Chapter 23 Aldehydes and Ketones are common solvents





















- Slides: 76

Chapter 23 Aldehydes and Ketones are common solvents for quickdrying paints. Introduction to General, Organic, and Biochemistry, 10 e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena

Course Outline 23. 1 Structures of Aldehydes and Ketones 23. 2 Naming Aldehydes and Ketones 23. 3 Bonding and Physical Properties 23. 4 Chemical Properties of Aldehydes and Ketones 23. 5 Common Aldehydes and Ketones 23. 6 Condensation Polymers Chapter 23 Summary 2

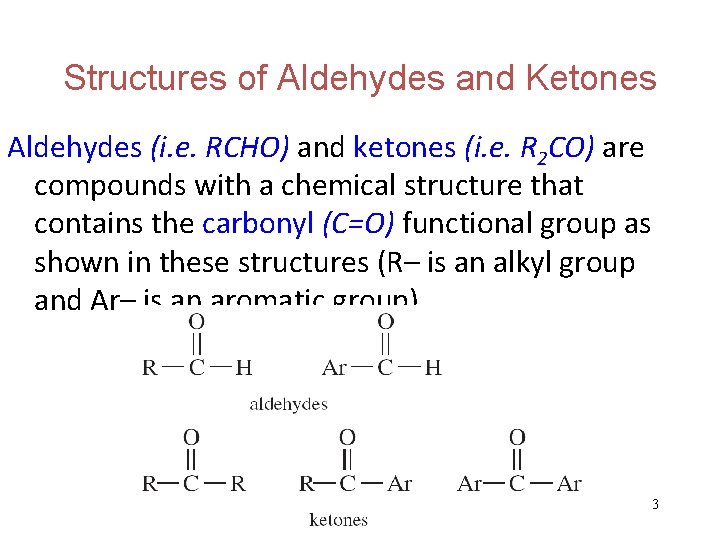
Structures of Aldehydes and Ketones Aldehydes (i. e. RCHO) and ketones (i. e. R 2 CO) are compounds with a chemical structure that contains the carbonyl (C=O) functional group as shown in these structures (R– is an alkyl group and Ar– is an aromatic group). 3

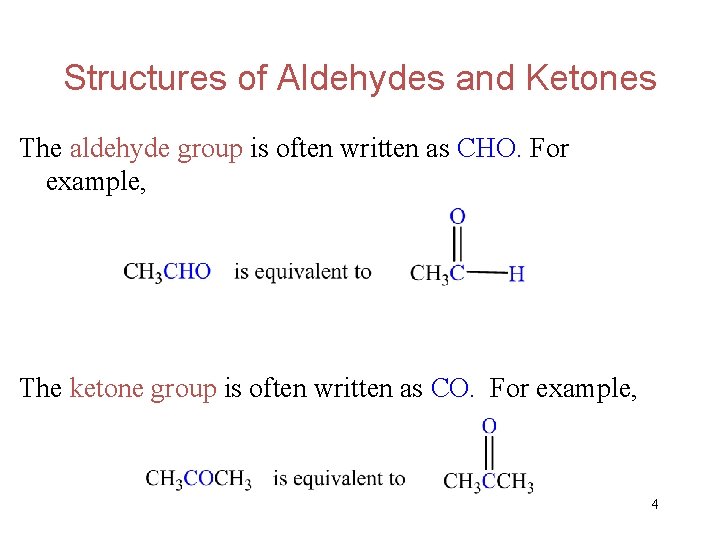
Structures of Aldehydes and Ketones The aldehyde group is often written as CHO. For example, The ketone group is often written as CO. For example, 4

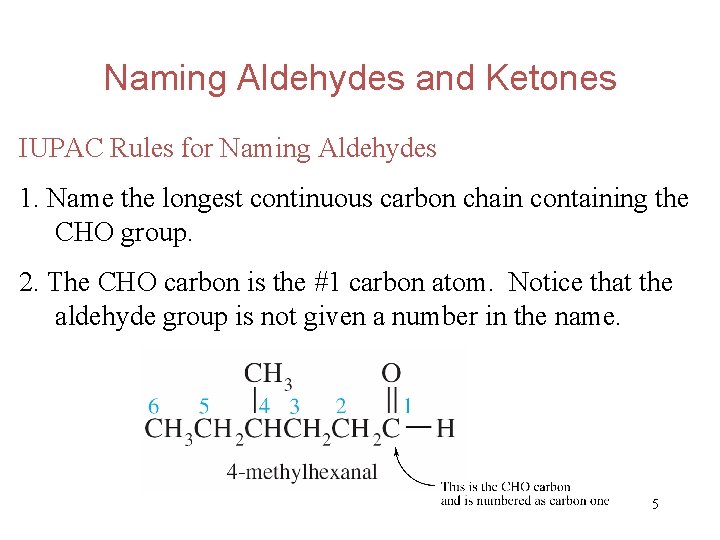
Naming Aldehydes and Ketones IUPAC Rules for Naming Aldehydes 1. Name the longest continuous carbon chain containing the CHO group. 2. The CHO carbon is the #1 carbon atom. Notice that the aldehyde group is not given a number in the name. 5

Naming Aldehydes and Ketones 3. Drop an –e from the corresponding alkane parent name and add the suffix –al. 4. Number and name groups attached to the longest carbon chain. 6

Naming Aldehydes and Ketones 7

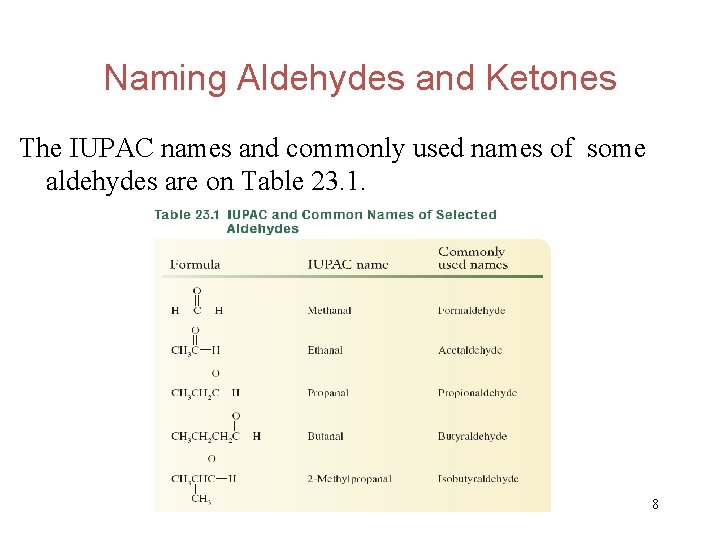
Naming Aldehydes and Ketones The IUPAC names and commonly used names of some aldehydes are on Table 23. 1. 8

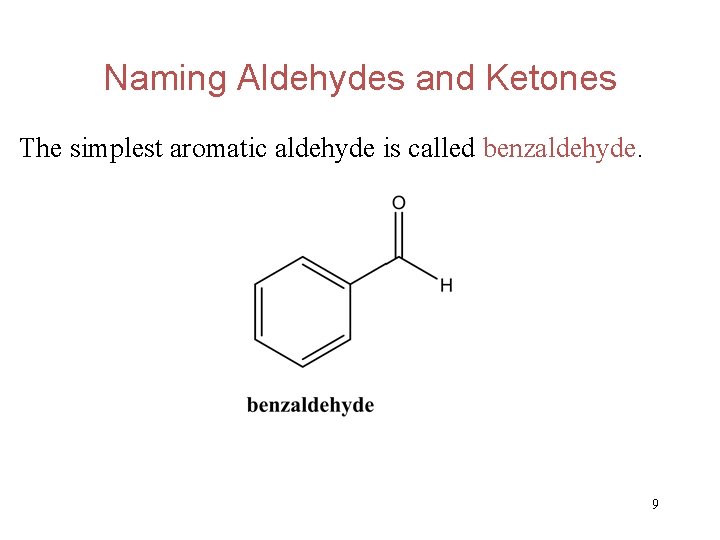
Naming Aldehydes and Ketones The simplest aromatic aldehyde is called benzaldehyde. 9

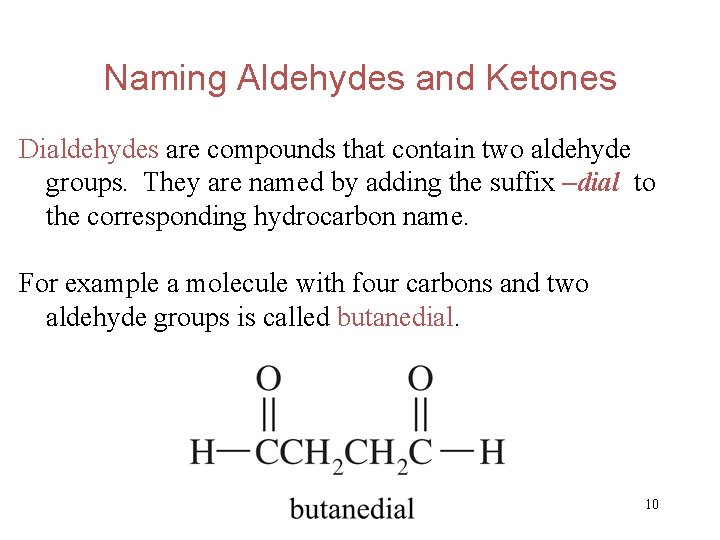
Naming Aldehydes and Ketones Dialdehydes are compounds that contain two aldehyde groups. They are named by adding the suffix –dial to the corresponding hydrocarbon name. For example a molecule with four carbons and two aldehyde groups is called butanedial. 10

Naming Aldehydes and Ketones IUPAC Rules for Naming Ketones 1. Name the longest continuous carbon chain containing the C=O group. 2. Drop the –e from the corresponding alkane parent name and add the suffix –one. 11

