States of Matter Section 1 Matter and Energy












































- Slides: 73

States of Matter Section 1: Matter and Energy Preview • Key Ideas • Bellringer • Kinetic Theory • States of Matter • Energy’s Role

States of Matter Section 1 Key Ideas 〉 What makes up matter? 〉 What is the difference between a solid, a liquid, and a gas? 〉 What kind of energy do all particles of matter have?

States of Matter Section 1 Kinetic Theory 〉 What makes up matter? 〉 According to the kinetic theory of matter, matter is made of atoms and molecules. These atoms and molecules act like tiny particles that are always in motion.

States of Matter Section 1 Kinetic Theory, continued • The following are observations of particles in motion: – The higher the temperature of the substance is, the faster the particles move. – At the same temperature, more massive particles move slower than less massive ones. • The kinetic theory helps to explain the differences between the three common states of matter: solid, liquid, and gas.

States of Matter Section 1 Visual Concept: Kinetic Molecular Theory

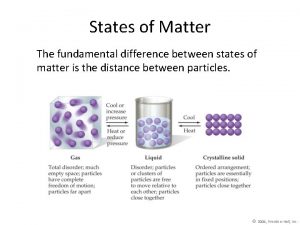
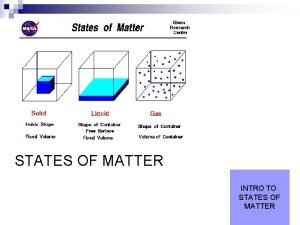
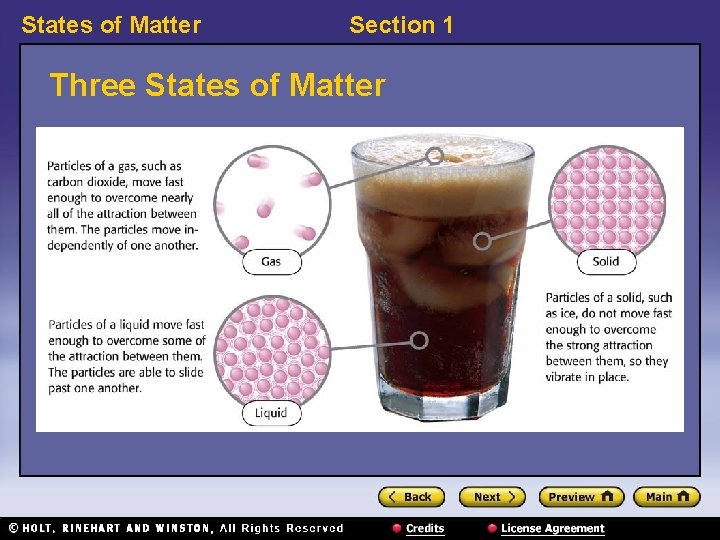
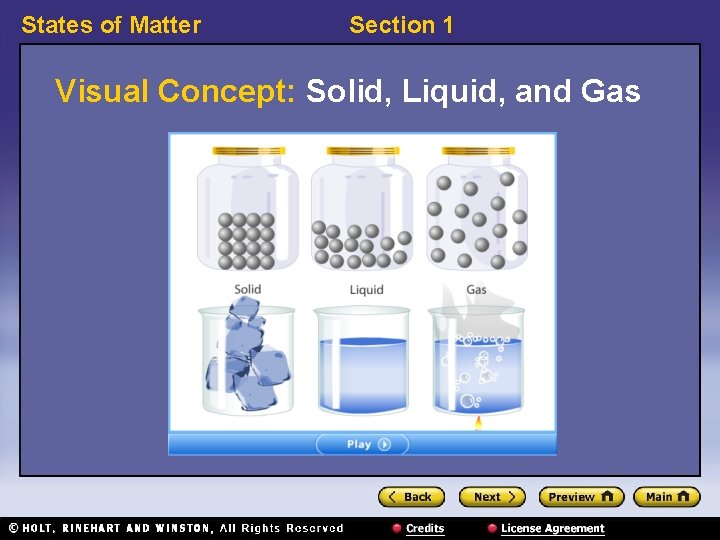
States of Matter Section 1 States of Matter 〉 What is the difference between a solid, a liquid, and a gas? 〉 You can classify matter as a solid, a liquid, or a gas by determining whether the shape and volume are definite or variable.

States of Matter Section 1 States of Matter, continued • Solids have a definite shape and volume. • Liquids change shape, not volume. • Gases change both shape and volume. – fluid: a nonsolid state of matter in which the atoms or molecules are free to move past each other, as in a gas or liquid • Plasma is the most common state of matter. – plasma: a state of matter that consists of free-moving ions and electrons

States of Matter Section 1 Three States of Matter

States of Matter Section 1 Visual Concept: Solid, Liquid, and Gas

States of Matter Section 1 Energy’s Role 〉 What kind of energy do all particles of matter have? 〉 Because they are in motion, all particles of matter have kinetic energy. • energy: the capacity to do work

States of Matter Section 1 Energy’s Role, continued • Temperature is a measure of average kinetic energy. – temperature: a measure of how hot (or cold) something is; specifically, a measure of the average kinetic energy of the particles in an object • Thermal energy depends on particle speed and number of particles. – thermal energy: the total kinetic energy of a substance’s atoms

States of Matter Section 1 Kinetic Energy and States of Matter

States of Matter Standardized Test Prep Understanding Concepts, continued 4. Plastic is put into molds to create specific shapes. In what state of matter should the plastic be when it is put in the mold, and why?

States of Matter Standardized Test Prep Understanding Concepts, continued 4. Plastic is put into molds to create specific shapes. In what state of matter should the plastic be when it is put in the mold, and why? Answer: The plastic should be in liquid form, because liquids conform to the shape of their containers.

