Organic Chemistry Second Edition David Klein Chapter 14





- Slides: 56

Organic Chemistry Second Edition David Klein Chapter 14 Ethers and Epoxides; Thiols and Sulfides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

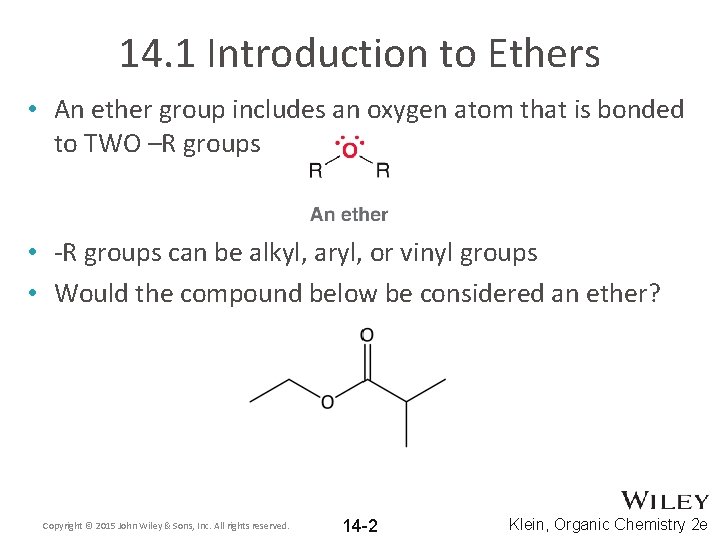
14. 1 Introduction to Ethers • An ether group includes an oxygen atom that is bonded to TWO –R groups • -R groups can be alkyl, aryl, or vinyl groups • Would the compound below be considered an ether? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -2 Klein, Organic Chemistry 2 e

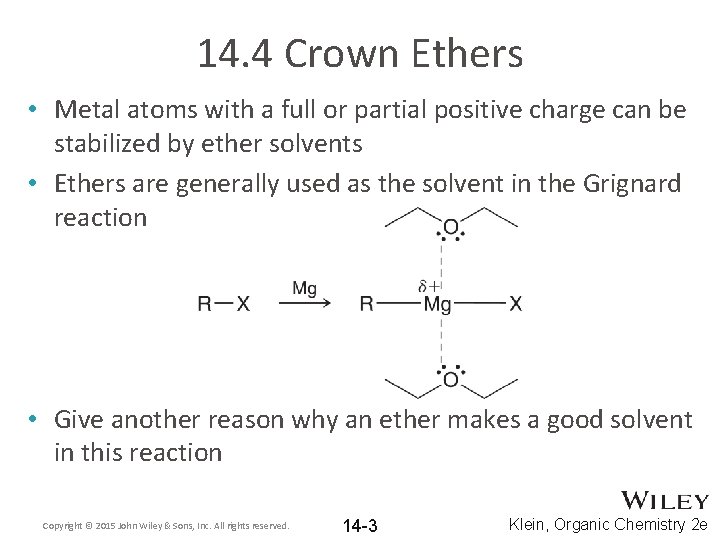
14. 4 Crown Ethers • Metal atoms with a full or partial positive charge can be stabilized by ether solvents • Ethers are generally used as the solvent in the Grignard reaction • Give another reason why an ether makes a good solvent in this reaction Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -3 Klein, Organic Chemistry 2 e

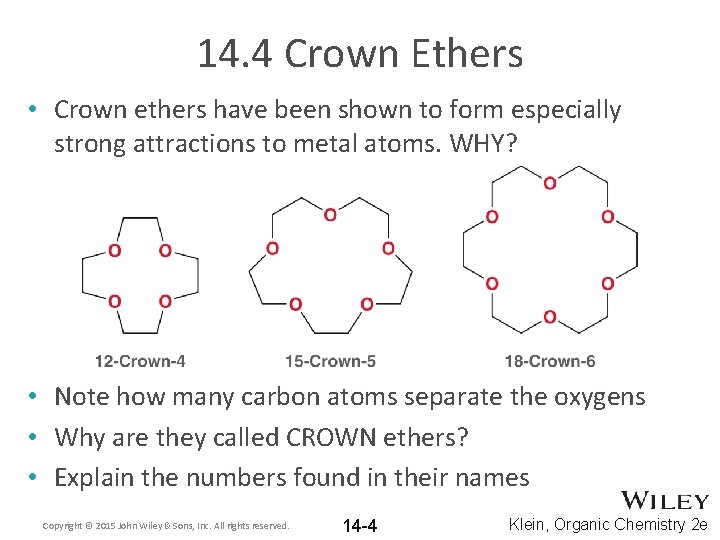
14. 4 Crown Ethers • Crown ethers have been shown to form especially strong attractions to metal atoms. WHY? • Note how many carbon atoms separate the oxygens • Why are they called CROWN ethers? • Explain the numbers found in their names Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -4 Klein, Organic Chemistry 2 e

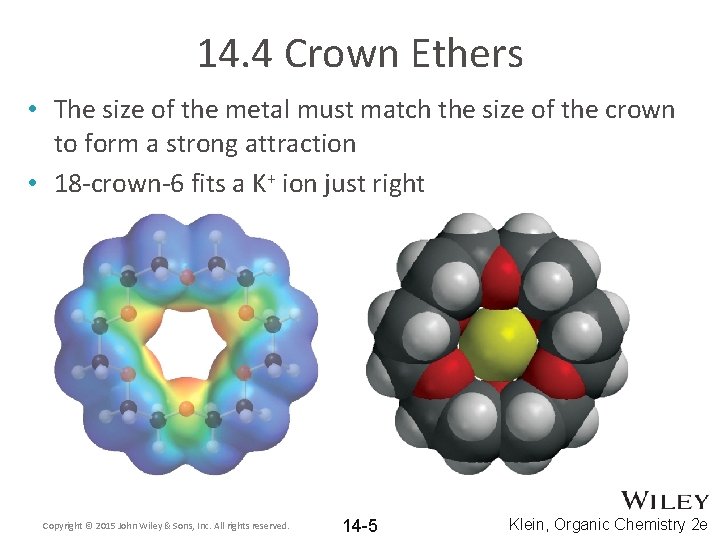
14. 4 Crown Ethers • The size of the metal must match the size of the crown to form a strong attraction • 18 -crown-6 fits a K+ ion just right Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -5 Klein, Organic Chemistry 2 e

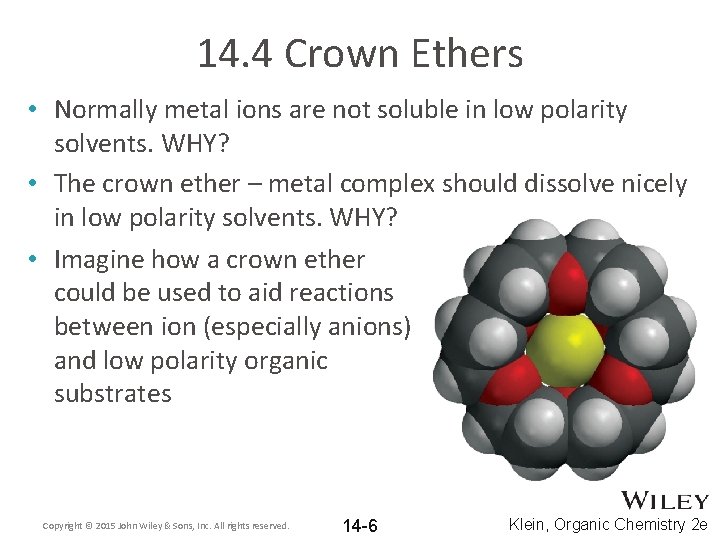
14. 4 Crown Ethers • Normally metal ions are not soluble in low polarity solvents. WHY? • The crown ether – metal complex should dissolve nicely in low polarity solvents. WHY? • Imagine how a crown ether could be used to aid reactions between ion (especially anions) and low polarity organic substrates Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -6 Klein, Organic Chemistry 2 e

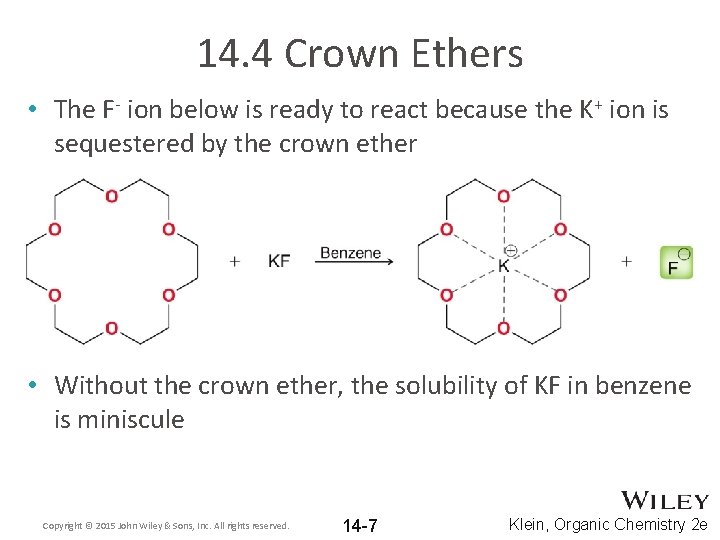
14. 4 Crown Ethers • The F- ion below is ready to react because the K+ ion is sequestered by the crown ether • Without the crown ether, the solubility of KF in benzene is miniscule Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -7 Klein, Organic Chemistry 2 e

