Organic Chemistry Second Edition David Klein Chapter 1








- Slides: 91

Organic Chemistry Second Edition David Klein Chapter 1 A Review of General Chemistry: Electrons, Bonds, and Molecular Properties Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

1. 1 Organic Chemistry • The study of carbon-containing molecules and their reactions • What happens to a molecule during a reaction? – A collision – Bonds break/form • The BIG question: WHY do reactions occur? – We will need at least 2 semesters of your time to answer this question – FOCUS ON THE ELECTRONS Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -2 Klein, Organic Chemistry 2 e

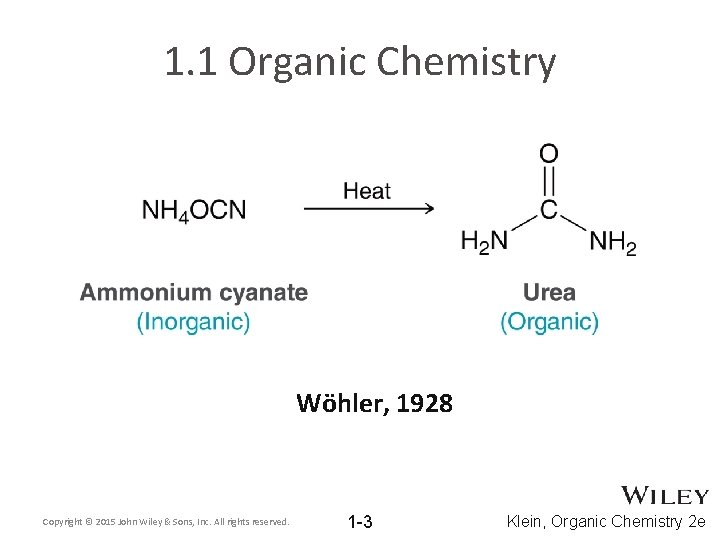
1. 1 Organic Chemistry Wöhler, 1928 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -3 Klein, Organic Chemistry 2 e

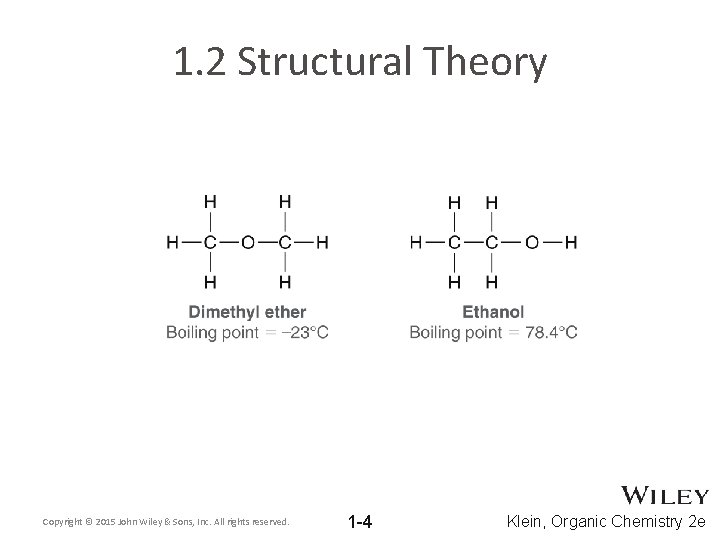
1. 2 Structural Theory Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -4 Klein, Organic Chemistry 2 e

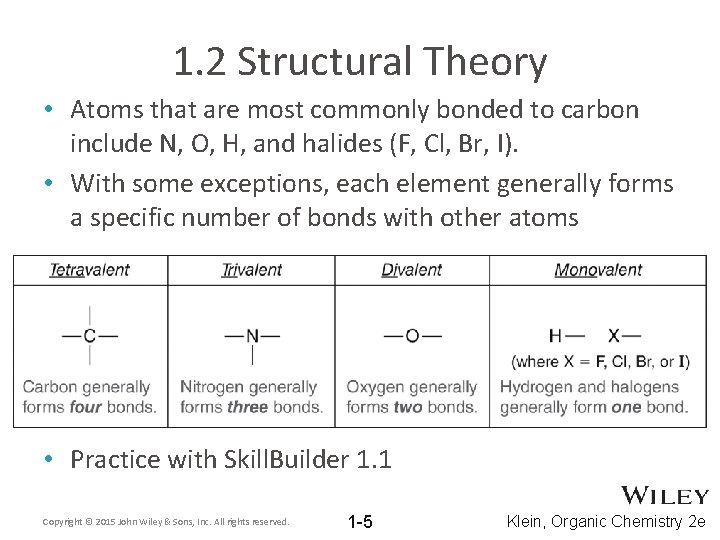
1. 2 Structural Theory • Atoms that are most commonly bonded to carbon include N, O, H, and halides (F, Cl, Br, I). • With some exceptions, each element generally forms a specific number of bonds with other atoms • Practice with Skill. Builder 1. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -5 Klein, Organic Chemistry 2 e

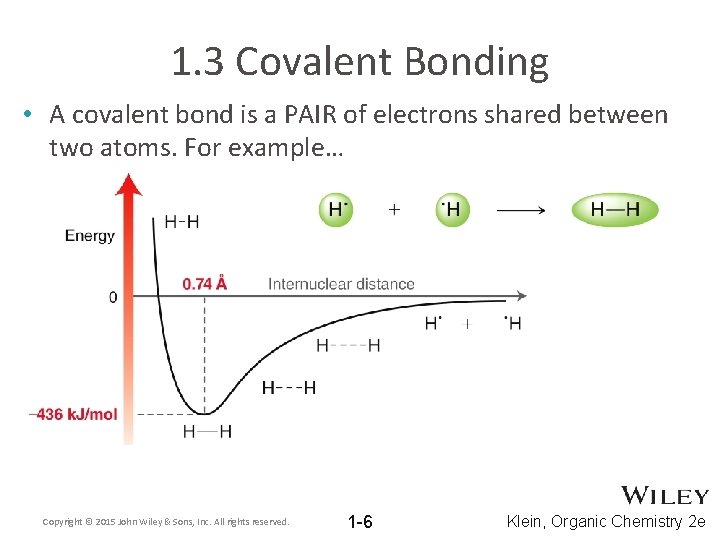
1. 3 Covalent Bonding • A covalent bond is a PAIR of electrons shared between two atoms. For example… Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -6 Klein, Organic Chemistry 2 e

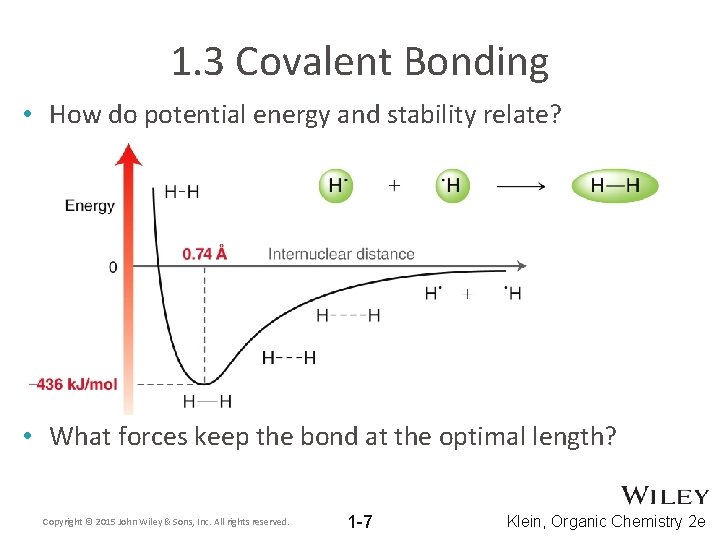
1. 3 Covalent Bonding • How do potential energy and stability relate? • What forces keep the bond at the optimal length? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -7 Klein, Organic Chemistry 2 e

1. 3 Atomic Structure • A review from General Chemistry – Protons (+1) and neutrons (neutral) reside in the nucleus – Electrons (-1) reside outside the nucleus. – Some electrons are close to the nucleus and others are far away. – Look at carbon for example. Which electrons are the valence electrons? – Why are valence electrons important? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -8 Klein, Organic Chemistry 2 e

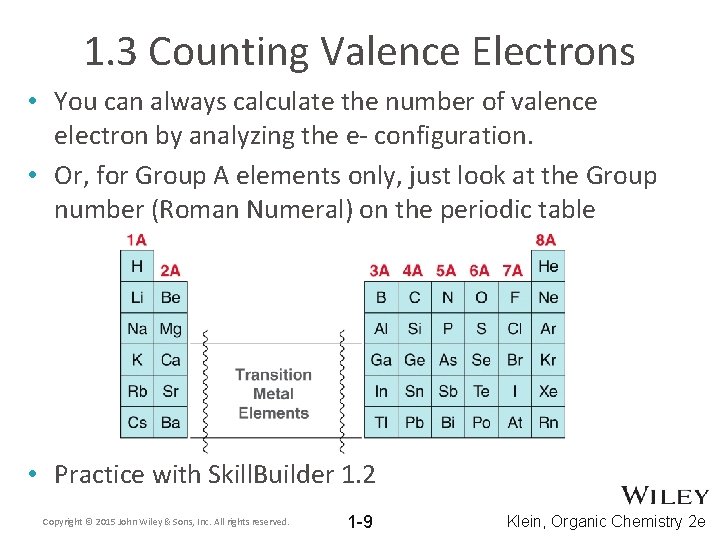
1. 3 Counting Valence Electrons • You can always calculate the number of valence electron by analyzing the e- configuration. • Or, for Group A elements only, just look at the Group number (Roman Numeral) on the periodic table • Practice with Skill. Builder 1. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -9 Klein, Organic Chemistry 2 e

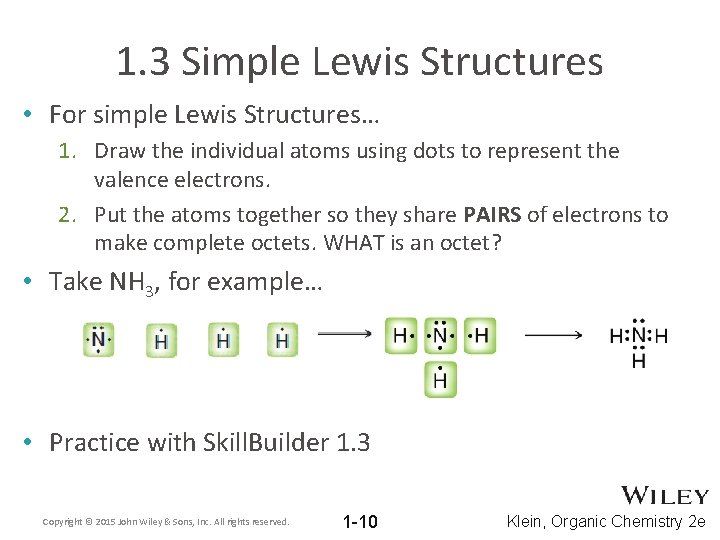
1. 3 Simple Lewis Structures • For simple Lewis Structures… 1. Draw the individual atoms using dots to represent the valence electrons. 2. Put the atoms together so they share PAIRS of electrons to make complete octets. WHAT is an octet? • Take NH 3, for example… • Practice with Skill. Builder 1. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -10 Klein, Organic Chemistry 2 e

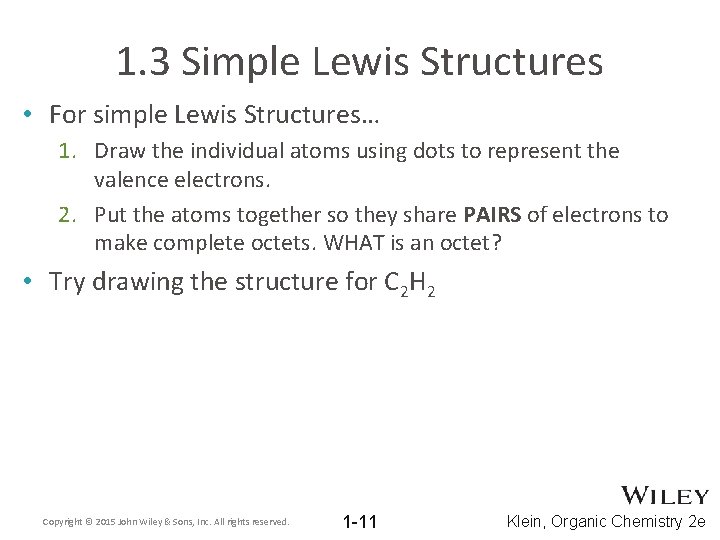
1. 3 Simple Lewis Structures • For simple Lewis Structures… 1. Draw the individual atoms using dots to represent the valence electrons. 2. Put the atoms together so they share PAIRS of electrons to make complete octets. WHAT is an octet? • Try drawing the structure for C 2 H 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -11 Klein, Organic Chemistry 2 e

1. 4 Formal Charge • What term do we use to describe atoms with an unbalanced or FORMAL charge? • How does formal charge affect the stability of an atom? • Atoms in molecules (sharing electrons) can also have unbalanced charge, which must be analyzed, because it affects stability • To calculate FORMAL charge for an atom, compare the number of valence electrons that should be associated with the atom to the number of valence electrons that are actually associated with an atom Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -12 Klein, Organic Chemistry 2 e

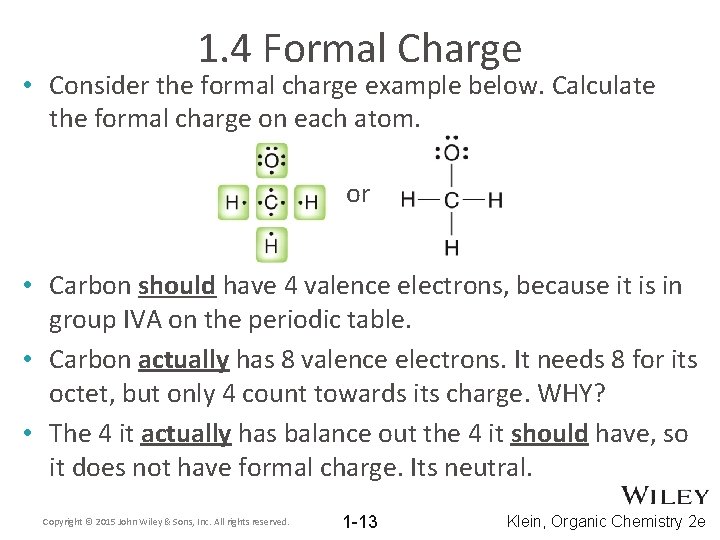
1. 4 Formal Charge • Consider the formal charge example below. Calculate the formal charge on each atom. or • Carbon should have 4 valence electrons, because it is in group IVA on the periodic table. • Carbon actually has 8 valence electrons. It needs 8 for its octet, but only 4 count towards its charge. WHY? • The 4 it actually has balance out the 4 it should have, so it does not have formal charge. Its neutral. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -13 Klein, Organic Chemistry 2 e

