Organic Chemistry Second Edition David Klein Chapter 15

- Slides: 74

Organic Chemistry Second Edition David Klein Chapter 15 Infrared Spectroscopy and Mass Spectrometry Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

15. 1 Introduction to Spectroscopy • Spectroscopy involves an interaction between matter and light (electromagnetic radiation) • Light can be thought of as waves of energy or packets (particles) of energy called photons • Properties of light waves include wavelength and frequency • Is wavelength directly or inversely proportional to energy? WHY? • Is frequency directly or inversely proportional to energy? WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -2 Klein, Organic Chemistry 2 e

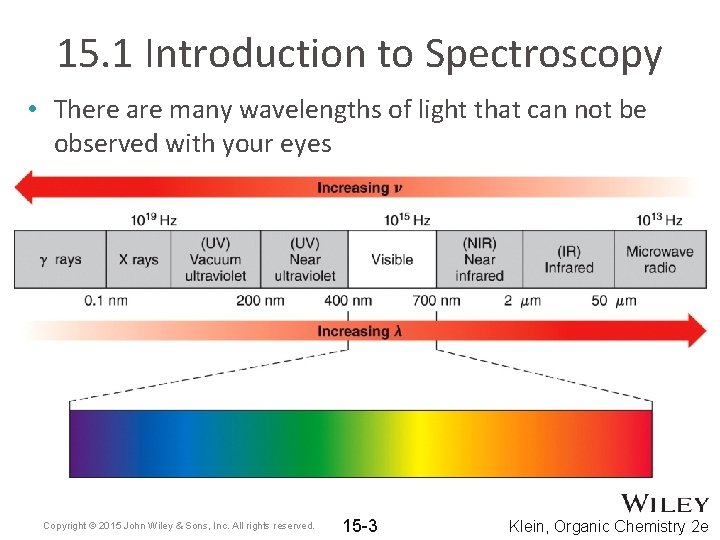
15. 1 Introduction to Spectroscopy • There are many wavelengths of light that can not be observed with your eyes Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -3 Klein, Organic Chemistry 2 e

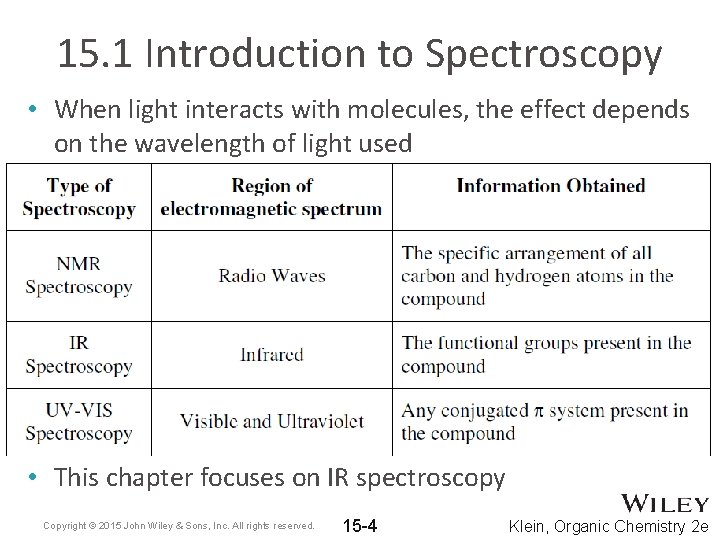
15. 1 Introduction to Spectroscopy • When light interacts with molecules, the effect depends on the wavelength of light used • This chapter focuses on IR spectroscopy Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -4 Klein, Organic Chemistry 2 e

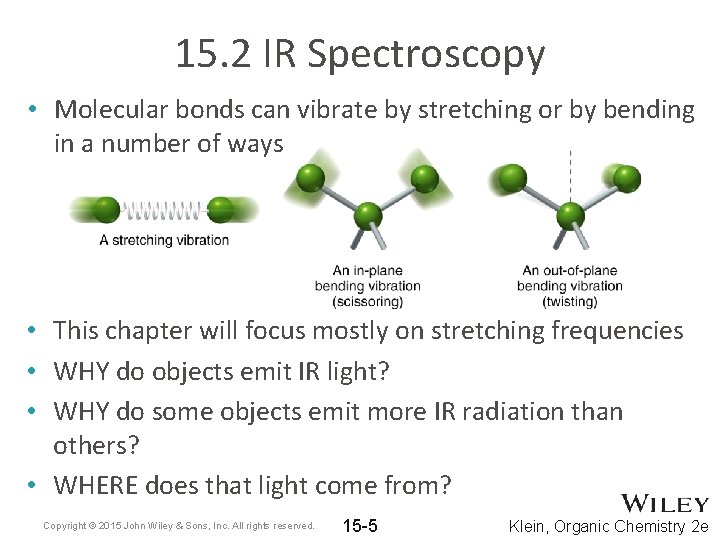
15. 2 IR Spectroscopy • Molecular bonds can vibrate by stretching or by bending in a number of ways • This chapter will focus mostly on stretching frequencies • WHY do objects emit IR light? • WHY do some objects emit more IR radiation than others? • WHERE does that light come from? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -5 Klein, Organic Chemistry 2 e

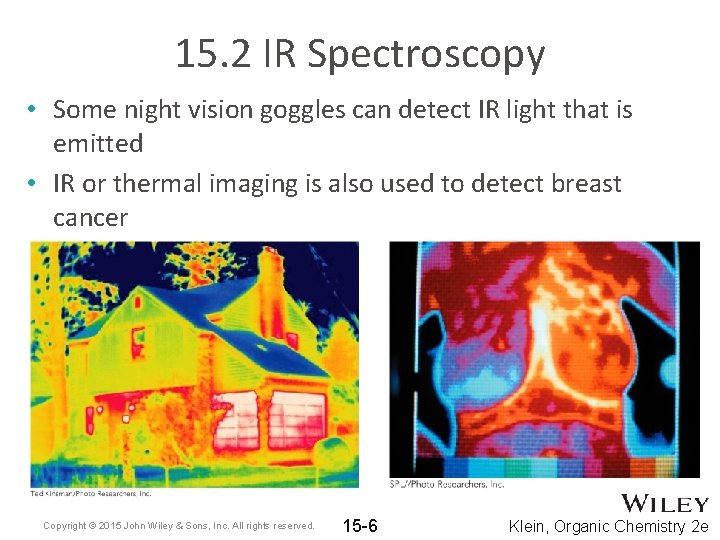
15. 2 IR Spectroscopy • Some night vision goggles can detect IR light that is emitted • IR or thermal imaging is also used to detect breast cancer Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -6 Klein, Organic Chemistry 2 e

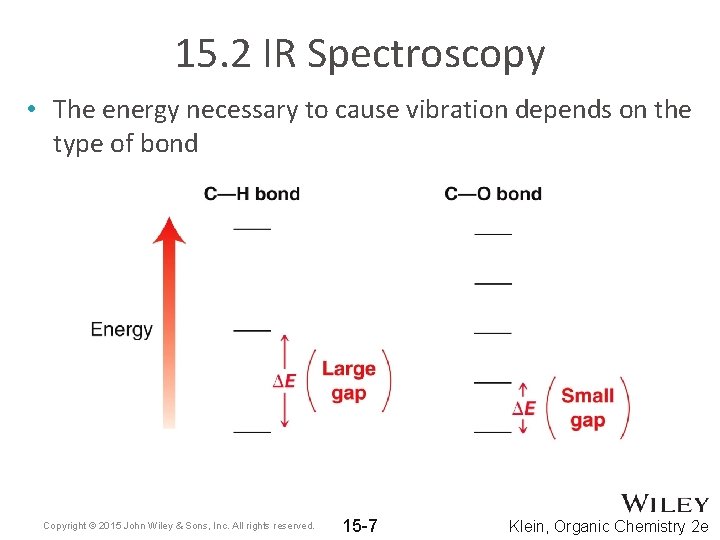
15. 2 IR Spectroscopy • The energy necessary to cause vibration depends on the type of bond Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -7 Klein, Organic Chemistry 2 e

15. 2 IR Spectroscopy • An IR spectrophotometer irradiates a sample with all frequencies of IR light • The frequencies that are absorbed by the sample tell us the types of bonds (functional groups) that are present • How do we measure the frequencies that are absorbed? • Most commonly, samples are deposited neat on a salt (Na. Cl) plate. WHY is salt used? • Alternatively, the compound may be dissolved in a solvent or embedded in a KBr pellet Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -8 Klein, Organic Chemistry 2 e

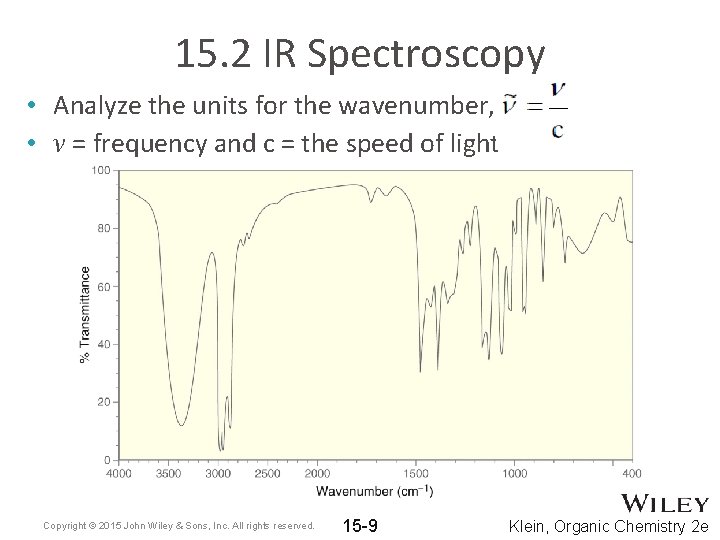
15. 2 IR Spectroscopy • Analyze the units for the wavenumber, • ν = frequency and c = the speed of light Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -9 Klein, Organic Chemistry 2 e

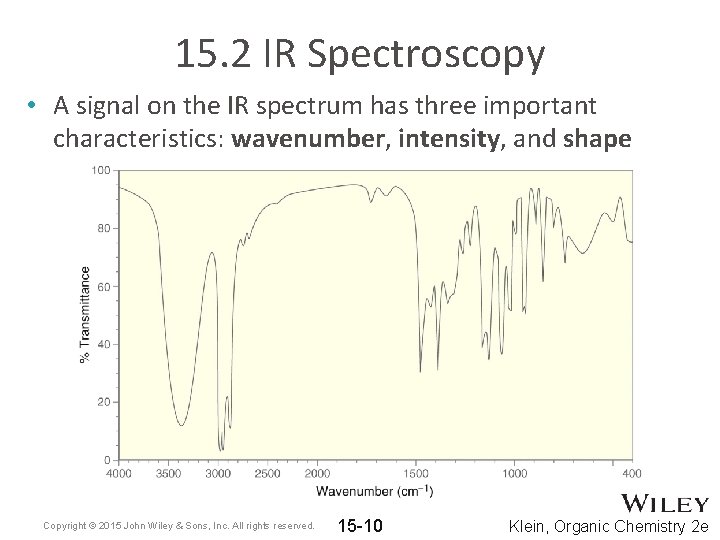
15. 2 IR Spectroscopy • A signal on the IR spectrum has three important characteristics: wavenumber, intensity, and shape Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -10 Klein, Organic Chemistry 2 e

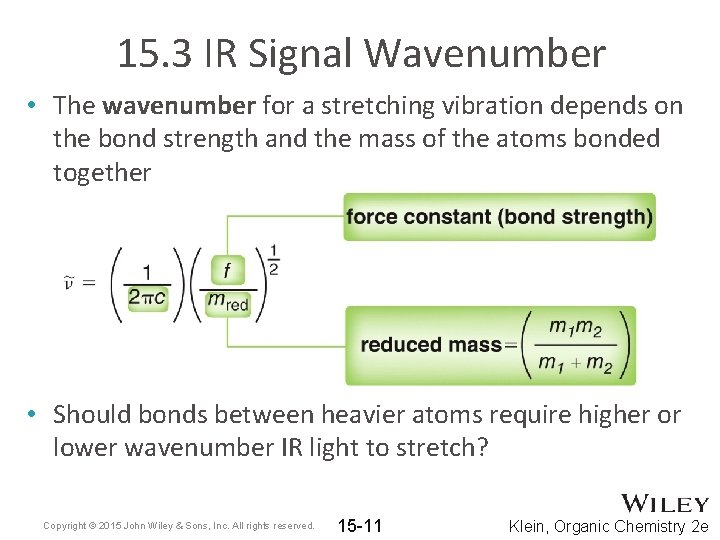
15. 3 IR Signal Wavenumber • The wavenumber for a stretching vibration depends on the bond strength and the mass of the atoms bonded together • Should bonds between heavier atoms require higher or lower wavenumber IR light to stretch? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -11 Klein, Organic Chemistry 2 e