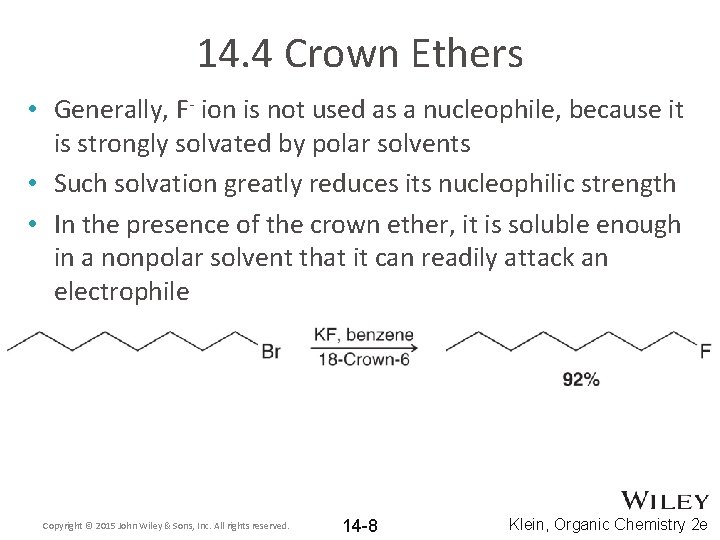
14. 4 Crown Ethers • Generally, F- ion is not used as a nucleophile, because it is strongly solvated by polar solvents • Such solvation greatly reduces its nucleophilic strength • In the presence of the crown ether, it is soluble enough in a nonpolar solvent that it can readily attack an electrophile Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -8 Klein, Organic Chemistry 2 e

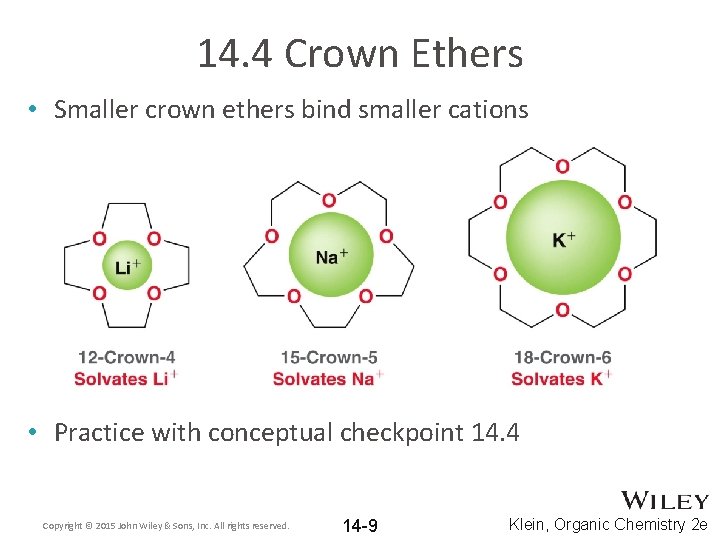
14. 4 Crown Ethers • Smaller crown ethers bind smaller cations • Practice with conceptual checkpoint 14. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -9 Klein, Organic Chemistry 2 e

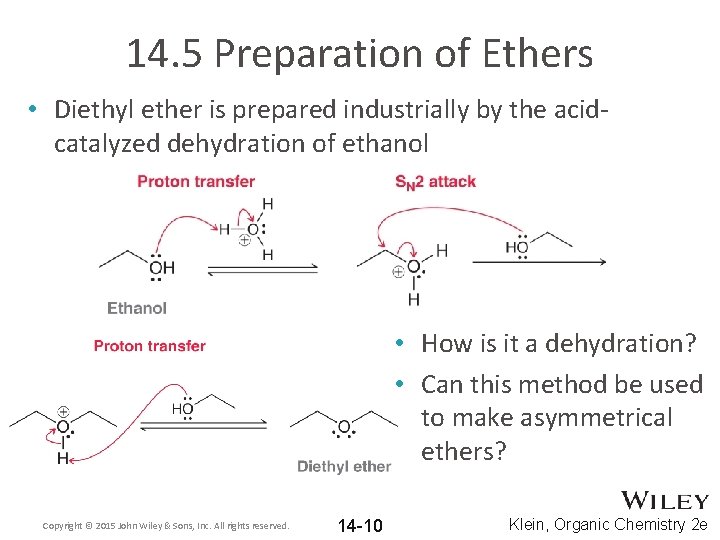
14. 5 Preparation of Ethers • Diethyl ether is prepared industrially by the acidcatalyzed dehydration of ethanol • How is it a dehydration? • Can this method be used to make asymmetrical ethers? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -10 Klein, Organic Chemistry 2 e

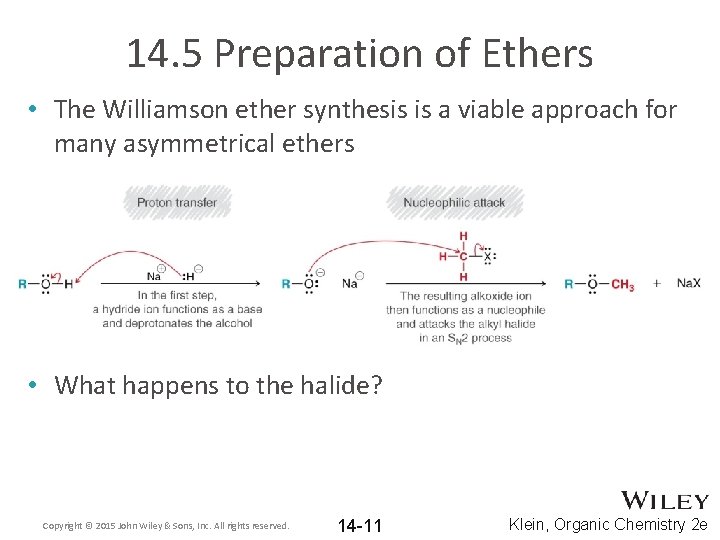
14. 5 Preparation of Ethers • The Williamson ether synthesis is a viable approach for many asymmetrical ethers • What happens to the halide? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -11 Klein, Organic Chemistry 2 e

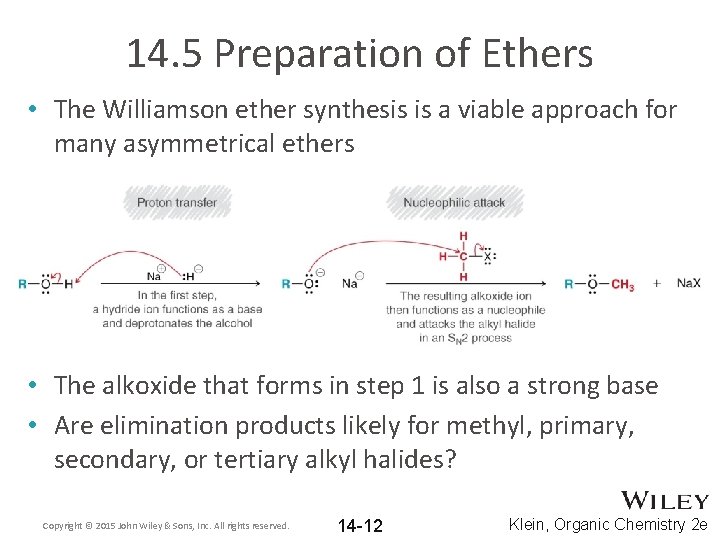
14. 5 Preparation of Ethers • The Williamson ether synthesis is a viable approach for many asymmetrical ethers • The alkoxide that forms in step 1 is also a strong base • Are elimination products likely for methyl, primary, secondary, or tertiary alkyl halides? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -12 Klein, Organic Chemistry 2 e

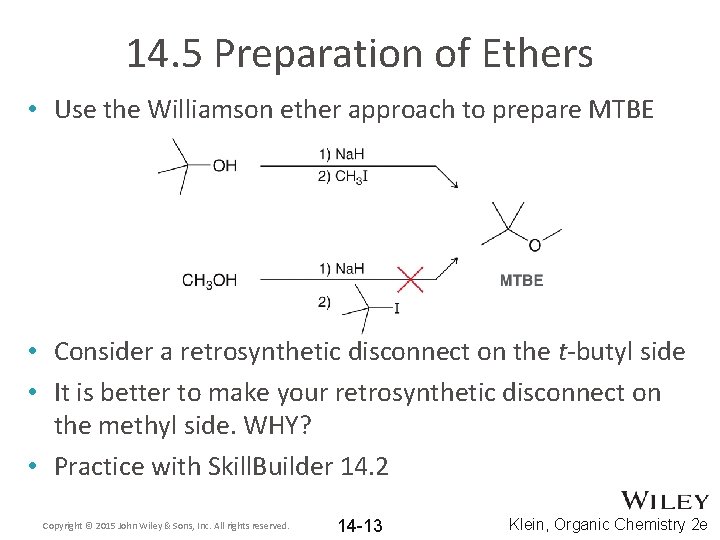
14. 5 Preparation of Ethers • Use the Williamson ether approach to prepare MTBE • Consider a retrosynthetic disconnect on the t-butyl side • It is better to make your retrosynthetic disconnect on the methyl side. WHY? • Practice with Skill. Builder 14. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -13 Klein, Organic Chemistry 2 e