States of Matter Section 2: Changes of State Preview • Key Ideas • Bellringer • Energy and Changes of State • Conservation of Mass and Energy

States of Matter Section 2 Key Ideas 〉 What happens when a substance changes from one state of matter to another? 〉 What happens to mass and energy during physical and chemical changes?

States of Matter Section 2 Energy and Changes of State 〉 What happens when a substance changes from one state of matter to another? 〉 The identity of a substance does not change during a change of state, but the energy of a substance does change.

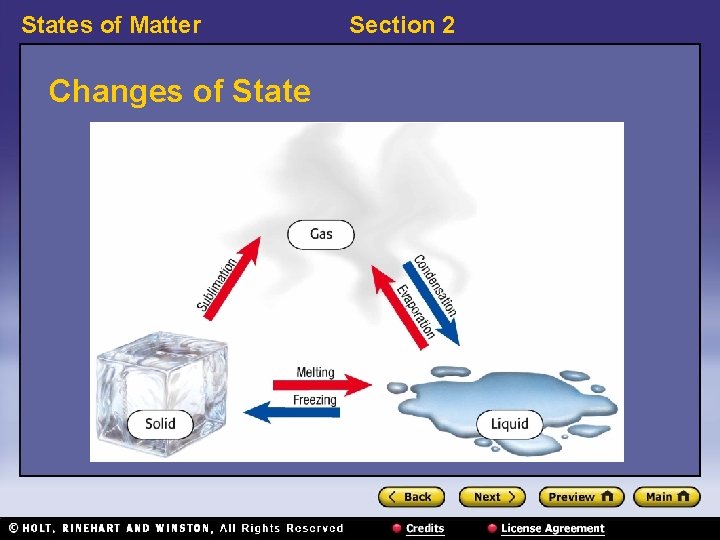
States of Matter Changes of State Section 2

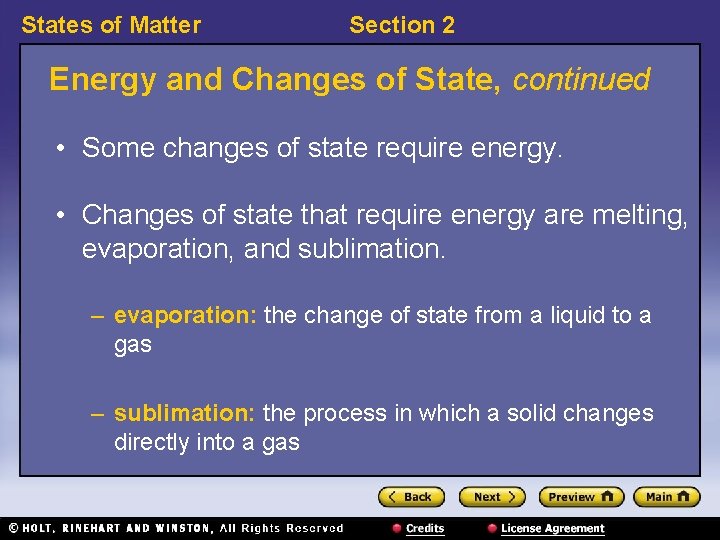
States of Matter Section 2 Energy and Changes of State, continued • Some changes of state require energy. • Changes of state that require energy are melting, evaporation, and sublimation. – evaporation: the change of state from a liquid to a gas – sublimation: the process in which a solid changes directly into a gas

States of Matter Section 2 Energy and Changes of State, continued • Energy is released in some changes of state. • Changes of state that release energy are freezing and condensation. – condensation: the change of state from a gas to a liquid

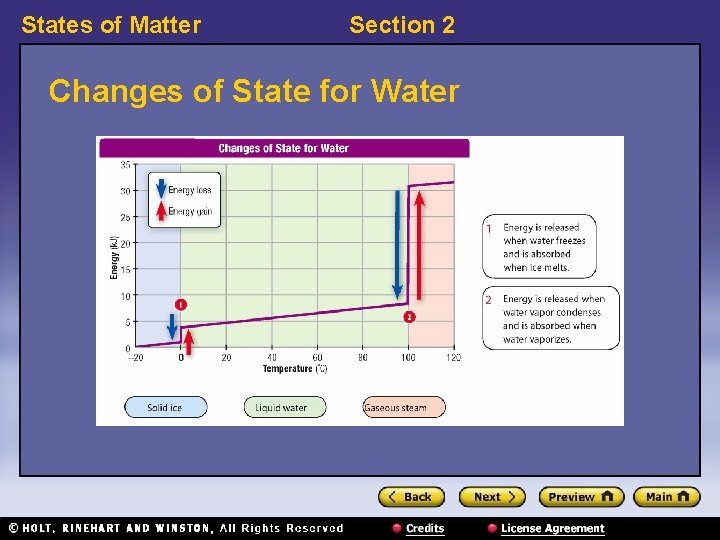
States of Matter Section 2 Changes of State for Water

