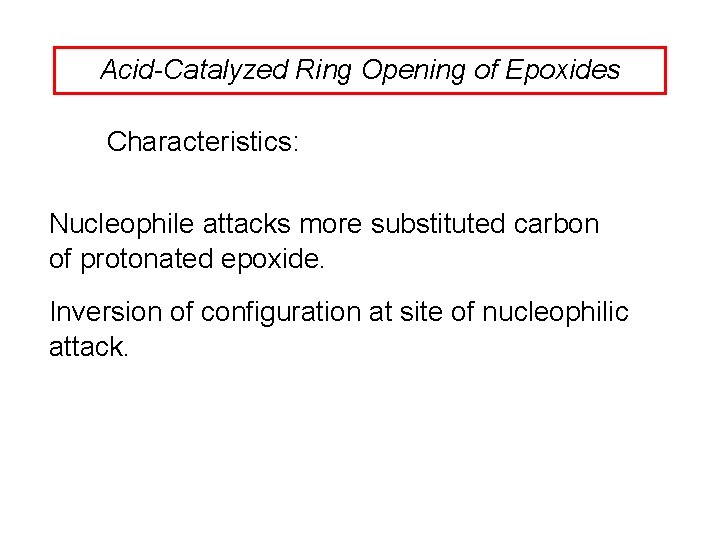
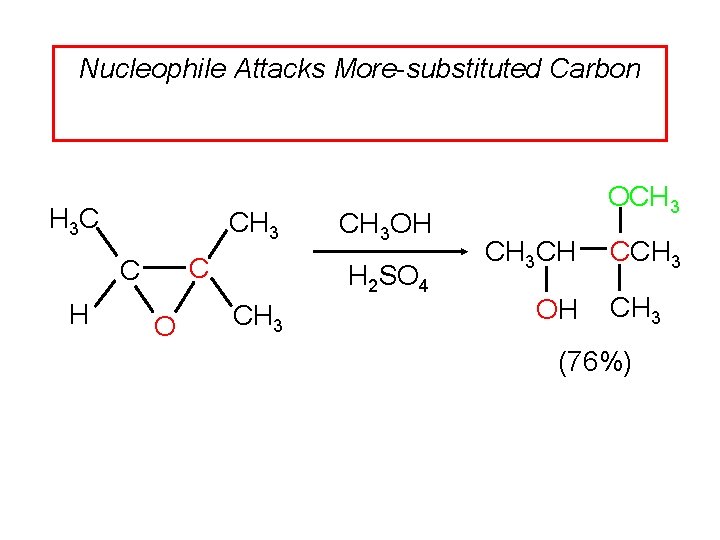
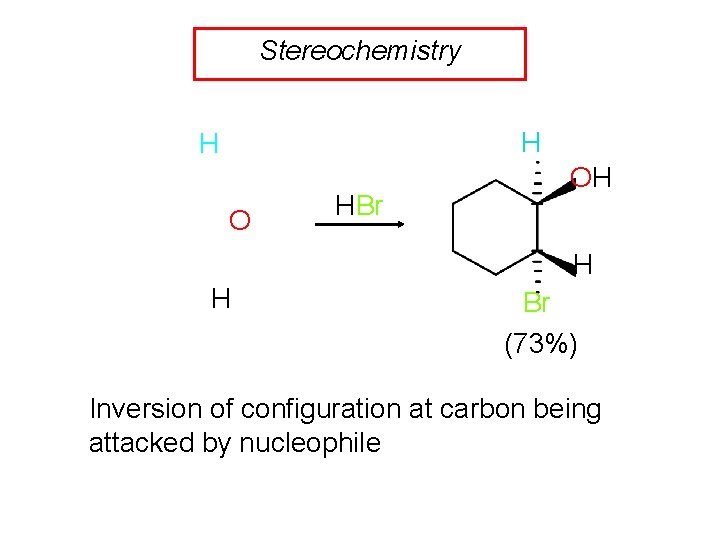
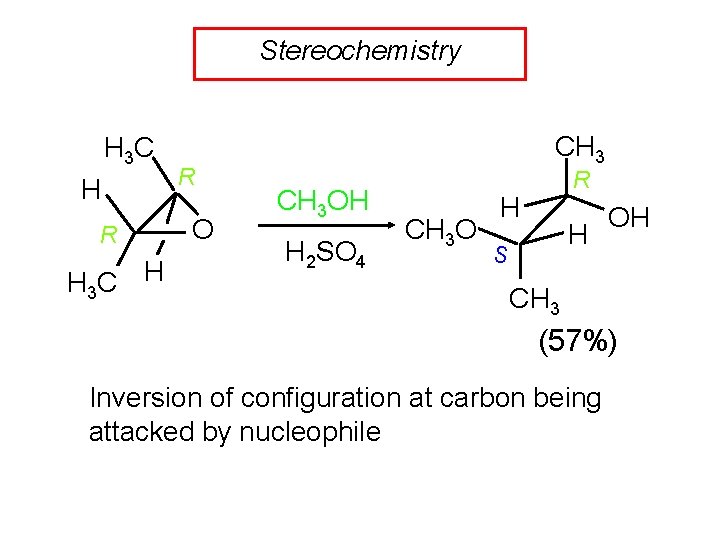
Acid-Catalyzed Ring Opening of Epoxides Characteristics: Nucleophile attacks more substituted carbon of protonated epoxide. Inversion of configuration at site of nucleophilic attack.