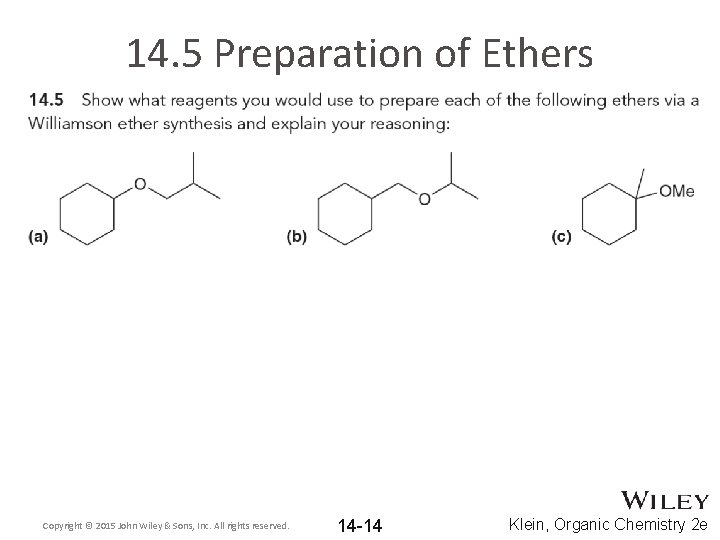
14. 5 Preparation of Ethers Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -14 Klein, Organic Chemistry 2 e

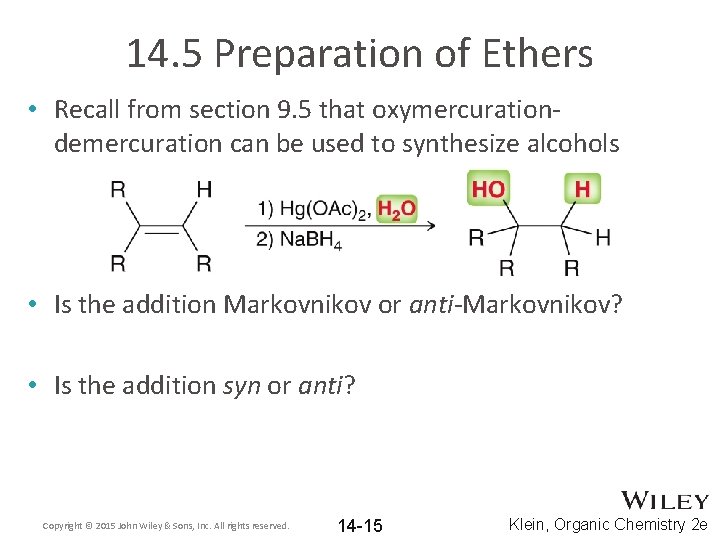
14. 5 Preparation of Ethers • Recall from section 9. 5 that oxymercurationdemercuration can be used to synthesize alcohols • Is the addition Markovnikov or anti-Markovnikov? • Is the addition syn or anti? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -15 Klein, Organic Chemistry 2 e

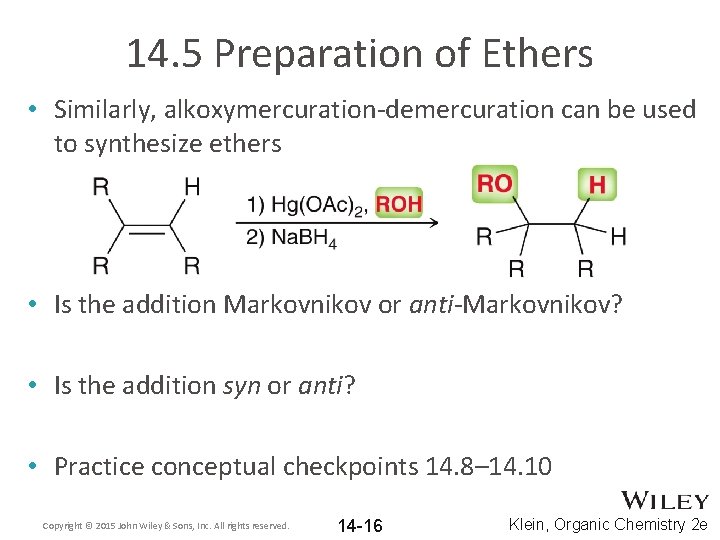
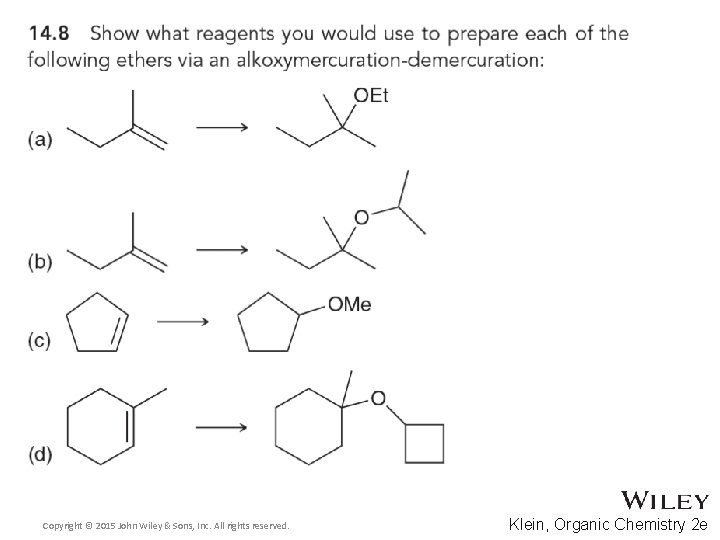
14. 5 Preparation of Ethers • Similarly, alkoxymercuration-demercuration can be used to synthesize ethers • Is the addition Markovnikov or anti-Markovnikov? • Is the addition syn or anti? • Practice conceptual checkpoints 14. 8– 14. 10 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -16 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

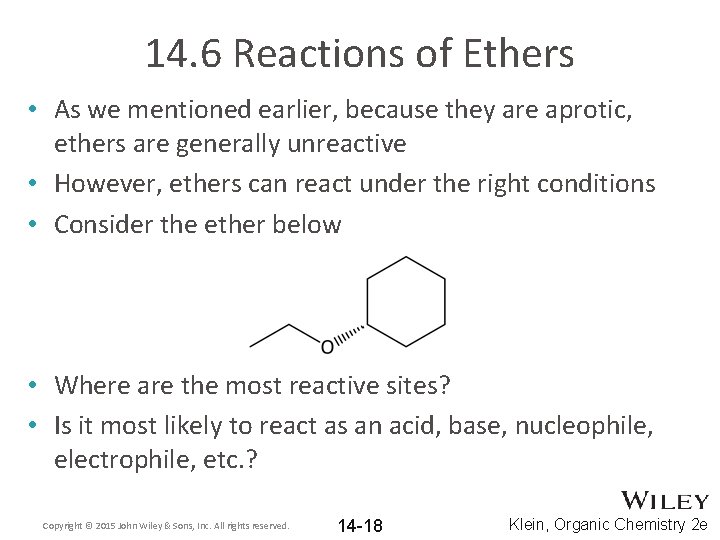
14. 6 Reactions of Ethers • As we mentioned earlier, because they are aprotic, ethers are generally unreactive • However, ethers can react under the right conditions • Consider the ether below • Where are the most reactive sites? • Is it most likely to react as an acid, base, nucleophile, electrophile, etc. ? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -18 Klein, Organic Chemistry 2 e

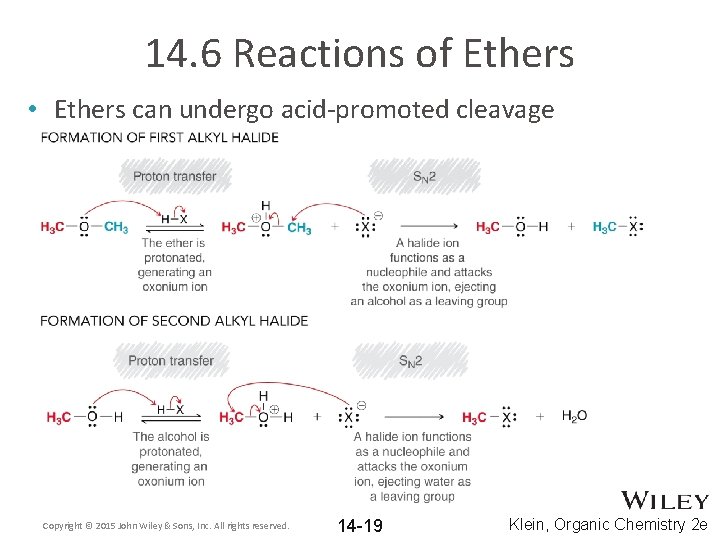
14. 6 Reactions of Ethers • Ethers can undergo acid-promoted cleavage Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -19 Klein, Organic Chemistry 2 e

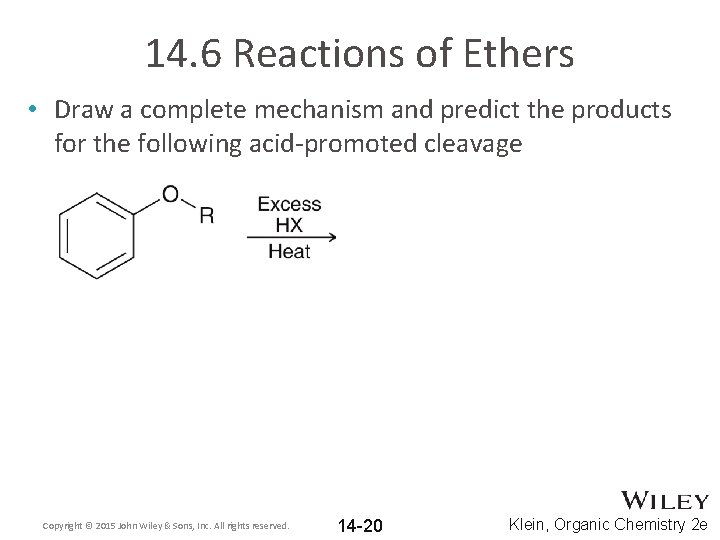
14. 6 Reactions of Ethers • Draw a complete mechanism and predict the products for the following acid-promoted cleavage Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -20 Klein, Organic Chemistry 2 e