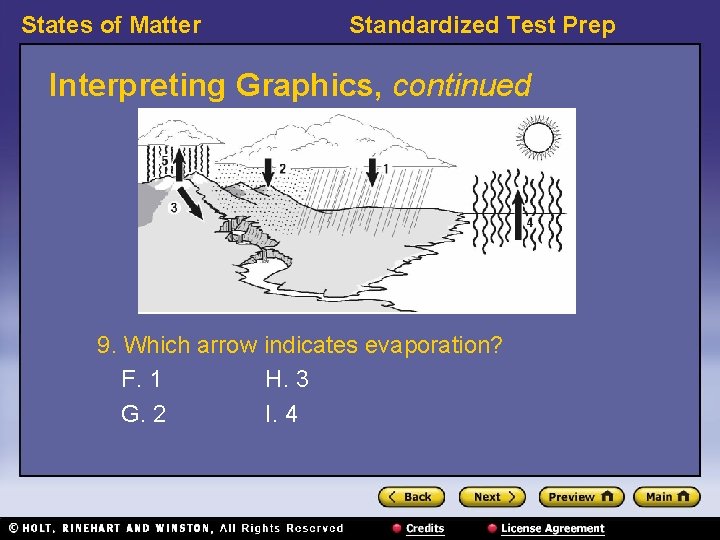
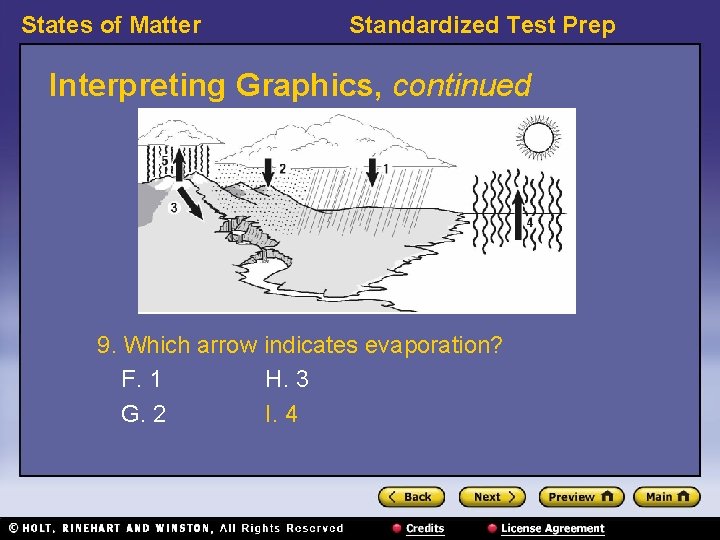
States of Matter Standardized Test Prep Interpreting Graphics, continued 9. Which arrow indicates evaporation? F. 1 H. 3 G. 2 I. 4

States of Matter Standardized Test Prep Interpreting Graphics, continued 9. Which arrow indicates evaporation? F. 1 H. 3 G. 2 I. 4

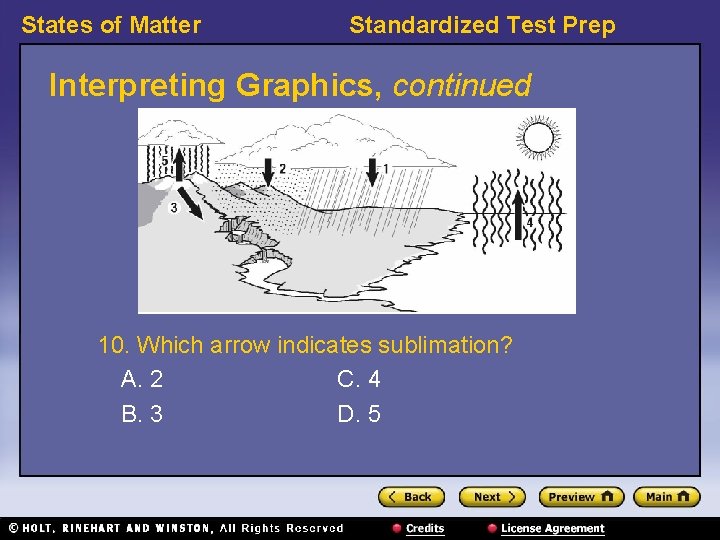
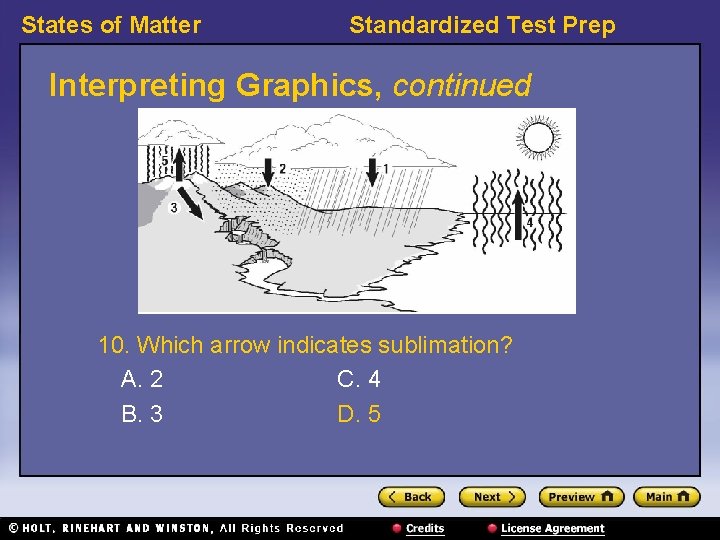
States of Matter Standardized Test Prep Interpreting Graphics, continued 10. Which arrow indicates sublimation? A. 2 C. 4 B. 3 D. 5

States of Matter Standardized Test Prep Interpreting Graphics, continued 10. Which arrow indicates sublimation? A. 2 C. 4 B. 3 D. 5