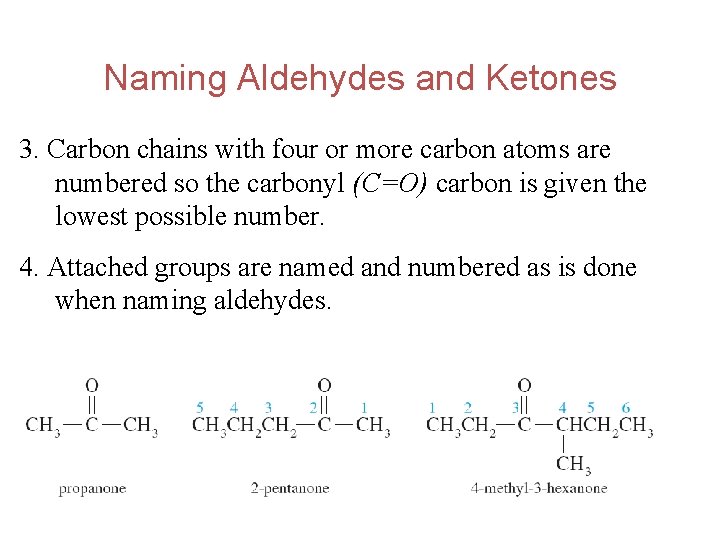
Naming Aldehydes and Ketones 3. Carbon chains with four or more carbon atoms are numbered so the carbonyl (C=O) carbon is given the lowest possible number. 4. Attached groups are named and numbered as is done when naming aldehydes. 12

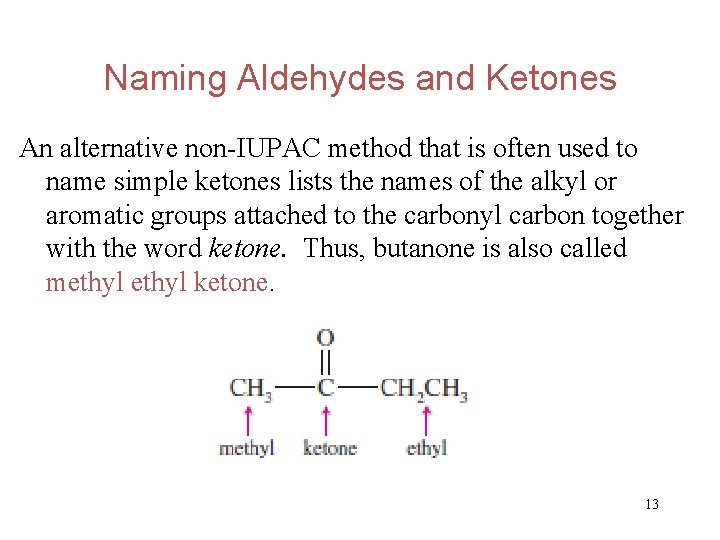
Naming Aldehydes and Ketones An alternative non-IUPAC method that is often used to name simple ketones lists the names of the alkyl or aromatic groups attached to the carbonyl carbon together with the word ketone. Thus, butanone is also called methyl ketone. 13

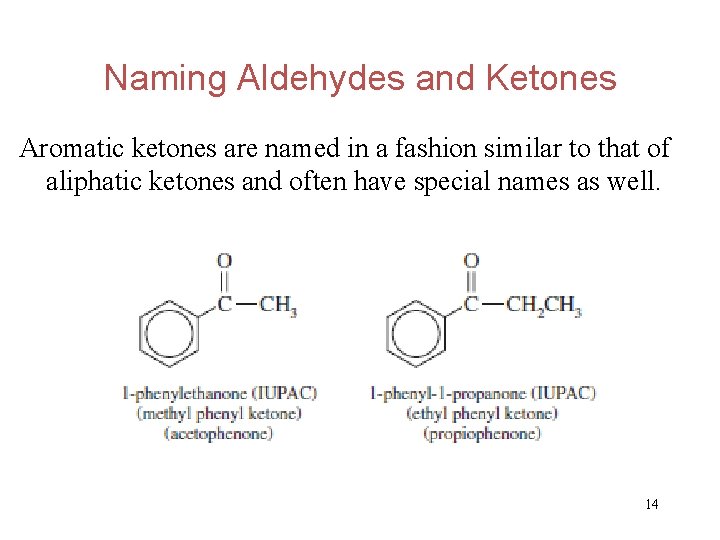
Naming Aldehydes and Ketones Aromatic ketones are named in a fashion similar to that of aliphatic ketones and often have special names as well. 14

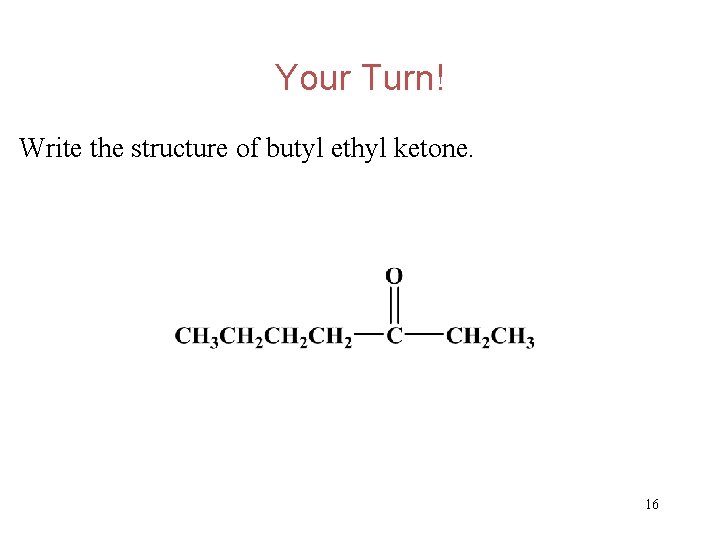
Your Turn! Write the structure of butyl ethyl ketone. 15

Your Turn! Write the structure of butyl ethyl ketone. 16

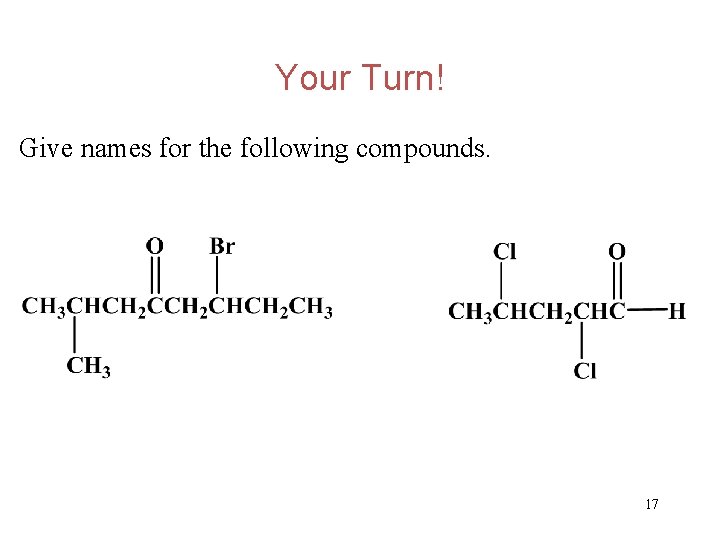
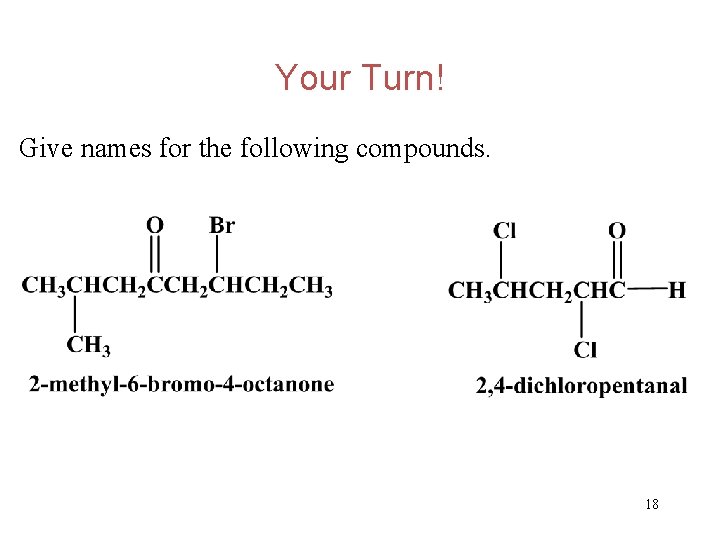
Your Turn! Give names for the following compounds. 17

Your Turn! Give names for the following compounds. 18

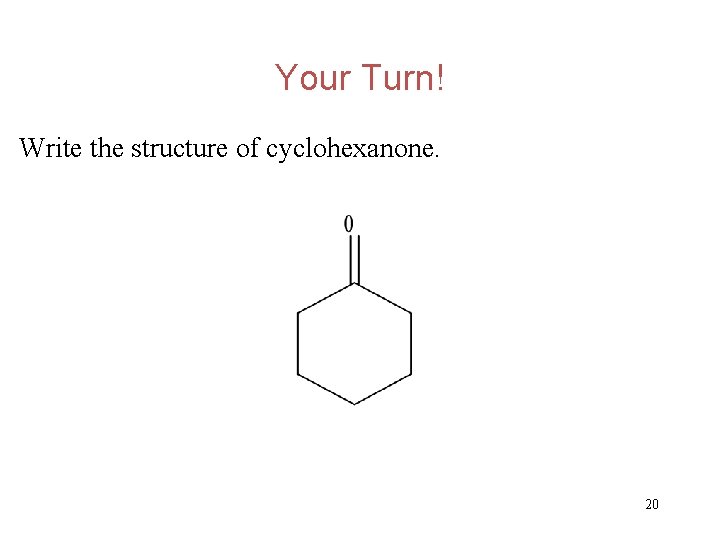
Your Turn! Write the structure of cyclohexanone. 19