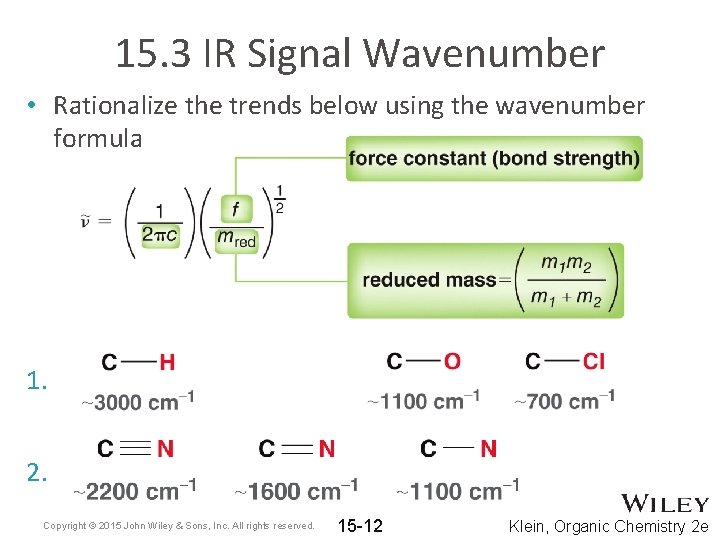
15. 3 IR Signal Wavenumber • Rationalize the trends below using the wavenumber formula 1. 2. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -12 Klein, Organic Chemistry 2 e

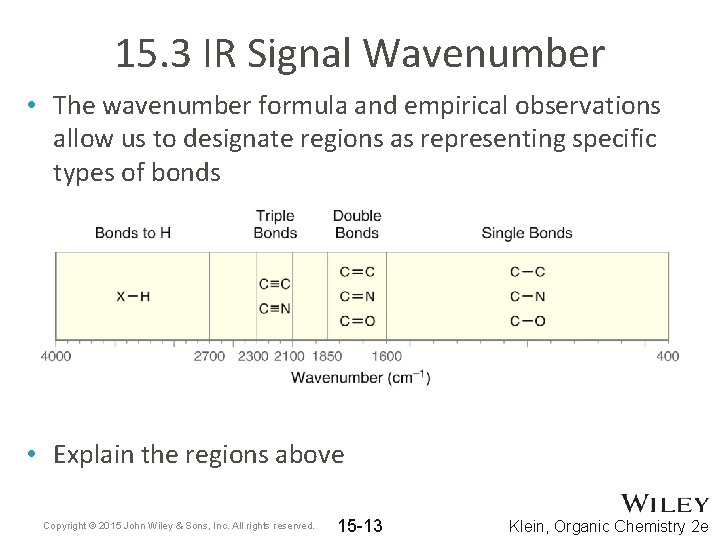
15. 3 IR Signal Wavenumber • The wavenumber formula and empirical observations allow us to designate regions as representing specific types of bonds • Explain the regions above Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -13 Klein, Organic Chemistry 2 e

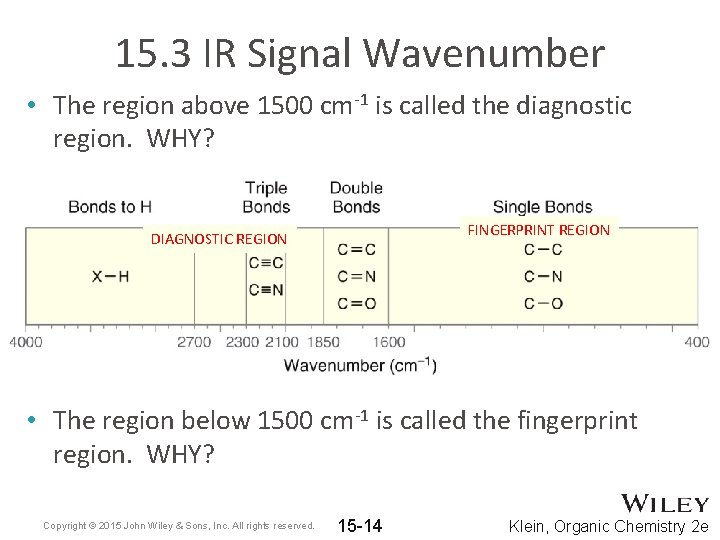
15. 3 IR Signal Wavenumber • The region above 1500 cm-1 is called the diagnostic region. WHY? FINGERPRINT REGION DIAGNOSTIC REGION • The region below 1500 cm-1 is called the fingerprint region. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -14 Klein, Organic Chemistry 2 e

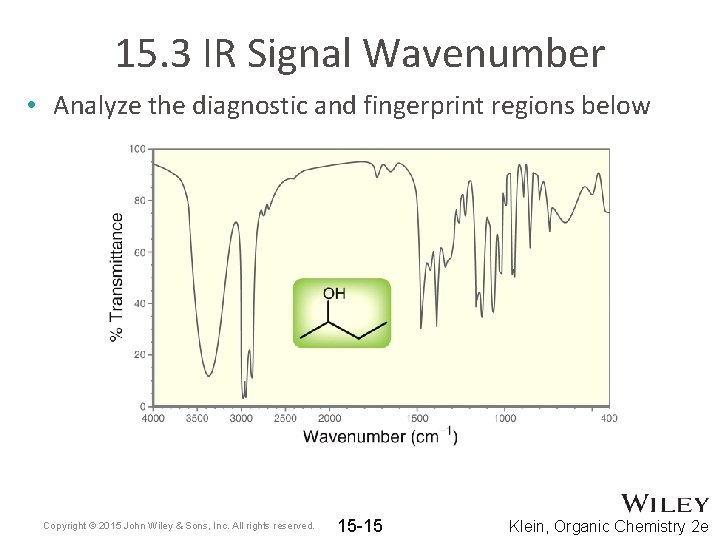
15. 3 IR Signal Wavenumber • Analyze the diagnostic and fingerprint regions below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -15 Klein, Organic Chemistry 2 e

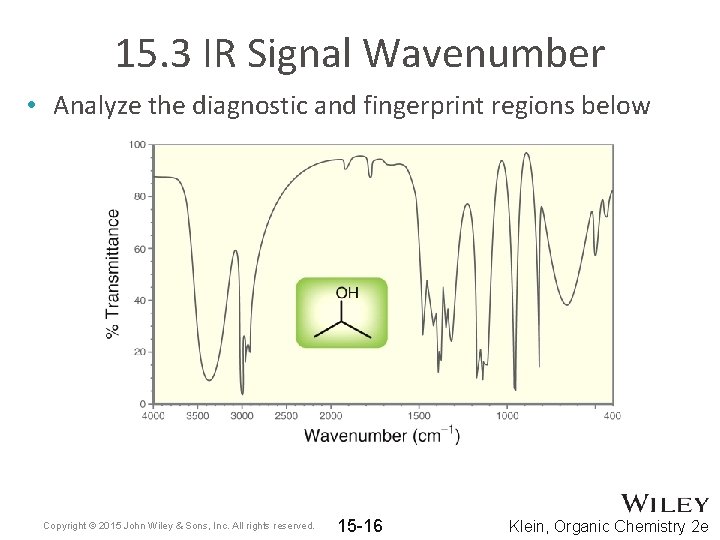
15. 3 IR Signal Wavenumber • Analyze the diagnostic and fingerprint regions below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -16 Klein, Organic Chemistry 2 e

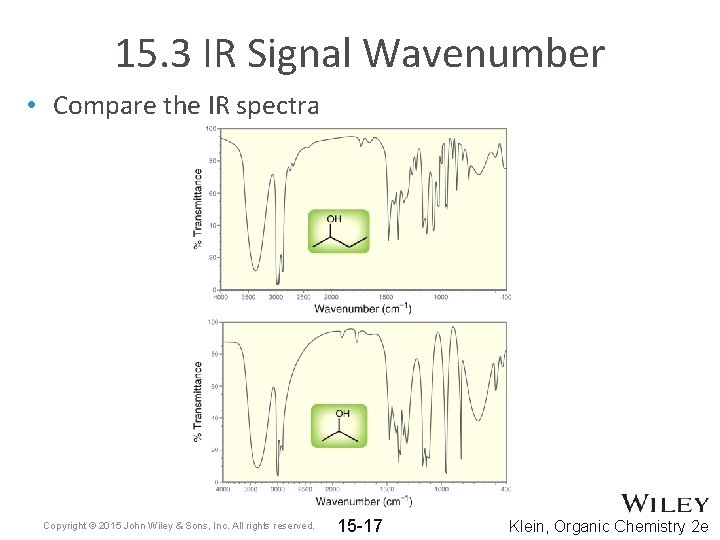
15. 3 IR Signal Wavenumber • Compare the IR spectra Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -17 Klein, Organic Chemistry 2 e

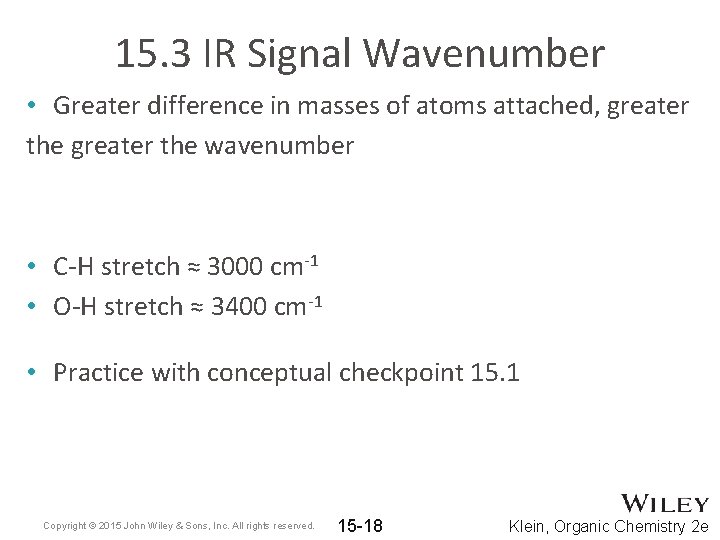
15. 3 IR Signal Wavenumber • Greater difference in masses of atoms attached, greater the wavenumber • C-H stretch ≈ 3000 cm-1 • O-H stretch ≈ 3400 cm-1 • Practice with conceptual checkpoint 15. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -18 Klein, Organic Chemistry 2 e

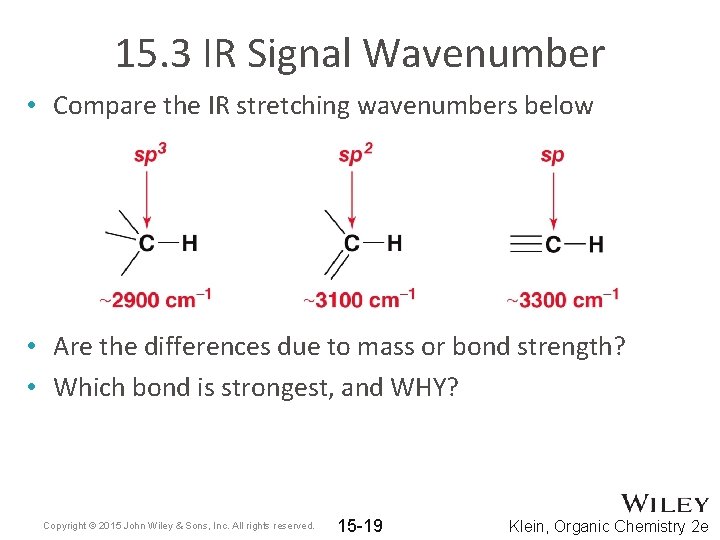
15. 3 IR Signal Wavenumber • Compare the IR stretching wavenumbers below • Are the differences due to mass or bond strength? • Which bond is strongest, and WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -19 Klein, Organic Chemistry 2 e