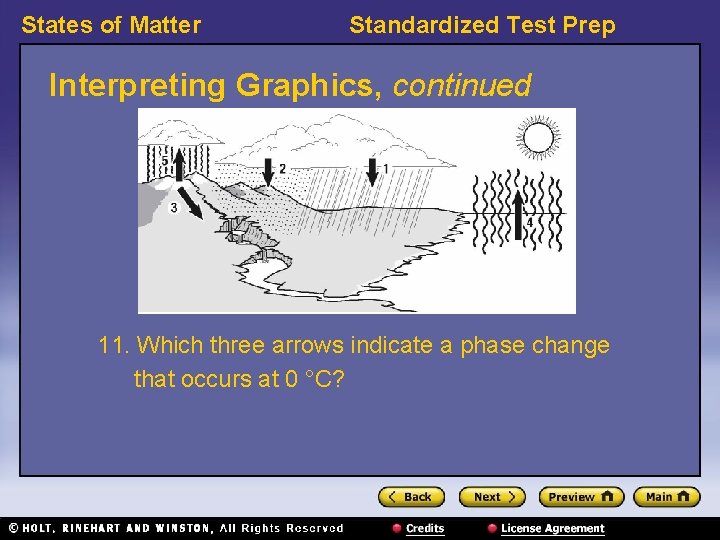
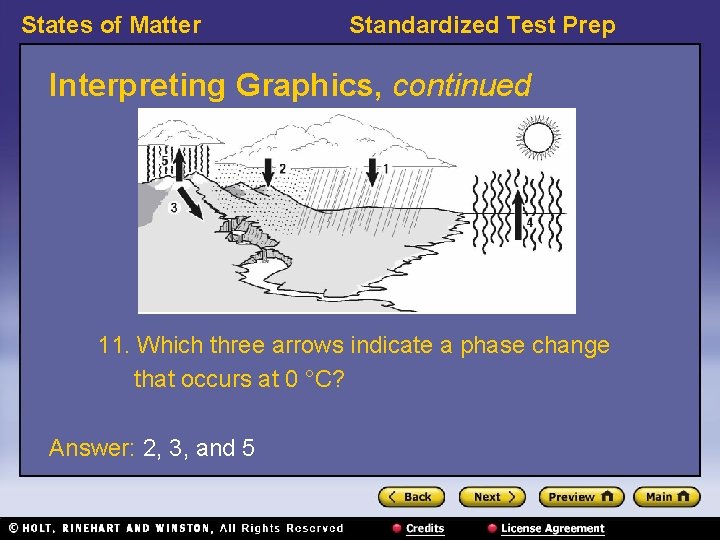
States of Matter Standardized Test Prep Interpreting Graphics, continued 11. Which three arrows indicate a phase change that occurs at 0 °C?

States of Matter Standardized Test Prep Interpreting Graphics, continued 11. Which three arrows indicate a phase change that occurs at 0 °C? Answer: 2, 3, and 5

States of Matter Section 2 Conservation of Mass and Energy 〉 What happens to mass and energy during physical and chemical changes? 〉 Mass and energy are both conserved. Neither mass nor energy can be created or destroyed.

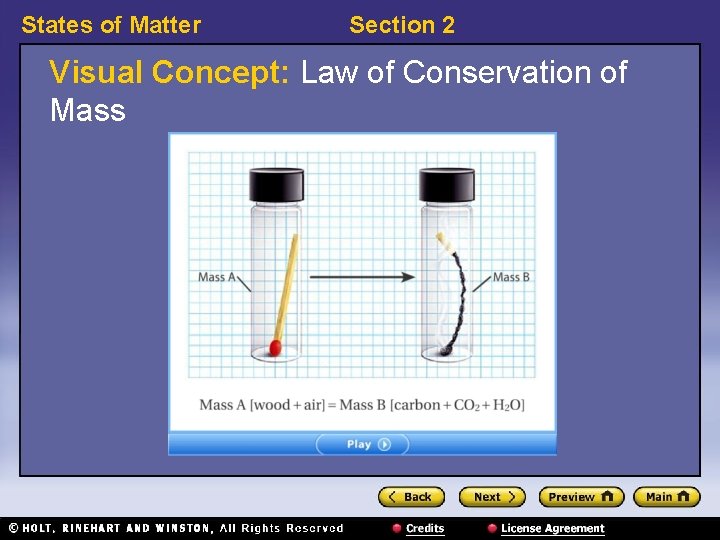
States of Matter Section 2 Conservation of Mass and Energy, continued • Mass cannot be created or destroyed. – In chemical changes, as well as in physical changes, the total mass of the substances undergoing the change stays the same before and after the change. – This is the law of conservation of mass.

States of Matter Standardized Test Prep Understanding Concepts, continued 3. In the year 2012, a space probe investigating Neptune scoops up a load of solid frozen oxygen from the planet’s atmosphere. Upon entry into Earth’s atmosphere, some of the solid oxygen immediately changes into a gas. Which of the following processes happened? A. evaporation C. sublimation B. condensation D. melting

States of Matter Standardized Test Prep Understanding Concepts, continued 3. In the year 2012, a space probe investigating Neptune scoops up a load of solid frozen oxygen from the planet’s atmosphere. Upon entry into Earth’s atmosphere, some of the solid oxygen immediately changes into a gas. Which of the following processes happened? A. evaporation C. sublimation B. condensation D. melting

States of Matter Section 2 Visual Concept: Law of Conservation of Mass

States of Matter Section 2 Conservation of Mass and Energy, continued • Energy cannot be created or destroyed. – Energy may be changed to another form during a physical or chemical change, but the total amount of energy present before and after the change is the same. – This is the law of conservation of energy.

States of Matter Section 2 Visual Concept: Law of Conservation of Energy

States of Matter Standardized Test Prep Understanding Concepts, continued 5. A kitchen scientist combines 5. 0 g of baking soda with 100. 0 g of vinegar, which causes a gas (carbon dioxide) to be given off. After all of the gas has escaped, the liquid has a mass of 102. 4 g. What is the mass of the escaped gas?

States of Matter Standardized Test Prep Understanding Concepts, continued 5. A kitchen scientist combines 5. 0 g of baking soda with 100. 0 g of vinegar, which causes a gas (carbon dioxide) to be given off. After all of the gas has escaped, the liquid has a mass of 102. 4 g. What is the mass of the escaped gas? Answer: 2. 6 g

States of Matter Section 3: Fluids Preview • Key Ideas • Bellringer • Pressure • Buoyant Force • Comparing Weight and Buoyant Force • Pascal’s Principle • Math Skills • Fluids in Motion

States of Matter Section 3 Key Ideas 〉 How do fluids exert pressure? 〉 What force makes a rubber duck float in a bathtub? 〉 What happens when pressure in a fluid changes? 〉 What affects the speed of a fluid in motion?