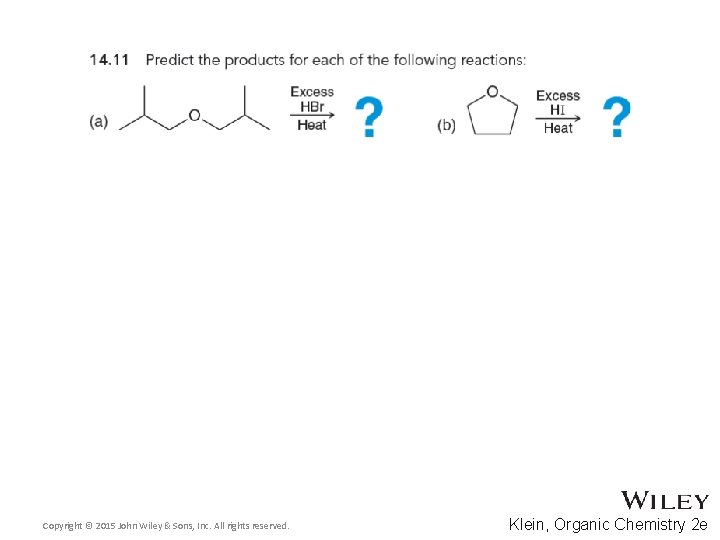
14. 6 Reactions of Ethers • To promote cleavage, HI and HBr are generally effective • HCl is less effective, and HF does not cause significant cleavage • Explain the trend above considering the relative strength of the halide nucleophiles • Why is the cleavage considered acid-promoted rather than acid-catalyzed? • Practice with conceptual checkpoint 14. 11 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -21 Klein, Organic Chemistry 2 e

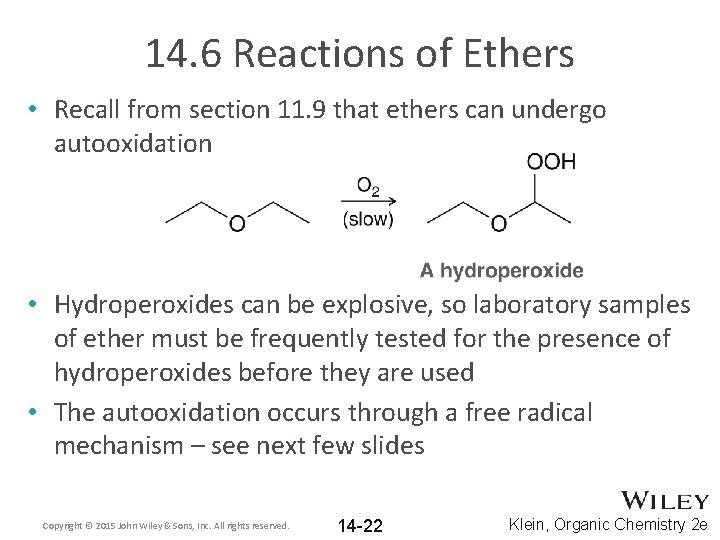
14. 6 Reactions of Ethers • Recall from section 11. 9 that ethers can undergo autooxidation • Hydroperoxides can be explosive, so laboratory samples of ether must be frequently tested for the presence of hydroperoxides before they are used • The autooxidation occurs through a free radical mechanism – see next few slides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -22 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

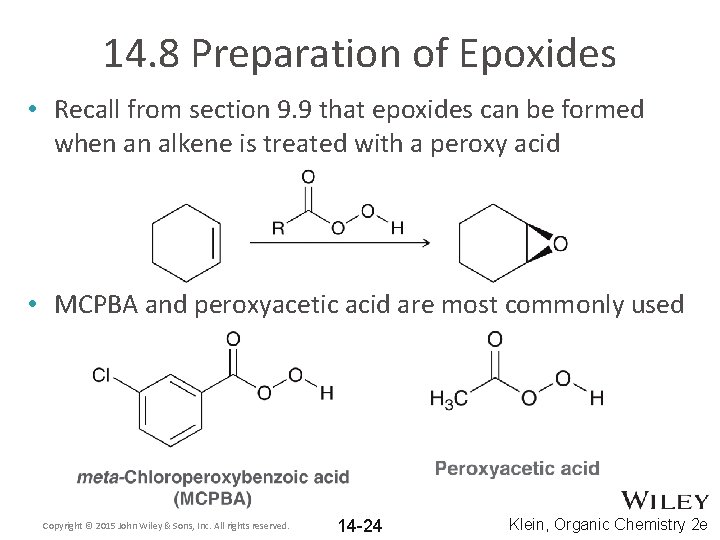
14. 8 Preparation of Epoxides • Recall from section 9. 9 that epoxides can be formed when an alkene is treated with a peroxy acid • MCPBA and peroxyacetic acid are most commonly used Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -24 Klein, Organic Chemistry 2 e

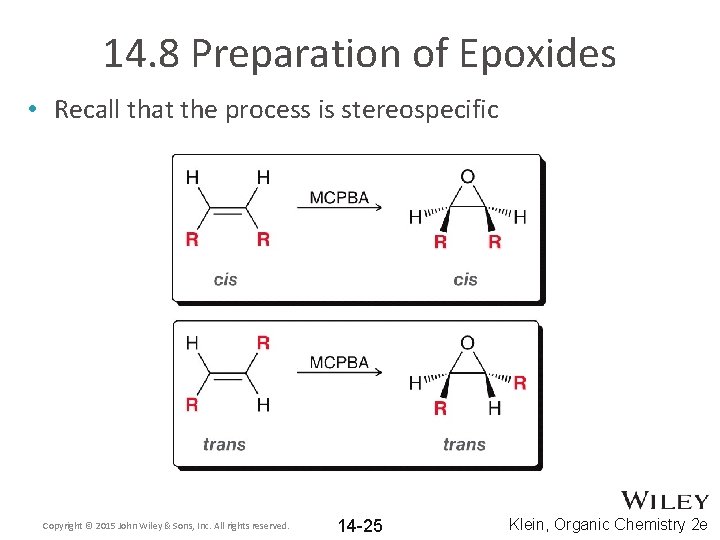
14. 8 Preparation of Epoxides • Recall that the process is stereospecific Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -25 Klein, Organic Chemistry 2 e

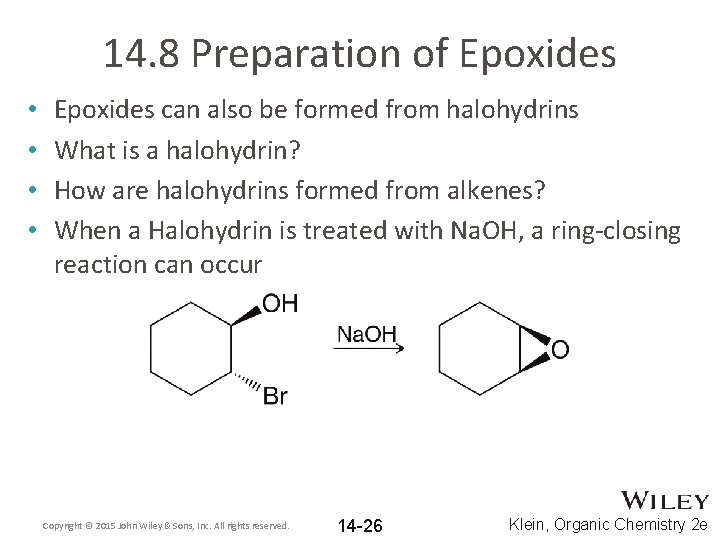
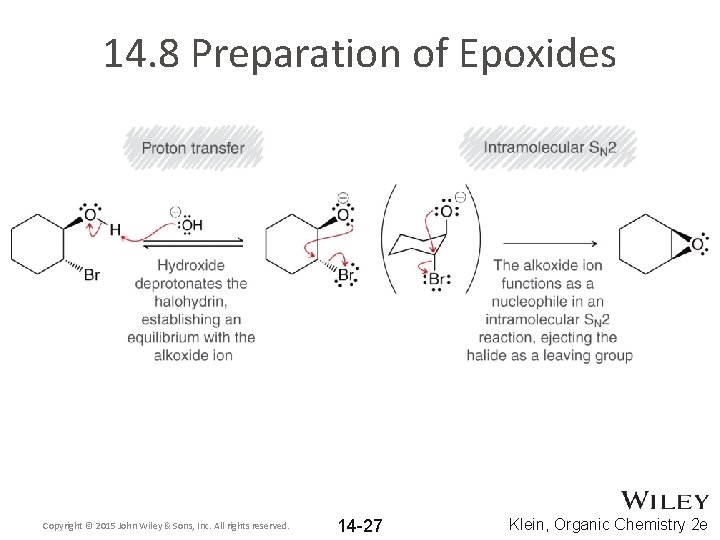
14. 8 Preparation of Epoxides • • Epoxides can also be formed from halohydrins What is a halohydrin? How are halohydrins formed from alkenes? When a Halohydrin is treated with Na. OH, a ring-closing reaction can occur Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -26 Klein, Organic Chemistry 2 e

14. 8 Preparation of Epoxides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -27 Klein, Organic Chemistry 2 e

