INTRA CANAL IRRIGANTS Contents INTRODUCTION DEFINITION HISTORY ROLE



































































- Slides: 102

“INTRA CANAL IRRIGANTS”

Contents: INTRODUCTION DEFINITION HISTORY ROLE OF IRRIGANTS IDEAL PROPERTIES CLASSIFICATIONS

FUNCTIONS ACIDS & CHELATORS SODIUM HYPOCHLORITE CHLORHEXIDINE GLUCONATE HYDROGEN PEROXIDE

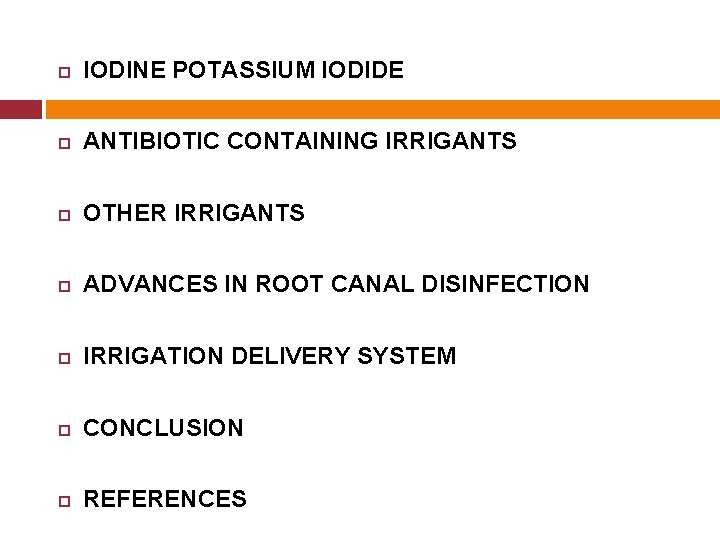
IODINE POTASSIUM IODIDE ANTIBIOTIC CONTAINING IRRIGANTS OTHER IRRIGANTS ADVANCES IN ROOT CANAL DISINFECTION IRRIGATION DELIVERY SYSTEM CONCLUSION REFERENCES

Introduction: Barret in 1925 had once said, “that of all the phases of anatomic study in the human system, one of the most complex is that of the pulp cavity morphology”.

Definition : Irrigation is a process to washout fragments of pulp tissue, dentinal shavings and microbes which results during access and biomechanical procedures.

History: Prior to 1940, water was the most commonly used endodontic irrigant During 1940’s - proteolytic enzymes like streptokinase, papain, enzymal etc were used b’coz of tissue

In 1943, Grossman introduced the concept of using oxidizing agents as irrigants In 1945, Daniel formulated an irrigating solution comprising of a aminoacridine

In 1955, Lorixzy showed periapical bone regeneration after mechanical instrumentation using tap water In 1970’s chelating agents were used increasingly b’coz of their biologically acceptable properties.

ROLE OF IRRIGANTS Loosens & flushes out tissue debris Dissolves tissues even in accessory canals Antibacterial action Lubricating effect Irrigants possess a bleaching

Ideal requirements: Effective & long lasting bactericidal along with spore destruction & fungicidal effect Non-toxic, odourless & tasteless Non-irritating to periapical tissue Active even in presence of body fluid

Shouldn't discolour the teeth Active & stable in solution form Should provide lubrication for easy instrumentation, lessening the hazard of instrument breakage.

Low surface tension Easily available Cost effective Convenient for use Good shelf life Easy storage

Classification: GROSSMAN 0 - Hot water-140 -176 F - Physiologic saline - 30% soln of urea - Urea peroxide in glycerin - Soln of chloramine - Na. OCl in with EDTA

COHEN: - Quarternary ammonium compound - Proteolytic material-eg: Na. OCl - Detergents-eg: Iodiphores - Decalcifying materials-eg: EDTA - Newer irrigants-eg: MTAD

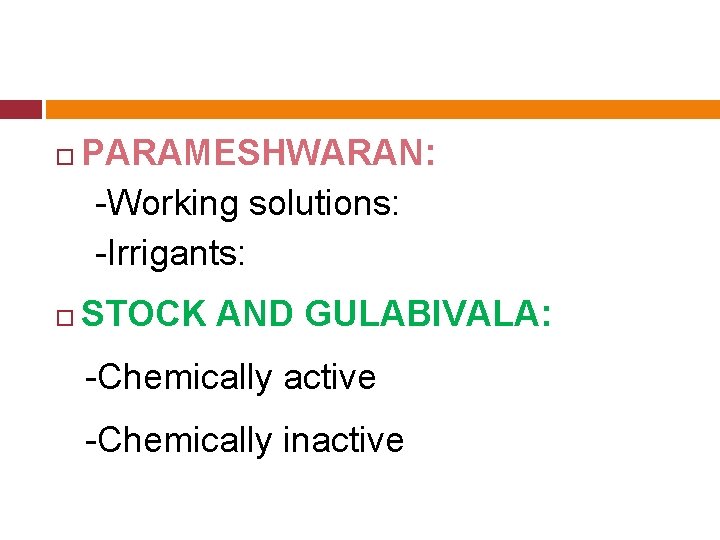
PARAMESHWARAN: -Working solutions: -Irrigants: STOCK AND GULABIVALA: -Chemically active -Chemically inactive

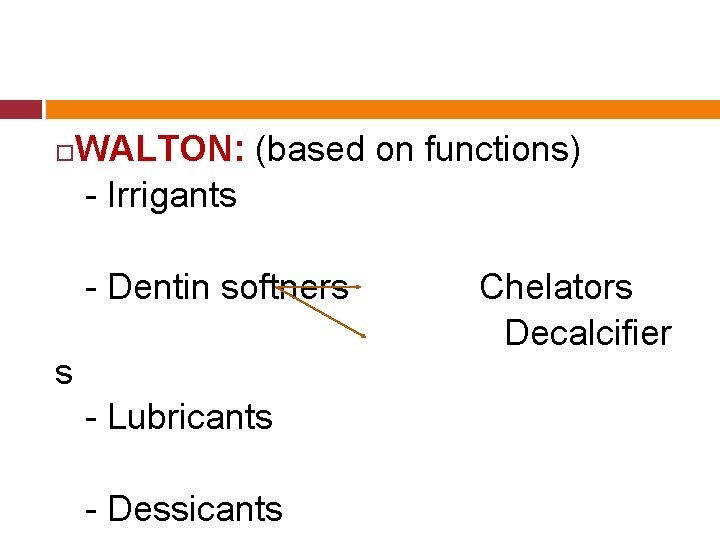
WALTON: (based on functions) - Irrigants - Dentin softners s - Lubricants - Dessicants Chelators Decalcifier

DCNA: ACIDS & CHELATING AGENTS PROTEOLYTIC ENZYMES OXIDIZING AGENTS ALKALINE SOLUTIONS OTHERS

Functions: Tissue dissolution Lubrication Chelation Antibacterial action Bleaching Aids in determining the presence of lateral/accessory canals

Sodium hypochlorite: Most commonly & widely used irrigant over 4 decades. Clear straw coloured reducing & hydrolyzing agent being used in conc B/W 0. 5 -7%.