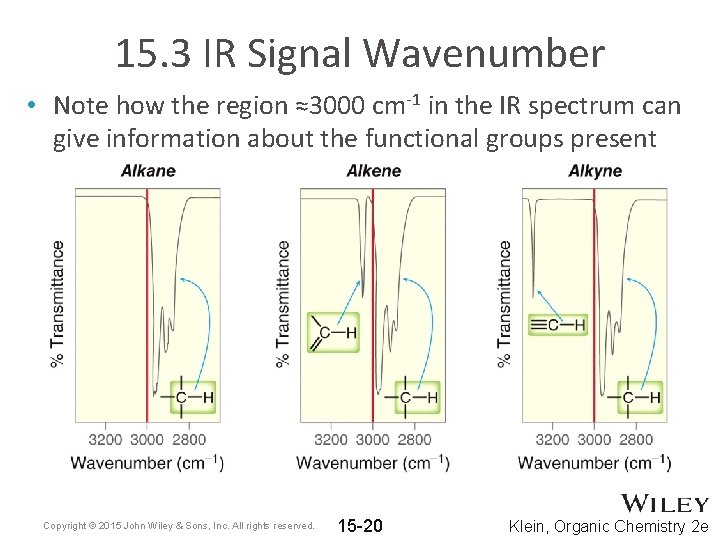
15. 3 IR Signal Wavenumber • Note how the region ≈3000 cm-1 in the IR spectrum can give information about the functional groups present Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -20 Klein, Organic Chemistry 2 e

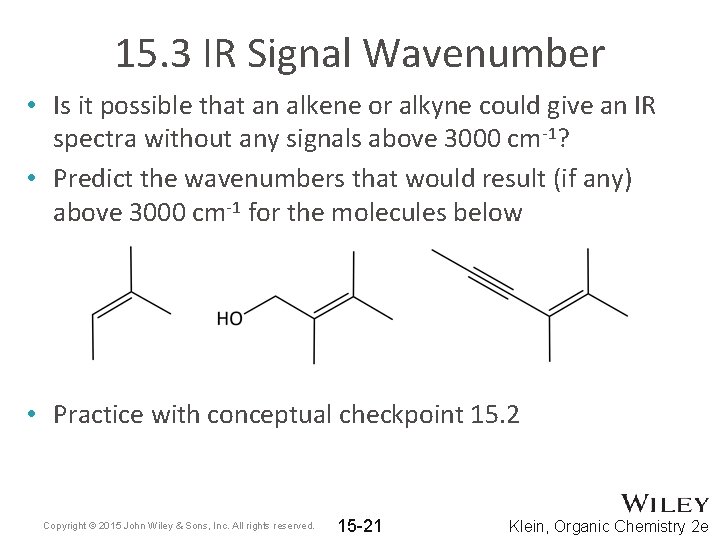
15. 3 IR Signal Wavenumber • Is it possible that an alkene or alkyne could give an IR spectra without any signals above 3000 cm-1? • Predict the wavenumbers that would result (if any) above 3000 cm-1 for the molecules below • Practice with conceptual checkpoint 15. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -21 Klein, Organic Chemistry 2 e

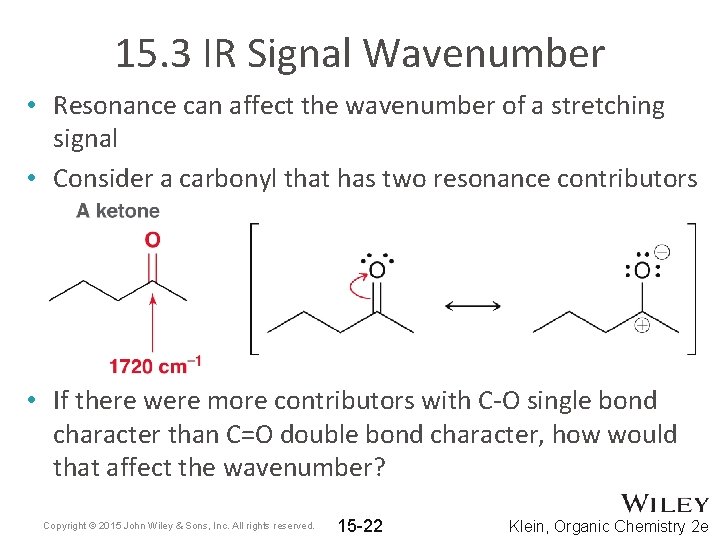
15. 3 IR Signal Wavenumber • Resonance can affect the wavenumber of a stretching signal • Consider a carbonyl that has two resonance contributors • If there were more contributors with C-O single bond character than C=O double bond character, how would that affect the wavenumber? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -22 Klein, Organic Chemistry 2 e

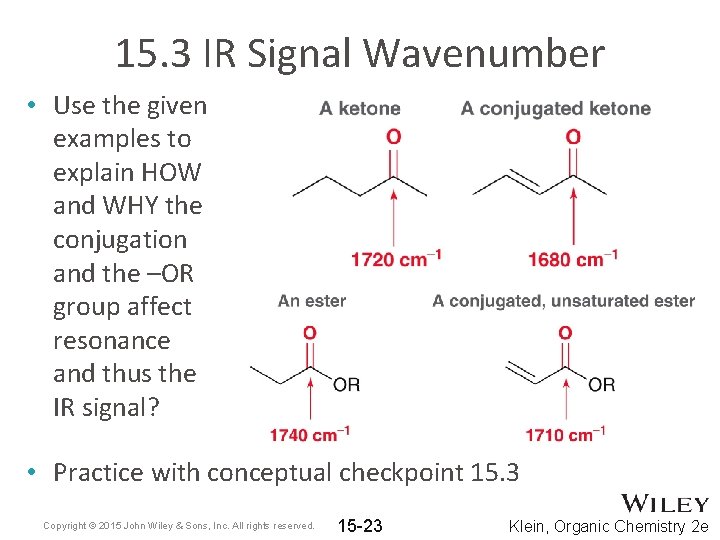
15. 3 IR Signal Wavenumber • Use the given examples to explain HOW and WHY the conjugation and the –OR group affect resonance and thus the IR signal? • Practice with conceptual checkpoint 15. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -23 Klein, Organic Chemistry 2 e

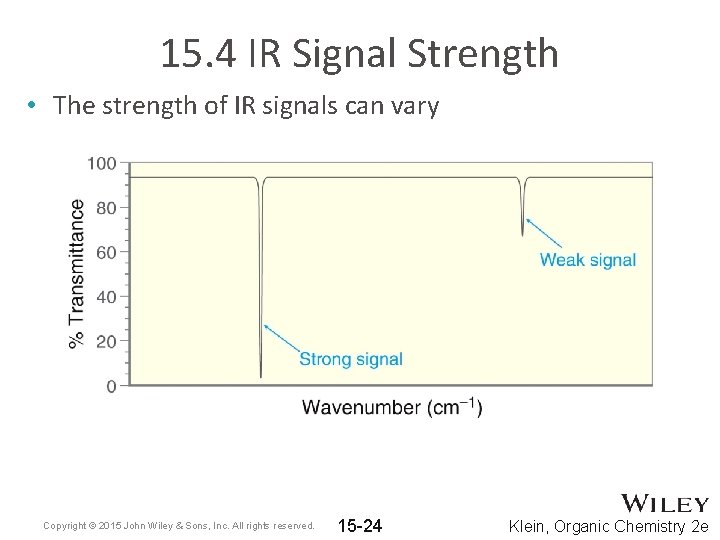
15. 4 IR Signal Strength • The strength of IR signals can vary Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -24 Klein, Organic Chemistry 2 e

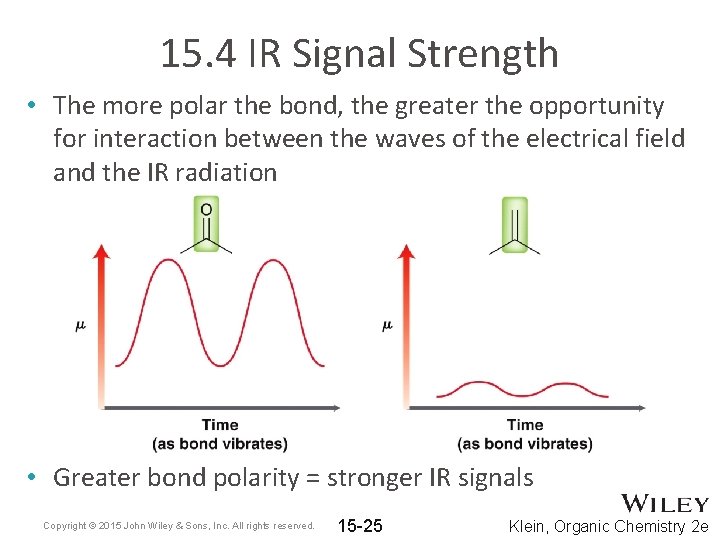
15. 4 IR Signal Strength • The more polar the bond, the greater the opportunity for interaction between the waves of the electrical field and the IR radiation • Greater bond polarity = stronger IR signals Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -25 Klein, Organic Chemistry 2 e

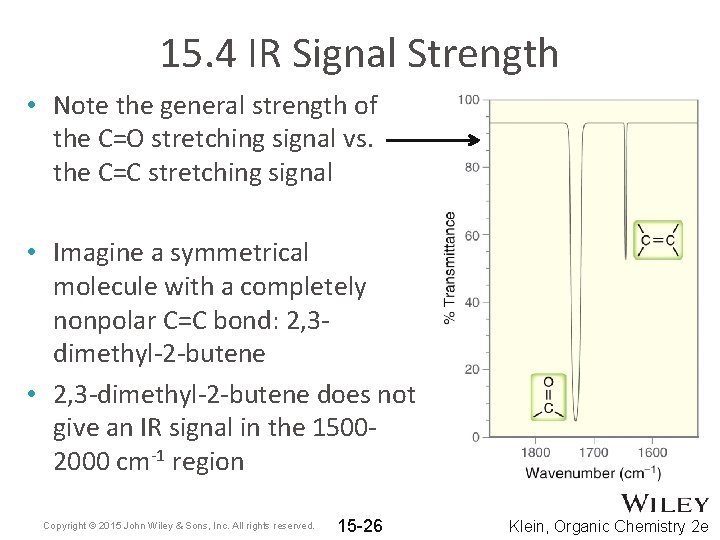
15. 4 IR Signal Strength • Note the general strength of the C=O stretching signal vs. the C=C stretching signal • Imagine a symmetrical molecule with a completely nonpolar C=C bond: 2, 3 dimethyl-2 -butene • 2, 3 -dimethyl-2 -butene does not give an IR signal in the 15002000 cm-1 region Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -26 Klein, Organic Chemistry 2 e

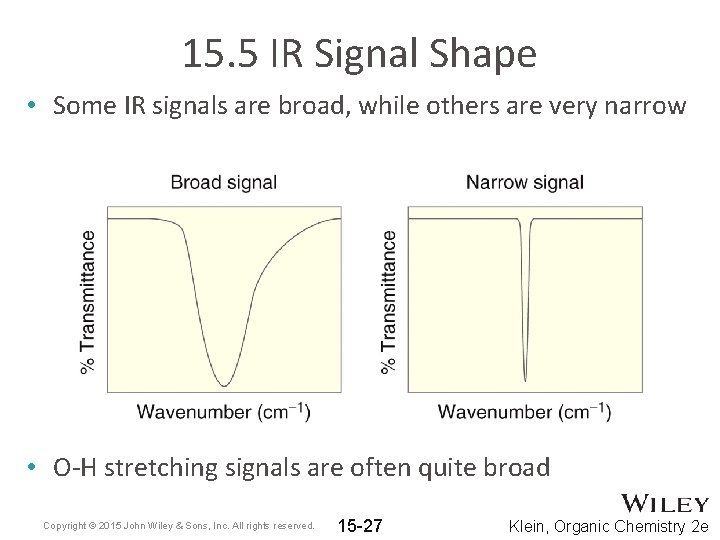
15. 5 IR Signal Shape • Some IR signals are broad, while others are very narrow • O-H stretching signals are often quite broad Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -27 Klein, Organic Chemistry 2 e