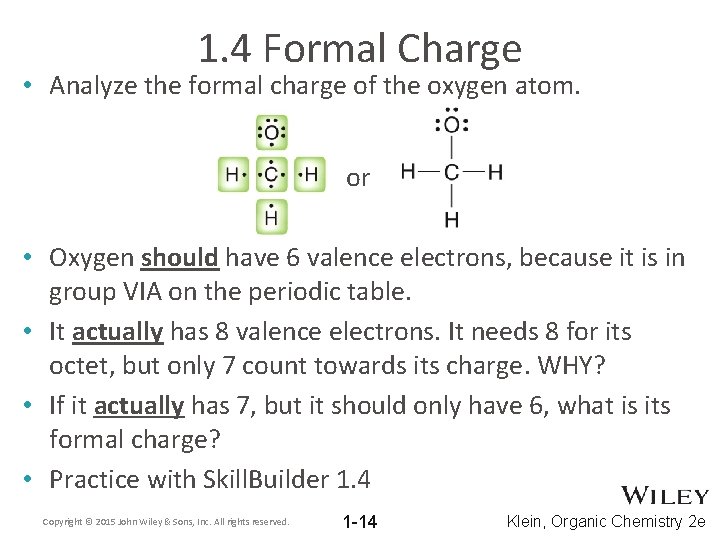
1. 4 Formal Charge • Analyze the formal charge of the oxygen atom. or • Oxygen should have 6 valence electrons, because it is in group VIA on the periodic table. • It actually has 8 valence electrons. It needs 8 for its octet, but only 7 count towards its charge. WHY? • If it actually has 7, but it should only have 6, what is its formal charge? • Practice with Skill. Builder 1. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -14 Klein, Organic Chemistry 2 e

1. 5 Polar Covalent Bonds • Covalent bonds are electrons pairs that exist in an orbital shared between two atoms. What do you think that orbital looks like? • Just like an atomic orbital, the electrons could be anywhere within that orbital region. • What factors determine which atom in the bond will attract the shared electrons more? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -15 Klein, Organic Chemistry 2 e

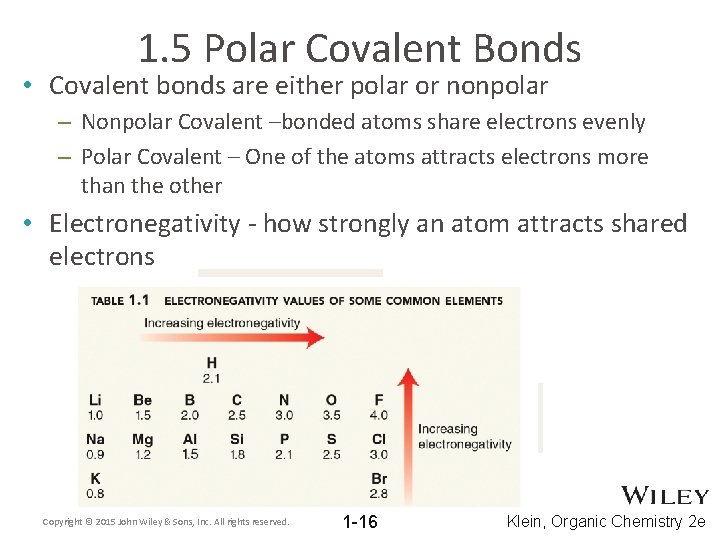
1. 5 Polar Covalent Bonds • Covalent bonds are either polar or nonpolar – Nonpolar Covalent –bonded atoms share electrons evenly – Polar Covalent – One of the atoms attracts electrons more than the other • Electronegativity - how strongly an atom attracts shared electrons Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -16 Klein, Organic Chemistry 2 e

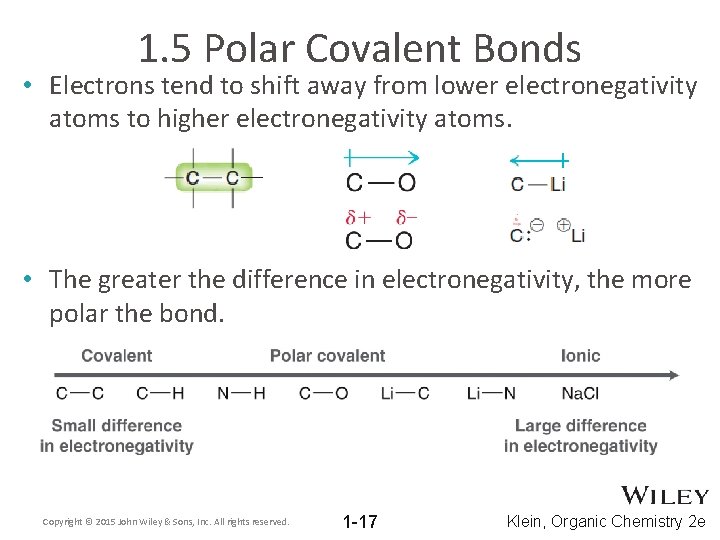
1. 5 Polar Covalent Bonds • Electrons tend to shift away from lower electronegativity atoms to higher electronegativity atoms. • The greater the difference in electronegativity, the more polar the bond. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -17 Klein, Organic Chemistry 2 e

1. 5 Polar Covalent Bonds • Can a bond have both covalent and ionic character? • Practice with Skill. Builder 1. 5 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -18 Klein, Organic Chemistry 2 e

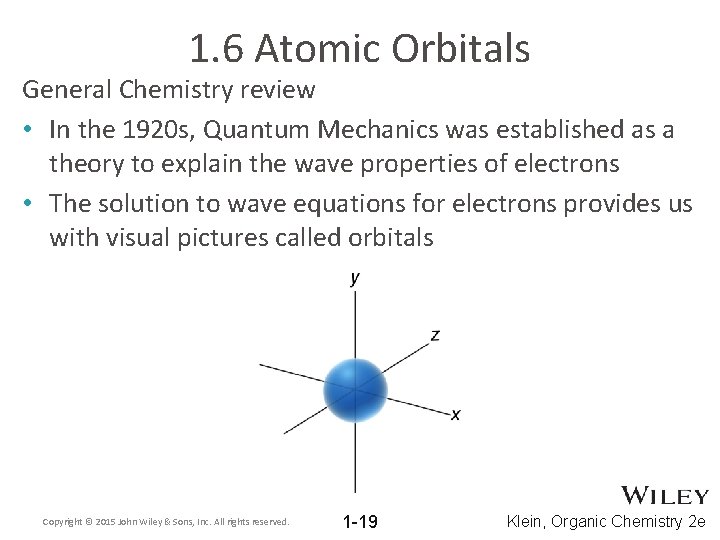
1. 6 Atomic Orbitals General Chemistry review • In the 1920 s, Quantum Mechanics was established as a theory to explain the wave properties of electrons • The solution to wave equations for electrons provides us with visual pictures called orbitals Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -19 Klein, Organic Chemistry 2 e

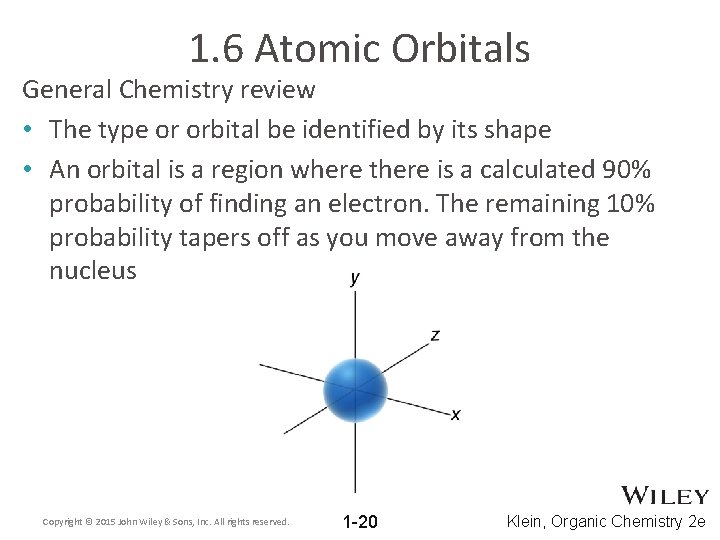
1. 6 Atomic Orbitals General Chemistry review • The type or orbital be identified by its shape • An orbital is a region where there is a calculated 90% probability of finding an electron. The remaining 10% probability tapers off as you move away from the nucleus Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -20 Klein, Organic Chemistry 2 e

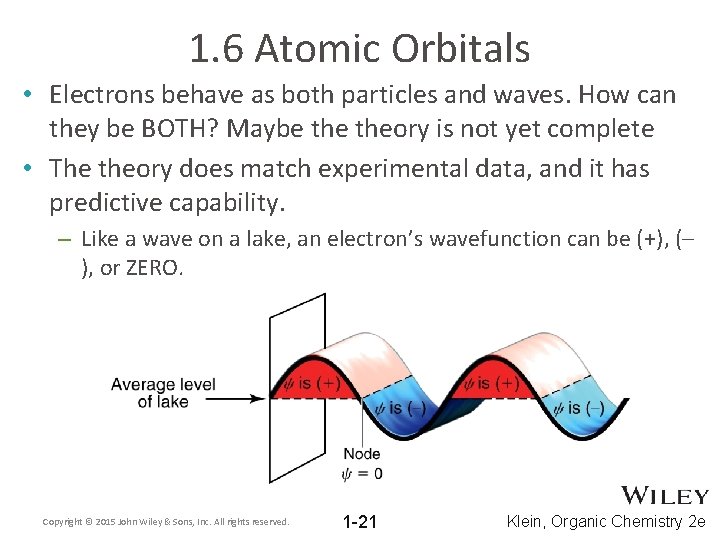
1. 6 Atomic Orbitals • Electrons behave as both particles and waves. How can they be BOTH? Maybe theory is not yet complete • The theory does match experimental data, and it has predictive capability. – Like a wave on a lake, an electron’s wavefunction can be (+), (– ), or ZERO. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -21 Klein, Organic Chemistry 2 e

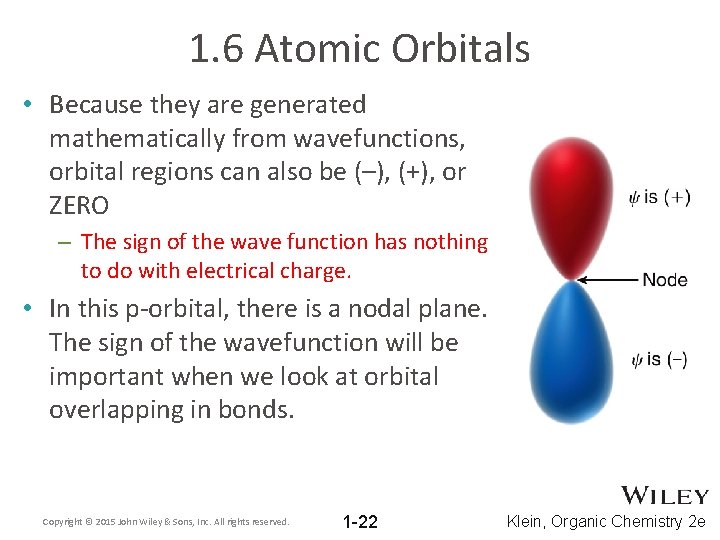
1. 6 Atomic Orbitals • Because they are generated mathematically from wavefunctions, orbital regions can also be (–), (+), or ZERO – The sign of the wave function has nothing to do with electrical charge. • In this p-orbital, there is a nodal plane. The sign of the wavefunction will be important when we look at orbital overlapping in bonds. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -22 Klein, Organic Chemistry 2 e

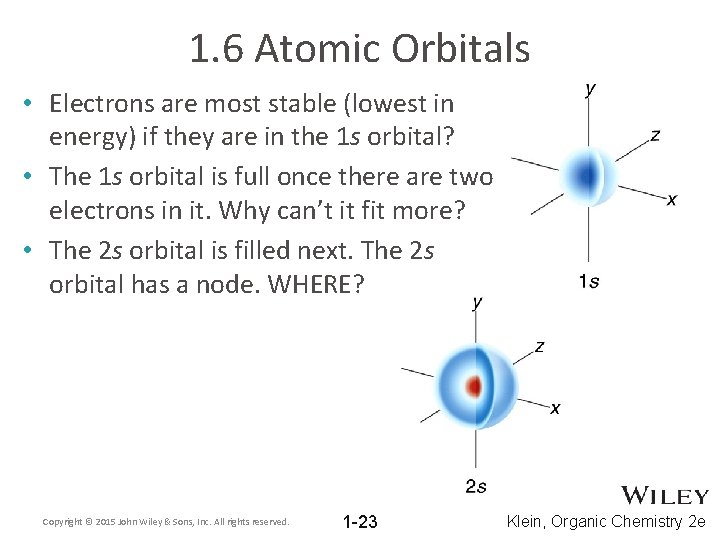
1. 6 Atomic Orbitals • Electrons are most stable (lowest in energy) if they are in the 1 s orbital? • The 1 s orbital is full once there are two electrons in it. Why can’t it fit more? • The 2 s orbital is filled next. The 2 s orbital has a node. WHERE? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -23 Klein, Organic Chemistry 2 e

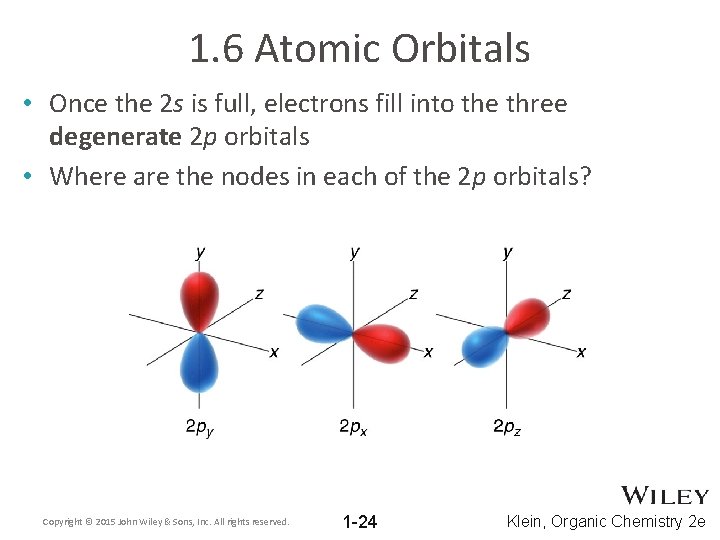
1. 6 Atomic Orbitals • Once the 2 s is full, electrons fill into the three degenerate 2 p orbitals • Where are the nodes in each of the 2 p orbitals? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -24 Klein, Organic Chemistry 2 e