Potent antimicrobial agent & effectively dissolving pulp remnants & organic components of dentin.

Na. OCl alone is not effective in removing smear layer, but in conjunction with EDTA/ultrasonics shown to remove smear layer effectively.

M. O. A: Na. OCl ionizes to produce Na & hypochlorite ions, that estb equilibrium with Hypochlorous acid is responsible for bacterial inactivation, by disrupting oxidative phosphorylation, DNA syn & other activities.

Used both as unbuffered soln & buffered soln: Unbuffered soln- p. H 11 Buffered soln-is its combination with bicarbonates, p. H 9, usually as 0. 5%(Dakin’s soln)/1% soln.

Houseold liquid bleach (clorox, linco)has 5. 25%Na. OCl & therefore requires dilution with distilled water to lower the incidence of periapical inflamation. Waltimo et al-C. albicans in vitro were killed in 30 sec by 5% & 0. 5%

Gomes et al-E. faecalis were killed in 30 sec by 5. 2% hypo, while 10 -30 sec was req for killing all bacteria by 2. 50. 5% hypo. Studies on Na. OCL cytotoxicity have indicated greater cytotoxicity and caustic effect on healthy tissue with 5. 25% than 1 and 0. 5%

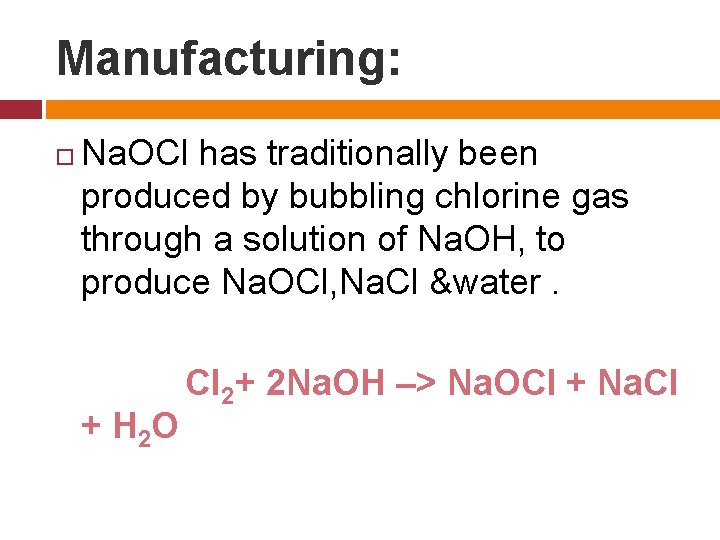
Manufacturing: Na. OCl has traditionally been produced by bubbling chlorine gas through a solution of Na. OH, to produce Na. OCl, Na. Cl &water. + H 2 O Cl 2+ 2 Na. OH –> Na. OCl + Na. Cl

Alternative method: Electrolysis of a saturated brine solution to produce Na & Cl ions. Na ions diffuse through a membrane, where they combine with H 2 O to produce Na. OH. The Cl ions from 1 st compartment combine to give chlorine gas which is

Function : Tissue solvent Lubrication Antimicrobial Bleaching action

Yamoda et al: final flushing with 17%EDTA, followed by 10 ml of 5. 25% Na. OCl, was more effective in removing both organic & inorganic debris. They concluded that flushing with chealating agents removed final calcific sludges that remained on the canal walls.

Weakness: Unpleasant taste and odour Toxicity High surface tension-dec dentin wettability Can cause inflammation of gingival tissue if leaked

Corrode equipments Stains clothes if spilt Pharyngeal oedema and oesophageal burns if swallowed Unable to remove inorganic part of smear layer

Effectiveness enhanced: Cunningham & Balekijan. Inc temp b/w 220 C-370 C of 2. 6% Na. OCl soln inc its tissue dissolving properties which were equivalent to those of 5. 25% soln at room temp

Ultrasonic activation: accelerate chemical rxn, creating cavitation effect & achieving a superior cleansing action.

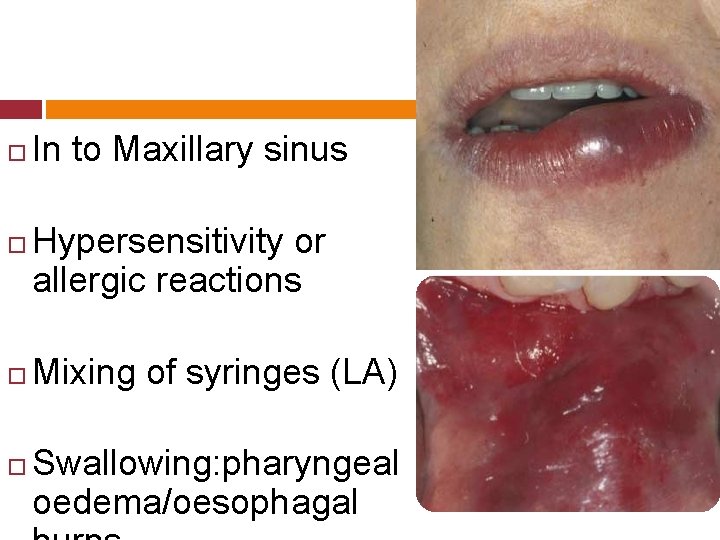
In to Maxillary sinus Hypersensitivity or allergic reactions Mixing of syringes (LA) Swallowing: pharyngeal oedema/oesophagal