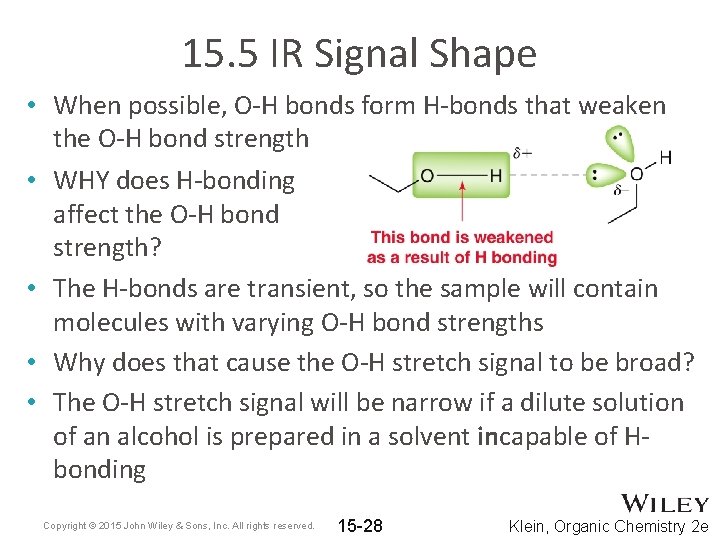
15. 5 IR Signal Shape • When possible, O-H bonds form H-bonds that weaken the O-H bond strength • WHY does H-bonding affect the O-H bond strength? • The H-bonds are transient, so the sample will contain molecules with varying O-H bond strengths • Why does that cause the O-H stretch signal to be broad? • The O-H stretch signal will be narrow if a dilute solution of an alcohol is prepared in a solvent incapable of Hbonding Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -28 Klein, Organic Chemistry 2 e

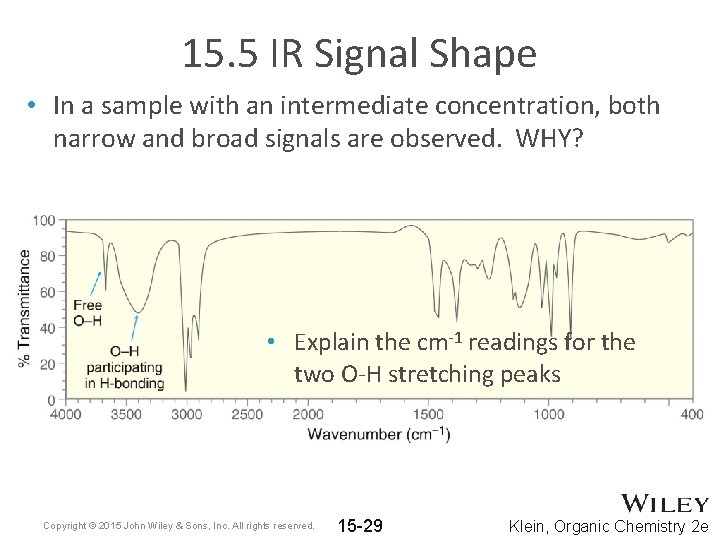
15. 5 IR Signal Shape • In a sample with an intermediate concentration, both narrow and broad signals are observed. WHY? • Explain the cm-1 readings for the two O-H stretching peaks Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -29 Klein, Organic Chemistry 2 e

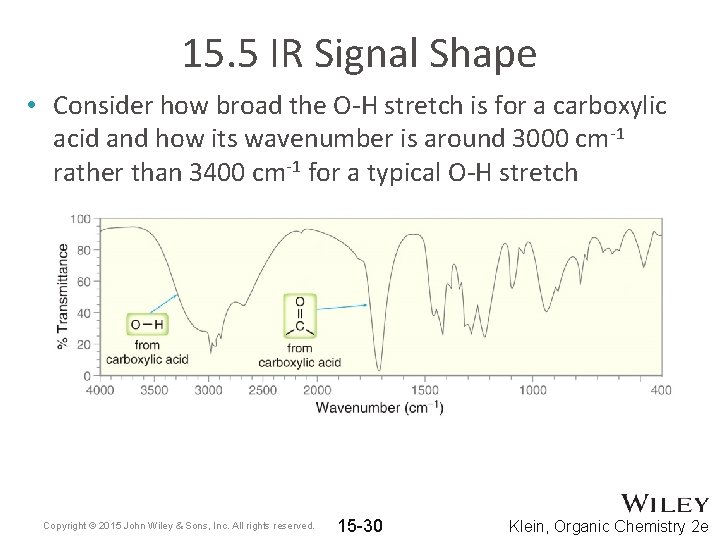
15. 5 IR Signal Shape • Consider how broad the O-H stretch is for a carboxylic acid and how its wavenumber is around 3000 cm-1 rather than 3400 cm-1 for a typical O-H stretch Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -30 Klein, Organic Chemistry 2 e

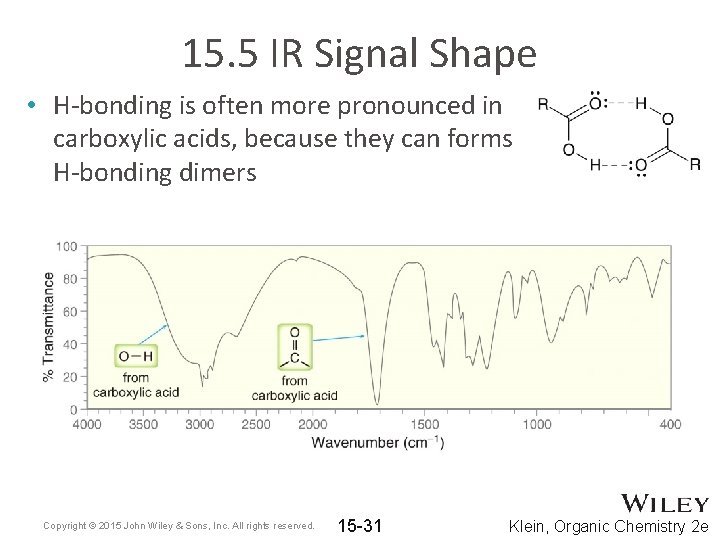
15. 5 IR Signal Shape • H-bonding is often more pronounced in carboxylic acids, because they can forms H-bonding dimers Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -31 Klein, Organic Chemistry 2 e

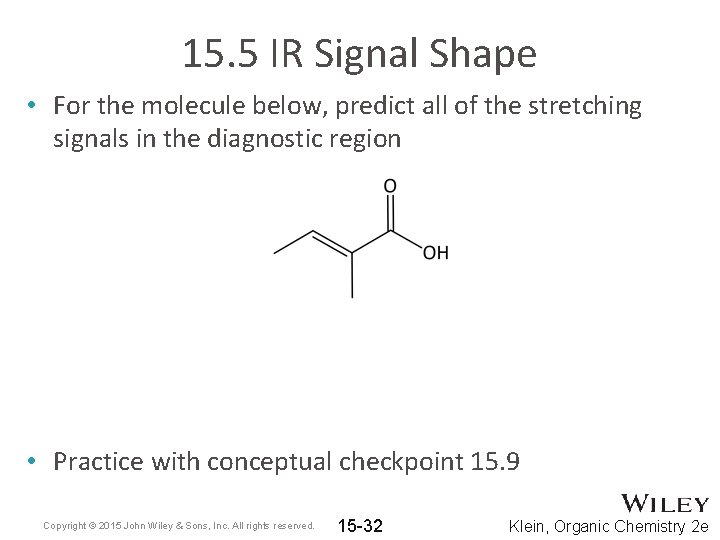
15. 5 IR Signal Shape • For the molecule below, predict all of the stretching signals in the diagnostic region • Practice with conceptual checkpoint 15. 9 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -32 Klein, Organic Chemistry 2 e

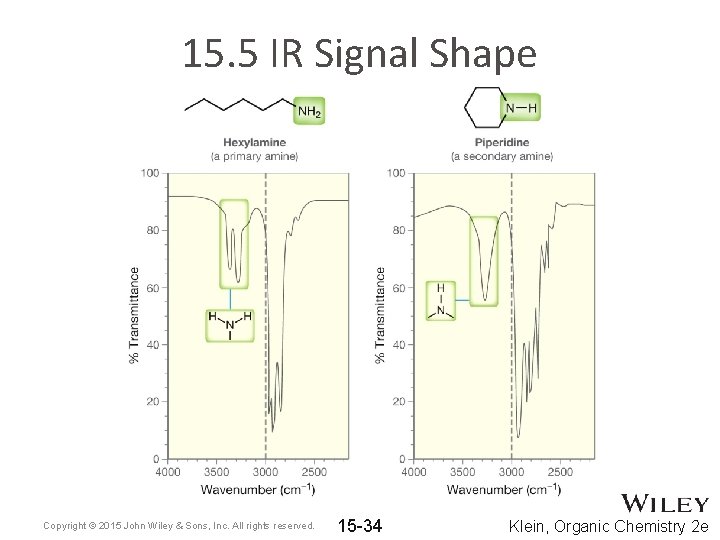
15. 5 IR Signal Shape • Primary and secondary amines exhibit N-H stretching signals. WHY not tertiary amines? • Because N-H bonds are capable of H-bonding, their stretching signals are often broadened • Which is generally more polar, an O-H or an N-H bond? • Do you expect N-H stretches to be strong or weak signals? • See example spectra on next slide Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -33 Klein, Organic Chemistry 2 e

15. 5 IR Signal Shape Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -34 Klein, Organic Chemistry 2 e

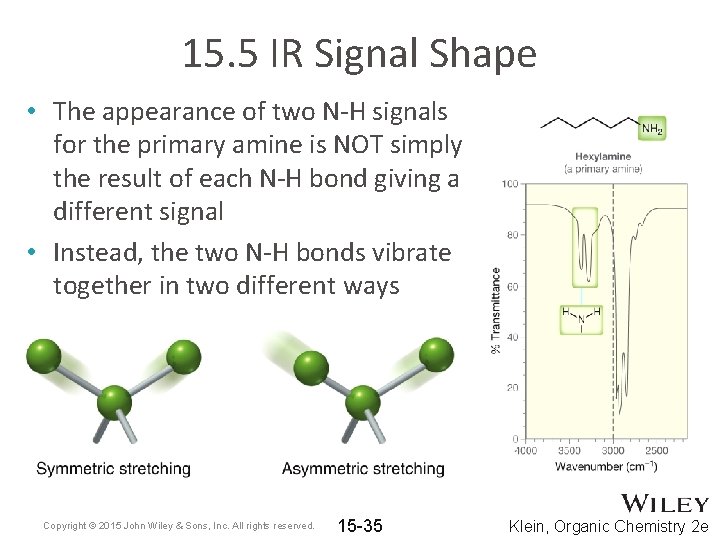
15. 5 IR Signal Shape • The appearance of two N-H signals for the primary amine is NOT simply the result of each N-H bond giving a different signal • Instead, the two N-H bonds vibrate together in two different ways Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -35 Klein, Organic Chemistry 2 e

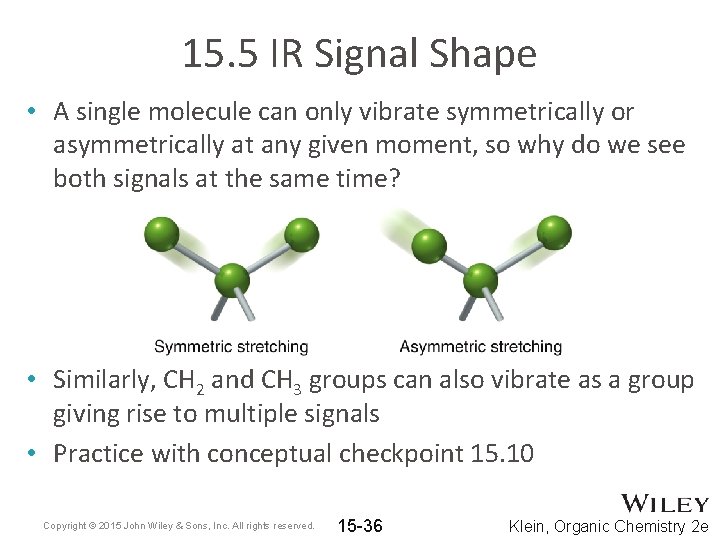
15. 5 IR Signal Shape • A single molecule can only vibrate symmetrically or asymmetrically at any given moment, so why do we see both signals at the same time? • Similarly, CH 2 and CH 3 groups can also vibrate as a group giving rise to multiple signals • Practice with conceptual checkpoint 15. 10 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -36 Klein, Organic Chemistry 2 e