States of Matter Section 3 Pressure 〉 How do fluids exert pressure? 〉 Fluids exert pressure evenly in all directions. – pressure: the amount of force exerted per unit area of a surface – example: when you pump up a bicycle tire, air particles constantly push against each other and against the tire walls

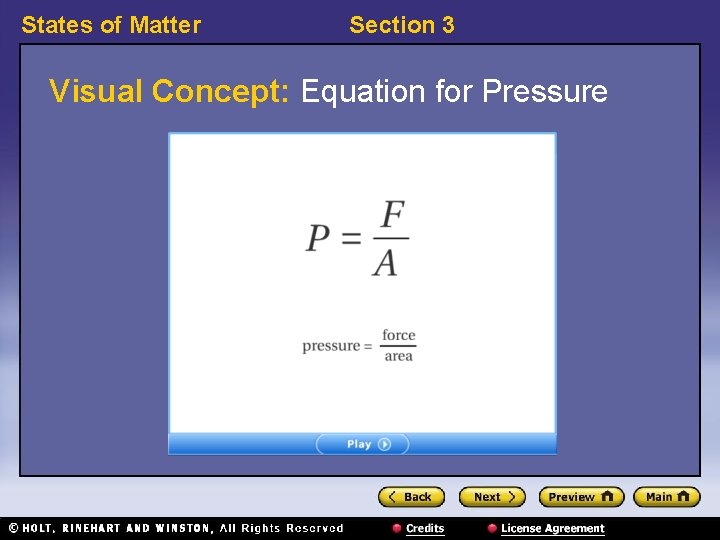
States of Matter Section 3 Pressure, continued • Pressure can be calculated by dividing force by the area over which the force is exerted: • The SI unit for pressure is the pascal. – pascal: the SI unit of pressure; equal to the force of 1 N exerted over an area of 1 m 2 (symbol, Pa)

States of Matter Section 3 Visual Concept: Equation for Pressure

States of Matter Standardized Test Prep Understanding Concepts 1. An industrial thermometer is heated until the mercury inside it is exerting 400 N of force against the inner surface. That surface has a total area of 200 cm 2. How much pressure is the mercury exerting against the inner surface of thermometer? Note that 1 Pa = 1 N/m 2. A. 800 Pa B. 2, 000 Pa C. 8, 000 Pa D. 20, 000 Pa

States of Matter Standardized Test Prep Understanding Concepts 1. An industrial thermometer is heated until the mercury inside it is exerting 400 N of force against the inner surface. That surface has a total area of 200 cm 2. How much pressure is the mercury exerting against the inner surface of thermometer? Note that 1 Pa = 1 N/m 2. A. 800 Pa B. 2, 000 Pa C. 8, 000 Pa D. 20, 000 Pa

States of Matter Standardized Test Prep Interpreting Graphics, continued 12. In which tank is the greatest pressure being exerted on the tank’s inner surface?

States of Matter Standardized Test Prep Interpreting Graphics, continued 12. In which tank is the greatest pressure being exerted on the tank’s inner surface? Answer: Tank A

States of Matter Section 3 Buoyant Force 〉 What force makes a rubber duck float in a bathtub? 〉 All fluids exert an upward buoyant force on matter. • buoyant force: the upward force that keeps an object immersed in or floating on a fluid

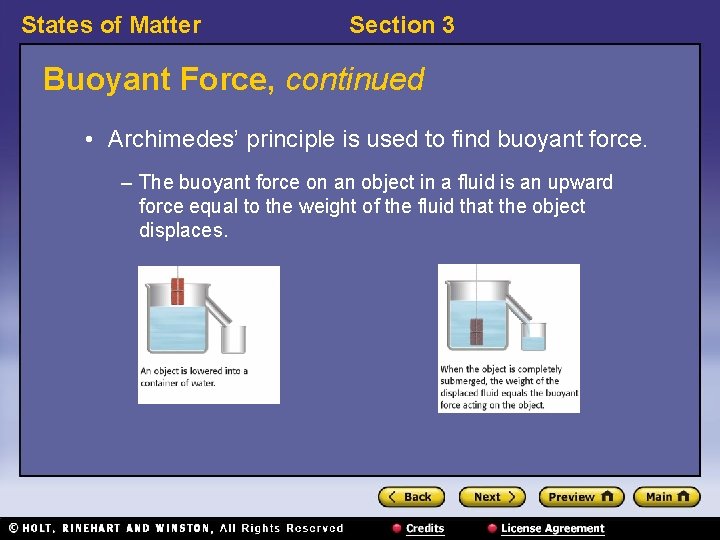
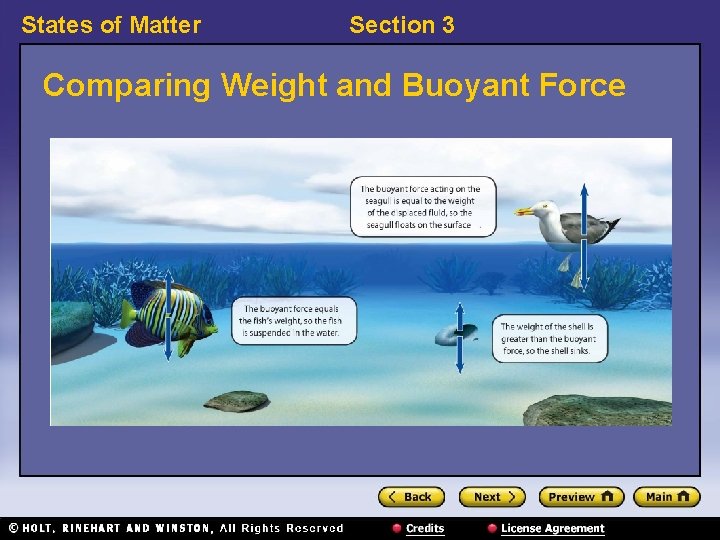
States of Matter Section 3 Buoyant Force, continued • Archimedes’ principle is used to find buoyant force. – The buoyant force on an object in a fluid is an upward force equal to the weight of the fluid that the object displaces.