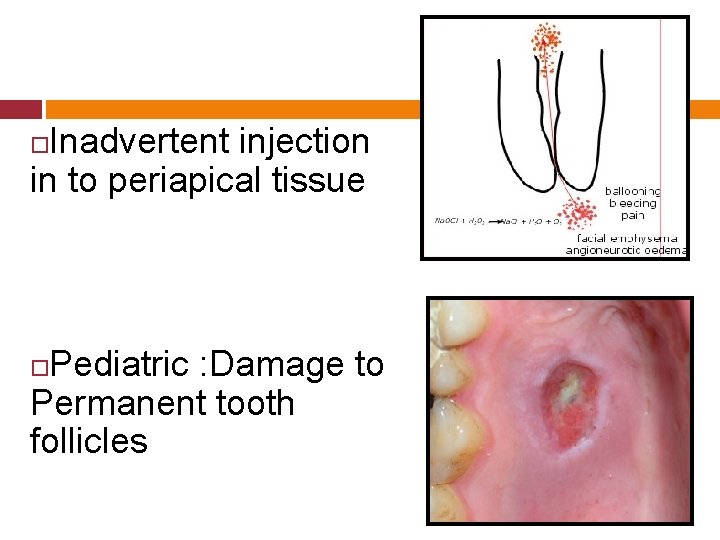
Inadvertent injection in to periapical tissue Pediatric : Damage to Permanent tooth follicles

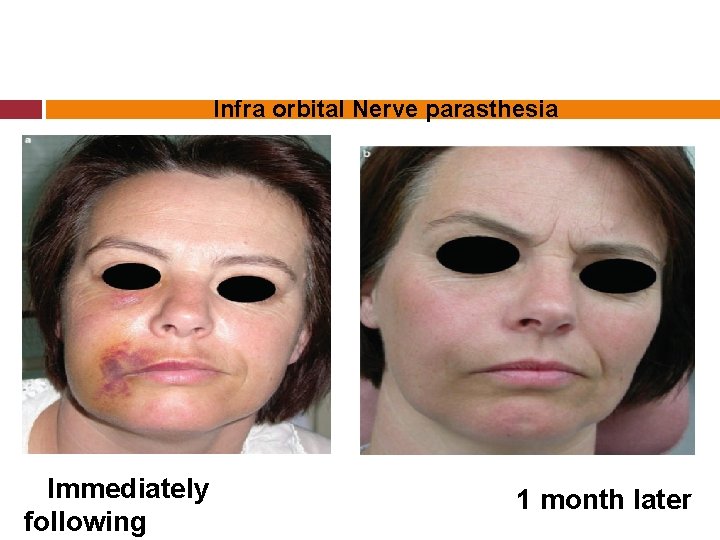
Infra orbital Nerve parasthesia Immediately following 1 month later

Sodium hypochlorite is a cytotoxic agent (Gatot et al. 1991, Gernhardtet al. 2004). When it comes into contact with vital tissue, it causes haemolysis, ulceration, inhibits neutrophil migration & damages endothelial & fibroblast

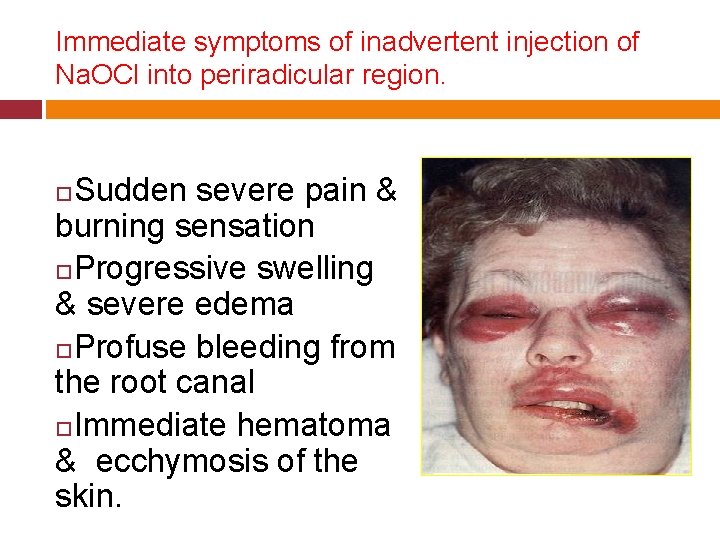
Immediate symptoms of inadvertent injection of Na. OCl into periradicular region. Sudden severe pain & burning sensation Progressive swelling & severe edema Profuse bleeding from the root canal Immediate hematoma & ecchymosis of the skin.

Protocol suggested by Hales & colleagues: Provide immediate pain relief by administering LA to the affected area. Allow the drainage of the inflammatory exudate & dilute the Na. OCl by irrigating the canal system with normal saline solution

Reassure the patient & inform him/her of the cause & severity of the complications. Reduce swelling by applying cold compresses in 15 min intervals for 1 st 24 hours, followed by warm compression thereafter.

Prescribe acetaminophenbased analgesics to control pain, Prophylactic antibiotics to prevent secondary infection Steroid medication to control inflammatory reaction

Set up regular recall appointments to monitor the patient’s recovery & complete the endodontic therapy upon resolution of the acute symptoms.

Hydrogen peroxide: Is a biocide used for disinfection & sterilization Available in 1 – 30% conc-Superoxol,

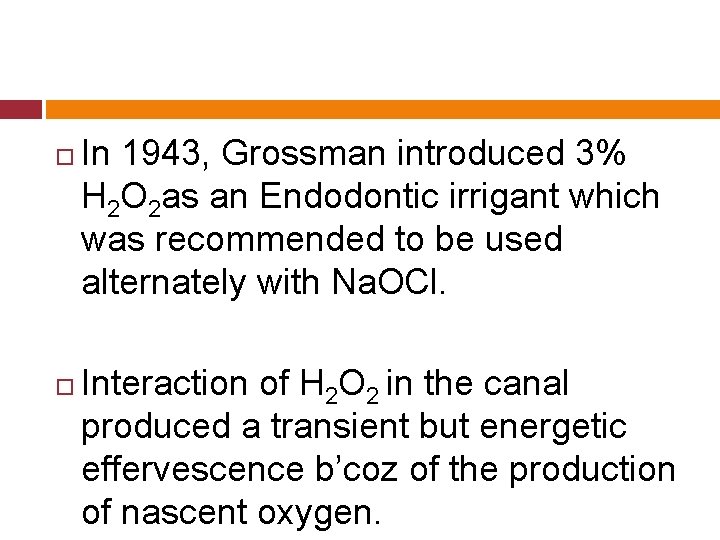
In 1943, Grossman introduced 3% H 2 O 2 as an Endodontic irrigant which was recommended to be used alternately with Na. OCl. Interaction of H 2 O 2 in the canal produced a transient but energetic effervescence b’coz of the production of nascent oxygen.