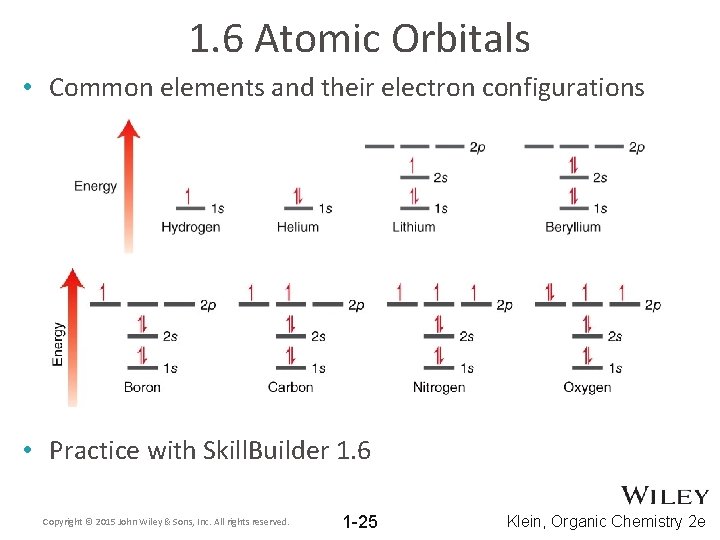
1. 6 Atomic Orbitals • Common elements and their electron configurations • Practice with Skill. Builder 1. 6 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -25 Klein, Organic Chemistry 2 e

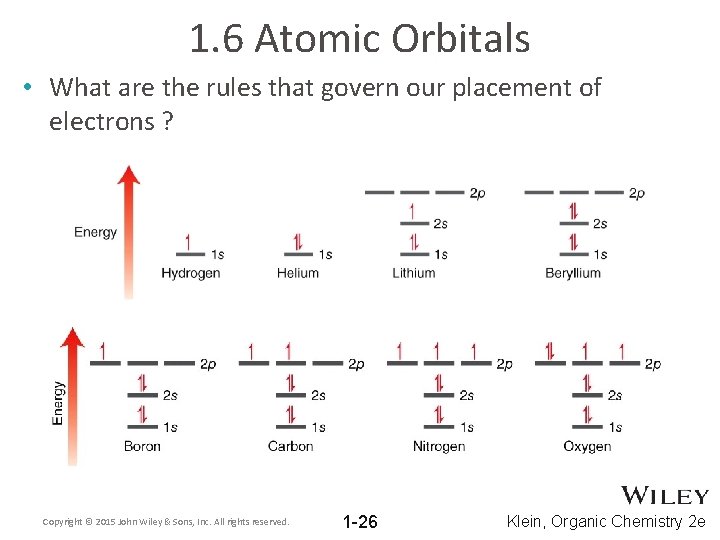
1. 6 Atomic Orbitals • What are the rules that govern our placement of electrons ? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -26 Klein, Organic Chemistry 2 e

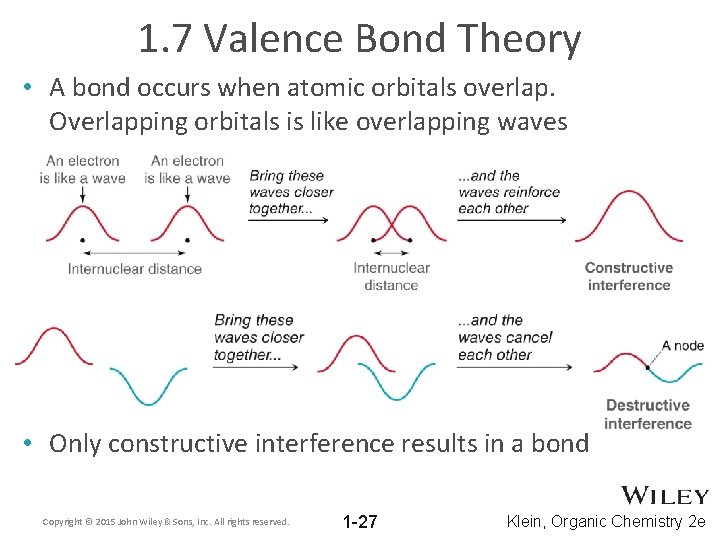
1. 7 Valence Bond Theory • A bond occurs when atomic orbitals overlap. Overlapping orbitals is like overlapping waves • Only constructive interference results in a bond Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -27 Klein, Organic Chemistry 2 e

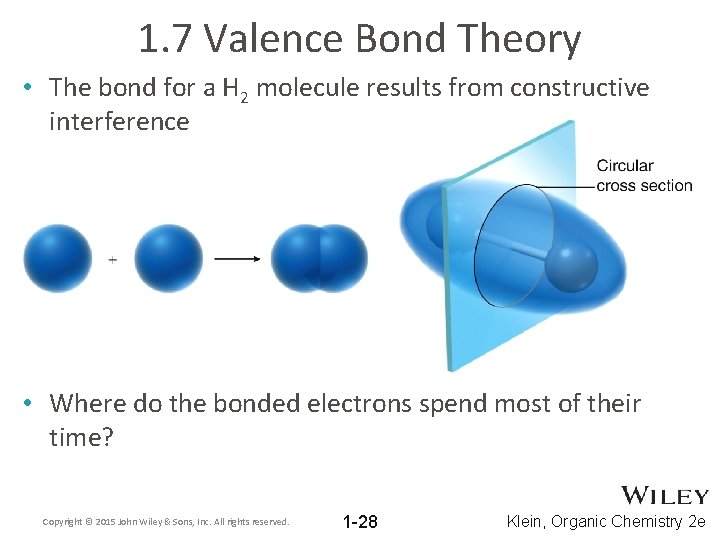
1. 7 Valence Bond Theory • The bond for a H 2 molecule results from constructive interference • Where do the bonded electrons spend most of their time? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -28 Klein, Organic Chemistry 2 e

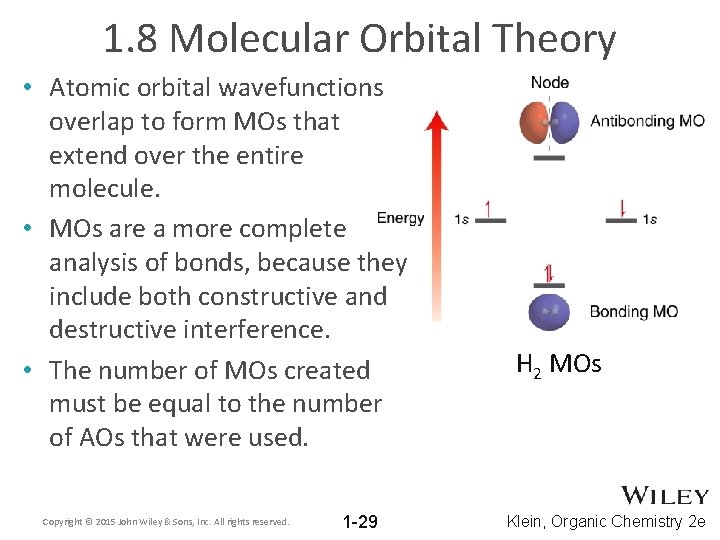
1. 8 Molecular Orbital Theory • Atomic orbital wavefunctions overlap to form MOs that extend over the entire molecule. • MOs are a more complete analysis of bonds, because they include both constructive and destructive interference. • The number of MOs created must be equal to the number of AOs that were used. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -29 H 2 MOs Klein, Organic Chemistry 2 e

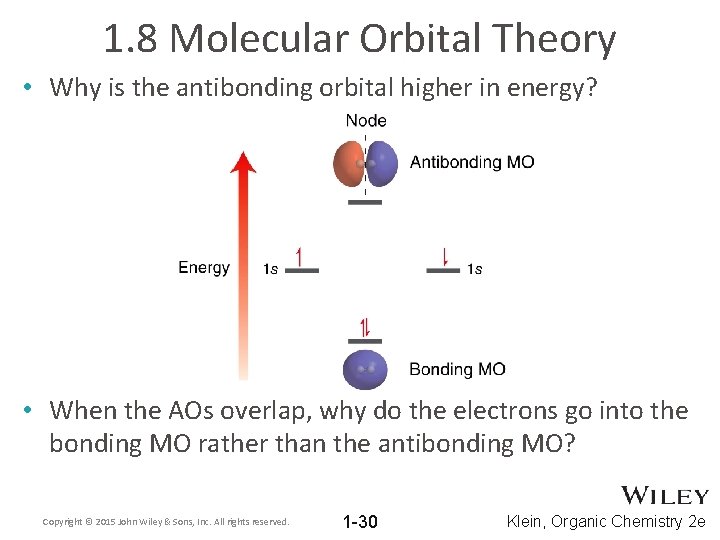
1. 8 Molecular Orbital Theory • Why is the antibonding orbital higher in energy? • When the AOs overlap, why do the electrons go into the bonding MO rather than the antibonding MO? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -30 Klein, Organic Chemistry 2 e

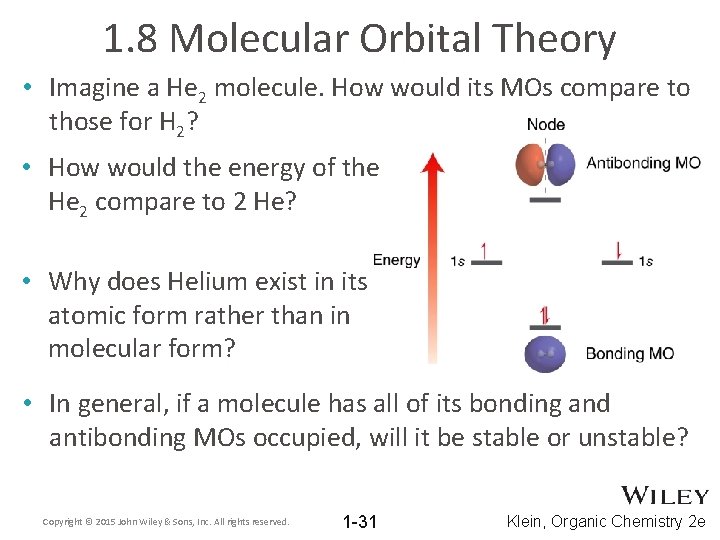
1. 8 Molecular Orbital Theory • Imagine a He 2 molecule. How would its MOs compare to those for H 2? • How would the energy of the He 2 compare to 2 He? • Why does Helium exist in its atomic form rather than in molecular form? • In general, if a molecule has all of its bonding and antibonding MOs occupied, will it be stable or unstable? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -31 Klein, Organic Chemistry 2 e

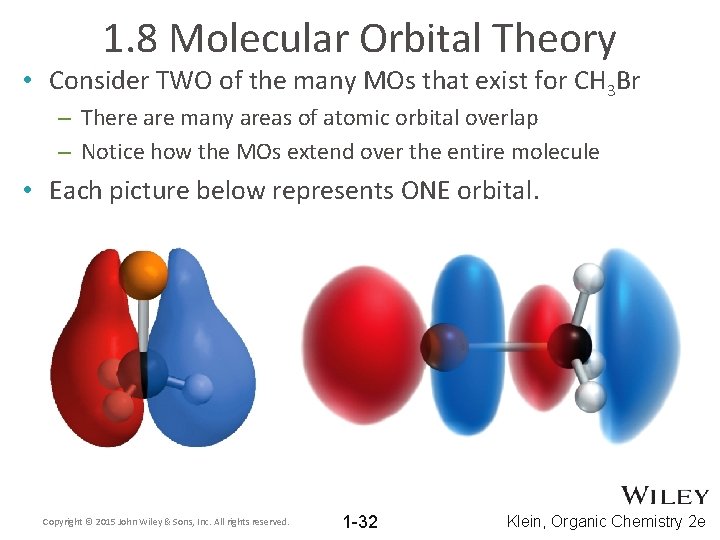
1. 8 Molecular Orbital Theory • Consider TWO of the many MOs that exist for CH 3 Br – There are many areas of atomic orbital overlap – Notice how the MOs extend over the entire molecule • Each picture below represents ONE orbital. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -32 Klein, Organic Chemistry 2 e

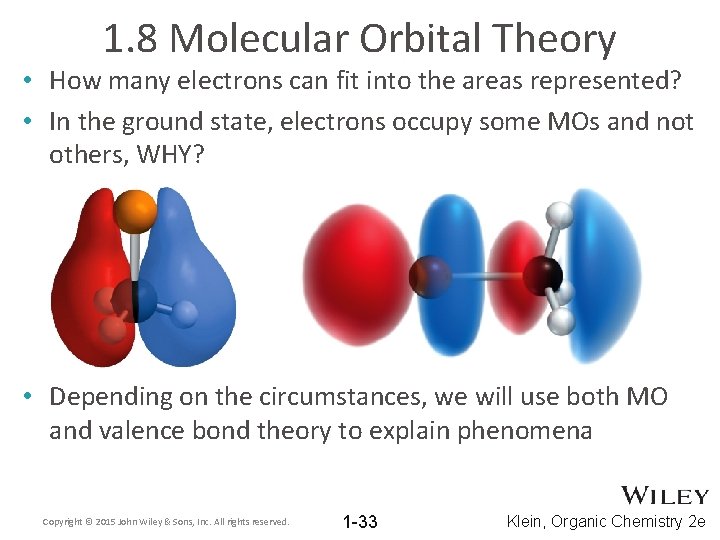
1. 8 Molecular Orbital Theory • How many electrons can fit into the areas represented? • In the ground state, electrons occupy some MOs and not others, WHY? • Depending on the circumstances, we will use both MO and valence bond theory to explain phenomena Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -33 Klein, Organic Chemistry 2 e