States of Matter Section 3 Comparing Weight and Buoyant Force

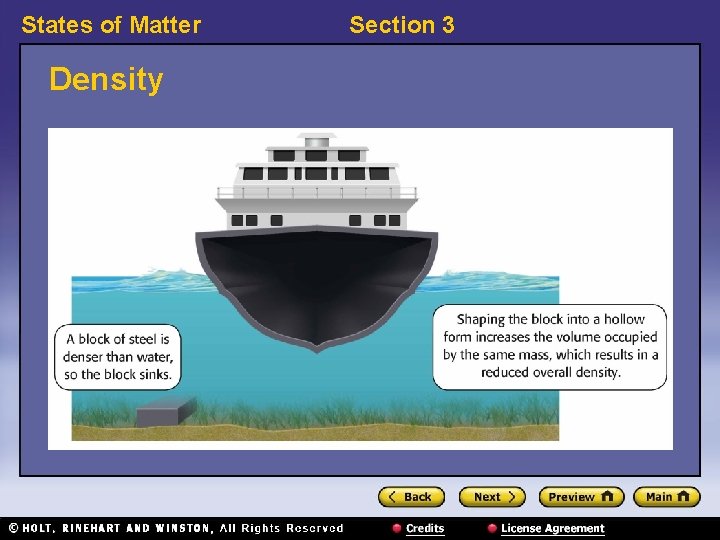
States of Matter Section 3 Buoyant Force, continued • An object will float or sink based on its density. – If an object is less dense than the fluid in which it is placed, it will float. – If an object is more dense than the fluid in which it is placed, it will sink.

States of Matter Density Section 3

States of Matter Section 3 Pascal’s Principle 〉 What happens when pressure in a fluid changes? 〉 Pascal’s principle states that a change in pressure at any point in an enclosed fluid will be transmitted equally to all parts of the fluid. In other words, if the pressure in a container is increased at any point, the pressure increases at all points by the same amount. – Mathematically, Pascal’s principle is stated as P 1 = P 2. – Because P = F/A, Pascal’s principle can also be expressed as F 1/A 1 = F 2/A 2.

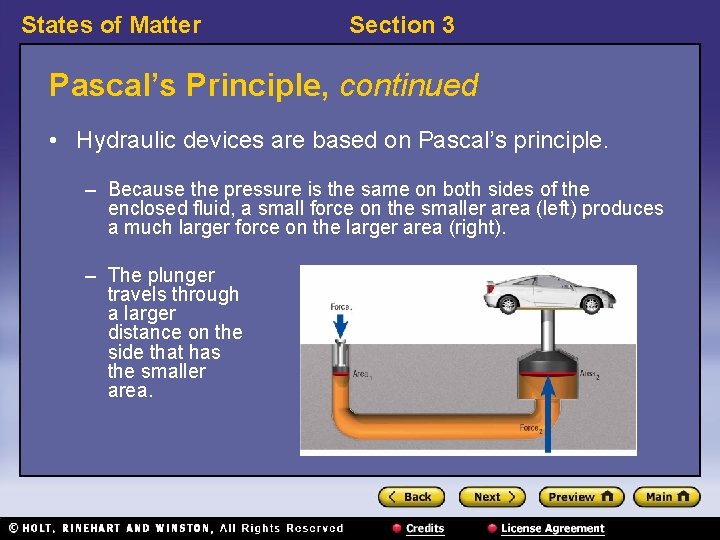
States of Matter Section 3 Pascal’s Principle, continued • Hydraulic devices are based on Pascal’s principle. – Because the pressure is the same on both sides of the enclosed fluid, a small force on the smaller area (left) produces a much larger force on the larger area (right). – The plunger travels through a larger distance on the side that has the smaller area.

States of Matter Section 3 Math Skills Pascal’s Principle A hydraulic lift uses Pascal’s principle to lift a 19, 000 N car. If the area of the small piston (A 1) equals 10. 5 cm 2 and the area of the large piston (A 2) equals 400 cm 2, what force needs to be exerted on the small piston to lift the car? 1. List the given and unknown values. Given: F 2 = 19, 000 N A 1 = 10. 5 cm 2 A 2 = 400 cm 2 Unknown: F 1

States of Matter Section 3 Math Skills, continued 2. Start with Pascal’s principle, and substitute the equation for pressure. Then, rearrange the equation to isolate the unknown value. P 1 = P 2

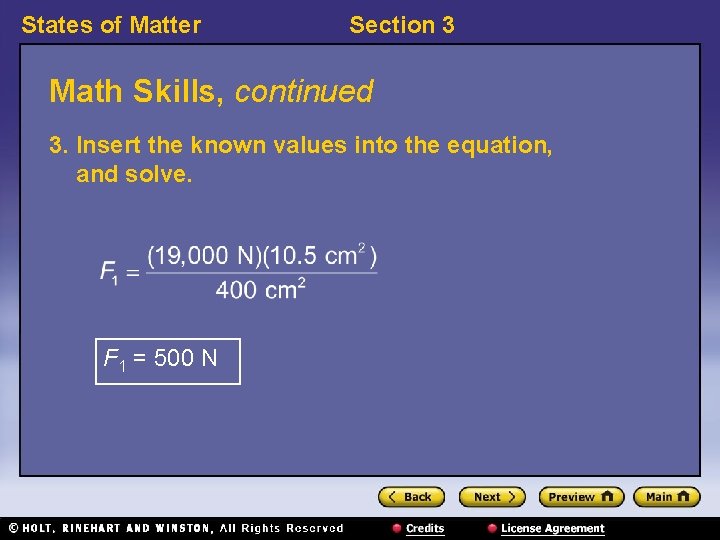
States of Matter Section 3 Math Skills, continued 3. Insert the known values into the equation, and solve. F 1 = 500 N

States of Matter Section 3 Fluids in Motion 〉 What affects the speed of a fluid in motion? 〉 Fluids move faster through small areas than through larger areas, if the overall flow rate remains constant. Fluids also vary in the rate at which they flow.