� This was responsible forcing debris & microorganism out of the canal. � Action to be especially useful in lifting debris from the canal system, almost defying gravity in mandibular teeth. � H 2 O 2 does’nt posses tissue dissolving properties, nor is it a lubricant, Limited antimicrobial action only.

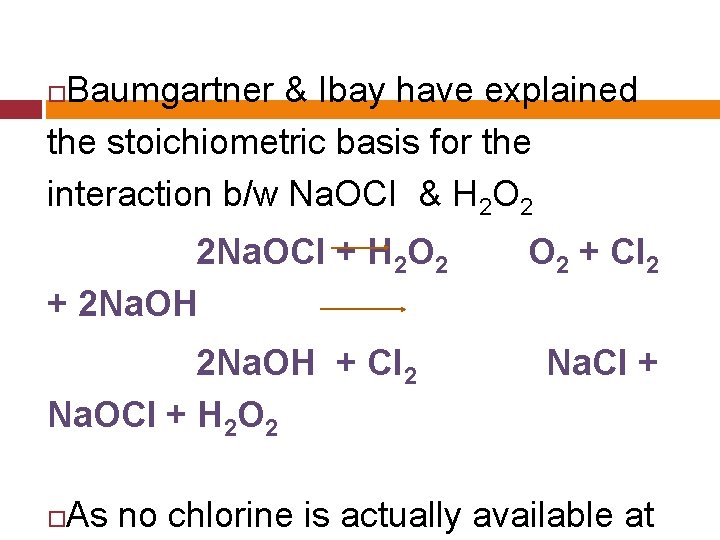
Baumgartner & Ibay have explained the stoichiometric basis for the interaction b/w Na. OCI & H 2 O 2 2 Na. OCI + H 2 O 2 + 2 Na. OH + CI 2 Na. OCI + H 2 O 2 + CI 2 Na. CI + As no chlorine is actually available at

Precaution: H 2 O 2 should’nt be the last irrigant, b’coz it liberates nacent oxygen causing build up of pressure & severe pain.

Heling & Chandler-strong synergism b/w H 2 O 2 & CHX in disinfecting the root canals. This combo at low conc was more effective in sterilizing dentin than any other medicaments alone.

Sequiera et al -Na. OCl & H 2 O 2 combo gave no much adv over Na. OCl alone against E. faecalis in contaminated root canals.

Acid irrigants: Acids & Chelating agents were used b’coz of their ability to soften dentin, making enlargement of canal easier. Acids : 30 -50%HCl 50%H 3 PO 4 30 -50%Citric acid

Acids act by removing mineral salts from dentin to aid canal preparation

Chelating agents: The term ‘chelate’ originates from Greek word chele (crab claw) Chelates are stable complexes of metal ions with organic substances as a result of ring shaped bonds.

M. O. A: Chelating agents act by substituting Ca ions in the dentin with Na ions & forms soluble salts which facilitate canal enlargement.

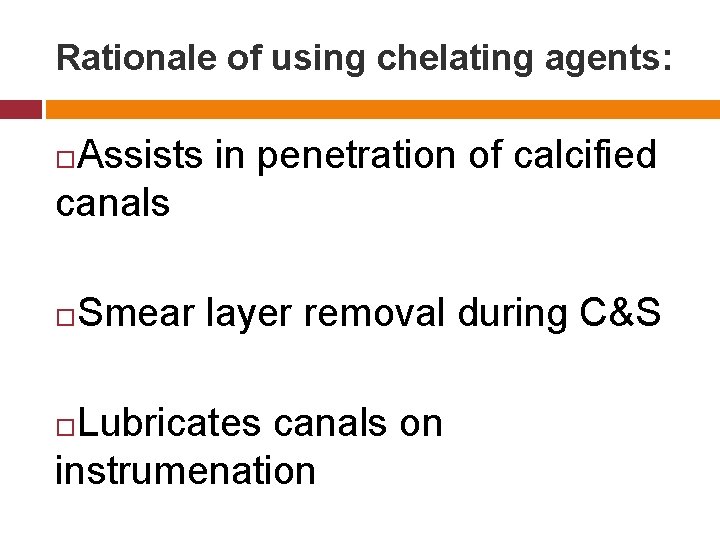
Rationale of using chelating agents: Assists in penetration of calcified canals Smear layer removal during C&S Lubricates canals on instrumenation

Chelator preparations: LIQUID CHELATORS: Calcinase v. REDTA v. EDTAC DTPAC v. EDTA-T v. EGTA v. Salvizol v. Decal v. Tubulicid Plus v. Hypaque v

PASTE TYPE: v. Calcinase slide v. RC-Prep v. Glyde file v. File. Care EDTA v. File-EZE

EDTA: Nygaard - Ostby in 1957 introduced EDTA with the following formulation as: Ethylene Diamine Tetracetic Acid (disodium salt)- 17. 0 gm Distilled water- 100 cc Na. OH- 9. 25 cc

EDTA is an insoluble, odorless. It is relatively non toxic & only slightly irritating in weak soln. Disodium salt of EDTA is employed in dentistry, adequately buffered to 1017%