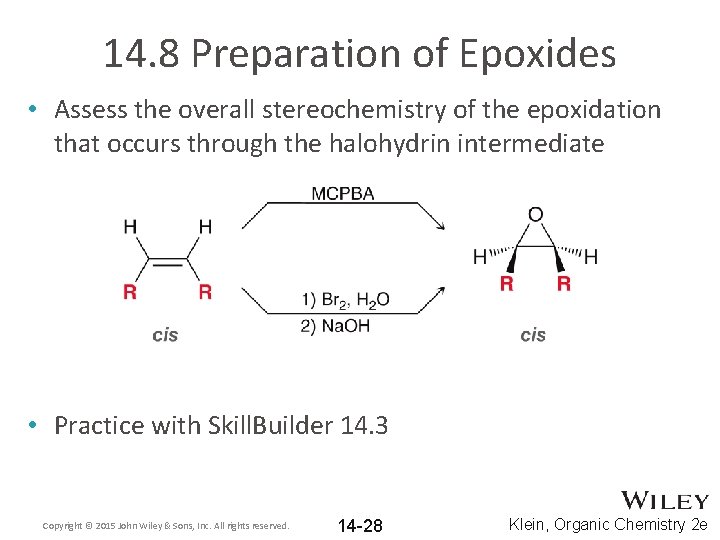
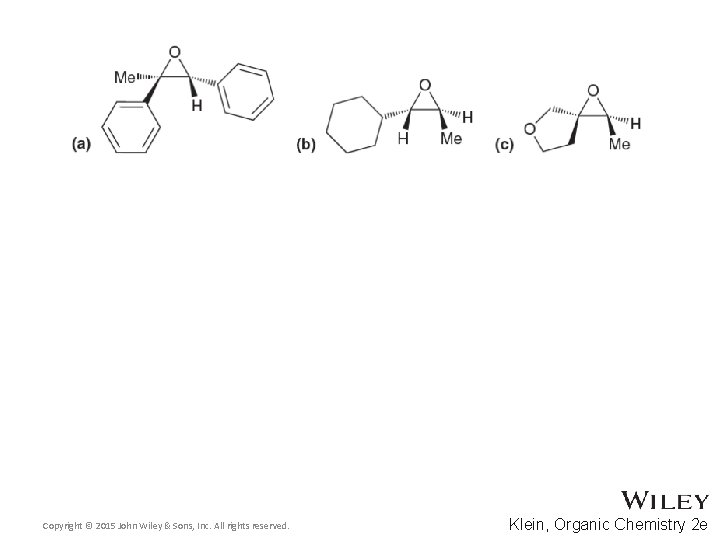
14. 8 Preparation of Epoxides • Assess the overall stereochemistry of the epoxidation that occurs through the halohydrin intermediate • Practice with Skill. Builder 14. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -28 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

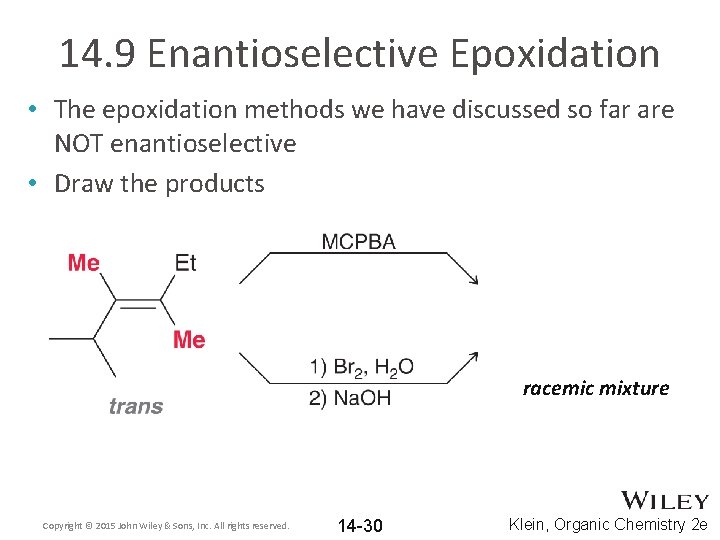
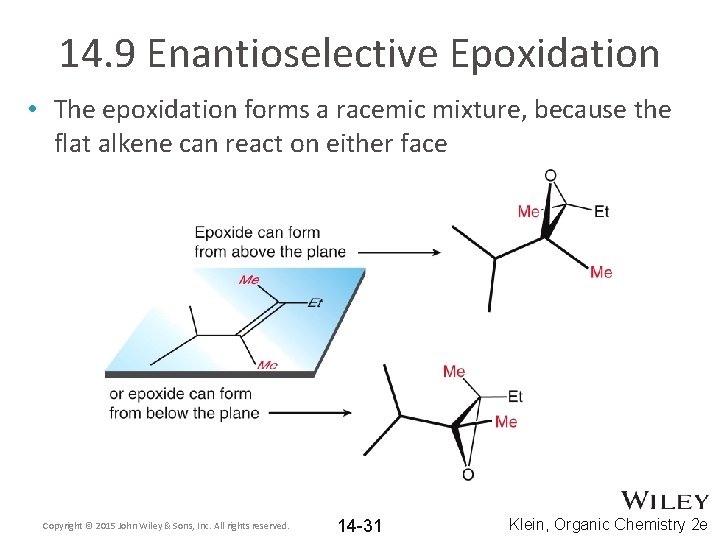
14. 9 Enantioselective Epoxidation • The epoxidation methods we have discussed so far are NOT enantioselective • Draw the products racemic mixture Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -30 Klein, Organic Chemistry 2 e

14. 9 Enantioselective Epoxidation • The epoxidation forms a racemic mixture, because the flat alkene can react on either face Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -31 Klein, Organic Chemistry 2 e

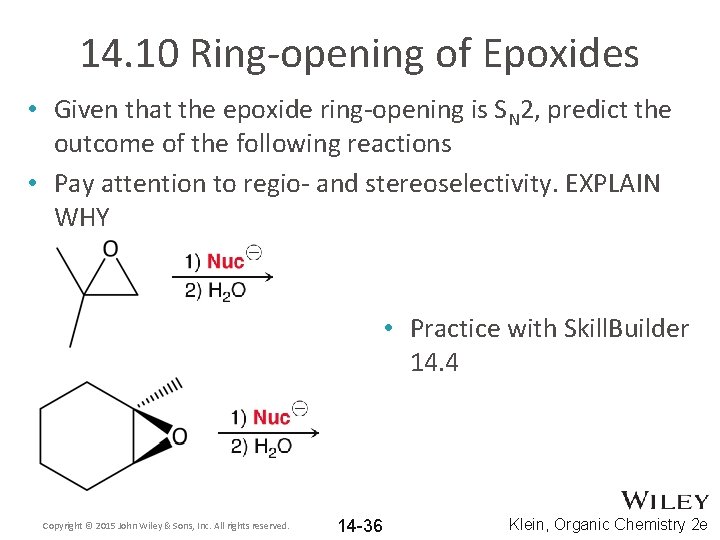
14. 10 Ring-opening of Epoxides • Because of their significant ring strain, epoxides have great synthetic utility as intermediates • Propose some reagents that might react with an epoxide to provide a specific functional group • Propose some reagents that might react with an epoxide to alter the carbon skeleton Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -32 Klein, Organic Chemistry 2 e

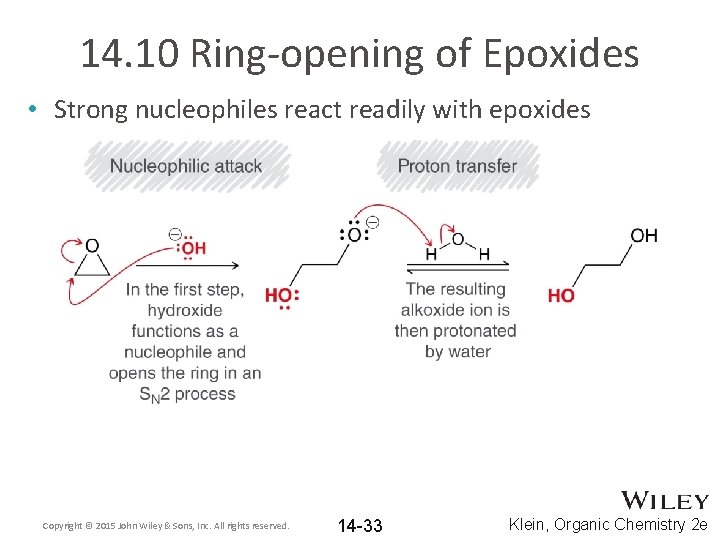
14. 10 Ring-opening of Epoxides • Strong nucleophiles react readily with epoxides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -33 Klein, Organic Chemistry 2 e

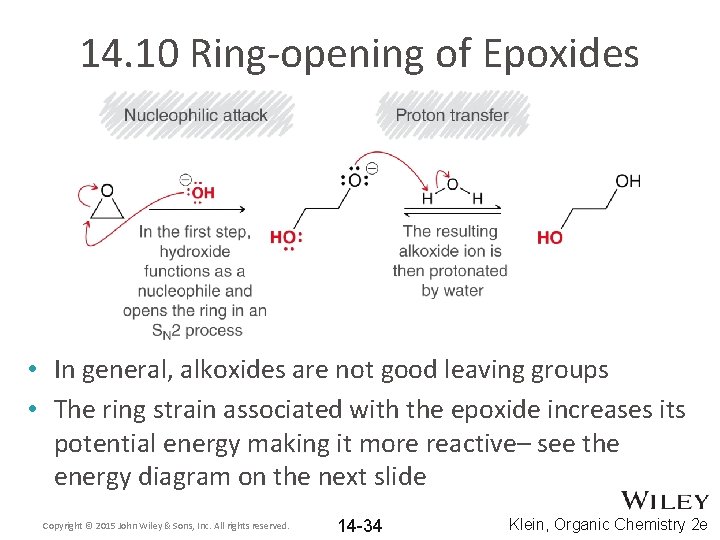
14. 10 Ring-opening of Epoxides • In general, alkoxides are not good leaving groups • The ring strain associated with the epoxide increases its potential energy making it more reactive– see the energy diagram on the next slide Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -34 Klein, Organic Chemistry 2 e