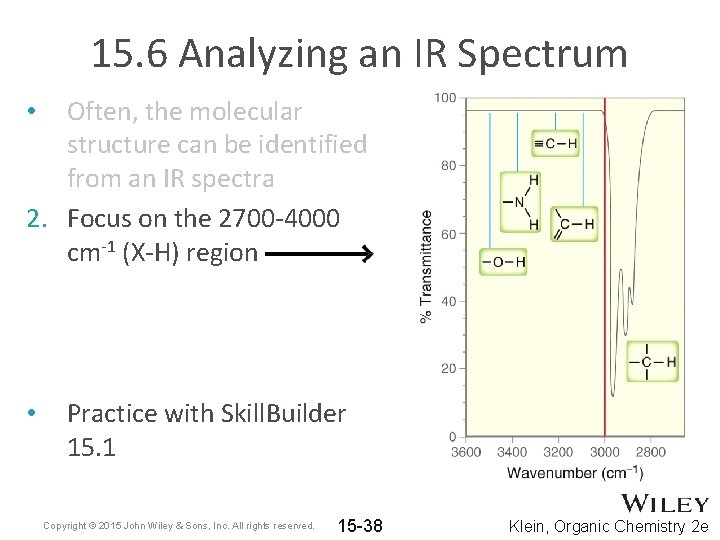
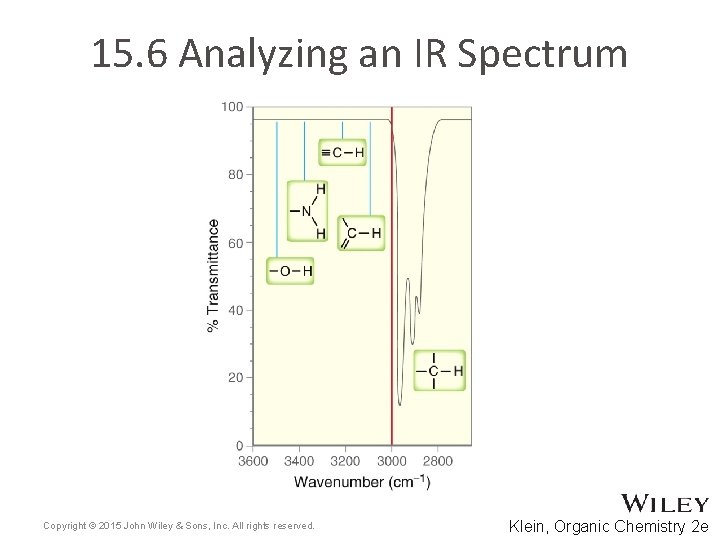
15. 6 Analyzing an IR Spectrum • Table 15. 2 summarizes some of the key signals that help us to identify functional groups present in molecules • Often, the molecular structure can be identified from an IR spectra 1. Focus on the diagnostic region (above 1500 cm-1) a) b) c) d) 1600 -1850 cm-1 – check for double bonds 2100 -2300 cm-1 – check for triple bonds 2700 -4000 cm-1 – check for X-H bonds Analyze wavenumber, intensity, and shape for each signal Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -37 Klein, Organic Chemistry 2 e

15. 6 Analyzing an IR Spectrum Often, the molecular structure can be identified from an IR spectra 2. Focus on the 2700 -4000 cm-1 (X-H) region • • Practice with Skill. Builder 15. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -38 Klein, Organic Chemistry 2 e

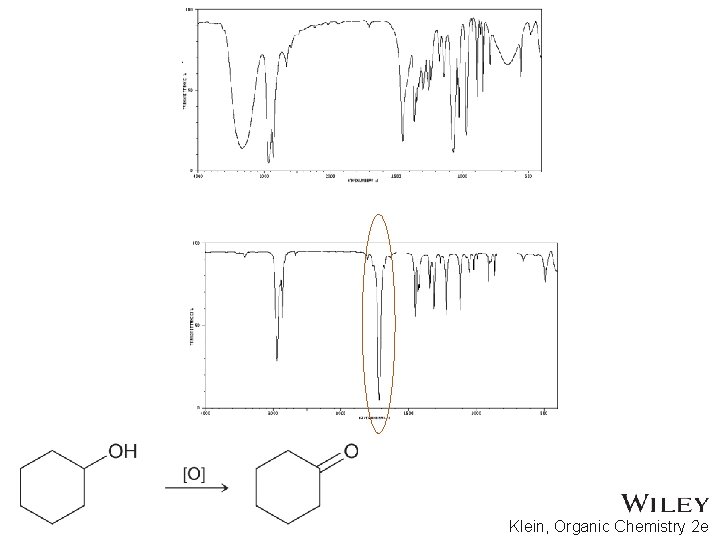
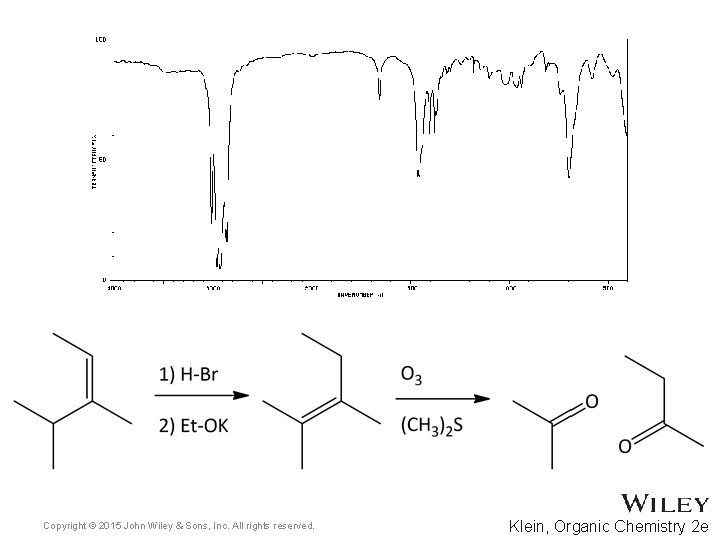
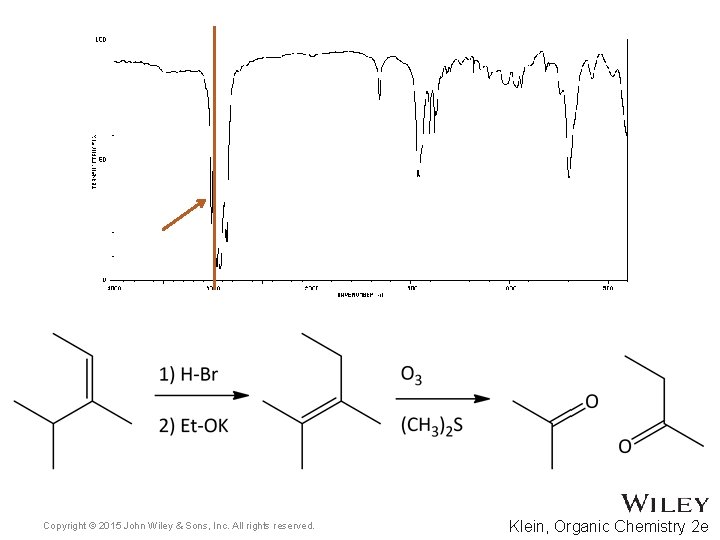
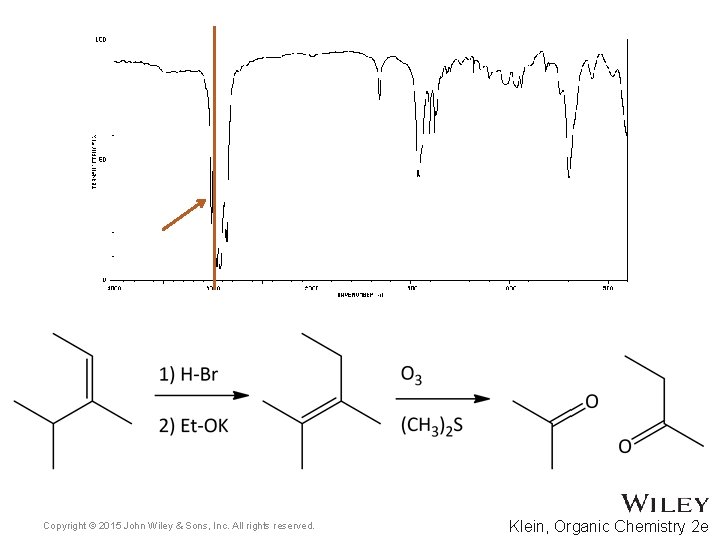
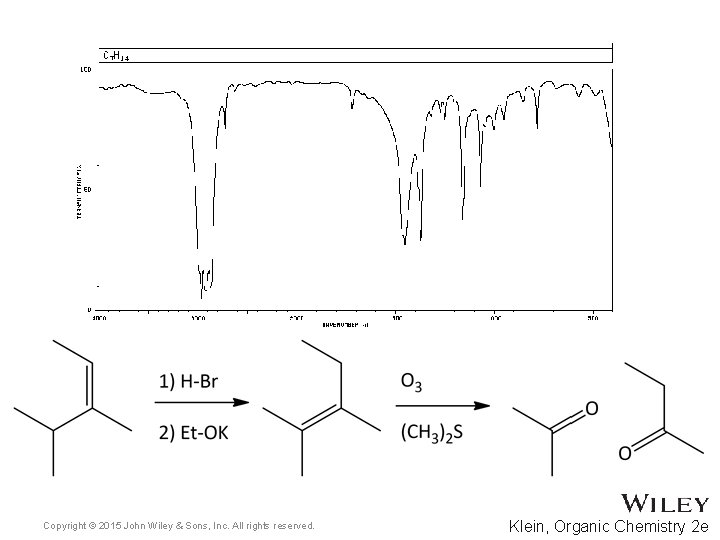
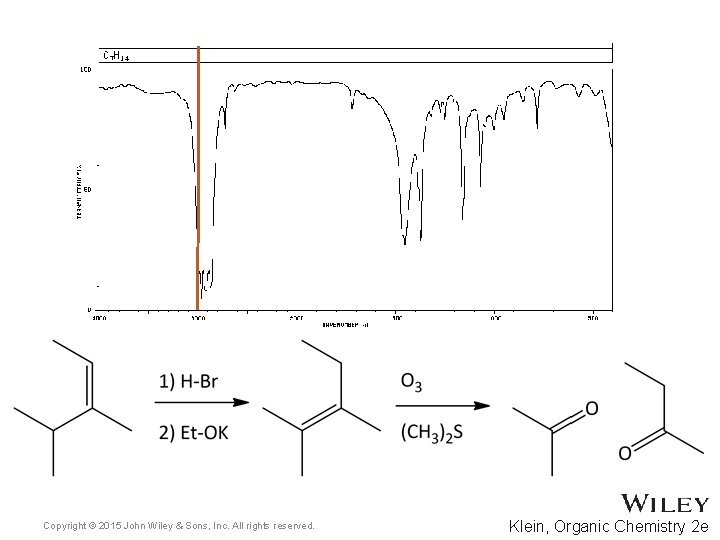
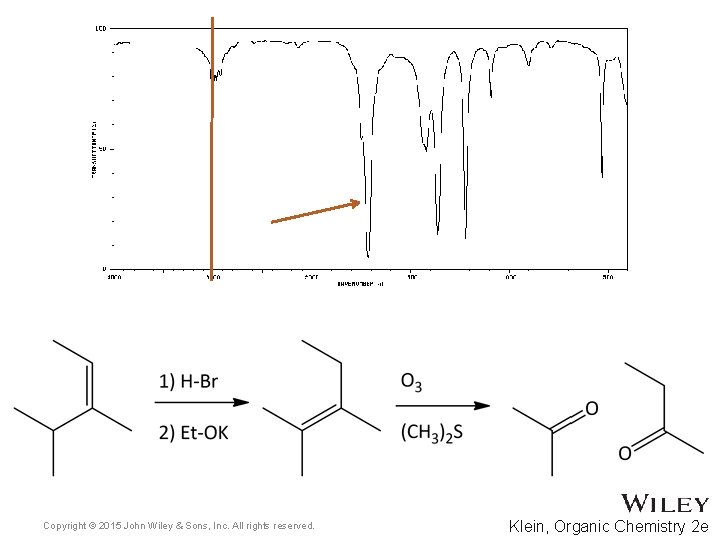
15. 7 Using IR to Distinguish Between Molecules • As we have learned in previous chapters, organic chemists often carry out reactions to convert one functional group into another • IR spectroscopy can often be used to determine the success of such reactions • For the reaction below, how might IR spectroscopy be used to analyze the reaction? • Practice with Skill. Builder 15. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -39 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