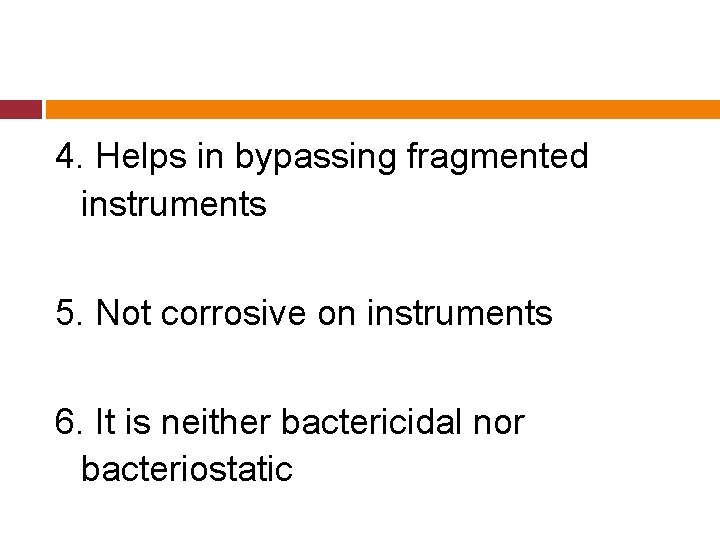
Properties: 1. Has dentin dissolving effects desirable in RC therapy 2. Reduces time necessary for debridement 3. Aids in enlarging narrow/obstructed canals

4. Helps in bypassing fragmented instruments 5. Not corrosive on instruments 6. It is neither bactericidal nor bacteriostatic

7. Self Limiting Action: � EDTA forms a stable bond with Ca & the deposited soln can dissolve only a certain amount of dentin, � When all chelating ions have reacted, an equilibrium will be reached; then no further dissolution will takes place. � This effect was found to be rapid during 1 st 1 hr & reached equilibrium by the end of 7 hrs.

Sen, Akdeniz & Denizci (OOO, 2000) have shown that this product may posses �ANTIFUNGAL ACTIVITY �CYTOTOXIC EFFECTS

• • • Antifungal activity is B’coz EDTA chelates to Ca 2+ & prevents binding of C. albicans to proteins in a dose dependent manner. It removes Ca 2+ from the cell walls & thereby causes the cell walls to collapse. Also inhibits enzymatic rxn

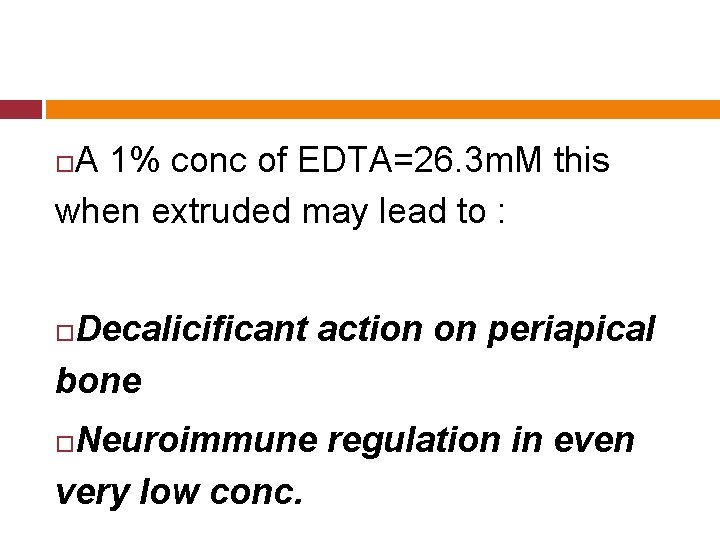
CYTOTOXIC EFFECTS: (JOE 1999) Koulaouzidou, showed alkaline EDTA soln to be moderately to severely cytotoxic. It has been reported that 5 -50 m. M of EDTA may be found in periapical tissues with the extrusion of EDTA.

A 1% conc of EDTA=26. 3 m. M this when extruded may lead to : Decalicificant action on periapical bone Neuroimmune regulation in even very low conc.

EDTA softens dentin effectively, Patterson reported that EDTA drastically reduced KHN of dentin from 42 at orifice & 70 at 1/3 rd distance from DEJ to as low as 7. Combo of Na. OCl & EDTA enhances both cleaning & antimicrobial properties.

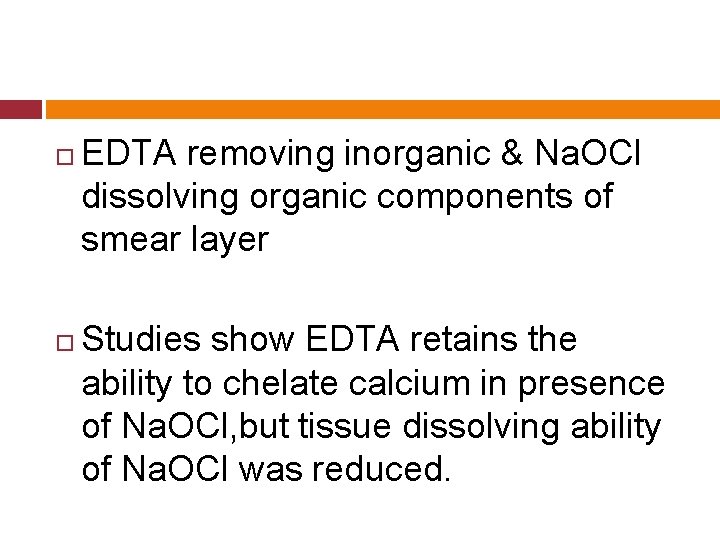
EDTA removing inorganic & Na. OCl dissolving organic components of smear layer Studies show EDTA retains the ability to chelate calcium in presence of Na. OCl, but tissue dissolving ability of Na. OCl was reduced.