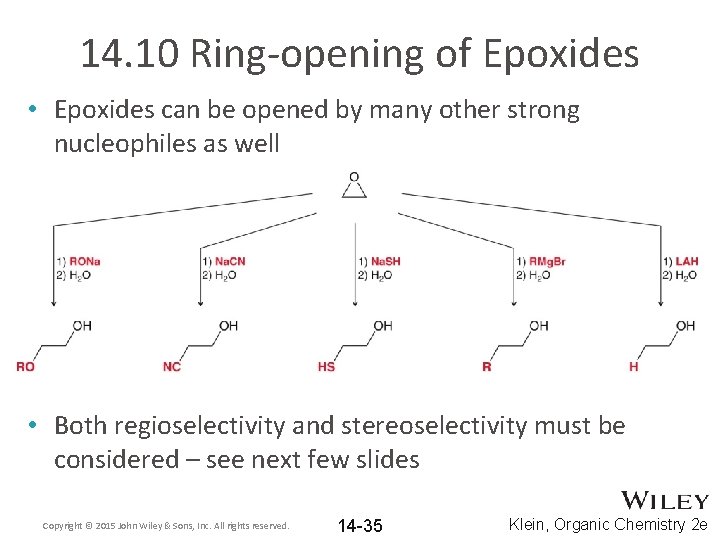
14. 10 Ring-opening of Epoxides • Epoxides can be opened by many other strong nucleophiles as well • Both regioselectivity and stereoselectivity must be considered – see next few slides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -35 Klein, Organic Chemistry 2 e

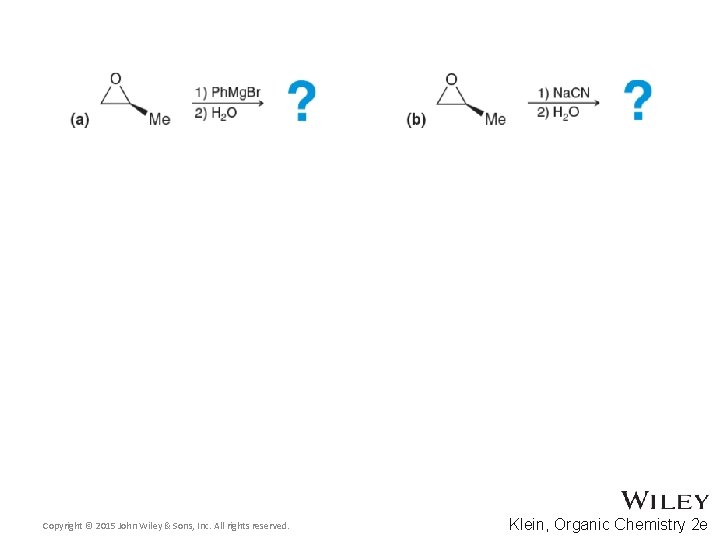
14. 10 Ring-opening of Epoxides • Given that the epoxide ring-opening is SN 2, predict the outcome of the following reactions • Pay attention to regio- and stereoselectivity. EXPLAIN WHY • Practice with Skill. Builder 14. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -36 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

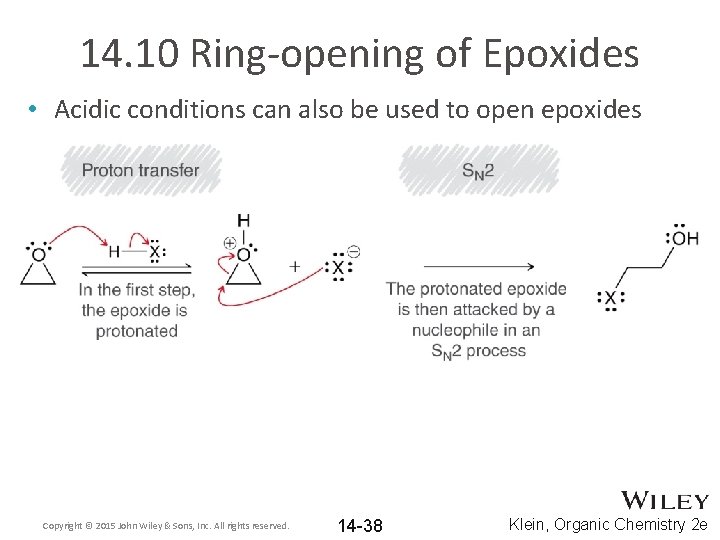
14. 10 Ring-opening of Epoxides • Acidic conditions can also be used to open epoxides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -38 Klein, Organic Chemistry 2 e

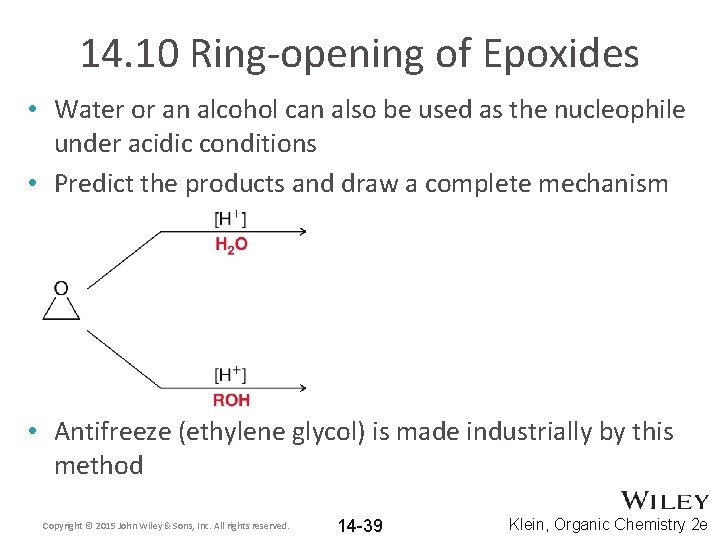
14. 10 Ring-opening of Epoxides • Water or an alcohol can also be used as the nucleophile under acidic conditions • Predict the products and draw a complete mechanism • Antifreeze (ethylene glycol) is made industrially by this method Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -39 Klein, Organic Chemistry 2 e

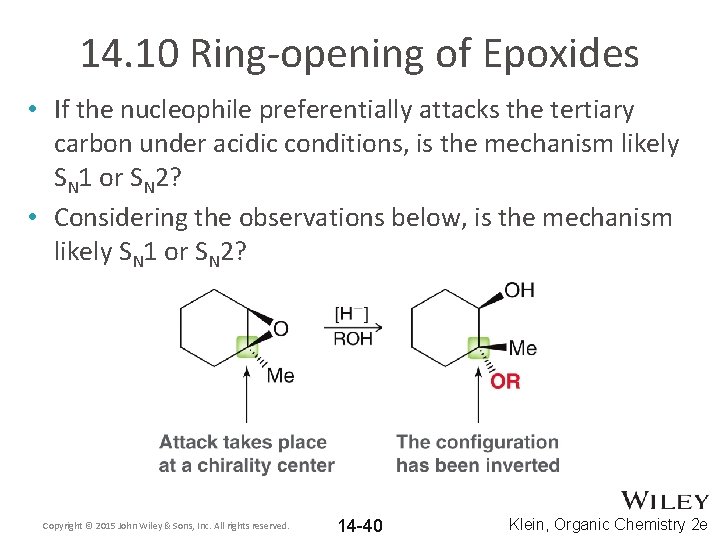
14. 10 Ring-opening of Epoxides • If the nucleophile preferentially attacks the tertiary carbon under acidic conditions, is the mechanism likely SN 1 or SN 2? • Considering the observations below, is the mechanism likely SN 1 or SN 2? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -40 Klein, Organic Chemistry 2 e

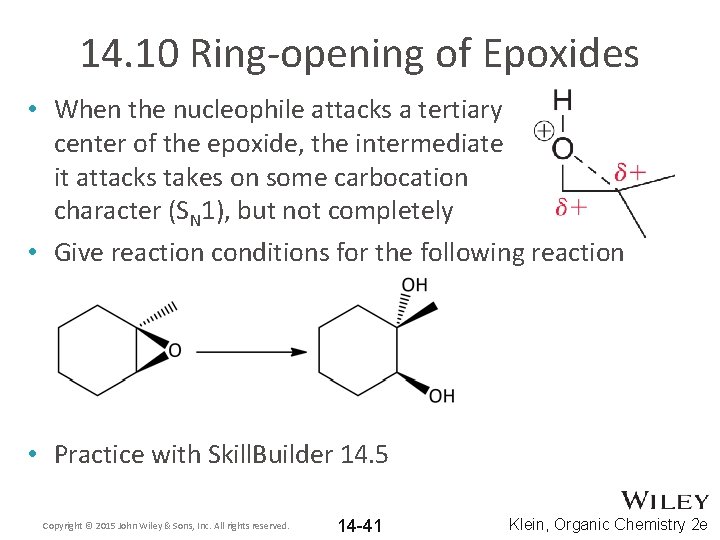
14. 10 Ring-opening of Epoxides • When the nucleophile attacks a tertiary center of the epoxide, the intermediate it attacks takes on some carbocation character (SN 1), but not completely • Give reaction conditions for the following reaction • Practice with Skill. Builder 14. 5 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -41 Klein, Organic Chemistry 2 e