States of Matter Section 3 Fluids in Motion, continued • Viscosity depends on particle attraction. – viscosity: the resistance of a gas or liquid to flow • Fluid pressure decreases as speed increases. – This is known as Bernoulli’s principle.

States of Matter Section 3 Visual Concept: Viscosity

States of Matter Section 4: Behavior of Gases Preview • Key Ideas • Bellringer • Properties of Gases • Gas Laws • Math Skills

States of Matter Section 4 Key Ideas 〉 What are some properties of gases? 〉 How can you predict the effects of pressure, temperature, and volume changes on gases?

States of Matter Section 4 Properties of Gases 〉 What are some properties of gases? 〉 Gases expand to fill their containers. They spread out easily and mix with one another. They have low densities and are compressible. Unlike solids and liquids, gases are mostly empty space. Also, gases exert pressure on their containers.

States of Matter Section 4 Visual Concept: Properties of Gases

States of Matter Section 4 Gas Laws 〉 How can you predict the effects of pressure, temperature, and volume changes on gases? 〉 The gas laws will help you understand predict the behavior of gases in specific situations. • gas laws: the laws that state the mathematical relationships between the volume, temperature, pressure, and quantity of a gas

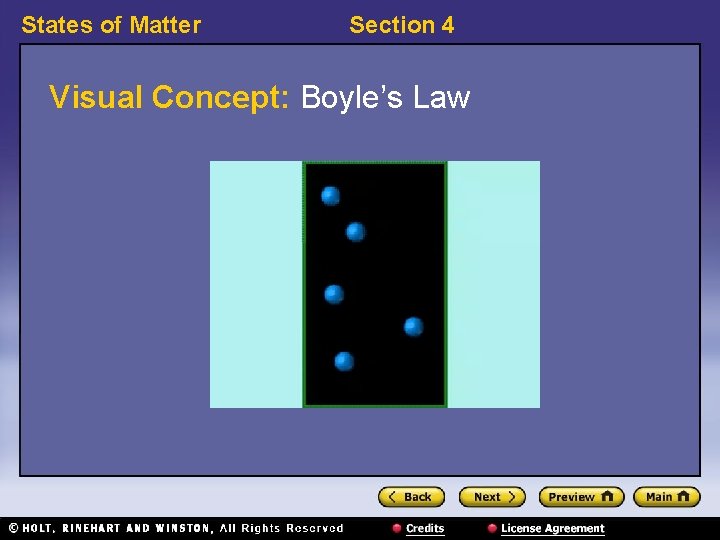
States of Matter Section 4 Gas Laws • Boyle’s law relates the pressure of a gas to its volume. – Boyle’s law: For a fixed amount of gas at a constant temperature, the volume of a gas increases as the gas’s pressure decreases. Likewise, the volume of a gas decreases as the gas’s pressure increases. – P 1 V 1 = P 2 V 2

States of Matter Section 4 Visual Concept: Boyle’s Law

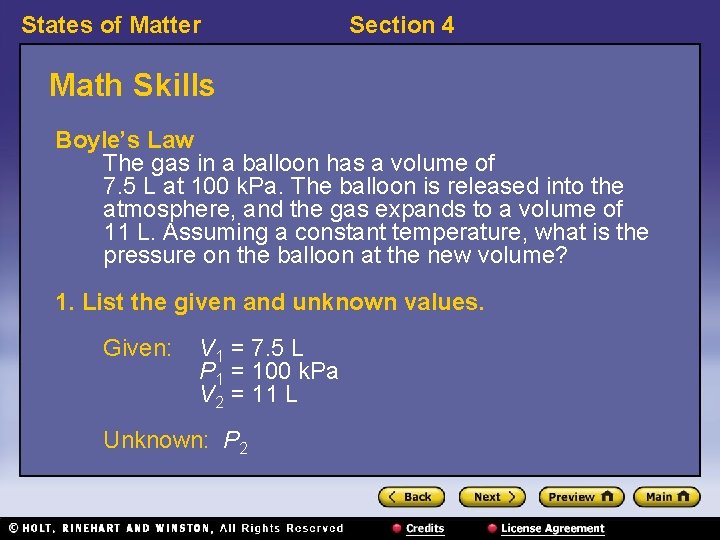
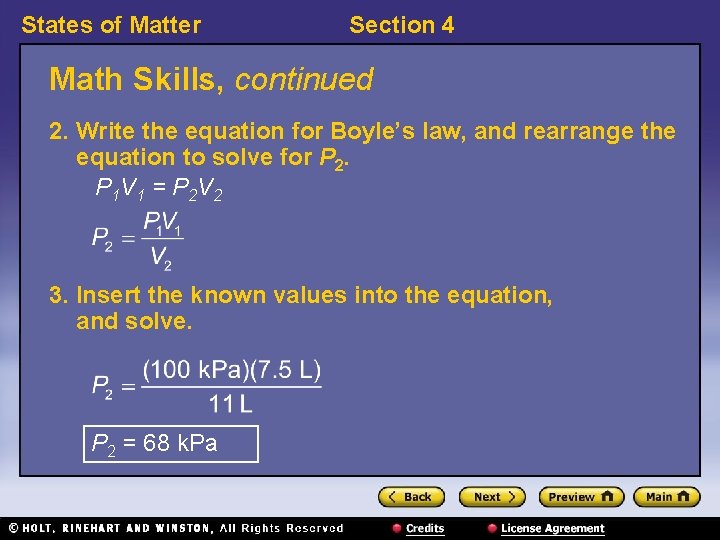
States of Matter Section 4 Math Skills Boyle’s Law The gas in a balloon has a volume of 7. 5 L at 100 k. Pa. The balloon is released into the atmosphere, and the gas expands to a volume of 11 L. Assuming a constant temperature, what is the pressure on the balloon at the new volume? 1. List the given and unknown values. Given: V 1 = 7. 5 L P 1 = 100 k. Pa V 2 = 11 L Unknown: P 2