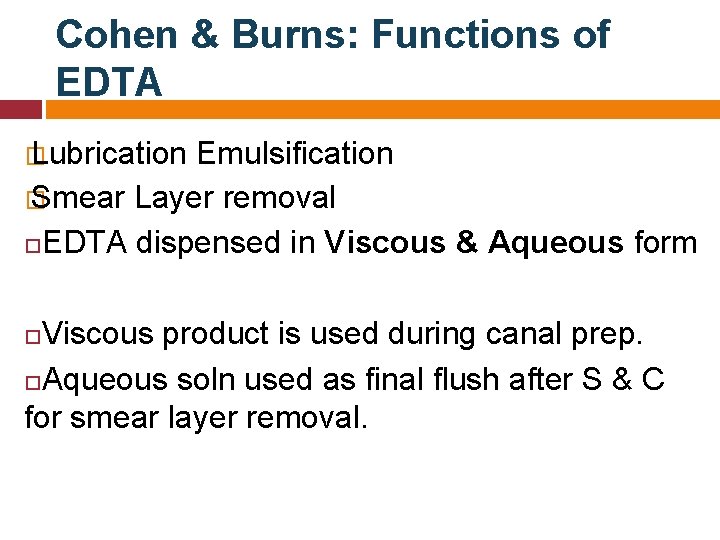
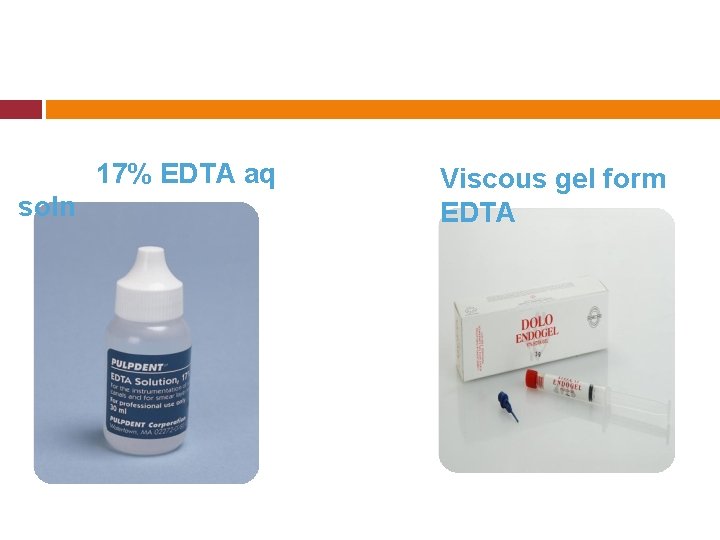
Cohen & Burns: Functions of EDTA � Lubrication Emulsification � Smear Layer removal EDTA dispensed in Viscous & Aqueous form Viscous product is used during canal prep. Aqueous soln used as final flush after S & C for smear layer removal.

17% EDTA aq soln Viscous gel form EDTA

RC-prep: 15% EDTA + 10% urea peroxide glycol in carbowax base. Intro-Stewart in 1969 It has a increased depth of penetration

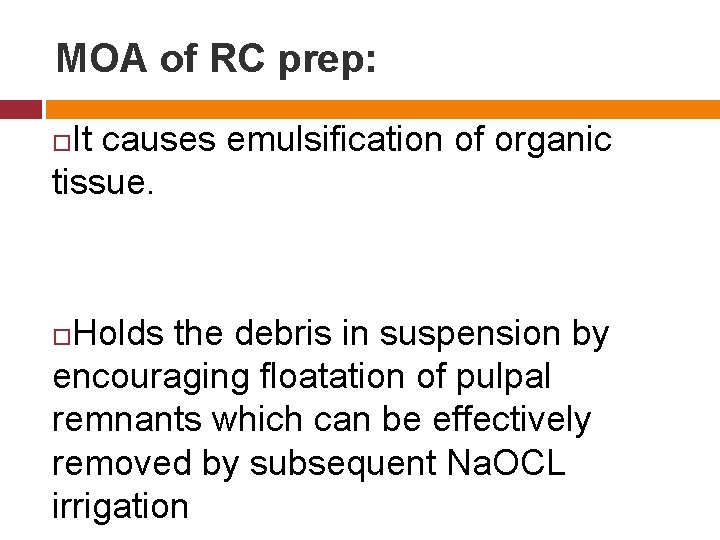
MOA of RC prep: It causes emulsification of organic tissue. Holds the debris in suspension by encouraging floatation of pulpal remnants which can be effectively removed by subsequent Na. OCL irrigation

Urea peroxide causes effervescence & release of O 2 on reacting with Na. OCL which again aids in bacterial elimination, Release of oxygen results in some bleaching effect due to urea peroxide, but no significant evidence to support the bleaching effect.

Goldberg & Abramovich added a quaternary ammonium bromide. Cetavlon to EDTA to reduce it’s surface tension & was called walls EDTAC.

This addition increased the wetting effect on the canal walls & permitted deeper penetration into the tubular irregularities. Cury, Bragotto & Valdriht evaluated the demineralizing efficiency of EDTA at different p. H & found that 0. 3 M soln showed greatest demineralizing efficiency b/w p. H 56.

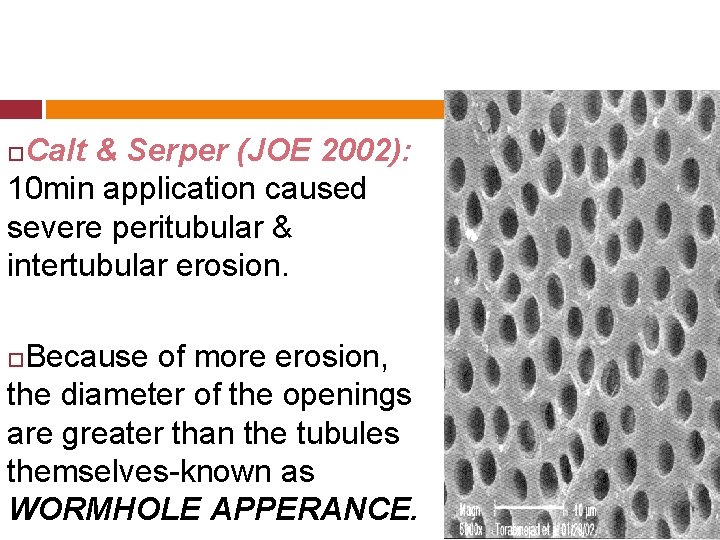
Calt & Serper (JOE 2002): 10 min application caused severe peritubular & intertubular erosion. Because of more erosion, the diameter of the openings are greater than the tubules themselves-known as WORMHOLE APPERANCE.

Chlorhexidine (CHX): It is a cationic bisbiguanide with optimal antimicrobial action b/w p. H 5. 5 -7. 0(Leonardo et al) 0. 5% used as mouth wash 2% used as endodontic irrigant Available as water based soln, gel, liquid mixture with surface active agents

M. A. O(Hennessey 1973): � High CHX Concentrations: Bactericidal penetrates the cell wall & causes precipitation or coagulation of cytoplasm probably caused by crosslinking.