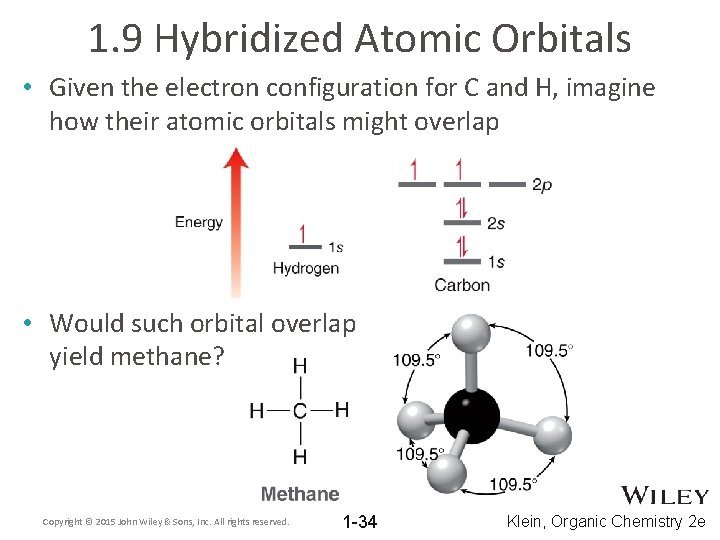
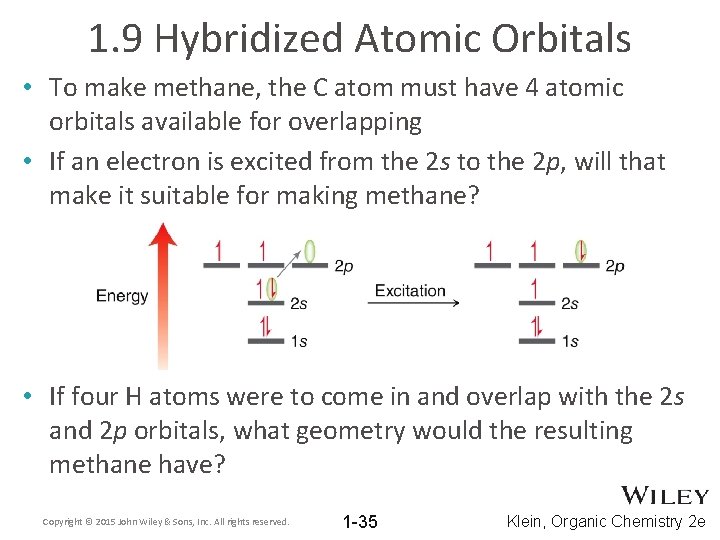
1. 9 Hybridized Atomic Orbitals • Given the electron configuration for C and H, imagine how their atomic orbitals might overlap • Would such orbital overlap yield methane? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -34 Klein, Organic Chemistry 2 e

1. 9 Hybridized Atomic Orbitals • To make methane, the C atom must have 4 atomic orbitals available for overlapping • If an electron is excited from the 2 s to the 2 p, will that make it suitable for making methane? • If four H atoms were to come in and overlap with the 2 s and 2 p orbitals, what geometry would the resulting methane have? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -35 Klein, Organic Chemistry 2 e

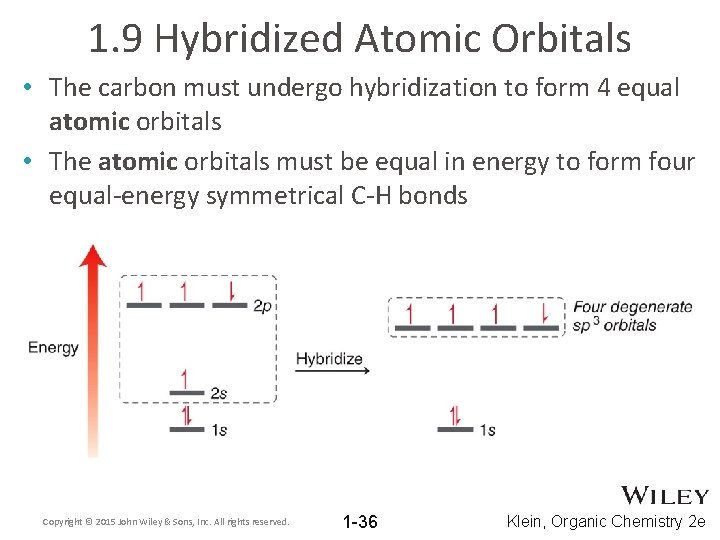
1. 9 Hybridized Atomic Orbitals • The carbon must undergo hybridization to form 4 equal atomic orbitals • The atomic orbitals must be equal in energy to form four equal-energy symmetrical C-H bonds Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -36 Klein, Organic Chemistry 2 e

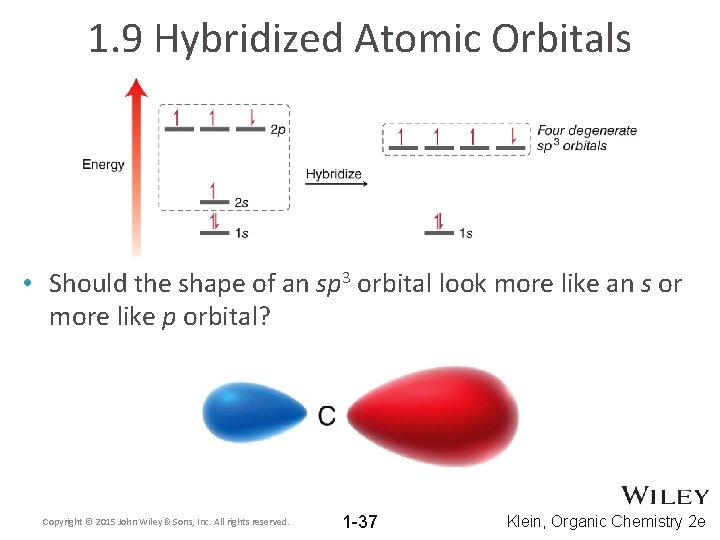
1. 9 Hybridized Atomic Orbitals • Should the shape of an sp 3 orbital look more like an s or more like p orbital? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -37 Klein, Organic Chemistry 2 e

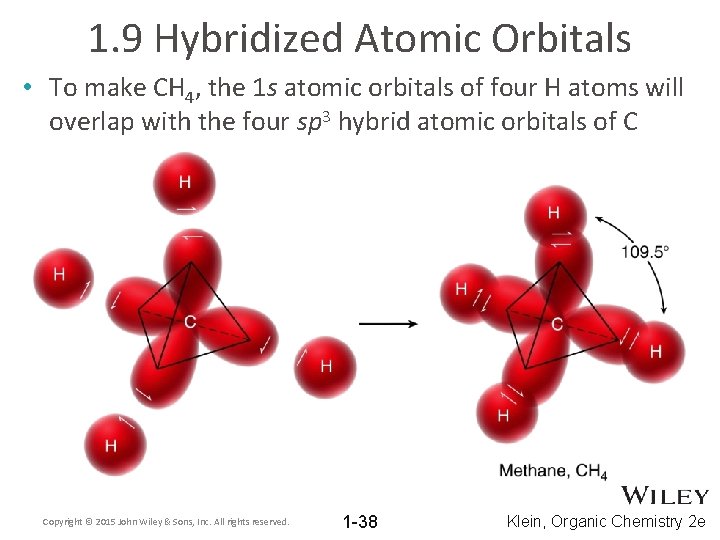
1. 9 Hybridized Atomic Orbitals • To make CH 4, the 1 s atomic orbitals of four H atoms will overlap with the four sp 3 hybrid atomic orbitals of C Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -38 Klein, Organic Chemistry 2 e

1. 9 Hybridized Atomic Orbitals • Draw a picture that shows the necessary atomic orbitals and their overlap to form ethane (C 2 H 6). • Draw a picture that shows the necessary atomic orbitals and their overlap to form water. • Practice with conceptual checkpoint 1. 19 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -39 Klein, Organic Chemistry 2 e

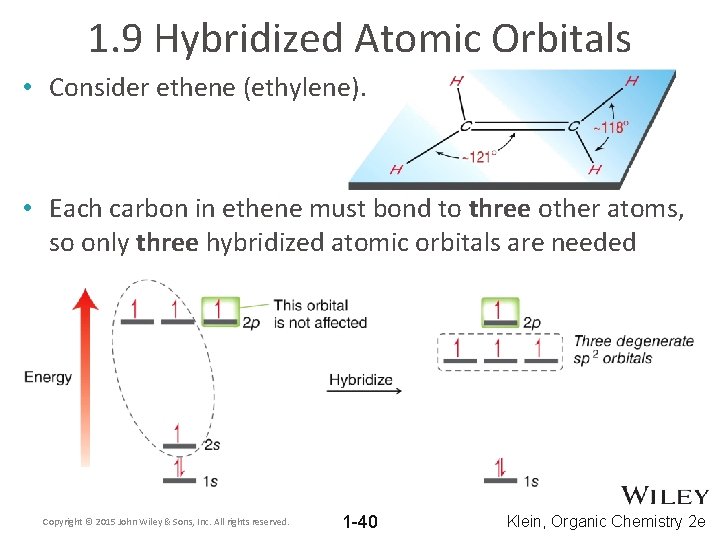
1. 9 Hybridized Atomic Orbitals • Consider ethene (ethylene). • Each carbon in ethene must bond to three other atoms, so only three hybridized atomic orbitals are needed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -40 Klein, Organic Chemistry 2 e

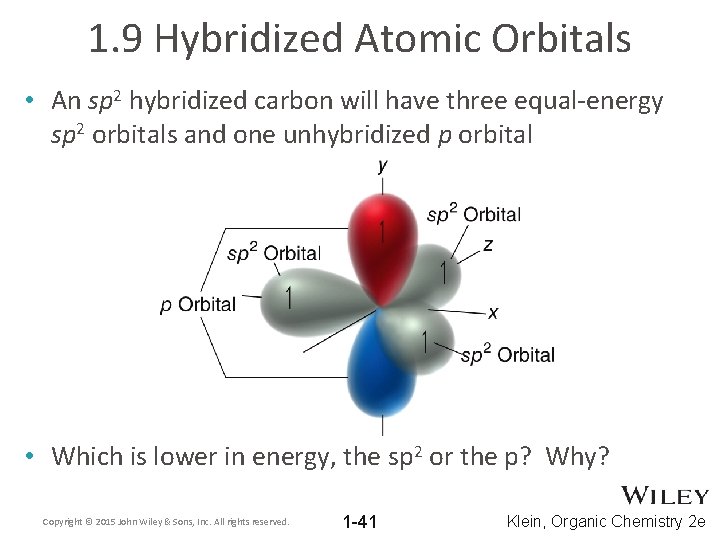
1. 9 Hybridized Atomic Orbitals • An sp 2 hybridized carbon will have three equal-energy sp 2 orbitals and one unhybridized p orbital • Which is lower in energy, the sp 2 or the p? Why? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -41 Klein, Organic Chemistry 2 e

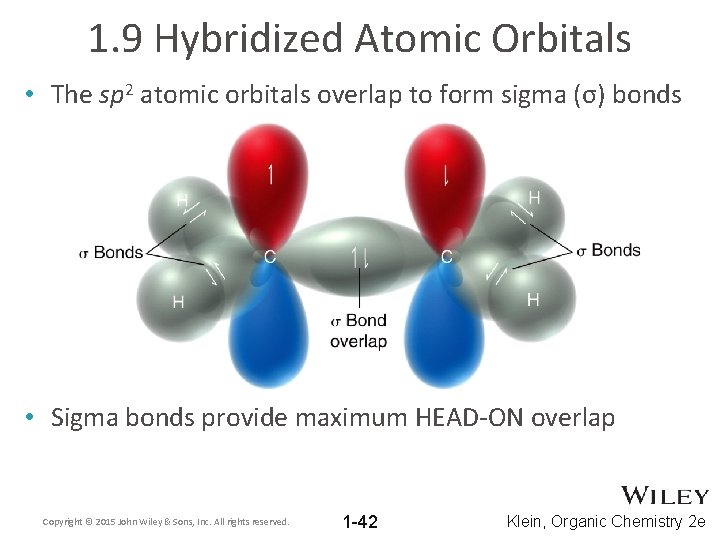
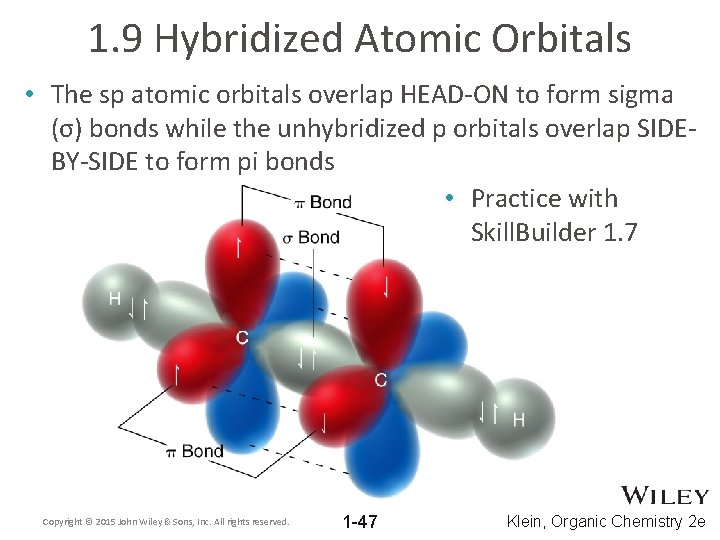
1. 9 Hybridized Atomic Orbitals • The sp 2 atomic orbitals overlap to form sigma (σ) bonds • Sigma bonds provide maximum HEAD-ON overlap Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -42 Klein, Organic Chemistry 2 e

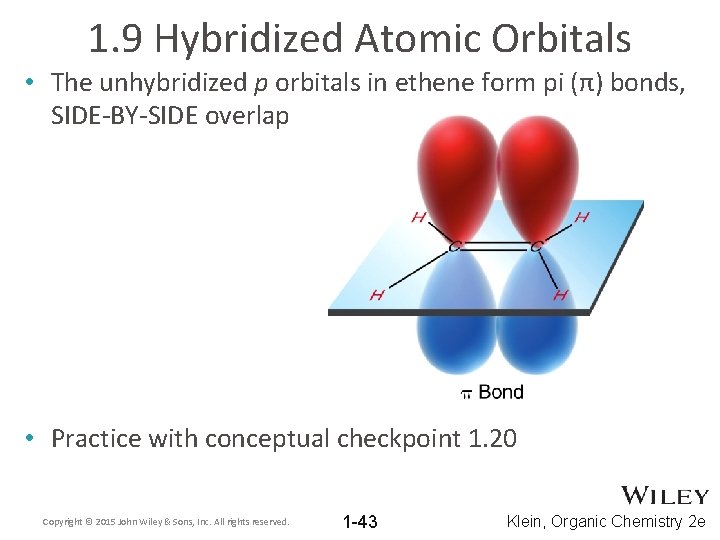
1. 9 Hybridized Atomic Orbitals • The unhybridized p orbitals in ethene form pi (π) bonds, SIDE-BY-SIDE overlap • Practice with conceptual checkpoint 1. 20 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -43 Klein, Organic Chemistry 2 e