Your Turn! Write the structure of cyclohexanone. 20

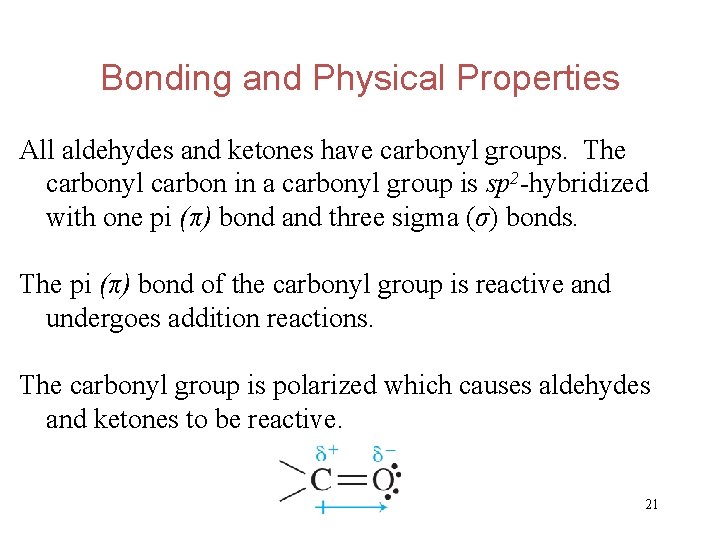
Bonding and Physical Properties All aldehydes and ketones have carbonyl groups. The carbonyl carbon in a carbonyl group is sp 2 -hybridized with one pi (π) bond and three sigma (σ) bonds. The pi (π) bond of the carbonyl group is reactive and undergoes addition reactions. The carbonyl group is polarized which causes aldehydes and ketones to be reactive. 21

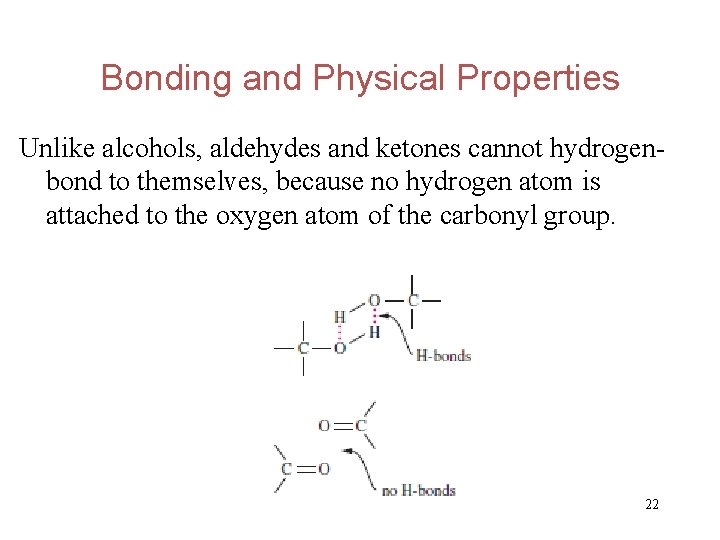
Bonding and Physical Properties Unlike alcohols, aldehydes and ketones cannot hydrogenbond to themselves, because no hydrogen atom is attached to the oxygen atom of the carbonyl group. 22

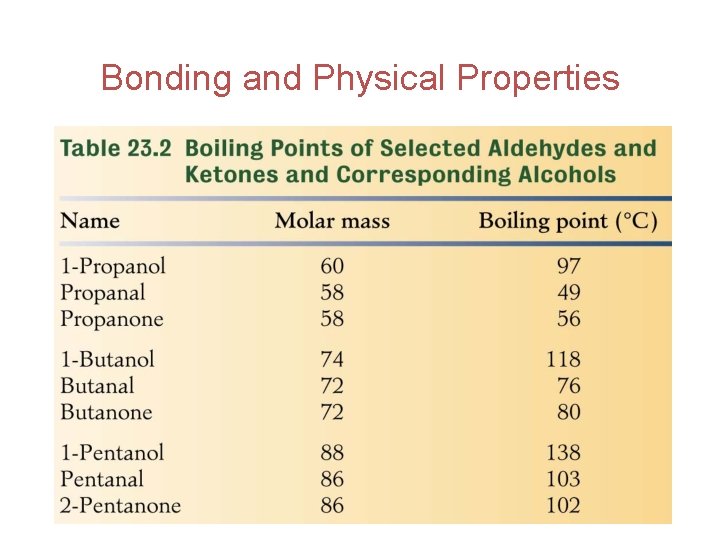
Bonding and Physical Properties Therefore aldehydes and ketones have lower boiling points than alcohols of comparable molar mass as seen on Table 23. 2 on the next slide. . . 23

Bonding and Physical Properties 24

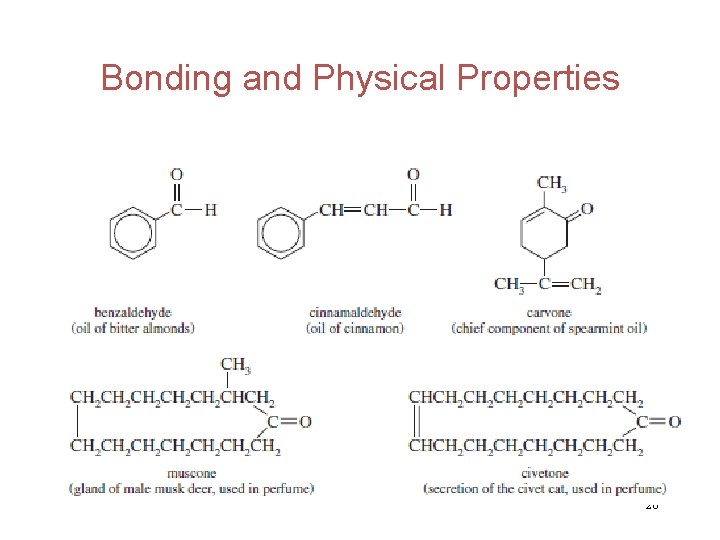
Bonding and Physical Properties Low-molar-mass aldehydes and ketones are soluble in water. The lower-molar-mass aldehydes have a penetrating, disagreeable odor. As the molar mass increases, the odor of both aldehydes and ketones—especially the aromatic ones—becomes more fragrant. Some aldehydes and ketons are used in flavorings and perfumes. . . 25

Bonding and Physical Properties 26

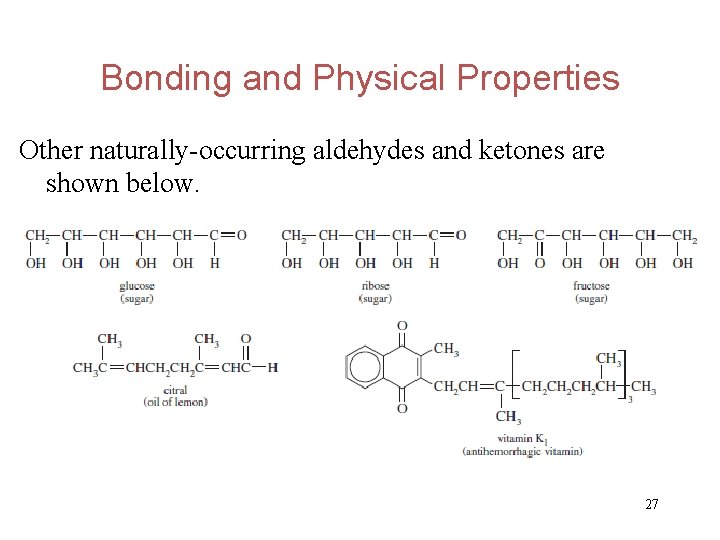
Bonding and Physical Properties Other naturally-occurring aldehydes and ketones are shown below. 27

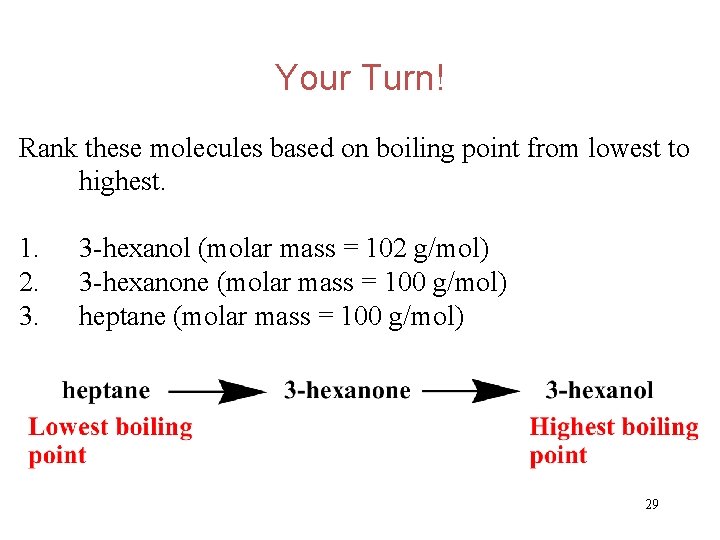
Your Turn! Rank these molecules based on boiling point from lowest to highest. 1. 2. 3. 3 -hexanol (molar mass = 102 g/mol) 3 -hexanone (molar mass = 100 g/mol) heptane (molar mass = 100 g/mol) 28

Your Turn! Rank these molecules based on boiling point from lowest to highest. 1. 2. 3. 3 -hexanol (molar mass = 102 g/mol) 3 -hexanone (molar mass = 100 g/mol) heptane (molar mass = 100 g/mol) 29

Chemical Properties of Aldehydes and Ketones The carbonyl functional group is the reactive site for aldehydes and ketones. These compounds undergo three broad classes of reactions. Reaction Type Aldehydes or Ketones Oxidation Aldehydes Only Reduction Both Addition Both 30