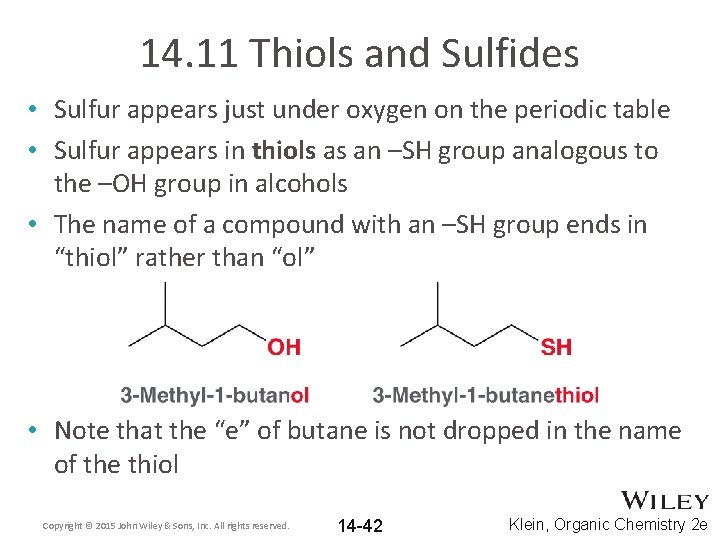
14. 11 Thiols and Sulfides • Sulfur appears just under oxygen on the periodic table • Sulfur appears in thiols as an –SH group analogous to the –OH group in alcohols • The name of a compound with an –SH group ends in “thiol” rather than “ol” • Note that the “e” of butane is not dropped in the name of the thiol Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -42 Klein, Organic Chemistry 2 e

14. 11 Thiols and Sulfides • Thiols are known for their unpleasant odor • Skunks use thiols as a defense mechanism • Methanethiol is added to natural gas (methane) so that gas leaks can be detected • Your nose is a very sensitive instrument • The hydrosulfide ion (HS-) is a strong nucleophile and a weak base • HS- promotes SN 2 rather than E 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -43 Klein, Organic Chemistry 2 e

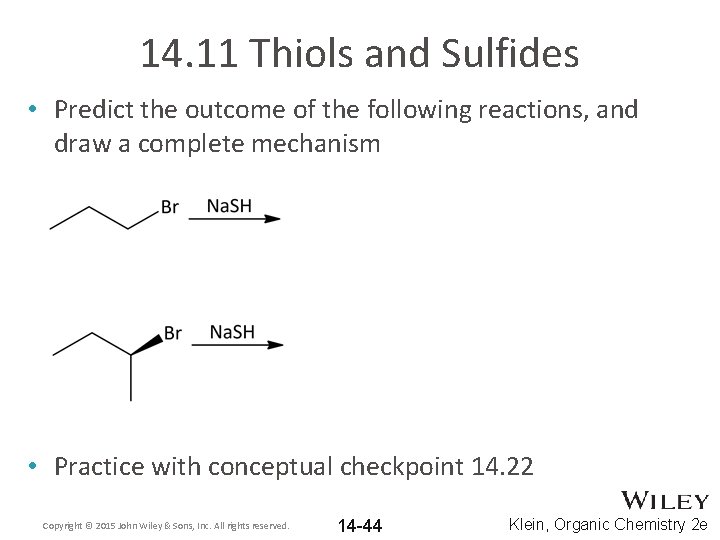
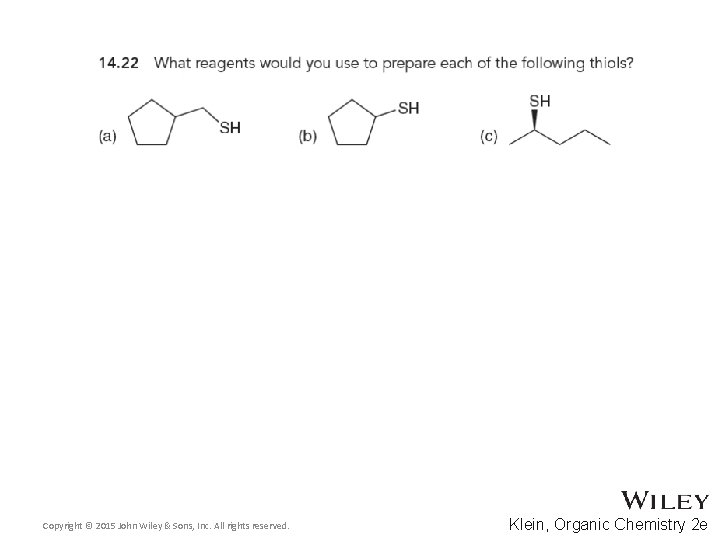
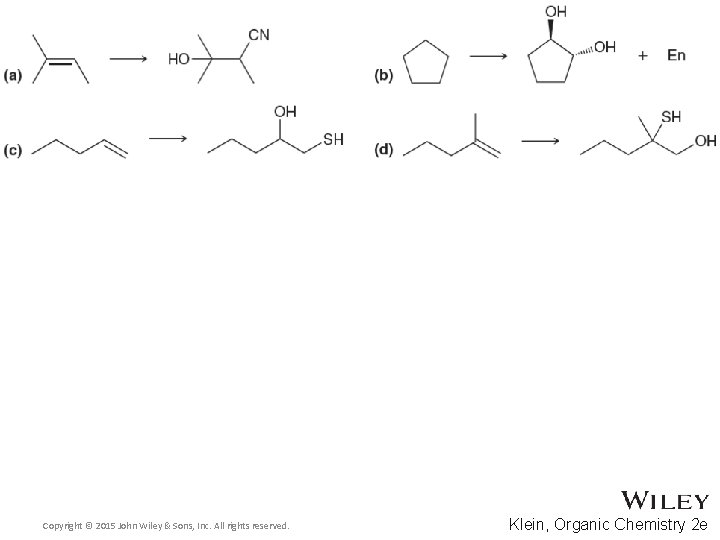
14. 11 Thiols and Sulfides • Predict the outcome of the following reactions, and draw a complete mechanism • Practice with conceptual checkpoint 14. 22 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -44 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

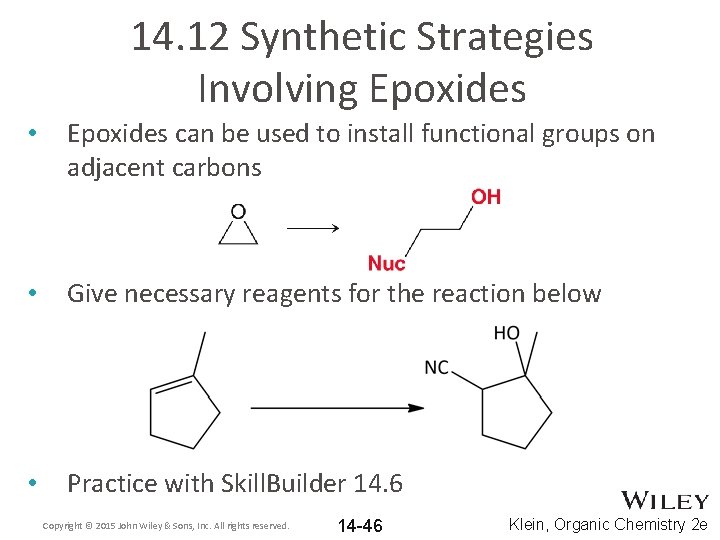
14. 12 Synthetic Strategies Involving Epoxides • Epoxides can be used to install functional groups on adjacent carbons • Give necessary reagents for the reaction below • Practice with Skill. Builder 14. 6 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -46 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

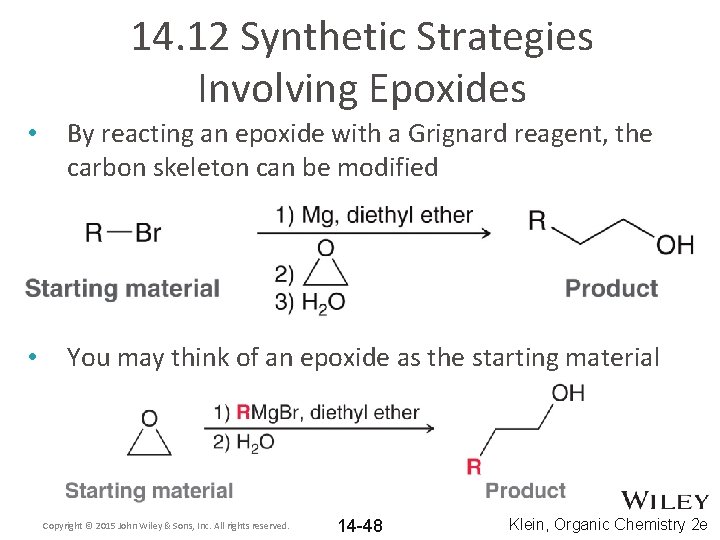
14. 12 Synthetic Strategies Involving Epoxides • By reacting an epoxide with a Grignard reagent, the carbon skeleton can be modified • You may think of an epoxide as the starting material Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -48 Klein, Organic Chemistry 2 e