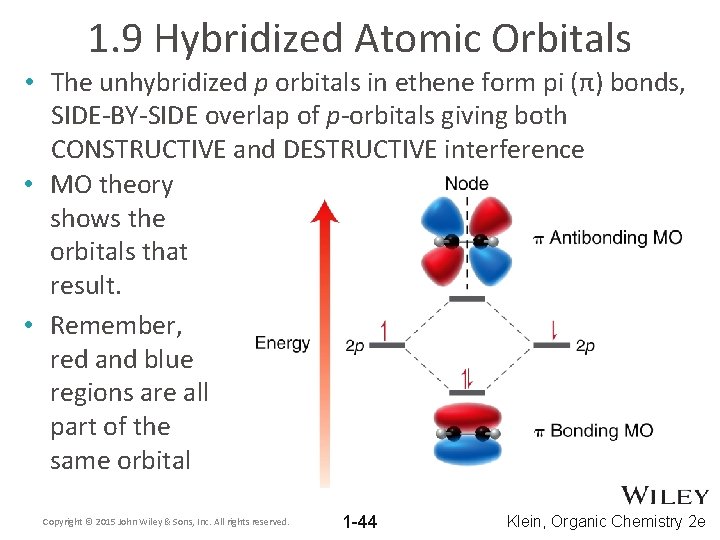
1. 9 Hybridized Atomic Orbitals • The unhybridized p orbitals in ethene form pi (π) bonds, SIDE-BY-SIDE overlap of p-orbitals giving both CONSTRUCTIVE and DESTRUCTIVE interference • MO theory shows the orbitals that result. • Remember, red and blue regions are all part of the same orbital Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -44 Klein, Organic Chemistry 2 e

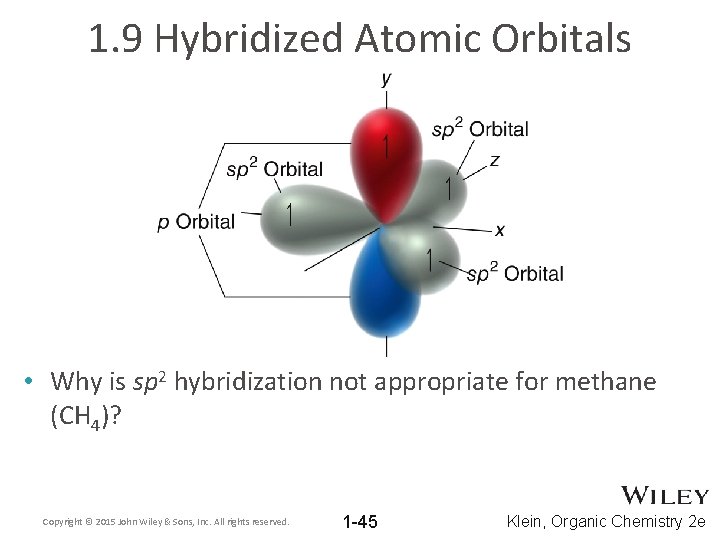
1. 9 Hybridized Atomic Orbitals • Why is sp 2 hybridization not appropriate for methane (CH 4)? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -45 Klein, Organic Chemistry 2 e

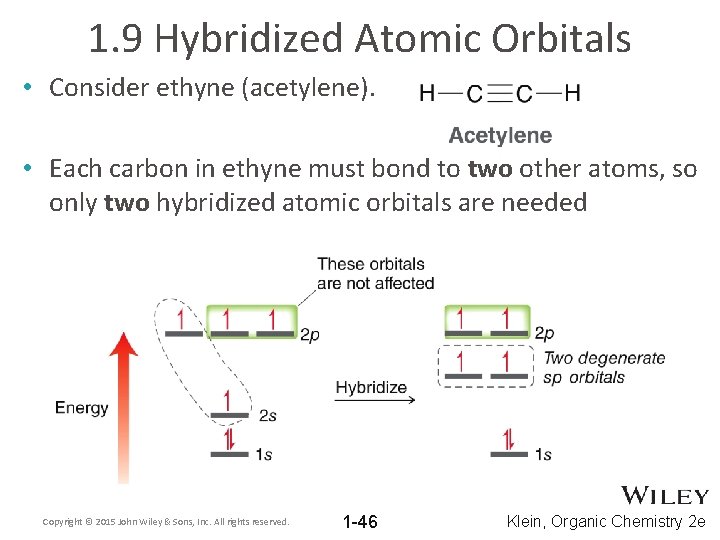
1. 9 Hybridized Atomic Orbitals • Consider ethyne (acetylene). • Each carbon in ethyne must bond to two other atoms, so only two hybridized atomic orbitals are needed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -46 Klein, Organic Chemistry 2 e

1. 9 Hybridized Atomic Orbitals • The sp atomic orbitals overlap HEAD-ON to form sigma (σ) bonds while the unhybridized p orbitals overlap SIDEBY-SIDE to form pi bonds • Practice with Skill. Builder 1. 7 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -47 Klein, Organic Chemistry 2 e

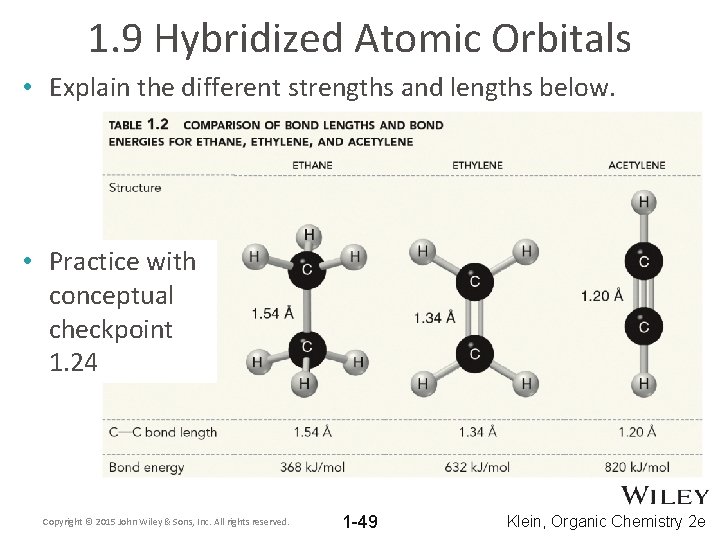
1. 9 Hybridized Atomic Orbitals • Which should be stronger, a pi bond or a sigma bond? WHY? • Which should be longer, an sp 3 – sp 3 sigma bond overlap or an sp – sp sigma bond overlap? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -48 Klein, Organic Chemistry 2 e

1. 9 Hybridized Atomic Orbitals • Explain the different strengths and lengths below. • Practice with conceptual checkpoint 1. 24 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -49 Klein, Organic Chemistry 2 e

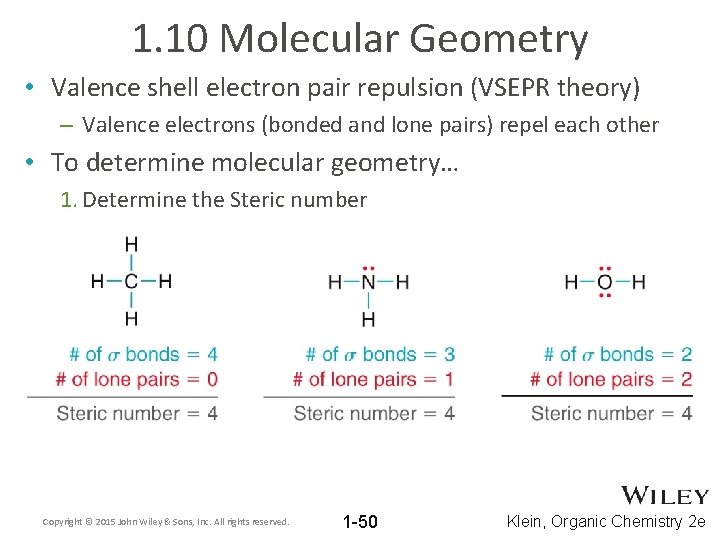
1. 10 Molecular Geometry • Valence shell electron pair repulsion (VSEPR theory) – Valence electrons (bonded and lone pairs) repel each other • To determine molecular geometry… 1. Determine the Steric number Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -50 Klein, Organic Chemistry 2 e

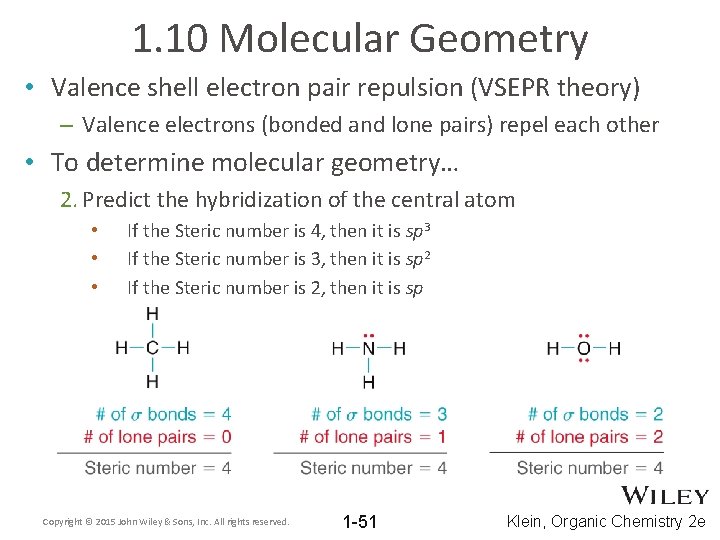
1. 10 Molecular Geometry • Valence shell electron pair repulsion (VSEPR theory) – Valence electrons (bonded and lone pairs) repel each other • To determine molecular geometry… 2. Predict the hybridization of the central atom • • • If the Steric number is 4, then it is sp 3 If the Steric number is 3, then it is sp 2 If the Steric number is 2, then it is sp Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -51 Klein, Organic Chemistry 2 e

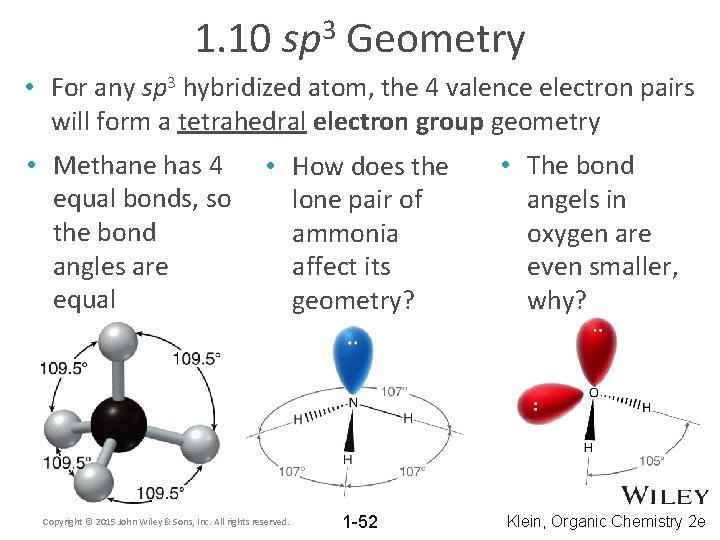
1. 10 3 sp Geometry • For any sp 3 hybridized atom, the 4 valence electron pairs will form a tetrahedral electron group geometry • Methane has 4 equal bonds, so the bond angles are equal • How does the lone pair of ammonia affect its geometry? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -52 • The bond angels in oxygen are even smaller, why? Klein, Organic Chemistry 2 e

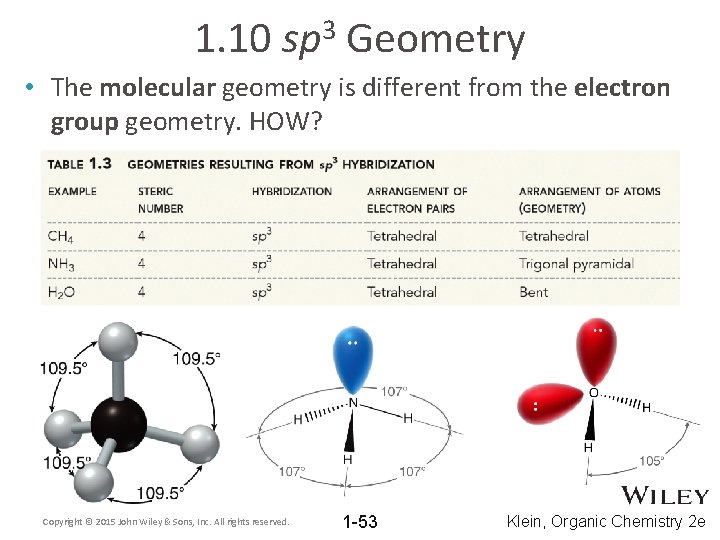
1. 10 3 sp Geometry • The molecular geometry is different from the electron group geometry. HOW? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -53 Klein, Organic Chemistry 2 e

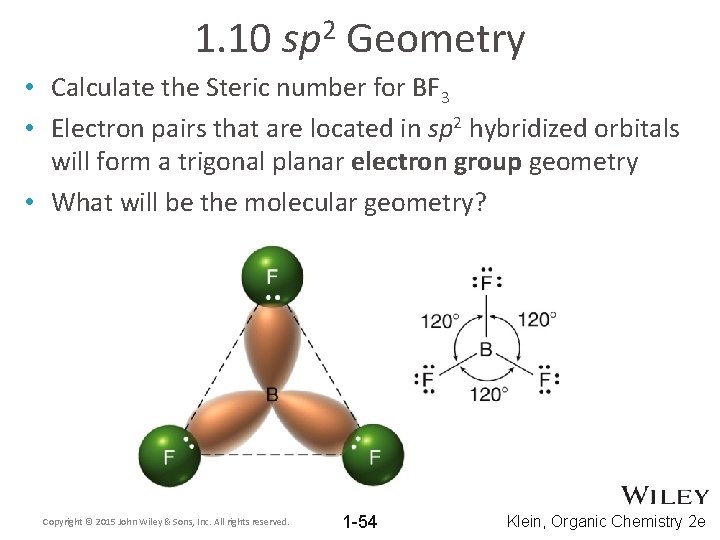
1. 10 2 sp Geometry • Calculate the Steric number for BF 3 • Electron pairs that are located in sp 2 hybridized orbitals will form a trigonal planar electron group geometry • What will be the molecular geometry? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -54 Klein, Organic Chemistry 2 e

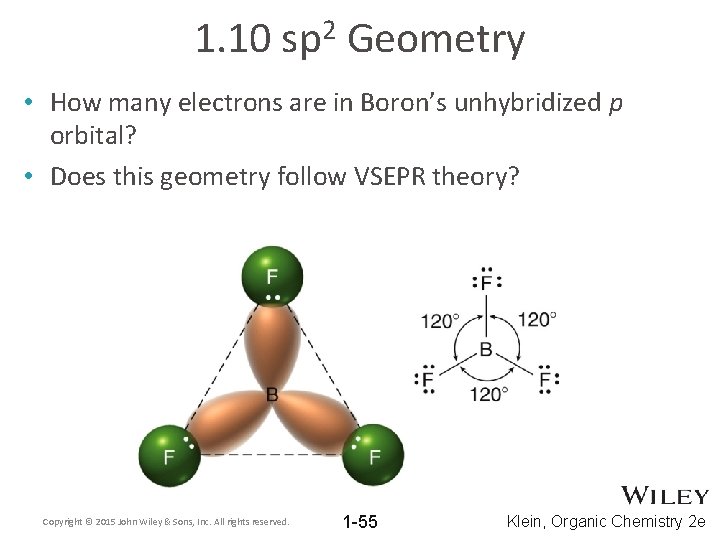
1. 10 2 sp Geometry • How many electrons are in Boron’s unhybridized p orbital? • Does this geometry follow VSEPR theory? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -55 Klein, Organic Chemistry 2 e