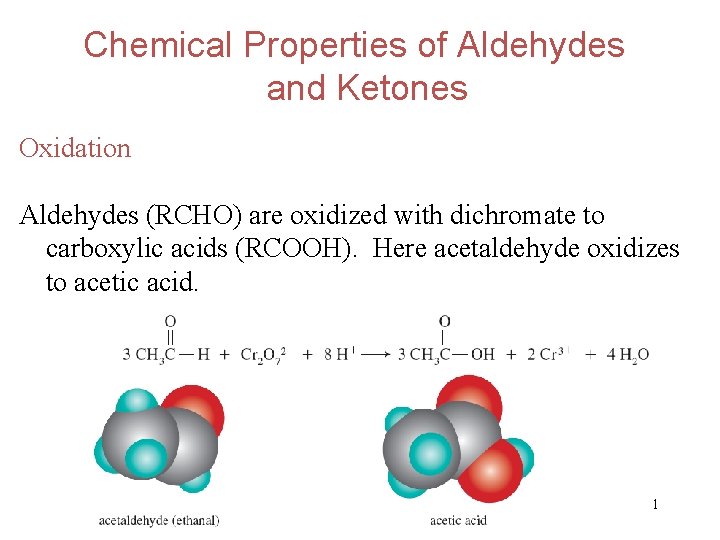
Chemical Properties of Aldehydes and Ketones Oxidation Aldehydes (RCHO) are oxidized with dichromate to carboxylic acids (RCOOH). Here acetaldehyde oxidizes to acetic acid. 31

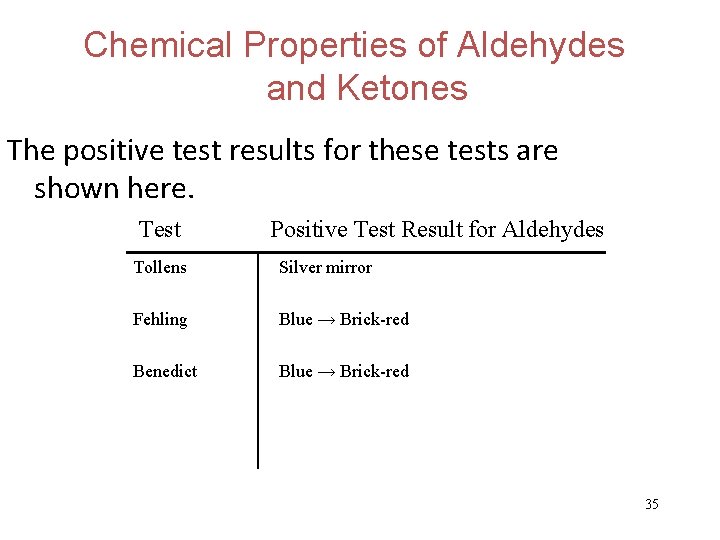
Chemical Properties of Aldehydes and Ketones Three well-known identification tests for aldehydes are based on the fact that aldehydes are much easier to oxidize than ketones. These tests are Tollens, Fehling, and Benedict tests. . . 32

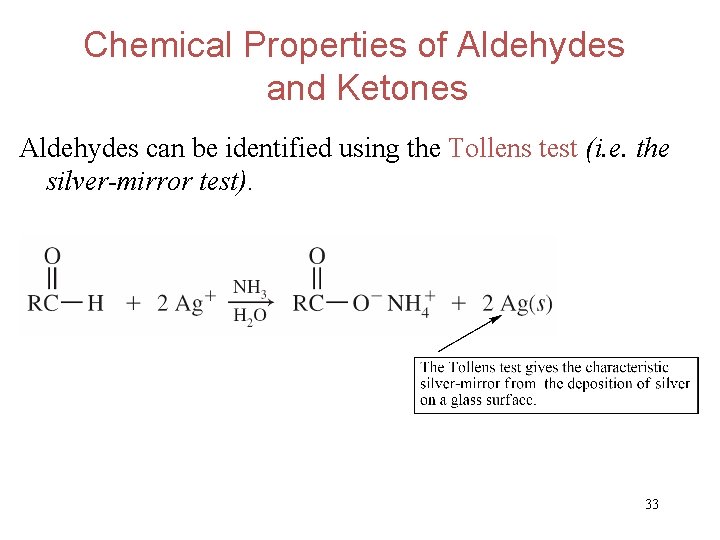
Chemical Properties of Aldehydes and Ketones Aldehydes can be identified using the Tollens test (i. e. the silver-mirror test). 33

Chemical Properties of Aldehydes and Ketones The aldehyde group (RCHO) is oxidized to a carboxylic acid by Cu 2+ ions in both the Fehling and Benedict tests. The tests are very similar except the Fehling test uses tartaric acid to complex Cu 2+ while the Benedict test uses citric acid. 34

Chemical Properties of Aldehydes and Ketones The positive test results for these tests are shown here. Test Positive Test Result for Aldehydes Tollens Silver mirror Fehling Blue → Brick-red Benedict Blue → Brick-red 35

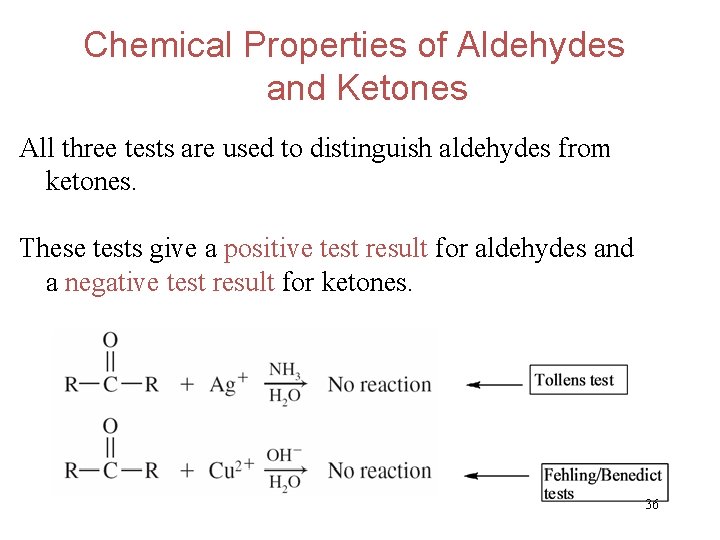
Chemical Properties of Aldehydes and Ketones All three tests are used to distinguish aldehydes from ketones. These tests give a positive test result for aldehydes and a negative test result for ketones. 36

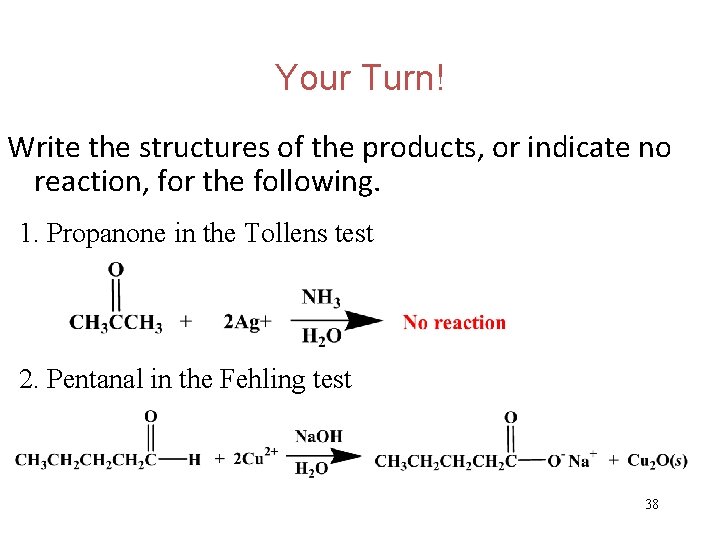
Your Turn! Write the structures of the products, or indicate no reaction, for the following. 1. Propanone in the Tollens test 2. Pentanal in the Fehling test 37

Your Turn! Write the structures of the products, or indicate no reaction, for the following. 1. Propanone in the Tollens test 2. Pentanal in the Fehling test 38

Chemical Properties of Aldehydes and Ketones Reduction Aldehydes and ketones are easily reduced to alcohols using Li. Al. H 4, Na. BH 4 , or H 2/Ni. Aldehydes yield primary alcohols and ketones yield secondary alcohols. 39

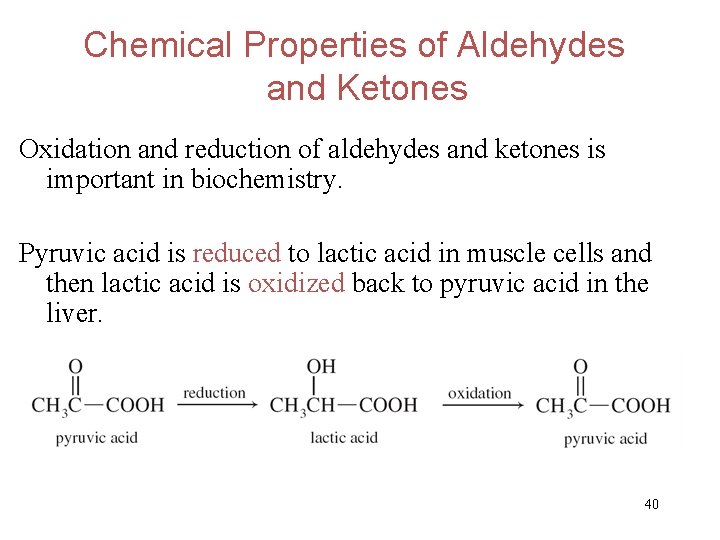
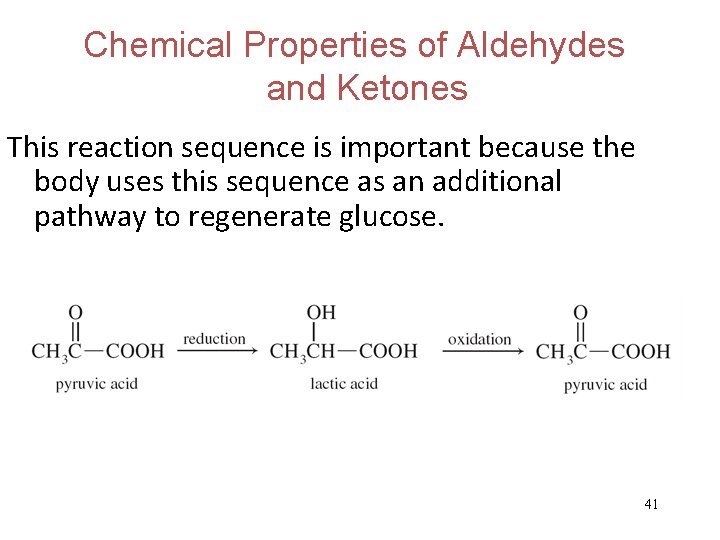
Chemical Properties of Aldehydes and Ketones Oxidation and reduction of aldehydes and ketones is important in biochemistry. Pyruvic acid is reduced to lactic acid in muscle cells and then lactic acid is oxidized back to pyruvic acid in the liver. 40