Low Concentrations: Bacteriostatic Positively charged molecules of CHX bind readily to the negatively charged cell wall, mainly to PO 4 groups in LPS, & coo groups in proteins. It therefore interferes with membrane

Acts by adsorbing into the cell walls of the micro-organisms & causing leakage of intracellular components. Bacteriostatic effect is considered to be more important since the bound CHX

When 2% & 0. 2% each of Na. OCI & CHX were compared, it was found that both showed equivalent antimicrobial effect (Vahdaty et al). Heating of CHX soln to 460 C enhanced the antimicrobial action of 0. 12% CHX

Buck & others have reported on the ability of a combination of CHX, Na. OCI & alcohol to detoxify LPS molecules by hydrolysis within the canal.

ANTIMICROBIAL SUBSTANTIVITY: RC treated with CHX result in the molecule being adsorbed into dentin. Root dentin Rx with CHX has shown to acquire substantivity, which extends to at least a period of 7 days.

Antimicrobial substantivity acquired by the root dentin after Rx with CHX could inhibit re-infection of the canal subsequent to Rx.

TISSUE DISSOLUTION PROPERTY CHX does’nt posses any tissue solvent property (Naenni, Thoma & Zehnder)

TOXICITY • Yesilsoy et al through their animal experiment demonstrated a moderate intensity foreign body rxn. • Jeansonne & White reported it’s non toxicity & suggested its use in cases of perforations, open apices or difficult isolation cases.

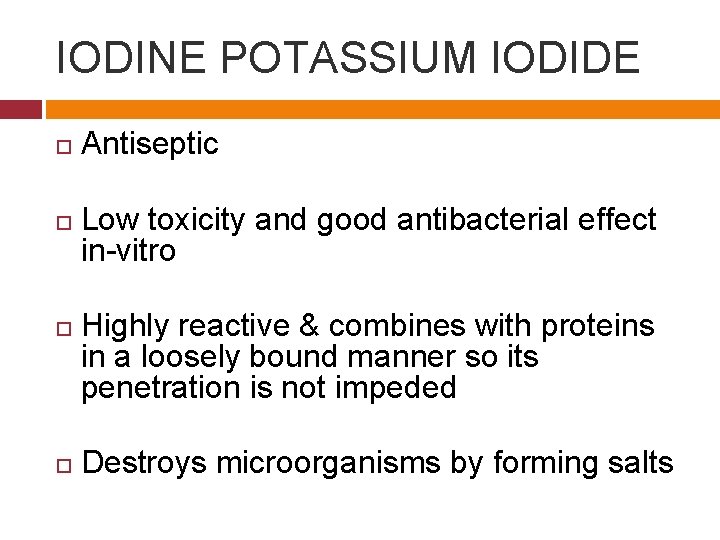
IODINE POTASSIUM IODIDE Antiseptic Low toxicity and good antibacterial effect in-vitro Highly reactive & combines with proteins in a loosely bound manner so its penetration is not impeded Destroys microorganisms by forming salts

9 -AMINOACRIDINE Reviewed by schmitz Properties: - Antibacterial action. - Low toxicity. - Osteogenic potential Not used because not tissue solvent nor chelator

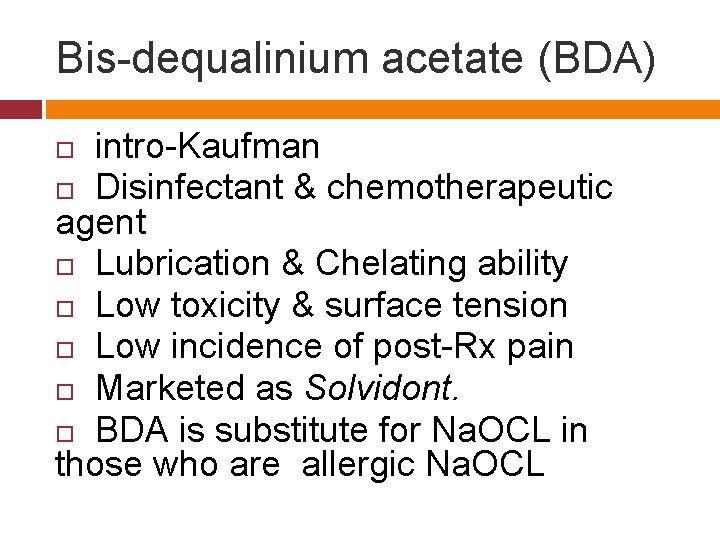
Bis-dequalinium acetate (BDA) intro-Kaufman Disinfectant & chemotherapeutic agent Lubrication & Chelating ability Low toxicity & surface tension Low incidence of post-Rx pain Marketed as Solvidont. BDA is substitute for Na. OCL in those who are allergic Na. OCL

2% glutaraldehyde Wemes & co-workers 1982 Causes irreversible fixation of tissues, they observed smooth layer of dentin material resulted in closure of apical and lateral canals and the dentinal tubules

Glyoxide 10% urea peroxide (Carbamide peroxide) in a vehicle of anhydrous glycerol 1961 Stewart proposed it Antibacterial activity & solvent action better than 3% H 2 O 2. Enhances root canal lubrication without softening the dentin

It is less toxic to periapical tissues than Na. OCl. Use: Best use of glyoxide is in narrow and curved canals.

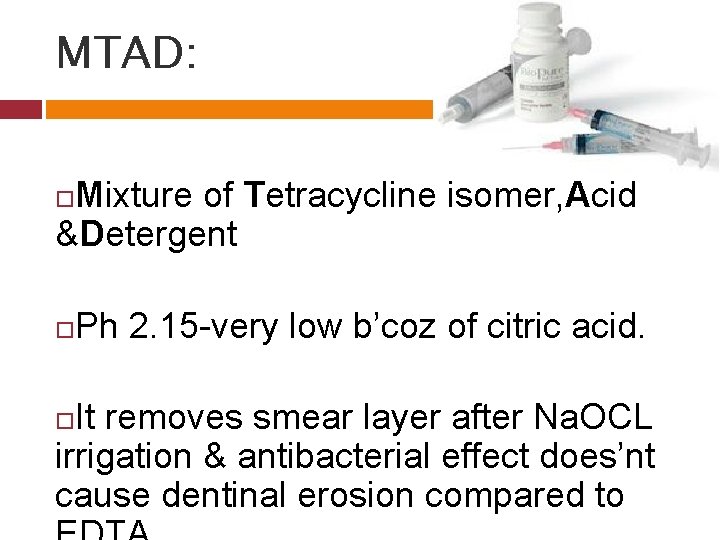
MTAD: Mixture of Tetracycline isomer, Acid &Detergent Ph 2. 15 -very low b’coz of citric acid. It removes smear layer after Na. OCL irrigation & antibacterial effect does’nt cause dentinal erosion compared to