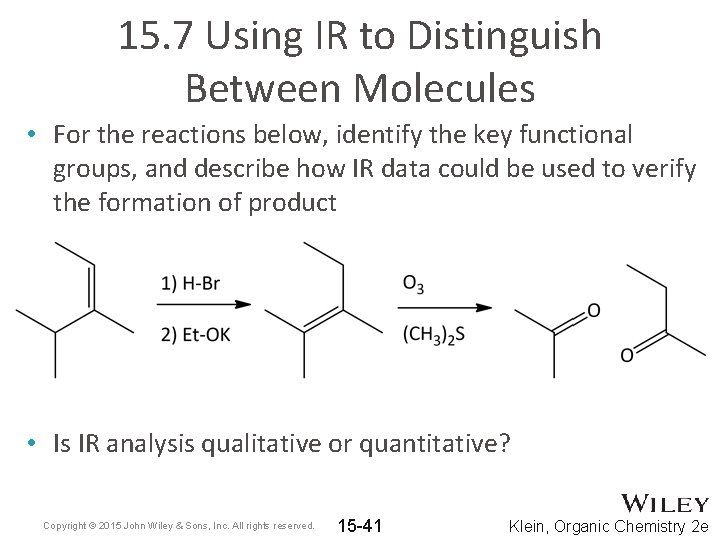
15. 7 Using IR to Distinguish Between Molecules • For the reactions below, identify the key functional groups, and describe how IR data could be used to verify the formation of product • Is IR analysis qualitative or quantitative? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -41 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

15. 6 Analyzing an IR Spectrum Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

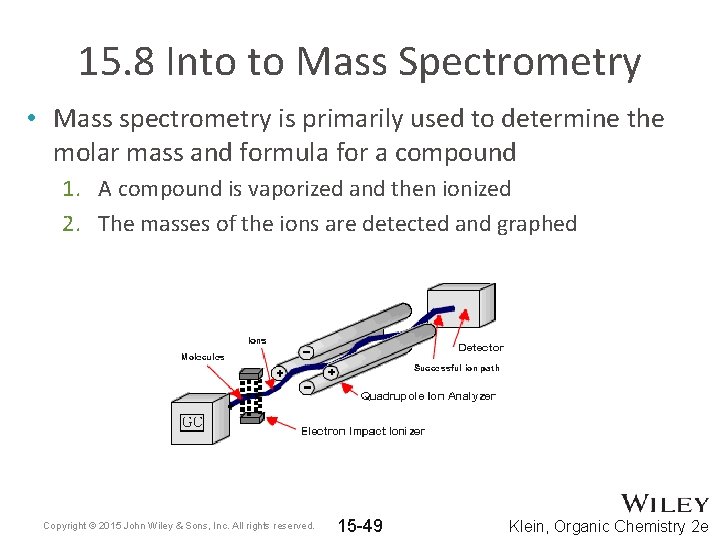
15. 8 Into to Mass Spectrometry • Mass spectrometry is primarily used to determine the molar mass and formula for a compound 1. A compound is vaporized and then ionized 2. The masses of the ions are detected and graphed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -49 Klein, Organic Chemistry 2 e

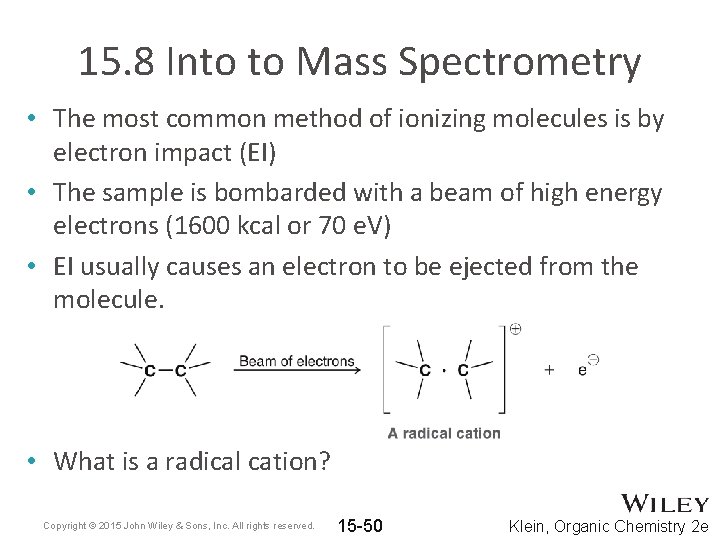
15. 8 Into to Mass Spectrometry • The most common method of ionizing molecules is by electron impact (EI) • The sample is bombarded with a beam of high energy electrons (1600 kcal or 70 e. V) • EI usually causes an electron to be ejected from the molecule. • What is a radical cation? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -50 Klein, Organic Chemistry 2 e

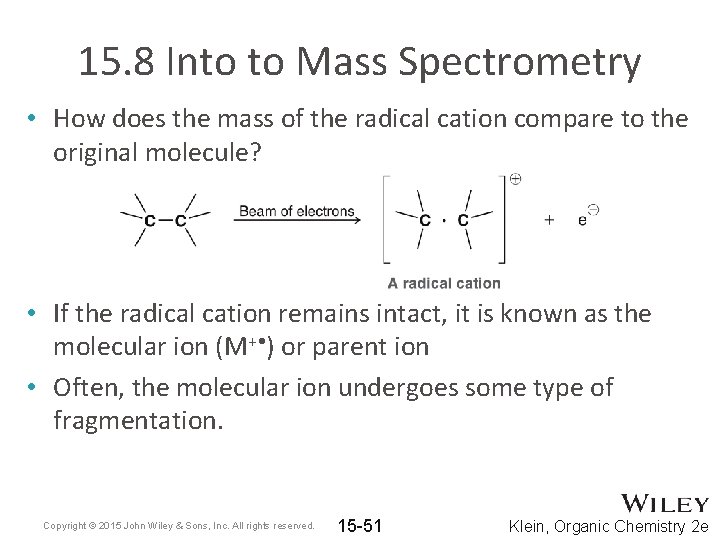
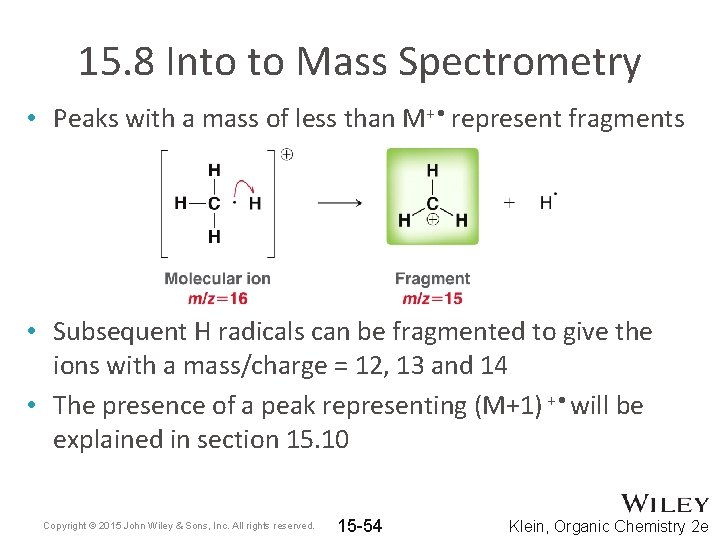
15. 8 Into to Mass Spectrometry • How does the mass of the radical cation compare to the original molecule? • If the radical cation remains intact, it is known as the molecular ion (M+ • ) or parent ion • Often, the molecular ion undergoes some type of fragmentation. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -51 Klein, Organic Chemistry 2 e

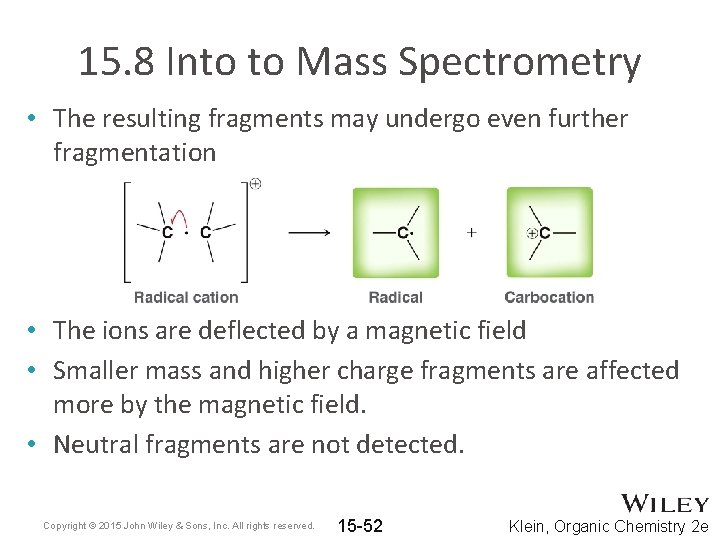
15. 8 Into to Mass Spectrometry • The resulting fragments may undergo even further fragmentation • The ions are deflected by a magnetic field • Smaller mass and higher charge fragments are affected more by the magnetic field. • Neutral fragments are not detected. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -52 Klein, Organic Chemistry 2 e

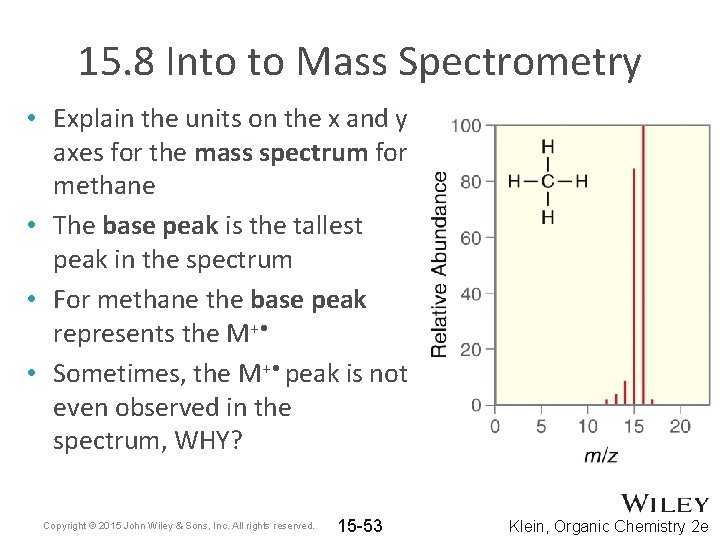
15. 8 Into to Mass Spectrometry • Explain the units on the x and y axes for the mass spectrum for methane • The base peak is the tallest peak in the spectrum • For methane the base peak represents the M+ • • Sometimes, the M+ • peak is not even observed in the spectrum, WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -53 Klein, Organic Chemistry 2 e

15. 8 Into to Mass Spectrometry • Peaks with a mass of less than M+ • represent fragments • Subsequent H radicals can be fragmented to give the ions with a mass/charge = 12, 13 and 14 • The presence of a peak representing (M+1) + • will be explained in section 15. 10 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -54 Klein, Organic Chemistry 2 e

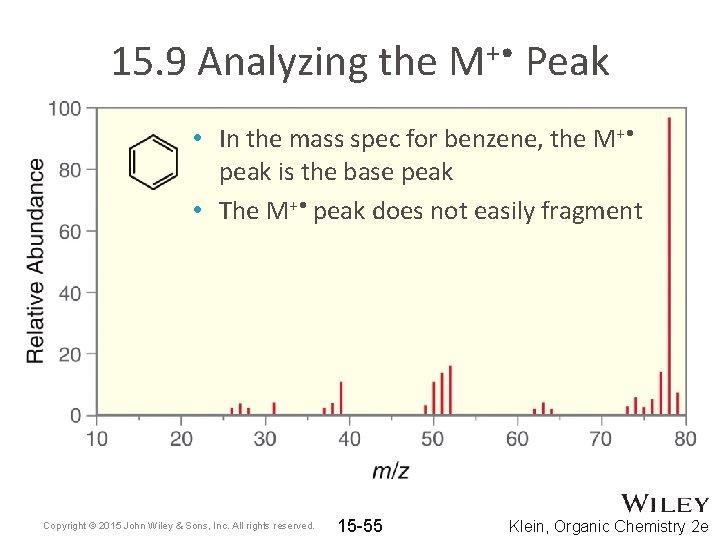
15. 9 Analyzing the M+ • Peak • In the mass spec for benzene, the M+ • peak is the base peak • The M+ • peak does not easily fragment Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -55 Klein, Organic Chemistry 2 e