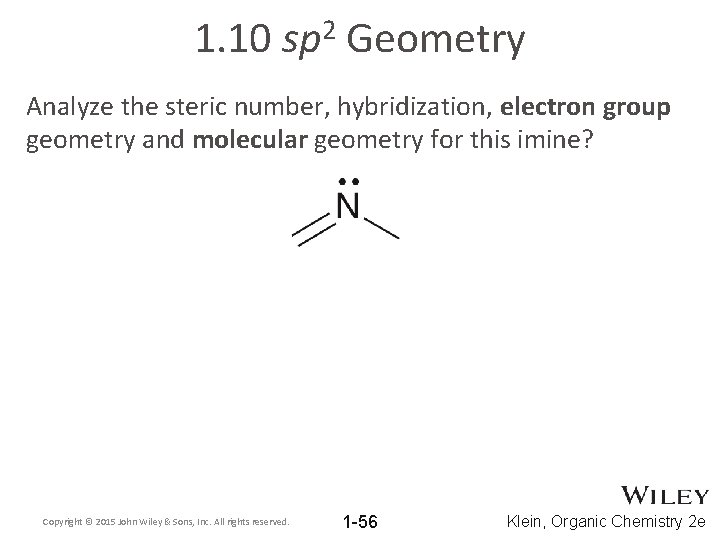
1. 10 2 sp Geometry Analyze the steric number, hybridization, electron group geometry and molecular geometry for this imine? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -56 Klein, Organic Chemistry 2 e

1. 10 sp Geometry • Analyze the Steric number, the hybridization, the electron group geometry, and the molecular geometry for the following molecules • Be. H 2 • CO 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -57 Klein, Organic Chemistry 2 e

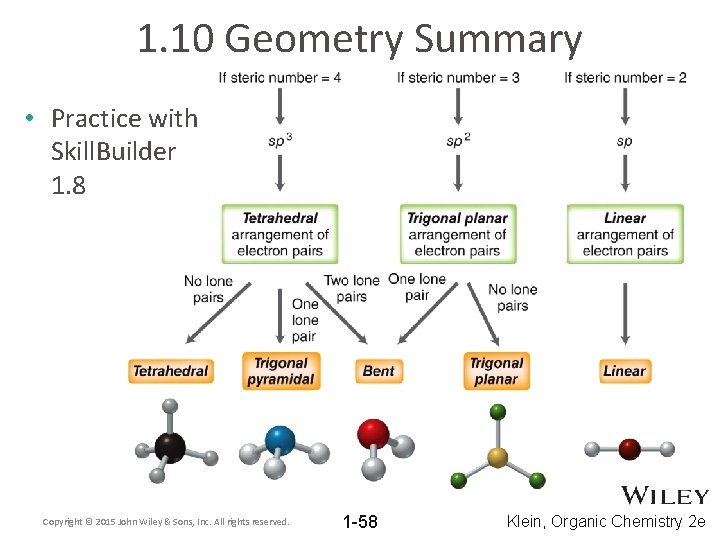
1. 10 Geometry Summary • Practice with Skill. Builder 1. 8 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -58 Klein, Organic Chemistry 2 e

1. 11 Molecular Polarity • Electronegativity Differences cause induction • Induction (shifting of electrons WITHIN their orbitals) results in a dipole moment. • Dipole moment = (the amount of partial charge) x (the distance the δ+ and δ- are separated) • Dipole moments are reported in units of debye (D) • 1 debye = 10 -18 esu ∙ cm – An esu is a unit of charge. 1 e- has a charge of 4. 80 x 10 -10 esu – cm are included in the unit, because the distance between the centers of + and – charges affects the dipole Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -59 Klein, Organic Chemistry 2 e

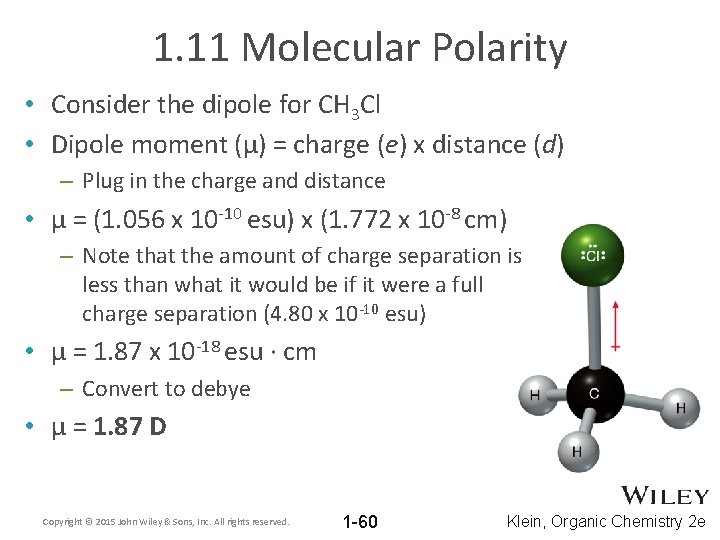
1. 11 Molecular Polarity • Consider the dipole for CH 3 Cl • Dipole moment (μ) = charge (e) x distance (d) – Plug in the charge and distance • μ = (1. 056 x 10 -10 esu) x (1. 772 x 10 -8 cm) – Note that the amount of charge separation is less than what it would be if it were a full charge separation (4. 80 x 10 -10 esu) • μ = 1. 87 x 10 -18 esu ∙ cm – Convert to debye • μ = 1. 87 D Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -60 Klein, Organic Chemistry 2 e

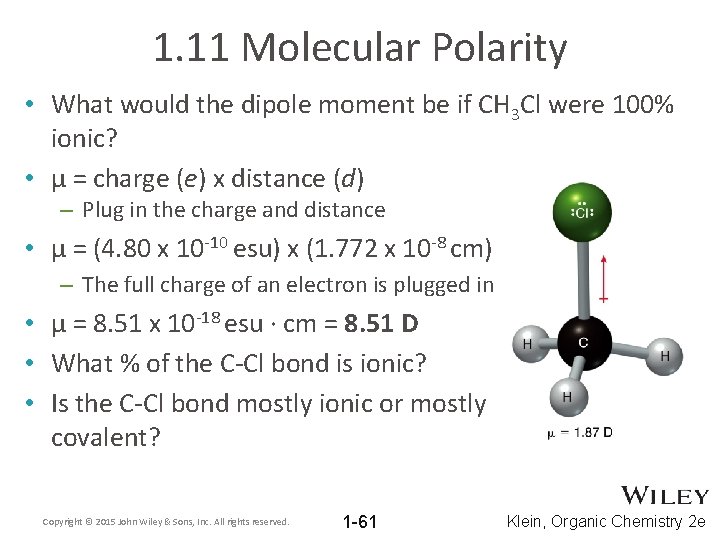
1. 11 Molecular Polarity • What would the dipole moment be if CH 3 Cl were 100% ionic? • μ = charge (e) x distance (d) – Plug in the charge and distance • μ = (4. 80 x 10 -10 esu) x (1. 772 x 10 -8 cm) – The full charge of an electron is plugged in • μ = 8. 51 x 10 -18 esu ∙ cm = 8. 51 D • What % of the C-Cl bond is ionic? • Is the C-Cl bond mostly ionic or mostly covalent? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -61 Klein, Organic Chemistry 2 e

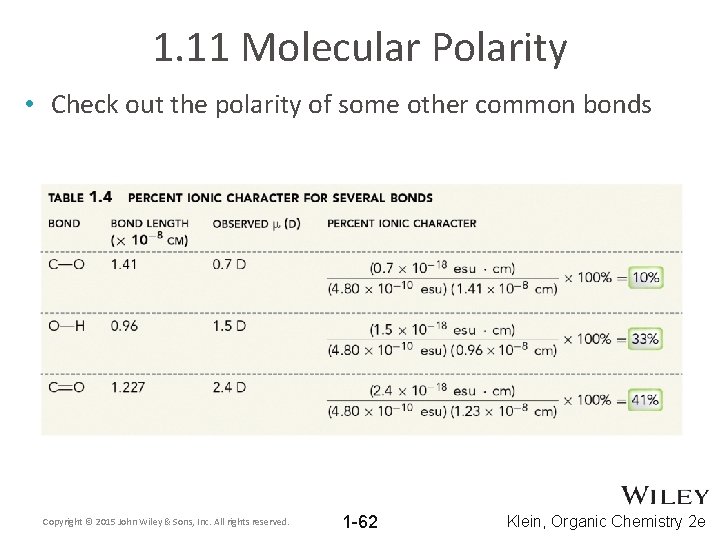
1. 11 Molecular Polarity • Check out the polarity of some other common bonds Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -62 Klein, Organic Chemistry 2 e

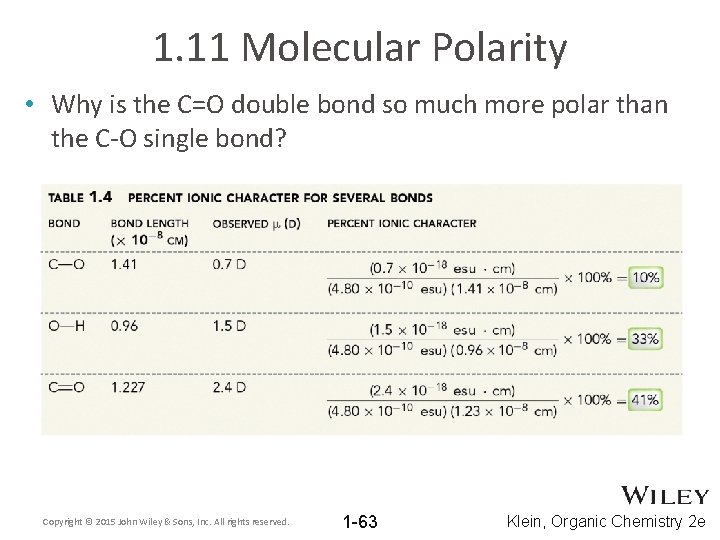
1. 11 Molecular Polarity • Why is the C=O double bond so much more polar than the C-O single bond? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -63 Klein, Organic Chemistry 2 e

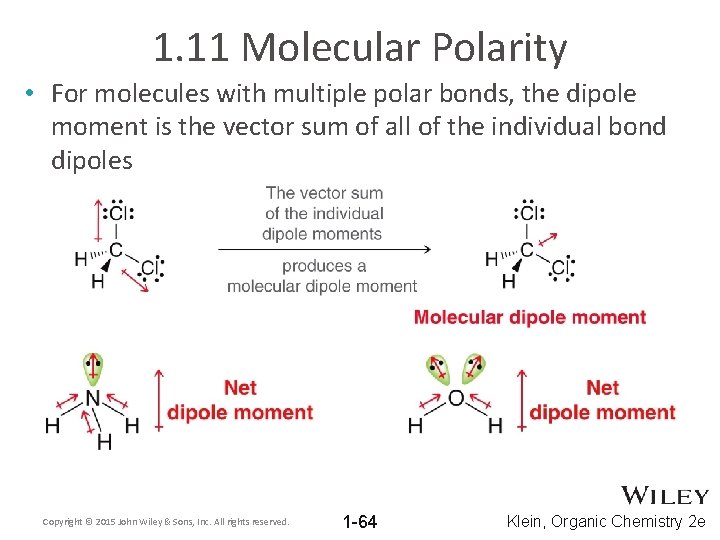
1. 11 Molecular Polarity • For molecules with multiple polar bonds, the dipole moment is the vector sum of all of the individual bond dipoles Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -64 Klein, Organic Chemistry 2 e

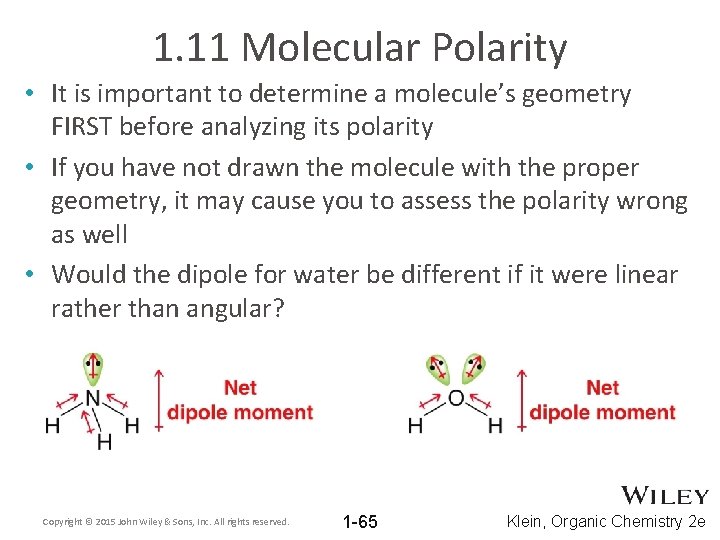
1. 11 Molecular Polarity • It is important to determine a molecule’s geometry FIRST before analyzing its polarity • If you have not drawn the molecule with the proper geometry, it may cause you to assess the polarity wrong as well • Would the dipole for water be different if it were linear rather than angular? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -65 Klein, Organic Chemistry 2 e

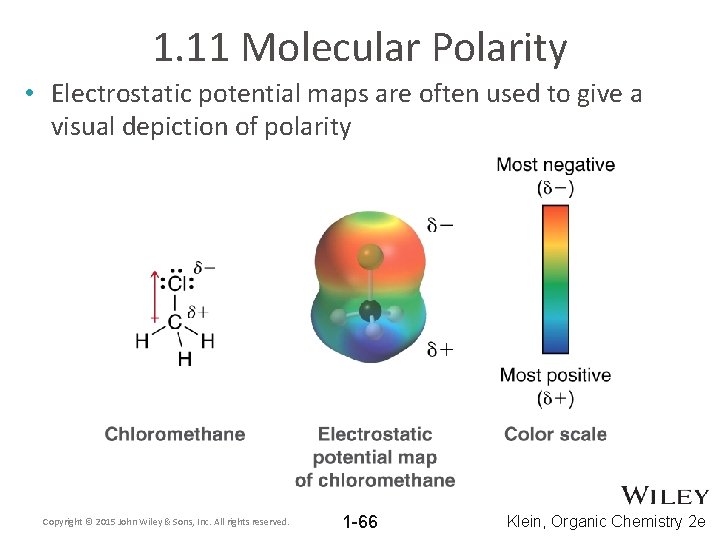
1. 11 Molecular Polarity • Electrostatic potential maps are often used to give a visual depiction of polarity Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -66 Klein, Organic Chemistry 2 e