States of Matter Section 4 Math Skills, continued 2. Write the equation for Boyle’s law, and rearrange the equation to solve for P 2. P 1 V 1 = P 2 V 2 3. Insert the known values into the equation, and solve. P 2 = 68 k. Pa

States of Matter Section 4 Gas Laws, continued • Gay-Lussac’s law relates gas pressure to temperature. – Gay-Lussac’s law: The pressure of a gas increases as the temperature increases, if the volume of the gas does not change. The pressure decreases as the temperature decreases. • Charles’s law relates temperature to volume. – Charles’s law: For a fixed amount of gas at a constant pressure, the volume of the gas increases as the gas’s temperature increases. Likewise, the volume of the gas decreases as the gas’s temperature decreases.

States of Matter Section 4 Visual Concept: Charles’s Law

States of Matter Standardized Test Prep Understanding Concepts, continued 2. A sealed refuse container is buried near a fault line, and seismic activity brings the container close to an underground source of geothermal heat. As the container gets warmer, what happens to the internal air pressure of the container? F. Particles are far apart and move freely. G. Particles are close together and vibrate in place. H. Particles are far apart and unable to change location. I. Particles are close together and move past each other easily.

States of Matter Standardized Test Prep Understanding Concepts, continued 2. A sealed refuse container is buried near a fault line, and seismic activity brings the container close to an underground source of geothermal heat. As the container gets warmer, what happens to the internal air pressure of the container? F. Particles are far apart and move freely. G. Particles are close together and vibrate in place. H. Particles are far apart and unable to change location. I. Particles are close together and move past each other easily.

States of Matter Standardized Test Prep Interpreting Graphics, continued 13. As more helium is released from the tank, the person who is inflating the balloons notices that the tank has become cold to the touch. Why does this happen?

States of Matter Standardized Test Prep Interpreting Graphics, continued 13. As more helium is released from the tank, the person who is inflating the balloons notices that the tank has become cold to the touch. Why does this happen? Answer: Given a constant volume, as the pressure of a gas decreases, so does the temperature.
 Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 Thermal energy states of matter
Thermal energy states of matter What is heat energy?
What is heat energy? Uses of heat
Uses of heat Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Section 1 matter and thermal energy
Section 1 matter and thermal energy Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Energy naturally flows from warmer matter to cooler matter.
Energy naturally flows from warmer matter to cooler matter. Chemical potential energy images
Chemical potential energy images Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Solid
Solid Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids What were the 11 free states
What were the 11 free states Northern and southern states
Northern and southern states How does the constitution guard against tyranny
How does the constitution guard against tyranny Section 3 using thermal energy
Section 3 using thermal energy Chapter 8 section 1 how organisms obtain energy answer key
Chapter 8 section 1 how organisms obtain energy answer key Chapter 7 section 4 states rights and the national bank
Chapter 7 section 4 states rights and the national bank Gray matter in the brain
Gray matter in the brain Gyrus and sulcus function
Gyrus and sulcus function Gray matter and white matter
Gray matter and white matter What is gray matter
What is gray matter Whats the study of matter and energy
Whats the study of matter and energy Four states of matter
Four states of matter Four states of matter
Four states of matter States of matter
States of matter Changing states of matter
Changing states of matter Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Venn diagram of states of matter
Venn diagram of states of matter The kinetic theory of matter states that
The kinetic theory of matter states that Chapter 12 states of matter study guide
Chapter 12 states of matter study guide States of matter basics
States of matter basics States of matter foldable
States of matter foldable The fundamental difference between states of matter is the
The fundamental difference between states of matter is the States of matter graph
States of matter graph Solid liquid gas concept map
Solid liquid gas concept map States of matter concept map
States of matter concept map Better matter
Better matter States of matter
States of matter Properties of matter jeopardy
Properties of matter jeopardy Chemical equation states of matter
Chemical equation states of matter All matter is in constant motion
All matter is in constant motion 4 phases of matter
4 phases of matter States of matter solid liquid gas
States of matter solid liquid gas Interconversion of states of matter
Interconversion of states of matter Mixture flow chart
Mixture flow chart Five states of matter
Five states of matter Examples of gas
Examples of gas States of matter
States of matter States of matter
States of matter The change of phase from gas to solid *
The change of phase from gas to solid * Vocab
Vocab 5 states of matter
5 states of matter Jeopardy states of matter
Jeopardy states of matter Chapter 4 work and energy section 1 work and machines
Chapter 4 work and energy section 1 work and machines Lesson 3 energy and matter in ecosystems answer key
Lesson 3 energy and matter in ecosystems answer key Science matter and energy
Science matter and energy Matter energy and measurement
Matter energy and measurement Dark matter and dark energy presentation
Dark matter and dark energy presentation Unit 2 matter and energy
Unit 2 matter and energy Trophic level definition biology
Trophic level definition biology Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Mechanical waves and electromagnetic waves similarities
Mechanical waves and electromagnetic waves similarities Properties of waves
Properties of waves Energy pyramid labeled
Energy pyramid labeled The law of conservation of energy states that
The law of conservation of energy states that The law of conservation of energy states that
The law of conservation of energy states that Kinetic potential
Kinetic potential Energy states
Energy states