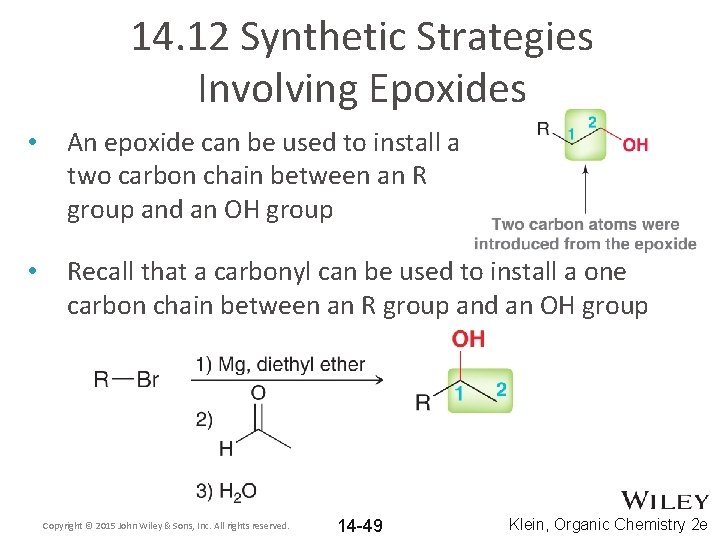
14. 12 Synthetic Strategies Involving Epoxides • An epoxide can be used to install a two carbon chain between an R group and an OH group • Recall that a carbonyl can be used to install a one carbon chain between an R group and an OH group Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -49 Klein, Organic Chemistry 2 e

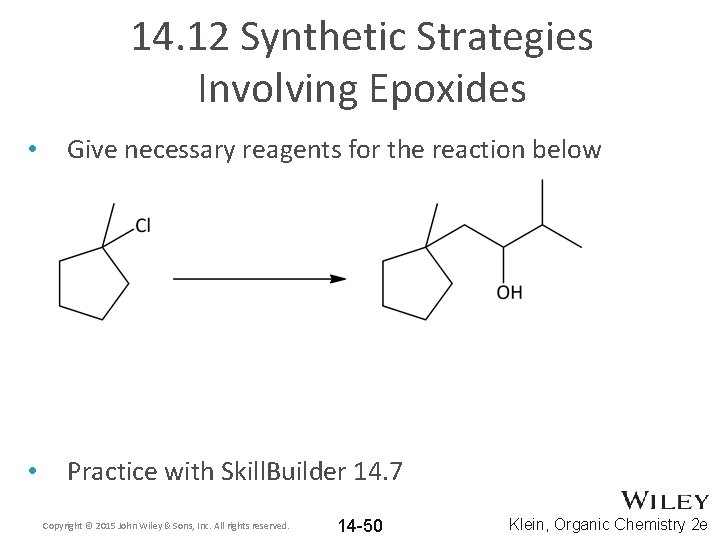
14. 12 Synthetic Strategies Involving Epoxides • Give necessary reagents for the reaction below • Practice with Skill. Builder 14. 7 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -50 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

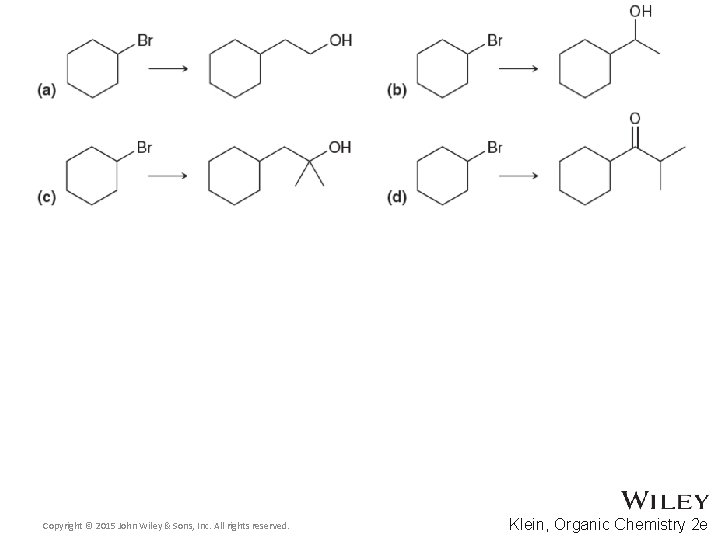
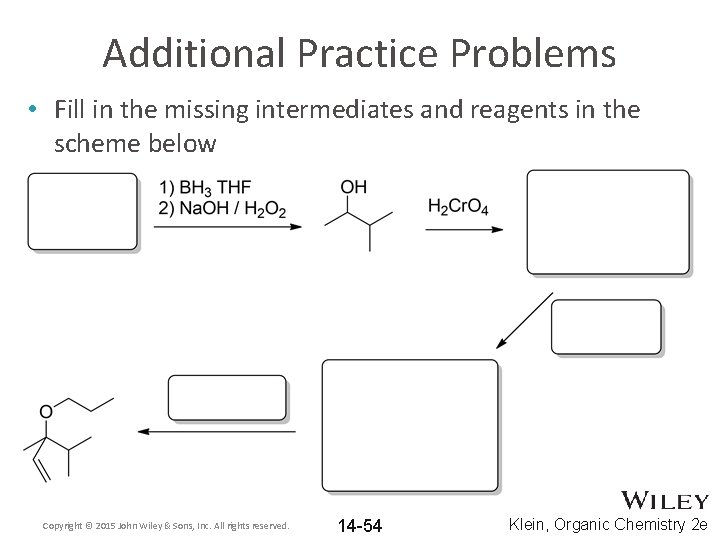
Additional Practice Problems • Fill in the missing intermediates and reagents in the scheme below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -54 Klein, Organic Chemistry 2 e

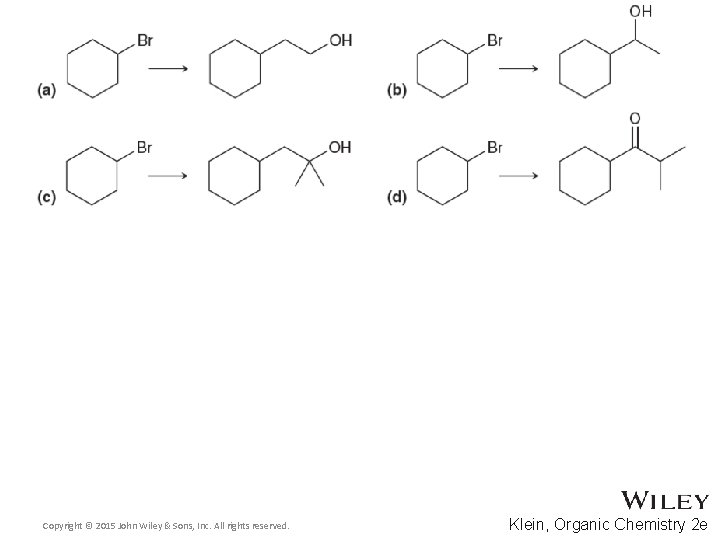
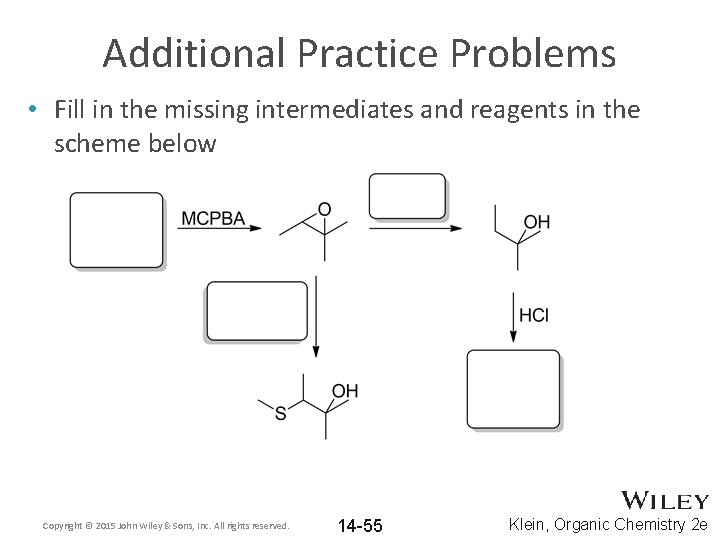
Additional Practice Problems • Fill in the missing intermediates and reagents in the scheme below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -55 Klein, Organic Chemistry 2 e

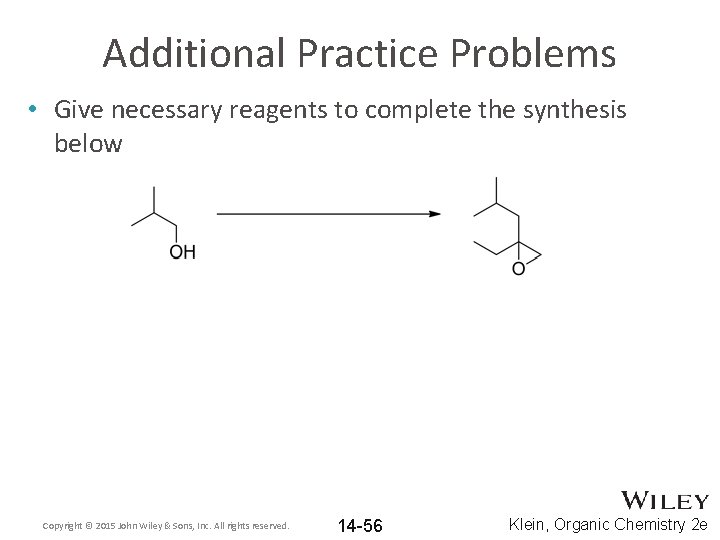
Additional Practice Problems • Give necessary reagents to complete the synthesis below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 14 -56 Klein, Organic Chemistry 2 e