Nucleophile Attacks More-substituted Carbon H 3 C CH 3 C C H O CH 3 OH H 2 SO 4 CH 3 OCH 3 CH OH CCH 3 (76%)

Stereochemistry H H O HBr OH H H Br (73%) Inversion of configuration at carbon being attacked by nucleophile

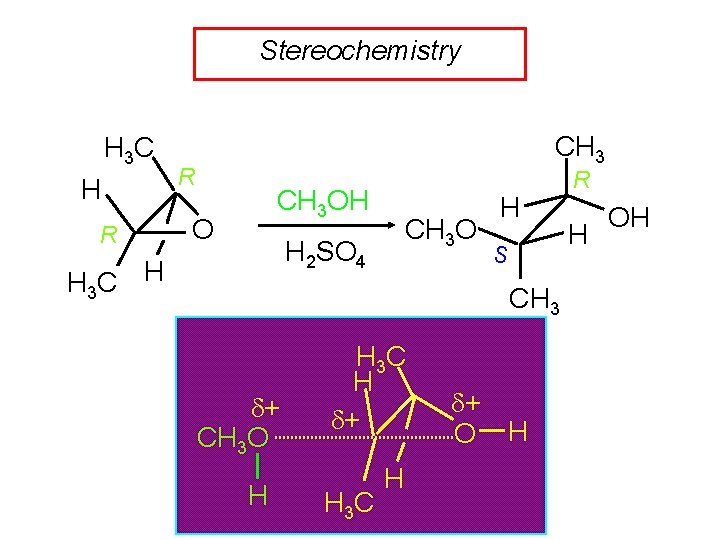
Stereochemistry H 3 C H R O CH 3 OH H 2 SO 4 CH 3 O R H H S OH CH 3 (57%) Inversion of configuration at carbon being attacked by nucleophile

Stereochemistry H 3 C H R CH 3 OH O CH 3 O H 2 SO 4 H 3 C H H S CH 3 + CH 3 O H H 3 C H + H 3 C H + O H R H OH

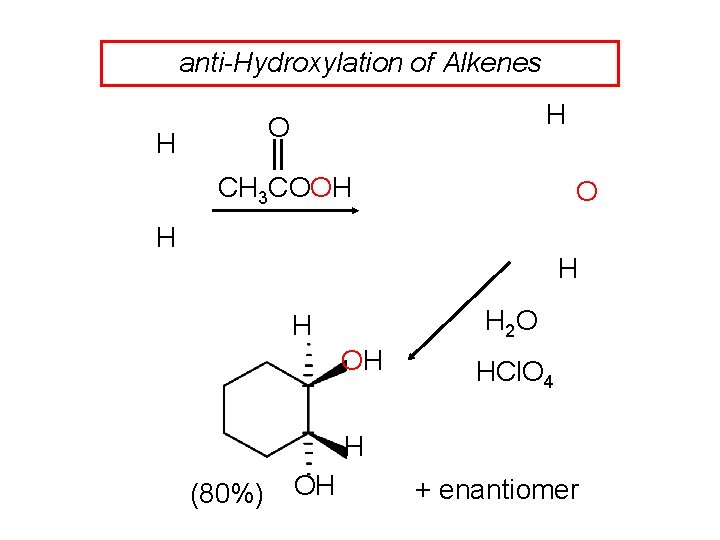
anti-Hydroxylation of Alkenes H O H CH 3 COOH O H H H 2 O H OH HCl. O 4 H (80%) OH + enantiomer