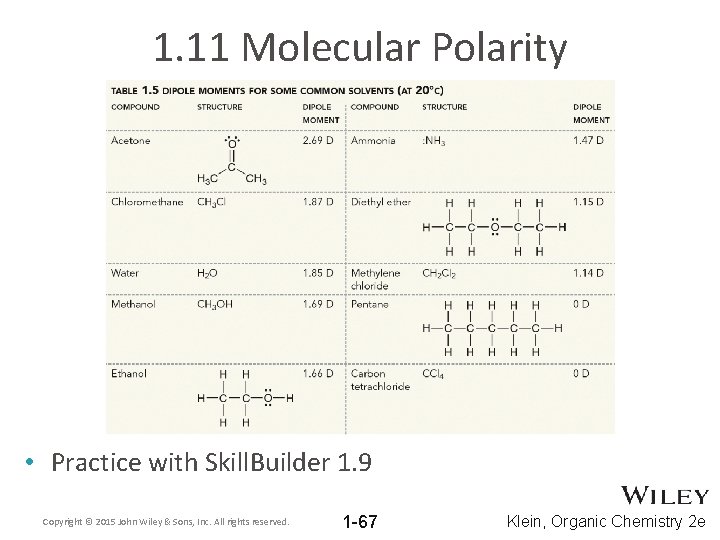
1. 11 Molecular Polarity • Practice with Skill. Builder 1. 9 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -67 Klein, Organic Chemistry 2 e

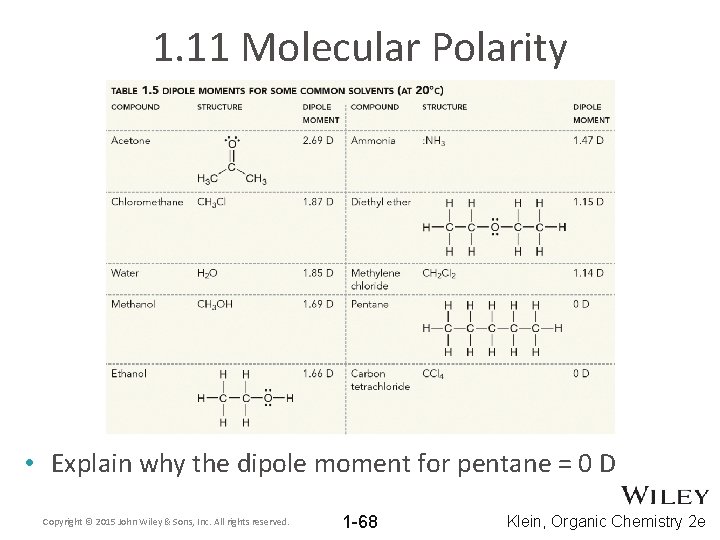
1. 11 Molecular Polarity • Explain why the dipole moment for pentane = 0 D Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -68 Klein, Organic Chemistry 2 e

1. 12 Intermolecular Forces • Many properties such as solubility, boiling point, density, state of matter, melting point, etc. are affected by the attractions BETWEEN molecules • Neutral molecules (polar and nonpolar) are attracted to one another through… – Dipole-dipole interactions – Hydrogen bonding – Dispersion forces (a. k. a. London forces or fleeting dipole forces) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -69 Klein, Organic Chemistry 2 e

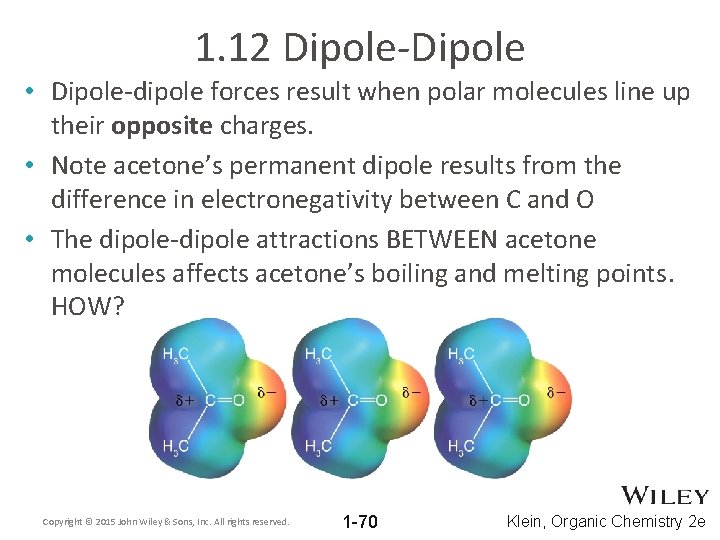
1. 12 Dipole-Dipole • Dipole-dipole forces result when polar molecules line up their opposite charges. • Note acetone’s permanent dipole results from the difference in electronegativity between C and O • The dipole-dipole attractions BETWEEN acetone molecules affects acetone’s boiling and melting points. HOW? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -70 Klein, Organic Chemistry 2 e

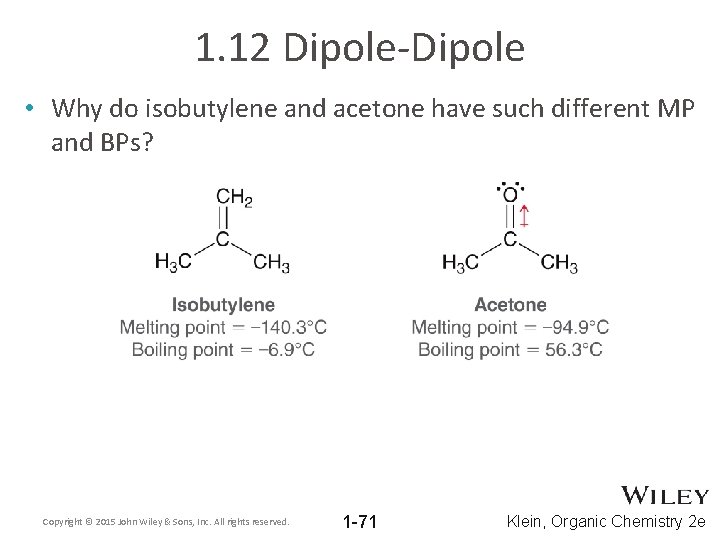
1. 12 Dipole-Dipole • Why do isobutylene and acetone have such different MP and BPs? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -71 Klein, Organic Chemistry 2 e

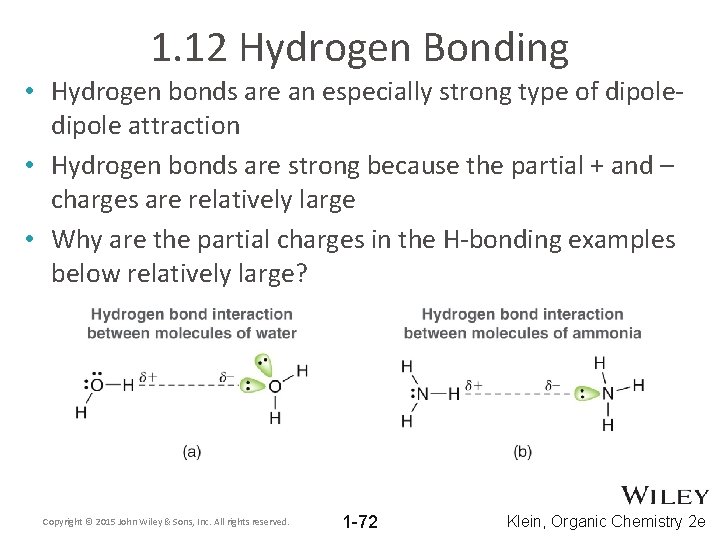
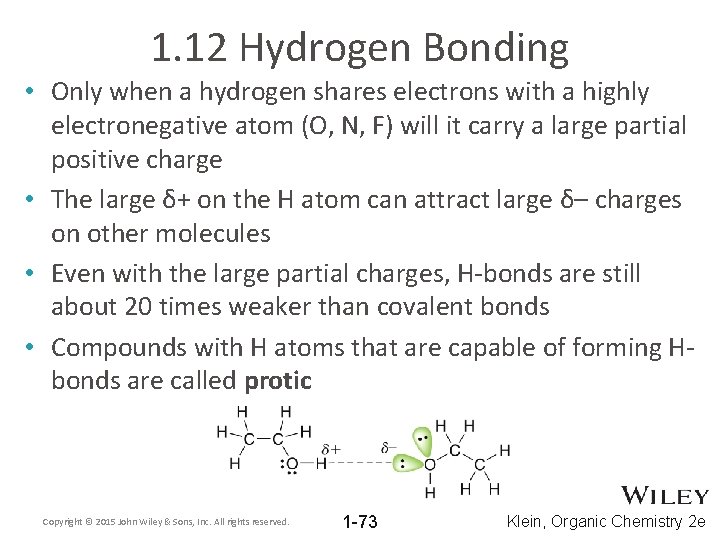
1. 12 Hydrogen Bonding • Hydrogen bonds are an especially strong type of dipole attraction • Hydrogen bonds are strong because the partial + and – charges are relatively large • Why are the partial charges in the H-bonding examples below relatively large? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -72 Klein, Organic Chemistry 2 e

1. 12 Hydrogen Bonding • Only when a hydrogen shares electrons with a highly electronegative atom (O, N, F) will it carry a large partial positive charge • The large δ+ on the H atom can attract large δ– charges on other molecules • Even with the large partial charges, H-bonds are still about 20 times weaker than covalent bonds • Compounds with H atoms that are capable of forming Hbonds are called protic Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -73 Klein, Organic Chemistry 2 e

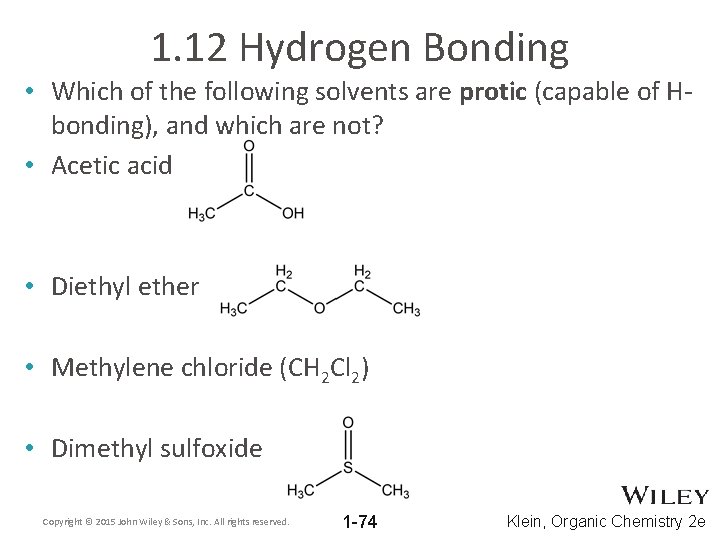
1. 12 Hydrogen Bonding • Which of the following solvents are protic (capable of Hbonding), and which are not? • Acetic acid • Diethyl ether • Methylene chloride (CH 2 Cl 2) • Dimethyl sulfoxide Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -74 Klein, Organic Chemistry 2 e

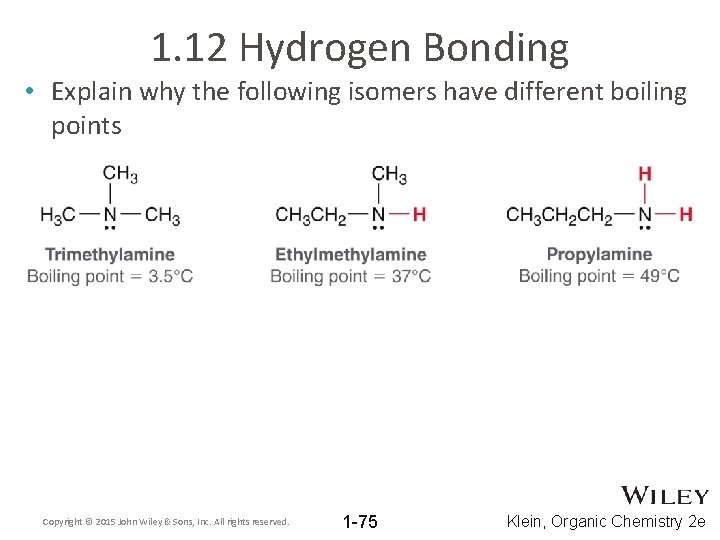
1. 12 Hydrogen Bonding • Explain why the following isomers have different boiling points Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -75 Klein, Organic Chemistry 2 e

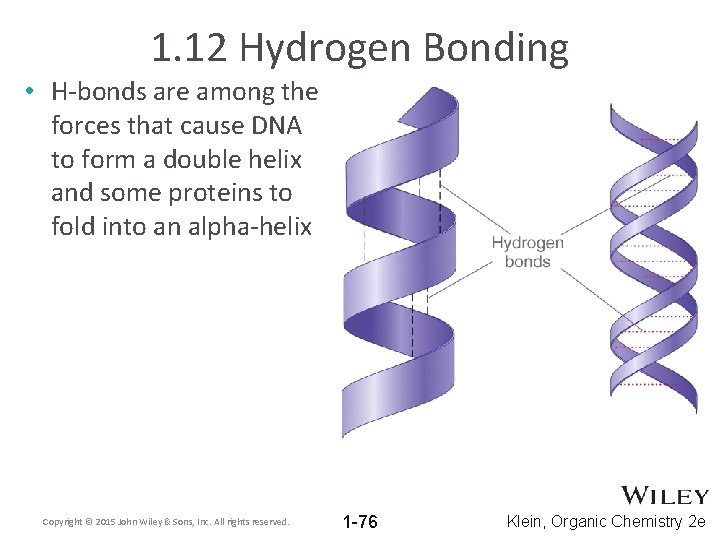
1. 12 Hydrogen Bonding • H-bonds are among the forces that cause DNA to form a double helix and some proteins to fold into an alpha-helix Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -76 Klein, Organic Chemistry 2 e

1. 12 London Dispersion Forces • If two molecules are nonpolar (dipole = 0 D), will they attract one another? – YES! HOW? • Nonpolar molecules normally have their electrons (–) spread out evenly around the nuclei (+) completely balancing the charge • However, the electrons are in constant random motion within their MOs Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -77 Klein, Organic Chemistry 2 e