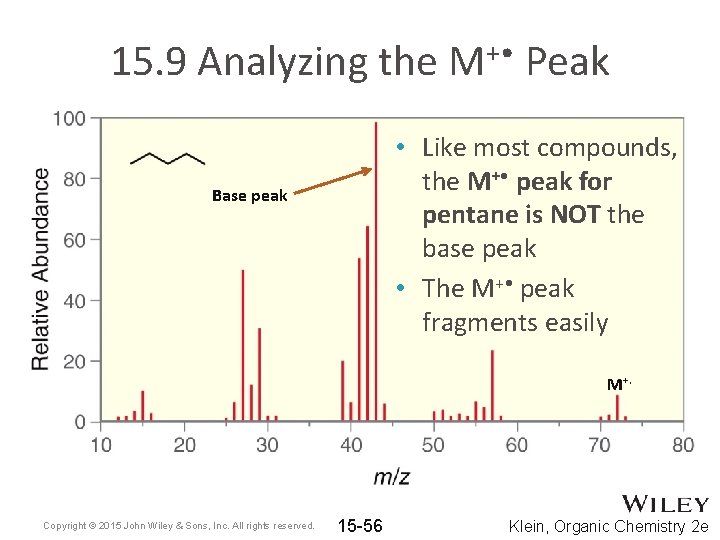
15. 9 Analyzing the M+ • Peak • Like most compounds, the M+ • peak for pentane is NOT the base peak • The M+ • peak fragments easily Base peak M+. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -56 Klein, Organic Chemistry 2 e

15. 9 Analyzing the M+ • Peak • The first step in analyzing a mass spec is to identify the M+ • peak – It will tell you the molar mass of the compound – An odd massed M+ • peak MAY indicate an odd number of N atoms in the molecule – An even massed M+ • peak MAY indicate an even number of N atoms or zero N atoms in the molecule • Give an alternative explanation for a M+ • peak with an odd mass • Practice with conceptual checkpoint 15. 19 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -57 Klein, Organic Chemistry 2 e

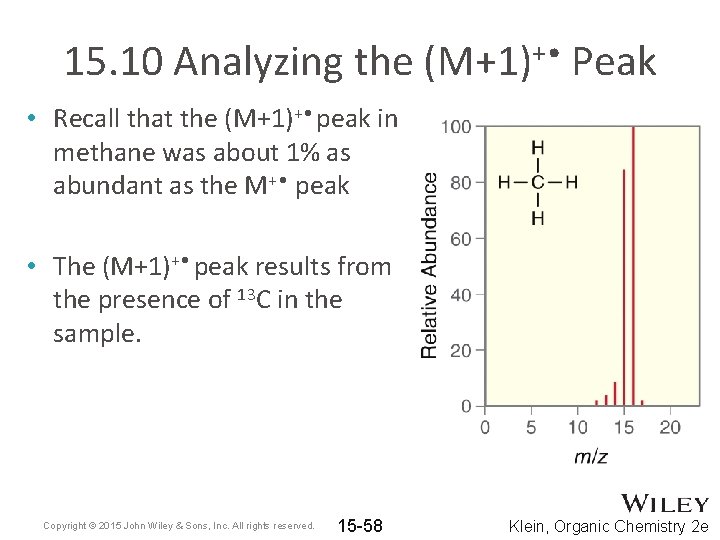
15. 10 Analyzing the (M+1)+ • Peak • Recall that the (M+1)+ • peak in methane was about 1% as abundant as the M+ • peak • The (M+1)+ • peak results from the presence of 13 C in the sample. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -58 Klein, Organic Chemistry 2 e

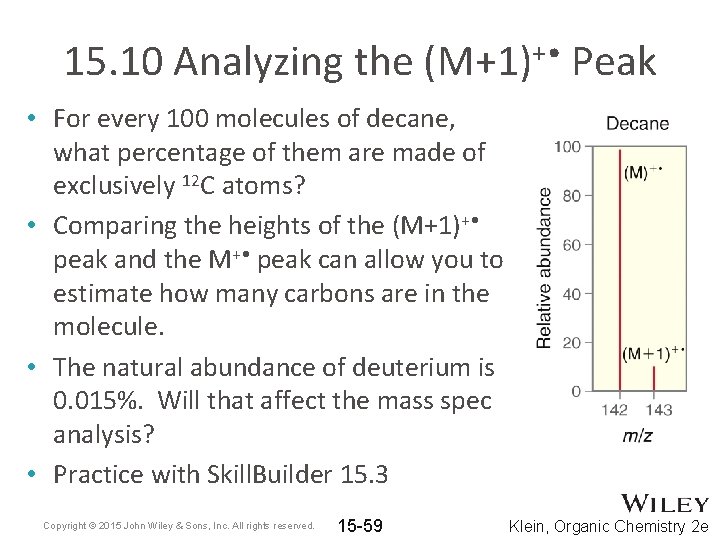
15. 10 Analyzing the (M+1)+ • Peak • For every 100 molecules of decane, what percentage of them are made of exclusively 12 C atoms? • Comparing the heights of the (M+1)+ • peak and the M+ • peak can allow you to estimate how many carbons are in the molecule. • The natural abundance of deuterium is 0. 015%. Will that affect the mass spec analysis? • Practice with Skill. Builder 15. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -59 Klein, Organic Chemistry 2 e

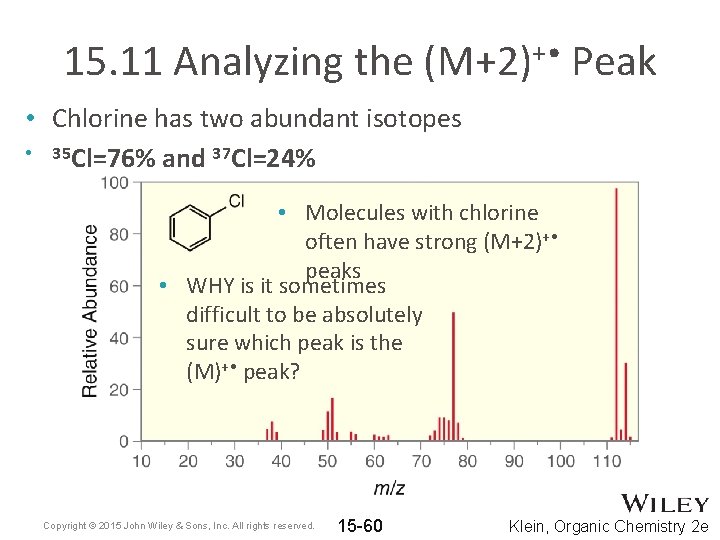
15. 11 Analyzing the (M+2)+ • Peak • Chlorine has two abundant isotopes • 35 Cl=76% and 37 Cl=24% • Molecules with chlorine often have strong (M+2)+ • peaks • WHY is it sometimes difficult to be absolutely sure which peak is the (M)+ • peak? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -60 Klein, Organic Chemistry 2 e

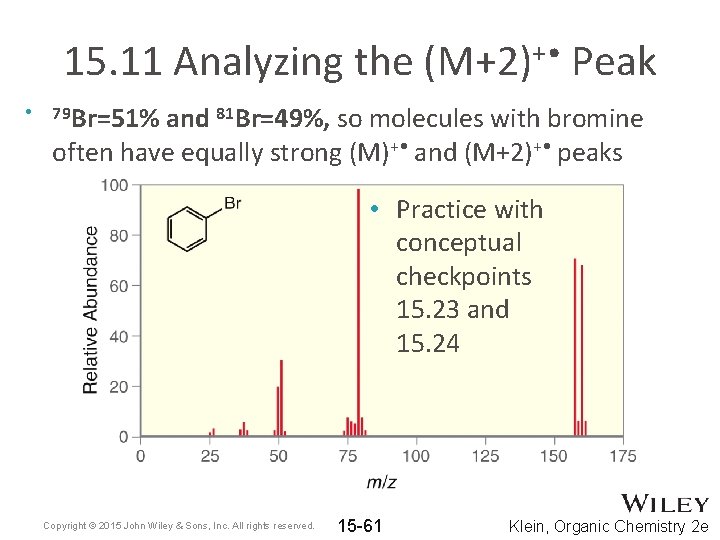
15. 11 Analyzing the (M+2)+ • Peak • 79 Br=51% and 81 Br=49%, so molecules with bromine often have equally strong (M)+ • and (M+2)+ • peaks • Practice with conceptual checkpoints 15. 23 and 15. 24 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -61 Klein, Organic Chemistry 2 e

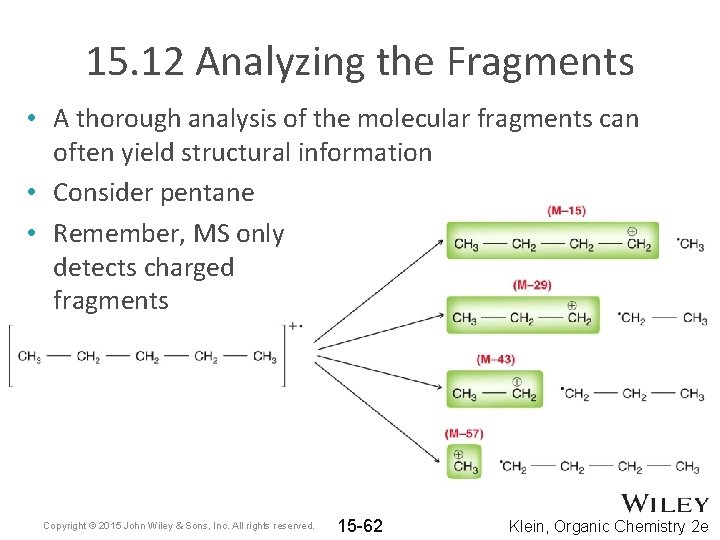
15. 12 Analyzing the Fragments • A thorough analysis of the molecular fragments can often yield structural information • Consider pentane • Remember, MS only detects charged fragments Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -62 Klein, Organic Chemistry 2 e

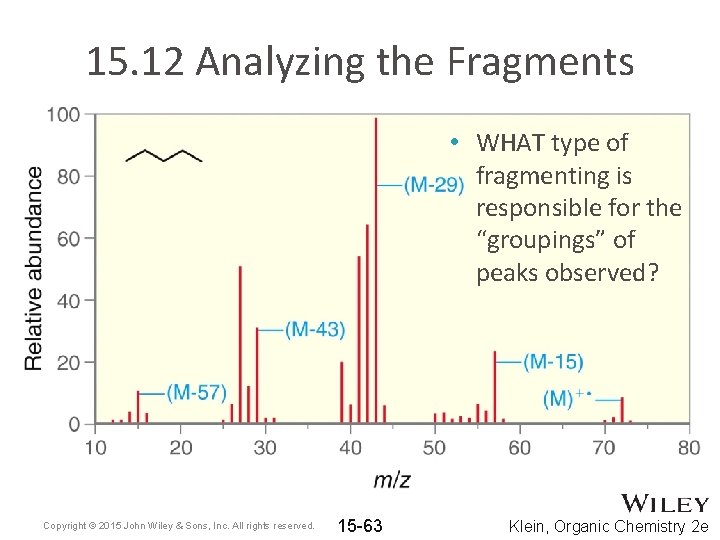
15. 12 Analyzing the Fragments • WHAT type of fragmenting is responsible for the “groupings” of peaks observed? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -63 Klein, Organic Chemistry 2 e

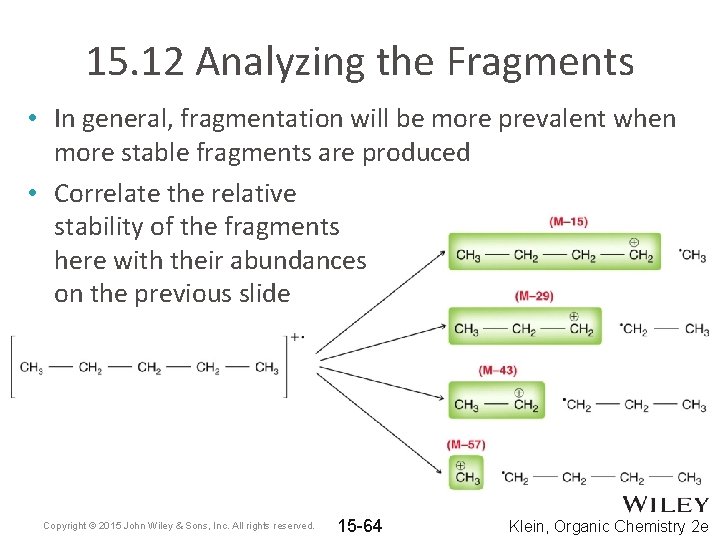
15. 12 Analyzing the Fragments • In general, fragmentation will be more prevalent when more stable fragments are produced • Correlate the relative stability of the fragments here with their abundances on the previous slide Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -64 Klein, Organic Chemistry 2 e