Chemical Properties of Aldehydes and Ketones This reaction sequence is important because the body uses this sequence as an additional pathway to regenerate glucose. 41

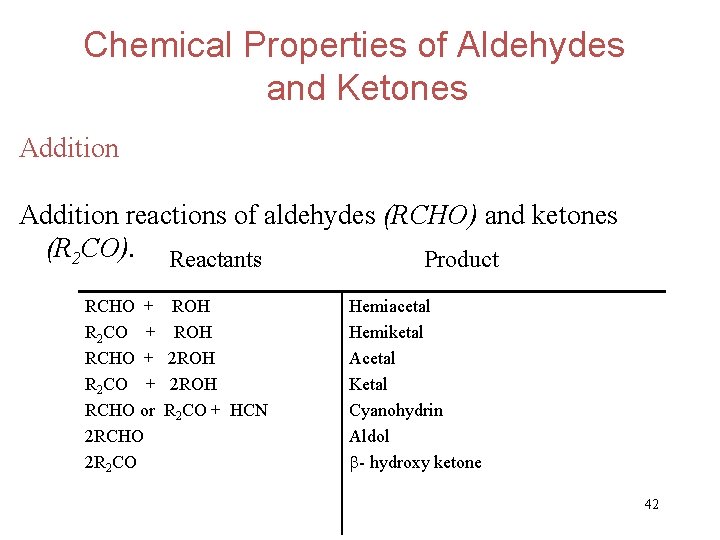
Chemical Properties of Aldehydes and Ketones Addition reactions of aldehydes (RCHO) and ketones (R 2 CO). Reactants Product RCHO + R 2 CO + RCHO or 2 RCHO 2 R 2 CO ROH 2 ROH R 2 CO + HCN Hemiacetal Hemiketal Acetal Ketal Cyanohydrin Aldol - hydroxy ketone 42

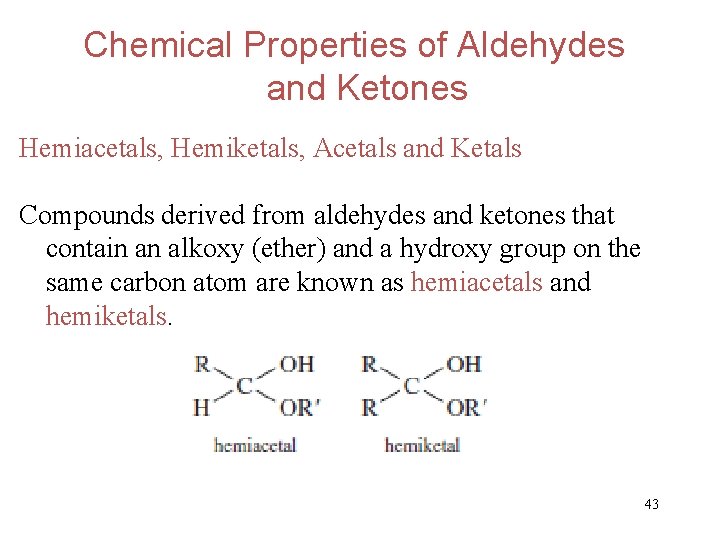
Chemical Properties of Aldehydes and Ketones Hemiacetals, Hemiketals, Acetals and Ketals Compounds derived from aldehydes and ketones that contain an alkoxy (ether) and a hydroxy group on the same carbon atom are known as hemiacetals and hemiketals. 43

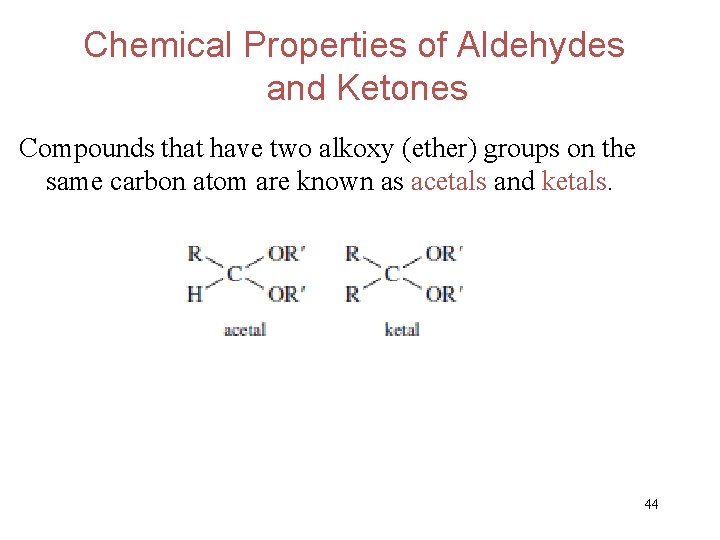
Chemical Properties of Aldehydes and Ketones Compounds that have two alkoxy (ether) groups on the same carbon atom are known as acetals and ketals. 44

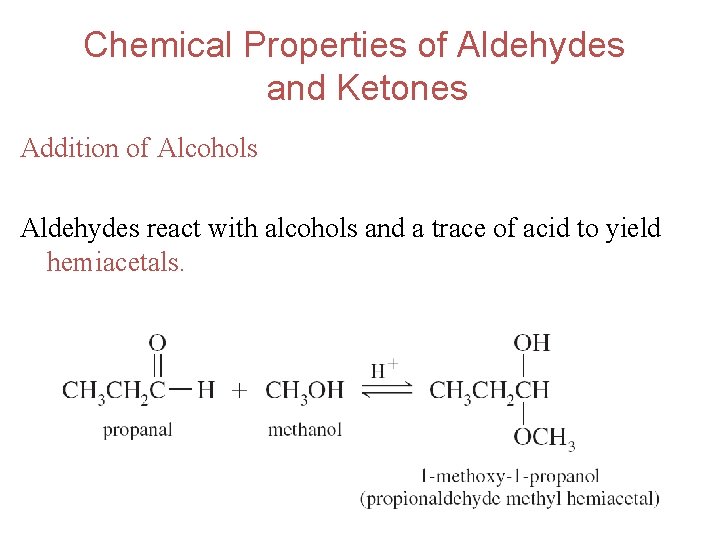
Chemical Properties of Aldehydes and Ketones Addition of Alcohols Aldehydes react with alcohols and a trace of acid to yield hemiacetals. 45

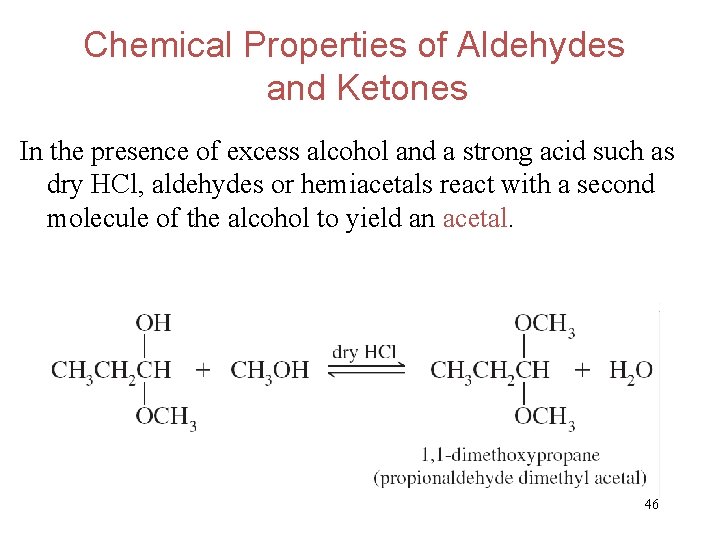
Chemical Properties of Aldehydes and Ketones In the presence of excess alcohol and a strong acid such as dry HCl, aldehydes or hemiacetals react with a second molecule of the alcohol to yield an acetal. 46

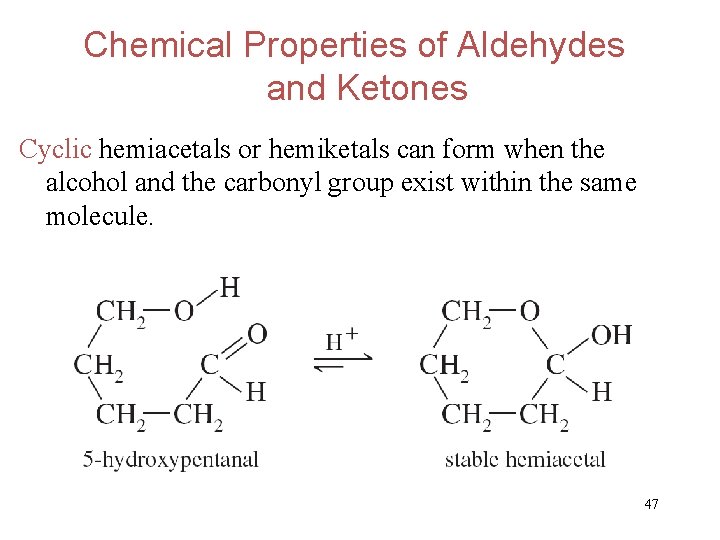
Chemical Properties of Aldehydes and Ketones Cyclic hemiacetals or hemiketals can form when the alcohol and the carbonyl group exist within the same molecule. 47