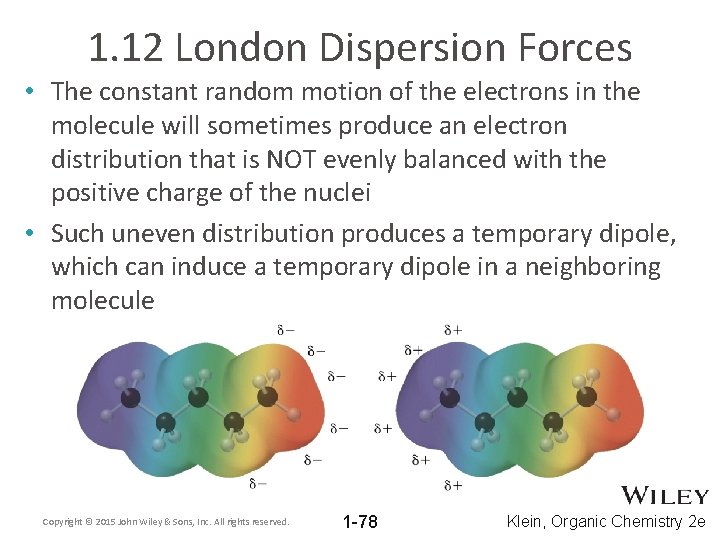
1. 12 London Dispersion Forces • The constant random motion of the electrons in the molecule will sometimes produce an electron distribution that is NOT evenly balanced with the positive charge of the nuclei • Such uneven distribution produces a temporary dipole, which can induce a temporary dipole in a neighboring molecule Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -78 Klein, Organic Chemistry 2 e

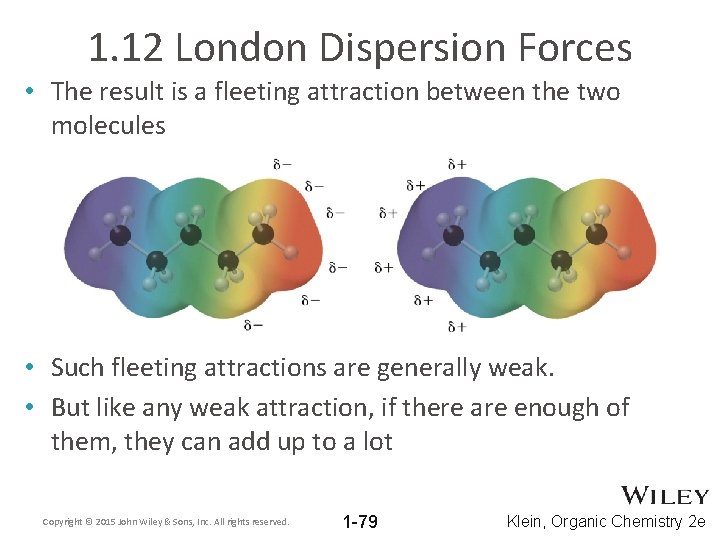
1. 12 London Dispersion Forces • The result is a fleeting attraction between the two molecules • Such fleeting attractions are generally weak. • But like any weak attraction, if there are enough of them, they can add up to a lot Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -79 Klein, Organic Chemistry 2 e

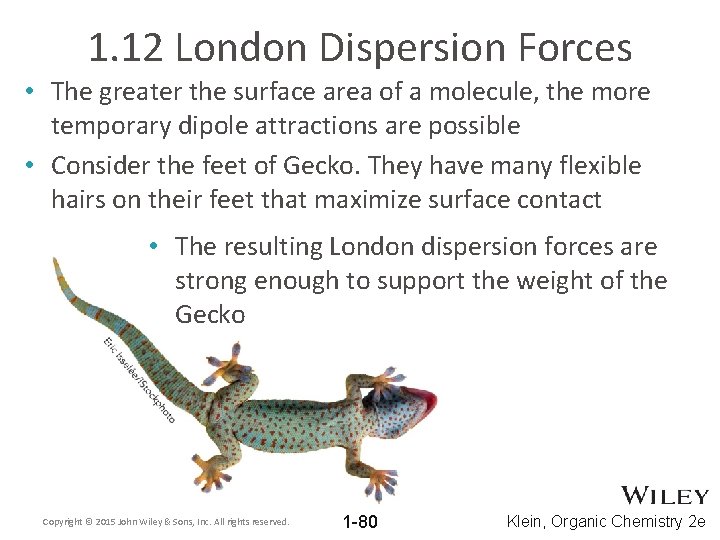
1. 12 London Dispersion Forces • The greater the surface area of a molecule, the more temporary dipole attractions are possible • Consider the feet of Gecko. They have many flexible hairs on their feet that maximize surface contact • The resulting London dispersion forces are strong enough to support the weight of the Gecko Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -80 Klein, Organic Chemistry 2 e

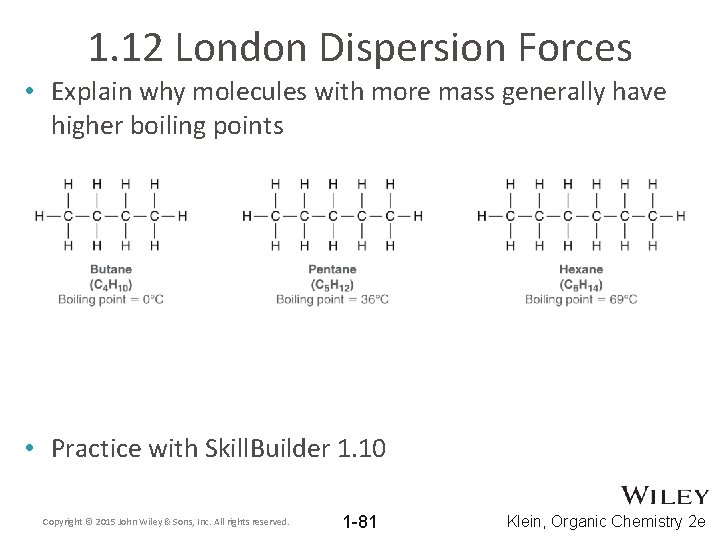
1. 12 London Dispersion Forces • Explain why molecules with more mass generally have higher boiling points • Practice with Skill. Builder 1. 10 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -81 Klein, Organic Chemistry 2 e

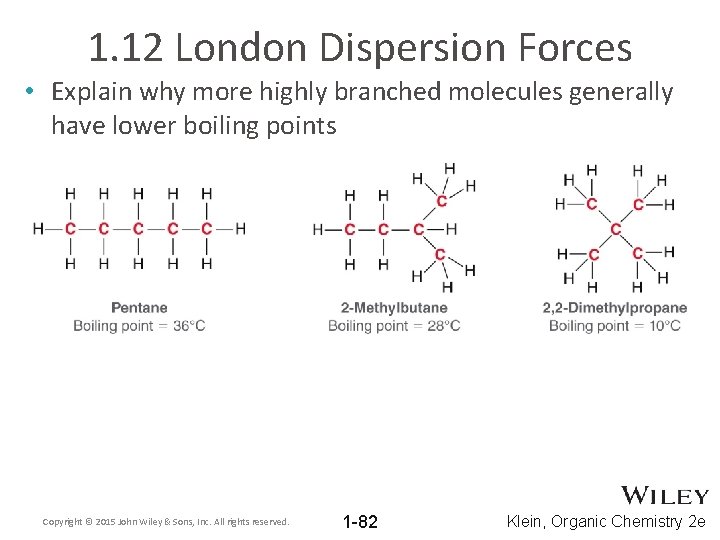
1. 12 London Dispersion Forces • Explain why more highly branched molecules generally have lower boiling points Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -82 Klein, Organic Chemistry 2 e

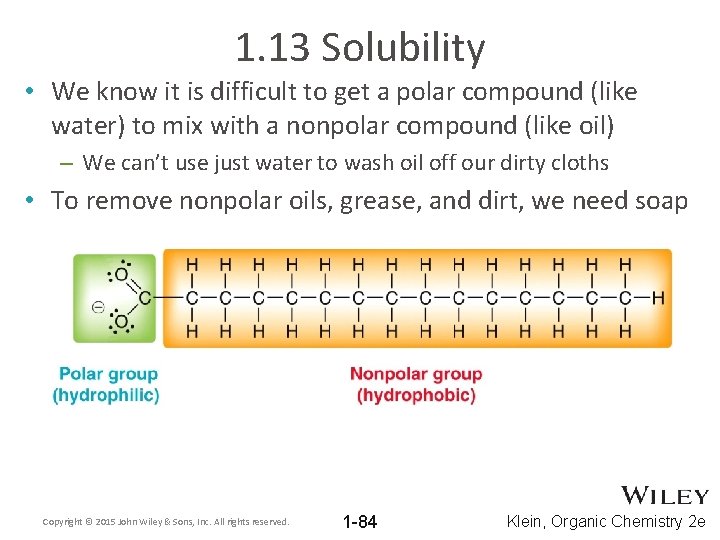
1. 13 Solubility • We use the principle, like-dissolves-like • Polar compounds generally mix well with other polar compounds – If the compounds mixing are all capable of H-bonding and/or strong dipole-dipole, then there is no reason why they shouldn’t mix • Nonpolar compounds generally mix well with other nonpolar compounds – If none of the compounds are capable of forming strong attractions, then no strong attractions would have to be broken to allow them to mix Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -83 Klein, Organic Chemistry 2 e

1. 13 Solubility • We know it is difficult to get a polar compound (like water) to mix with a nonpolar compound (like oil) – We can’t use just water to wash oil off our dirty cloths • To remove nonpolar oils, grease, and dirt, we need soap Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -84 Klein, Organic Chemistry 2 e

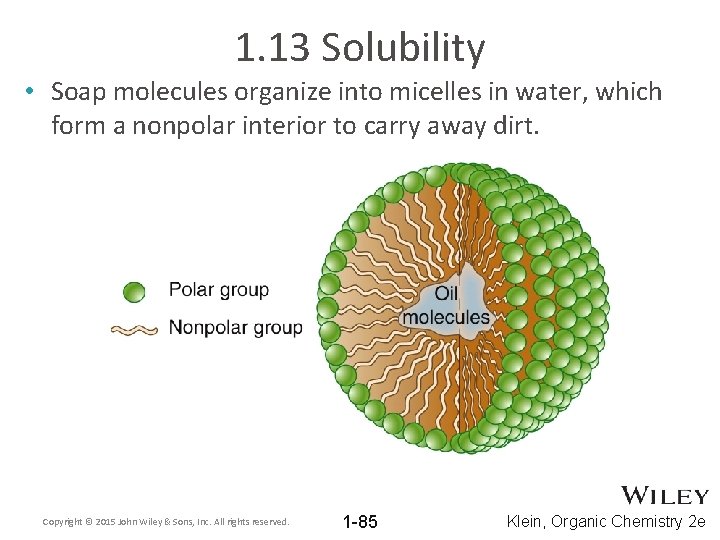
1. 13 Solubility • Soap molecules organize into micelles in water, which form a nonpolar interior to carry away dirt. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -85 Klein, Organic Chemistry 2 e

1. 13 Solubility • Which attraction is generally stronger? – The attraction between a permanent dipole and an induced dipole versus – The attraction between a temporary dipole and an induced dipole • Which attraction is generally stronger? – The attraction between a polar molecule and a nonpolar molecule versus – The attraction between two nonpolar molecules? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -86 Klein, Organic Chemistry 2 e

1. 13 Solubility • Why won’t a nonpolar compound readily dissolve in water? • Is it because the water molecules repel the nonpolar molecules? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -87 Klein, Organic Chemistry 2 e

Additional Example Problems • Draw the structure for C 2 Cl 3 N • Draw the structure for CH 2 O 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -88 Klein, Organic Chemistry 2 e

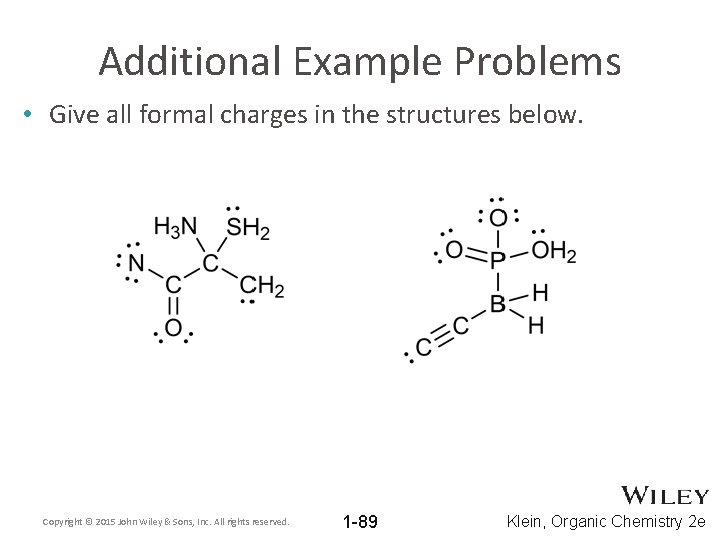
Additional Example Problems • Give all formal charges in the structures below. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -89 Klein, Organic Chemistry 2 e

Additional Example Problems • How many nodes are in the 3 s subshell? • How many nodes are in a typical sigma antibonding MO? • How many nodes are in a typical sp 3 orbital? • How many nodes are in a typical pi bonding MO? • How many nodes are in a typical pi antibonding MO Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -90 Klein, Organic Chemistry 2 e

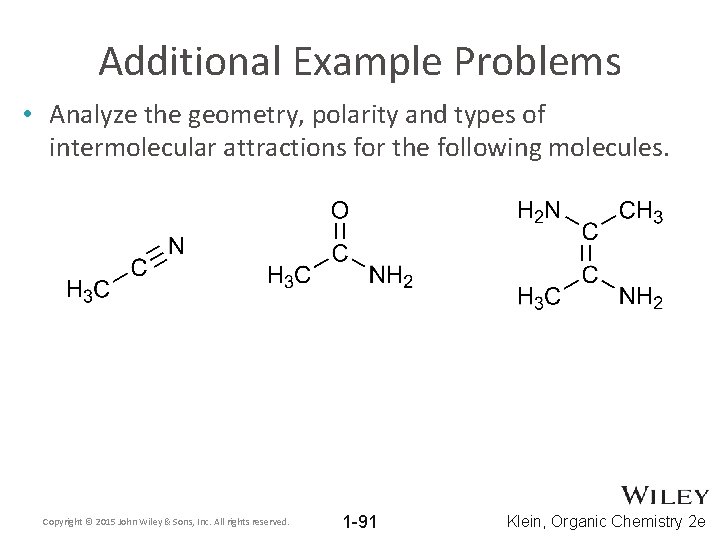
Additional Example Problems • Analyze the geometry, polarity and types of intermolecular attractions for the following molecules. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 1 -91 Klein, Organic Chemistry 2 e