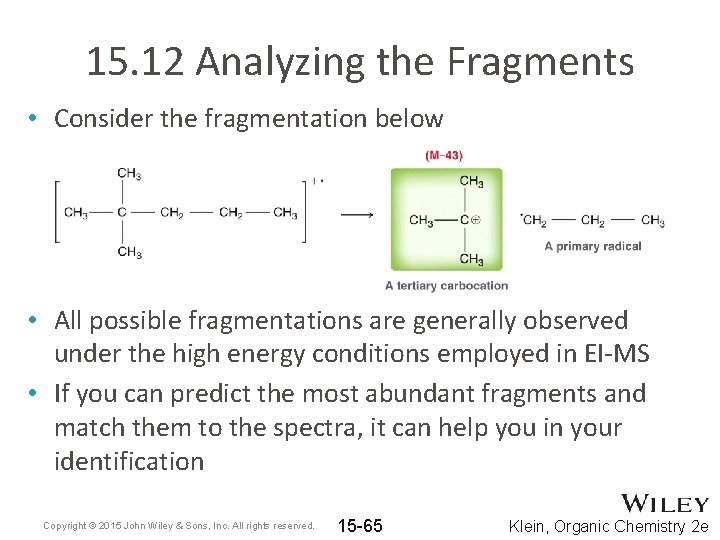
15. 12 Analyzing the Fragments • Consider the fragmentation below • All possible fragmentations are generally observed under the high energy conditions employed in EI-MS • If you can predict the most abundant fragments and match them to the spectra, it can help you in your identification Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -65 Klein, Organic Chemistry 2 e

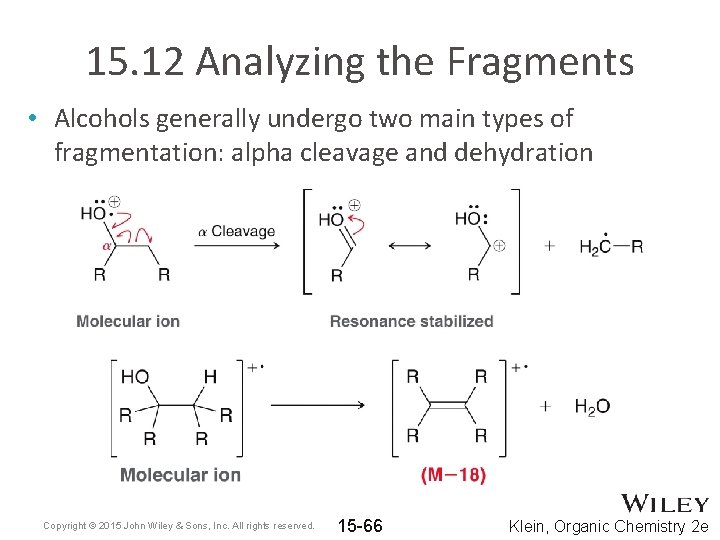
15. 12 Analyzing the Fragments • Alcohols generally undergo two main types of fragmentation: alpha cleavage and dehydration Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -66 Klein, Organic Chemistry 2 e

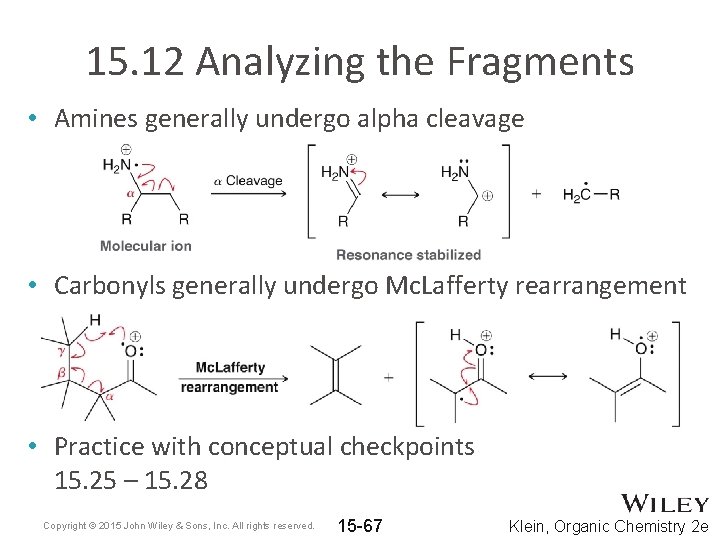
15. 12 Analyzing the Fragments • Amines generally undergo alpha cleavage • Carbonyls generally undergo Mc. Lafferty rearrangement • Practice with conceptual checkpoints 15. 25 – 15. 28 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -67 Klein, Organic Chemistry 2 e

15. 16 Degrees of Unsaturation • Mass spec can often be used to determine the formula for an organic compound • IR can often determine the functional groups present • Careful analysis of a molecule’s formula can yield a list of possible structures • Alkanes follow the formula below, because they are saturated Cn. H 2 n+2 • Verify the formula by drawing some isomers of pentane Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -68 Klein, Organic Chemistry 2 e

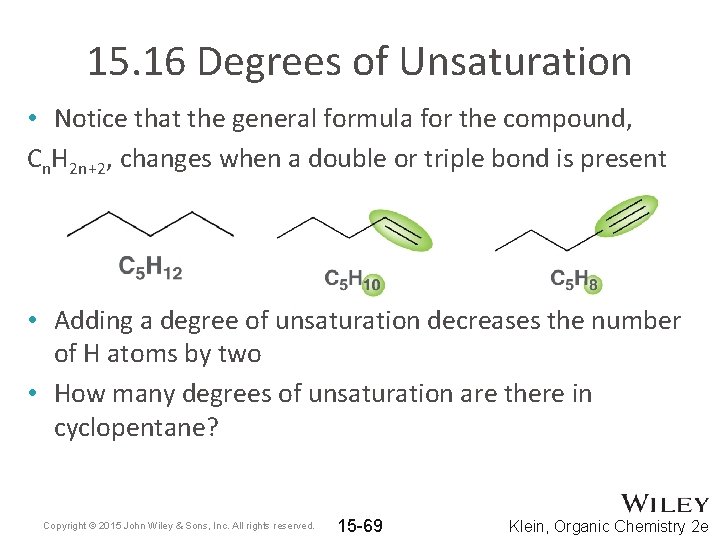
15. 16 Degrees of Unsaturation • Notice that the general formula for the compound, Cn. H 2 n+2, changes when a double or triple bond is present • Adding a degree of unsaturation decreases the number of H atoms by two • How many degrees of unsaturation are there in cyclopentane? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -69 Klein, Organic Chemistry 2 e

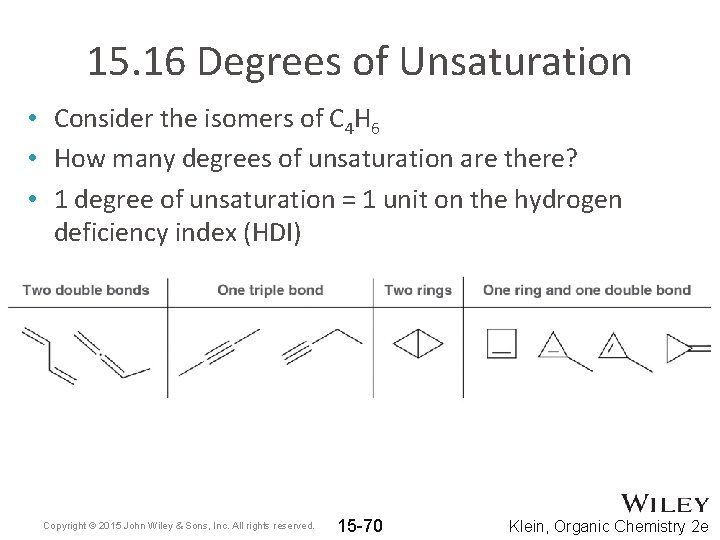
15. 16 Degrees of Unsaturation • Consider the isomers of C 4 H 6 • How many degrees of unsaturation are there? • 1 degree of unsaturation = 1 unit on the hydrogen deficiency index (HDI) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -70 Klein, Organic Chemistry 2 e

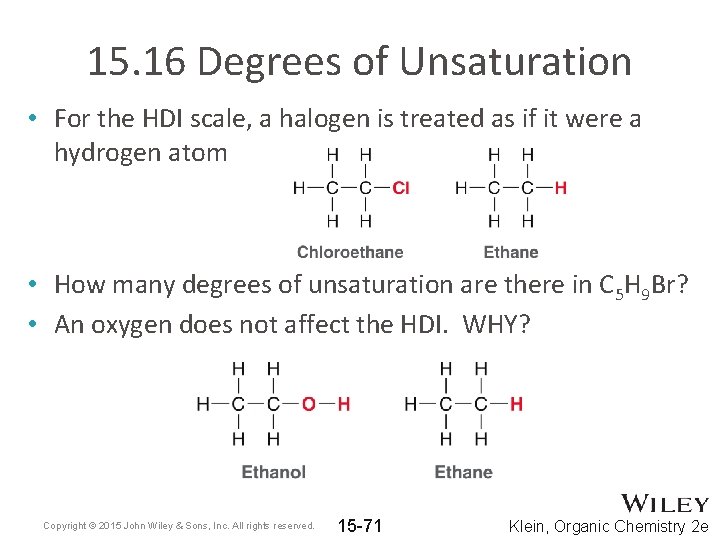
15. 16 Degrees of Unsaturation • For the HDI scale, a halogen is treated as if it were a hydrogen atom • How many degrees of unsaturation are there in C 5 H 9 Br? • An oxygen does not affect the HDI. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -71 Klein, Organic Chemistry 2 e

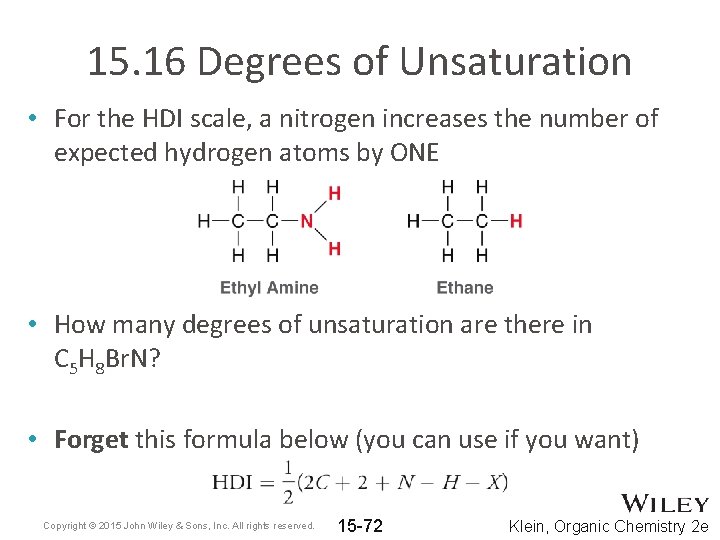
15. 16 Degrees of Unsaturation • For the HDI scale, a nitrogen increases the number of expected hydrogen atoms by ONE • How many degrees of unsaturation are there in C 5 H 8 Br. N? • Forget this formula below (you can use if you want) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -72 Klein, Organic Chemistry 2 e

15. 16 Degrees of Unsaturation • NOTrogen for Nitrogen • H alogen • 0 xygen (the O is a zero) Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -73 Klein, Organic Chemistry 2 e

15. 16 Degrees of Unsaturation • Calculating the HDI can be very useful. For example, if HDI=0, the molecule can NOT have any rings, double bonds, or triple bonds • Propose a structure for a molecule with the formula C 7 H 12 O. The molecule has the following IR peaks – A strong peak at 1687 cm-1 – NO IR peaks above 3000 cm-1 • Practice with Skill. Builder 15. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 15 -74 Klein, Organic Chemistry 2 e