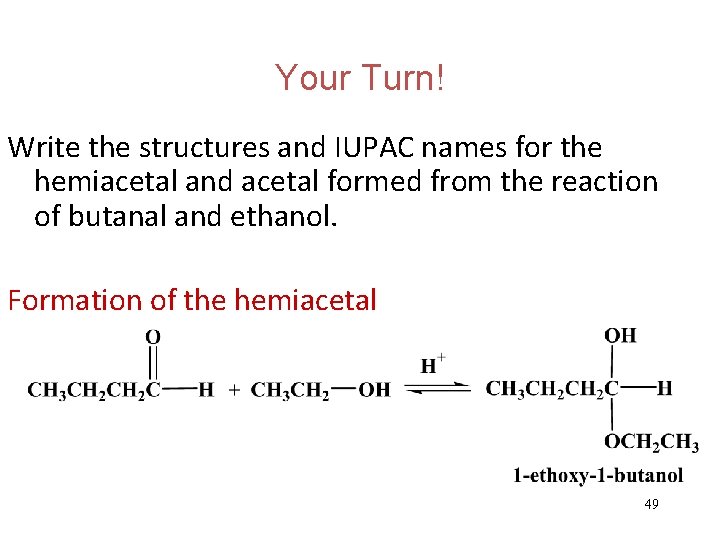
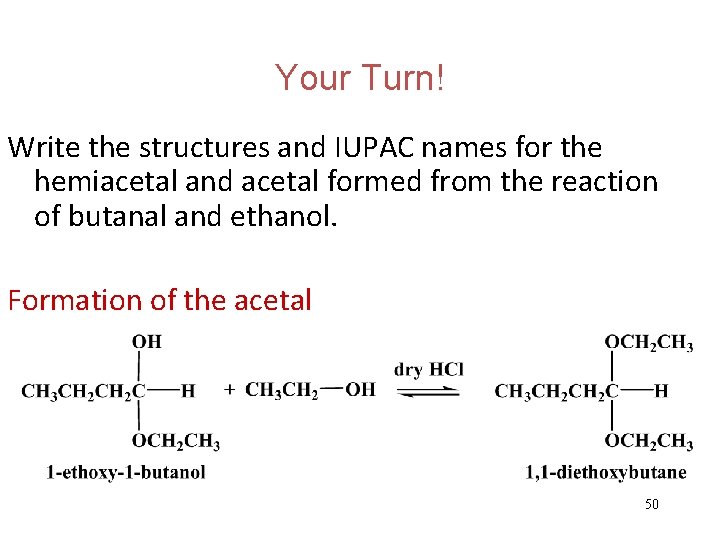
Your Turn! Write the structures and IUPAC names for the hemiacetal and acetal formed from the reaction of butanal and ethanol. 48

Your Turn! Write the structures and IUPAC names for the hemiacetal and acetal formed from the reaction of butanal and ethanol. Formation of the hemiacetal 49

Your Turn! Write the structures and IUPAC names for the hemiacetal and acetal formed from the reaction of butanal and ethanol. Formation of the acetal 50

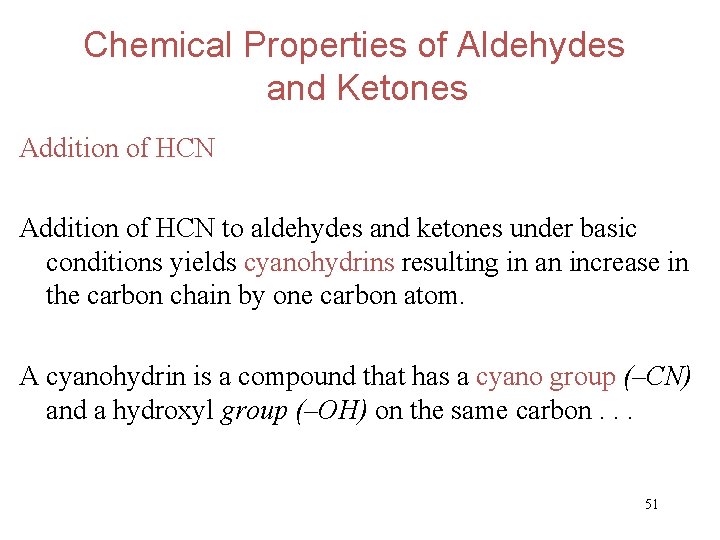
Chemical Properties of Aldehydes and Ketones Addition of HCN to aldehydes and ketones under basic conditions yields cyanohydrins resulting in an increase in the carbon chain by one carbon atom. A cyanohydrin is a compound that has a cyano group (–CN) and a hydroxyl group (–OH) on the same carbon. . . 51

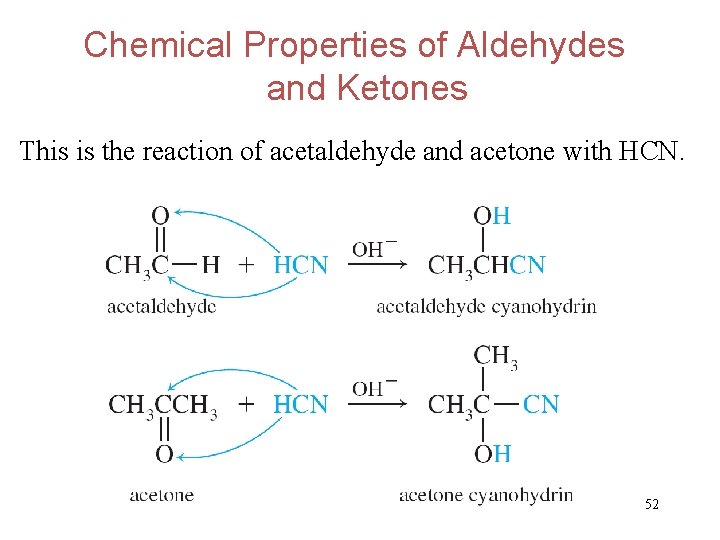
Chemical Properties of Aldehydes and Ketones This is the reaction of acetaldehyde and acetone with HCN. 52

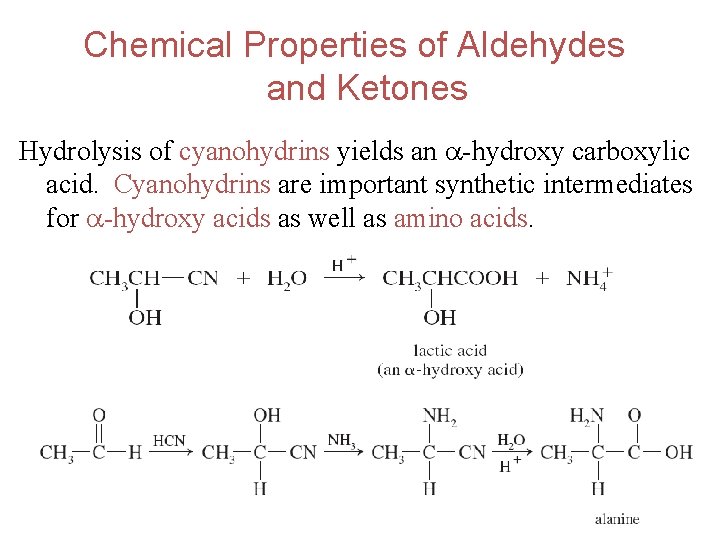
Chemical Properties of Aldehydes and Ketones Hydrolysis of cyanohydrins yields an -hydroxy carboxylic acid. Cyanohydrins are important synthetic intermediates for -hydroxy acids as well as amino acids. 53

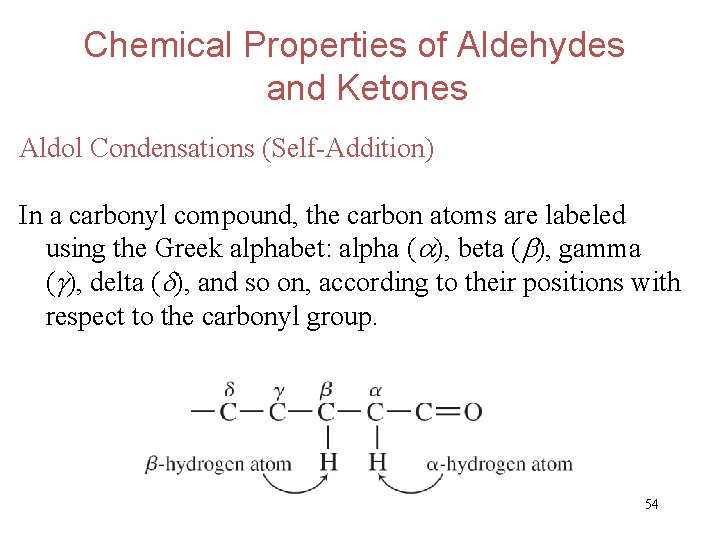
Chemical Properties of Aldehydes and Ketones Aldol Condensations (Self-Addition) In a carbonyl compound, the carbon atoms are labeled using the Greek alphabet: alpha ( ), beta ( ), gamma ( ), delta ( ), and so on, according to their positions with respect to the carbonyl group. 54

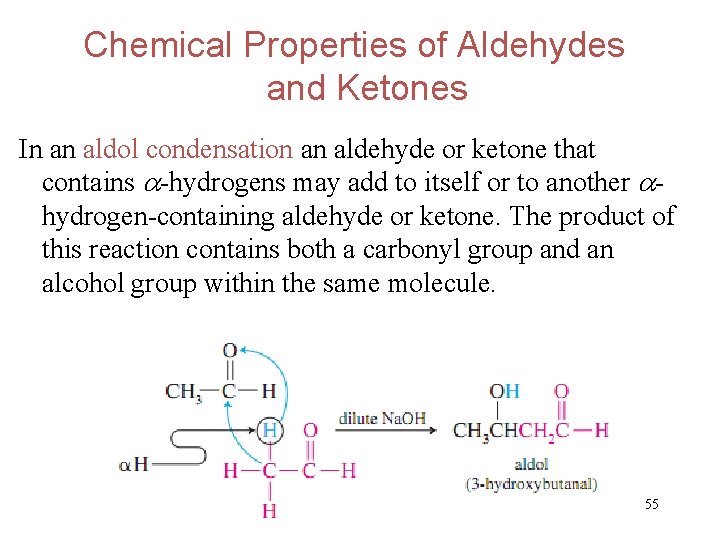
Chemical Properties of Aldehydes and Ketones In an aldol condensation an aldehyde or ketone that contains -hydrogens may add to itself or to another hydrogen-containing aldehyde or ketone. The product of this reaction contains both a carbonyl group and an alcohol group within the same molecule. 55

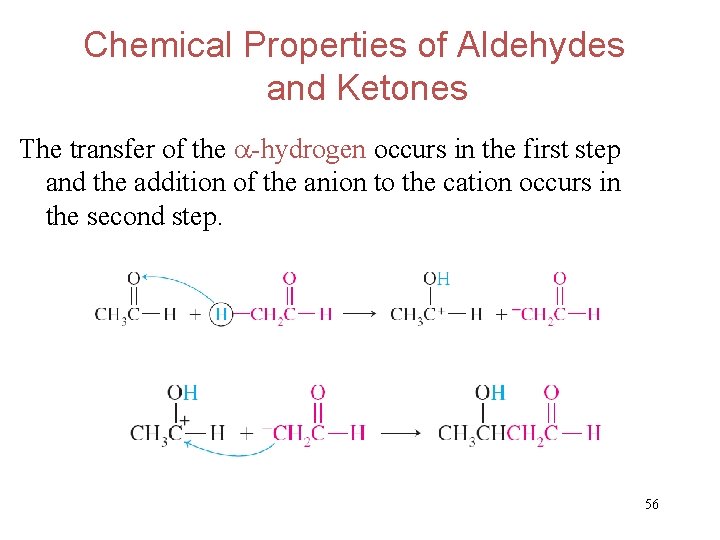
Chemical Properties of Aldehydes and Ketones The transfer of the -hydrogen occurs in the first step and the addition of the anion to the cation occurs in the second step. 56

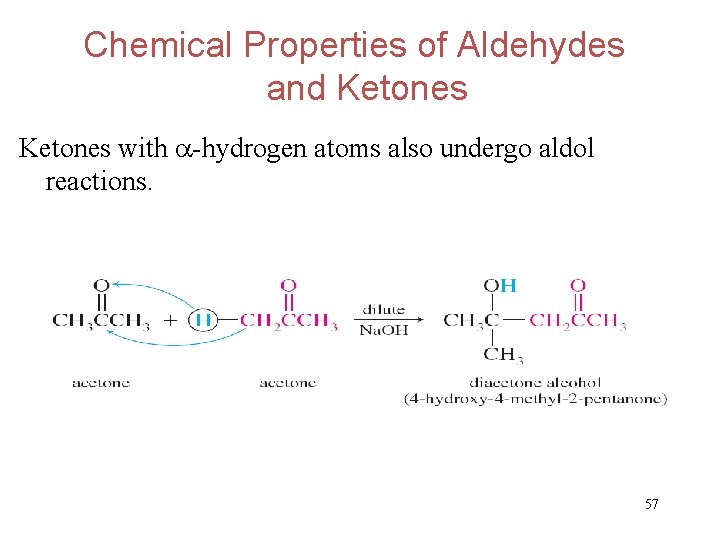
Chemical Properties of Aldehydes and Ketones with -hydrogen atoms also undergo aldol reactions. 57

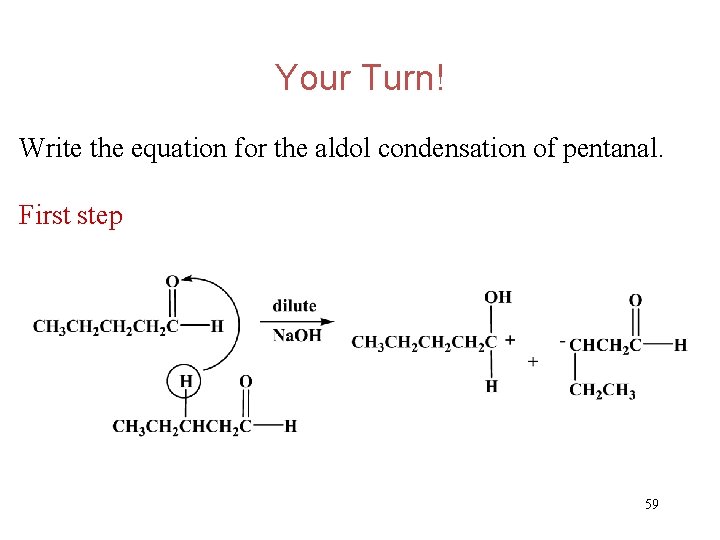
Your Turn! Write the equation for the aldol condensation of pentanal. 58

Your Turn! Write the equation for the aldol condensation of pentanal. First step 59

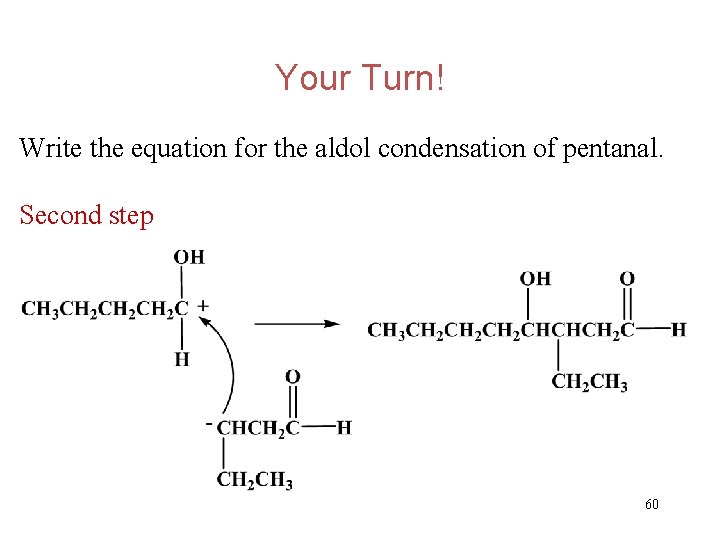
Your Turn! Write the equation for the aldol condensation of pentanal. Second step 60

Your Turn! The conversion of 2 -pentanone to 2 -pentanol is a(n): • • • Hydration Dehydration Hydrolysis Oxidation Reduction 61

Your Turn! The conversion of 2 -pentanone to 2 -pentanol is a(n): • • • Hydration Dehydration Hydrolysis Oxidation Reduction 2 -Pentanone is a ketone and 2 -pentanol is an alcohol. The conversion of a ketone to an alcohol is a reduction. 62

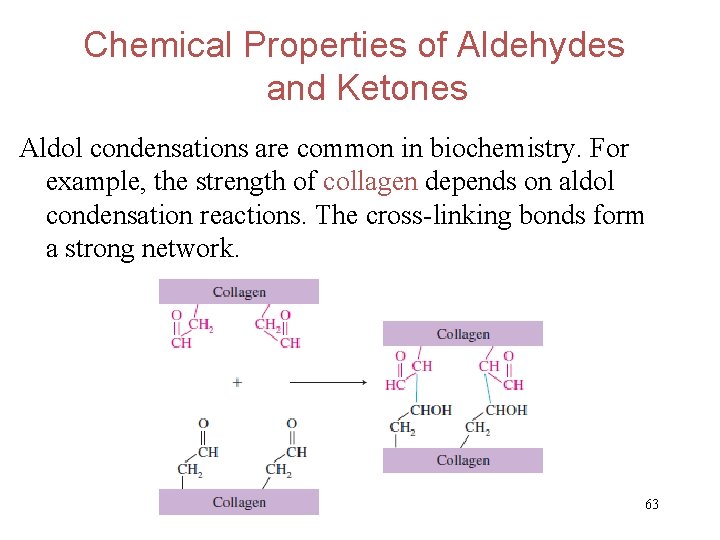
Chemical Properties of Aldehydes and Ketones Aldol condensations are common in biochemistry. For example, the strength of collagen depends on aldol condensation reactions. The cross-linking bonds form a strong network. 63

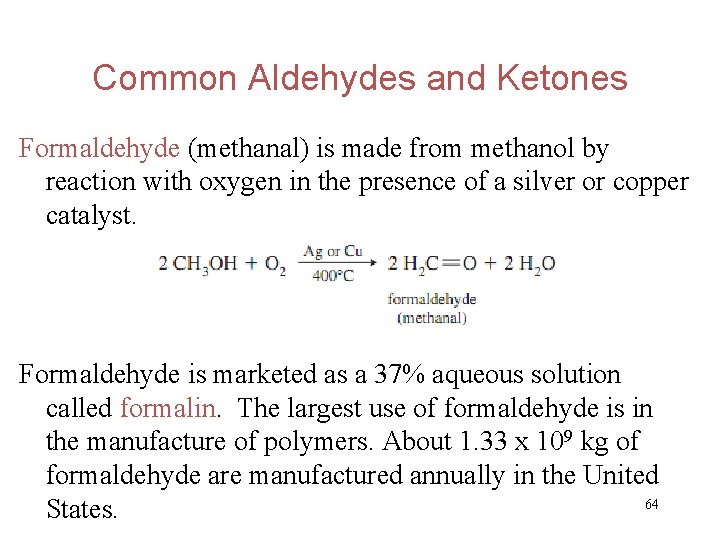
Common Aldehydes and Ketones Formaldehyde (methanal) is made from methanol by reaction with oxygen in the presence of a silver or copper catalyst. Formaldehyde is marketed as a 37% aqueous solution called formalin. The largest use of formaldehyde is in the manufacture of polymers. About 1. 33 x 109 kg of formaldehyde are manufactured annually in the United 64 States.

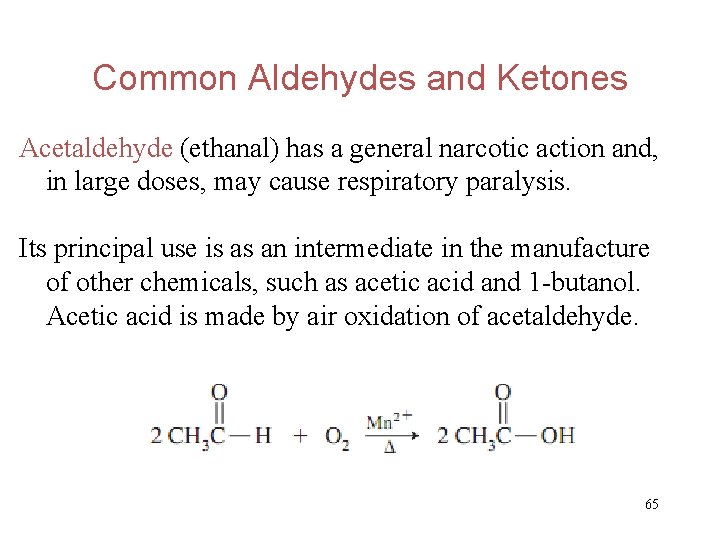
Common Aldehydes and Ketones Acetaldehyde (ethanal) has a general narcotic action and, in large doses, may cause respiratory paralysis. Its principal use is as an intermediate in the manufacture of other chemicals, such as acetic acid and 1 -butanol. Acetic acid is made by air oxidation of acetaldehyde. 65

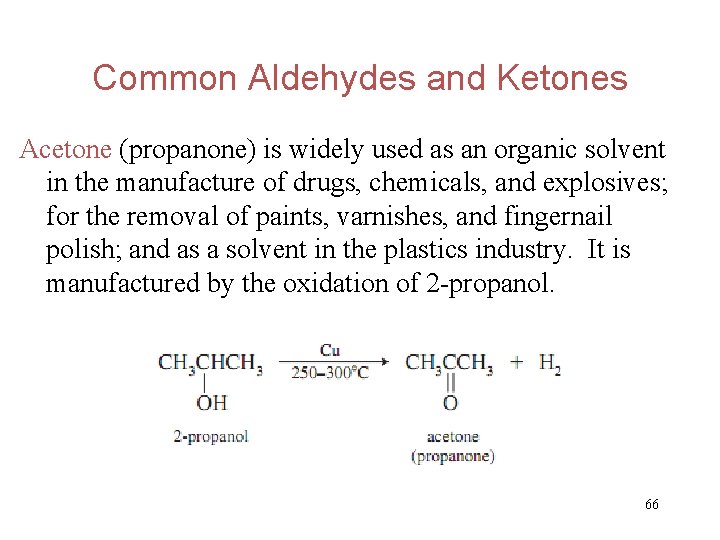
Common Aldehydes and Ketones Acetone (propanone) is widely used as an organic solvent in the manufacture of drugs, chemicals, and explosives; for the removal of paints, varnishes, and fingernail polish; and as a solvent in the plastics industry. It is manufactured by the oxidation of 2 -propanol. 66

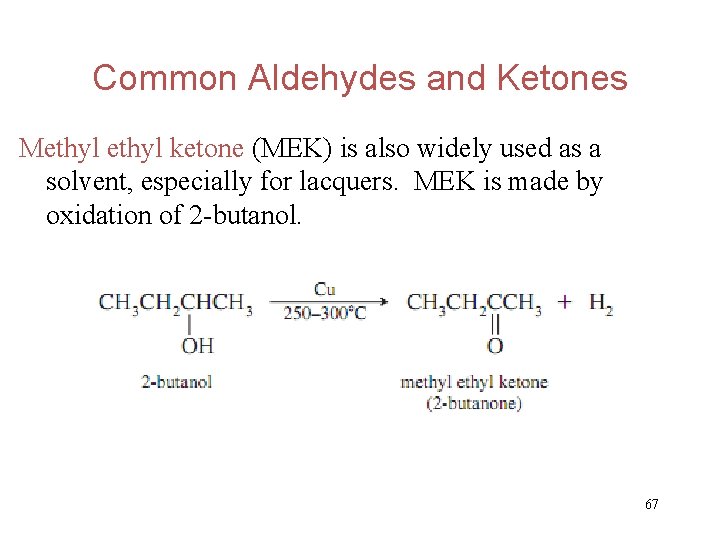
Common Aldehydes and Ketones Methyl ketone (MEK) is also widely used as a solvent, especially for lacquers. MEK is made by oxidation of 2 -butanol. 67

Condensation Polymers Condensation polymers are substances that are produced when a small molecule such as water is eliminated during polymerization. Polyesters, polyamides, polyurethanes, and phenolics represent four important classes of condensation polymers. 68

Condensation Polymers A phenol–formaldehyde condensation polymer (Bakelite) was first marketed a century ago. Polymers of this type are still widely used, especially in electrical equipment, because of their insulating and fire-resistant properties. Polymers made from phenol are known as phenolics. 69

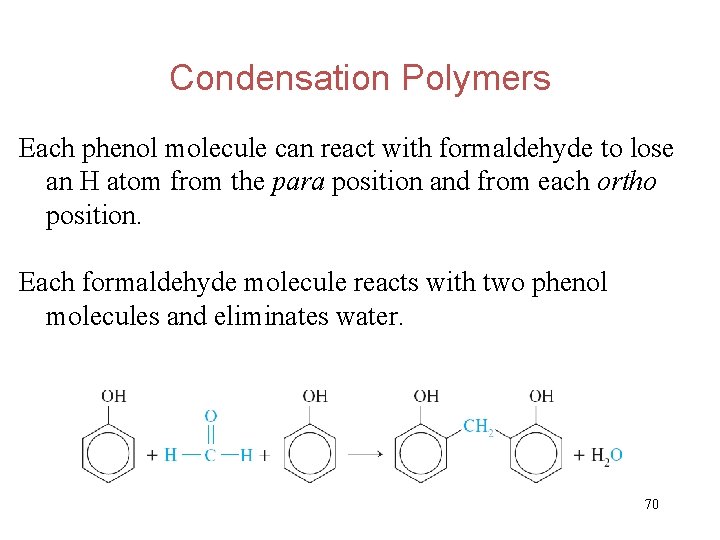
Condensation Polymers Each phenol molecule can react with formaldehyde to lose an H atom from the para position and from each ortho position. Each formaldehyde molecule reacts with two phenol molecules and eliminates water. 70

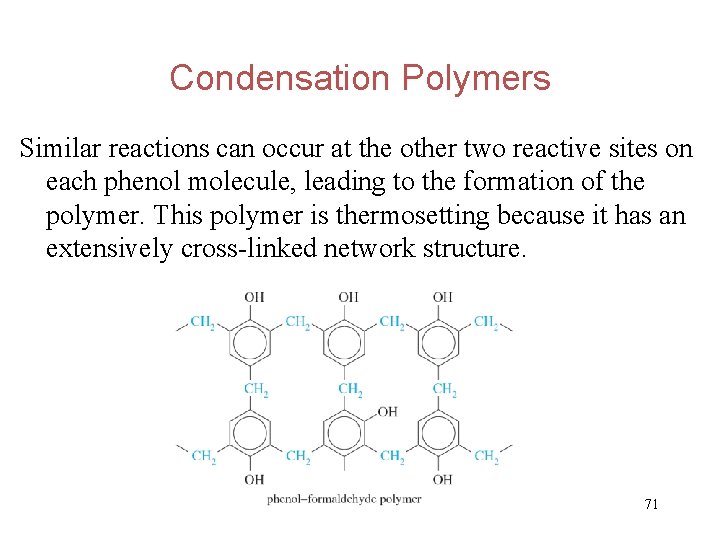
Condensation Polymers Similar reactions can occur at the other two reactive sites on each phenol molecule, leading to the formation of the polymer. This polymer is thermosetting because it has an extensively cross-linked network structure. 71

Chapter 23 Summary • Both aldehydes and ketones contain the carbonyl group. Aldehydes have at least one hydrogen atom bonded to the carbonyl carbon. Ketones have only alkyl or aryl groups bonded to the carbonyl carbon. • The IUPAC naming of aldehydes and ketones follows a similar process to that used for other organic molecules. • The carbonyl group in aldehydes and ketones is very polar, with the oxygen pulling electrons from the carbon. 72

Chapter 23 Summary • Aldehydes and ketones do not hydrogen-bond to themselves. They have lower boiling points than alcohols of comparable molar mass. • Aldehydes are easily oxidized to carboxylic acids. Ketones are unreactive under similar conditions. • Both aldehydes and ketones can be reduced. Aldehydes yield primary alcohols, while ketones yield secondary alcohols when reduced. 73

Chapter 23 Summary • A hemiacetal is formed when an alcohol adds across an aldehyde carbonyl double bond. If an alcohol adds to a ketone, a hemiketal is produced. • With an excess of alcohol, two alcohols can react with a carbonyl to form an acetal (from an aldehyde) and a ketal (from a ketone). • Cyanohydrins are formed by the addition of HCN to a carbonyl group. 74

Chapter 23 Summary • In an aldol condensation, two carbonyl-containing molecules (either aldehydes or ketones) connect together. • Common aldehydes are formaldehyde and acetaldehyde. Formaldehyde (methanal) is used primarily in the manufacture of polymers. Acetaldehyde (ethanal) is used to produce acetic acid and 1 -butanol. • Common ketones are acetone and methyl ketone (MEK). 75

Chapter 23 Summary • Condensation polymers form when monomers combine and eliminate a small-molecule product such as water. • Common examples of condensation polymers are the phenol–formaldehyde polymers, also known as phenolics. 76