Beltz et al found MTAD solubilized dentin, whereas organic pulp tissue is unaffected Shabahang et al found that MTAD was more effective in eliminating E. faecalis compared to 5. 25% Na. OCL Portenier et al showed MTAD

Torabinejad have recommended use of 1. 3% Na. OCL during instrumentation followed by MTAD to remove smear layer as final irrigant Antimicrobial substantivity of MTAD is higher than CHX. (zahed mohammadi; journal of dental science; 2007)

Order of sequence Na. OCL – EDTA – MTAD as final irrigant Should not be washed out , should be suctioned out

OXIDATIVE POTENTIAL WATER Was developed by Japanese who electrolyzed Na. OCL in a special machine called Aquacida Similar to ECA Antibacterial, biocompatible Removes smear layer

Conclusion There is a famous saying with regard to endodontic treatment- “It does not matter what you put in, it is more important what you take out from root canal” Irrigation plays a key role in disinfection & debridement of root canal space

Therefore for achieve successful endodontic Rx it is essential to practice chemomechanical preparation & not mechanical instrumentation alone.

References: Pathways of the Pulp 9 th Edition – Stephen Cohen & Kenneth M. Hargreaves. Endodontic Therapy 6 th Edition – Franklin S. Weine Endodontic practice 11 th edition- Louis i. Grossman Endodontics 5 th and 6 th Edition – John I. Ingle & Leif K. Bakland. Text book of endodontics- gulabivala and stock Problem solving in endodontics- gutman Color Atlas of Endodontology – Rudolf beer, baumann and syngcuk kim

Aust endod J, 2002; 34; 39 -42 OOO, 2003; 96; 578 -81 Journal of dental science, 2007; vol 5; no 2 Endodontic topic, 2005; 10; 77 -102 IEJ, 2007; 40; 415 -426 IEJ, 2000; 33; 320 -325 JOE, 2008; 34; 66 -70

Thank you
 Silver point dental
Silver point dental Indicate nerve passing through cruropopliteal canal:
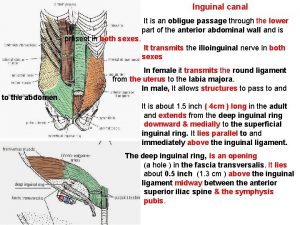
Indicate nerve passing through cruropopliteal canal: Inguinal line
Inguinal line Tabla de diámetros de tuberías en pulgadas y milímetros
Tabla de diámetros de tuberías en pulgadas y milímetros Contents introduction
Contents introduction Azure worker role
Azure worker role Rollendistanz krappmann beispiel
Rollendistanz krappmann beispiel Role conflict occurs when fulfilling the role expectations
Role conflict occurs when fulfilling the role expectations Canal syphon definition
Canal syphon definition Metakarpaali
Metakarpaali Verkkarit siunsote
Verkkarit siunsote Intraalveolar extraction
Intraalveolar extraction Inter vs intra personal
Inter vs intra personal Inter vs intra personal
Inter vs intra personal Srv intra
Srv intra Partial denture with retainer
Partial denture with retainer Intrapulmonary pressure
Intrapulmonary pressure Arcade dentaire parabolique
Arcade dentaire parabolique No+
No+ Https://www.cvlkra.com/kycpaninquiry.aspx
Https://www.cvlkra.com/kycpaninquiry.aspx Ligne bicanthale
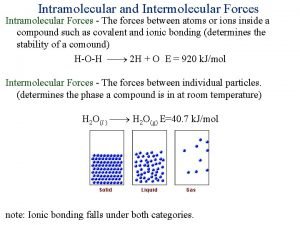
Ligne bicanthale Intramolecular vs intermolecular bonds
Intramolecular vs intermolecular bonds Intra vs intermolecular
Intra vs intermolecular Intra fallopian transfer
Intra fallopian transfer Inter intra extra
Inter intra extra Intranet kk
Intranet kk Aileron sacré
Aileron sacré Interaksi intraalelik
Interaksi intraalelik Missing trader intra-community
Missing trader intra-community Missing trader intra-community fraud
Missing trader intra-community fraud Arcade elliptique
Arcade elliptique Adrenaline intra tracheale
Adrenaline intra tracheale Laurea erillishaku
Laurea erillishaku Intra-elite schism
Intra-elite schism Describe explain predict control
Describe explain predict control Intra versus inter
Intra versus inter Ibpc certificate
Ibpc certificate Intra abdominal pressure monitoring
Intra abdominal pressure monitoring Brochage kapandji
Brochage kapandji Veinosus
Veinosus Intra entity transactions
Intra entity transactions Intra personal barrier
Intra personal barrier Webmail vtc
Webmail vtc Lshp intra
Lshp intra Intra-generational interaction
Intra-generational interaction Angiomammographie
Angiomammographie Intra building backbone cabling
Intra building backbone cabling Intra thoracique
Intra thoracique Intra sectoral coordination
Intra sectoral coordination Viad corp
Viad corp Belastningsovervågning
Belastningsovervågning Intra eip
Intra eip Intra action review
Intra action review Brachial cutdown
Brachial cutdown Jaakko rinne
Jaakko rinne Stress equalizer
Stress equalizer Requirements of direct retainers
Requirements of direct retainers Tige pituitaire
Tige pituitaire Vascolarizzazione intra e perinodulare
Vascolarizzazione intra e perinodulare Ganglion intra parotidien
Ganglion intra parotidien Wwqqaa history
Wwqqaa history Frcsed meaning
Frcsed meaning Intra community acquisition
Intra community acquisition Intra vgf
Intra vgf Habilidades intrapersonales
Habilidades intrapersonales Llums.intra.bt.com
Llums.intra.bt.com Oamk intra
Oamk intra Intra business e commerce
Intra business e commerce Kotatgent
Kotatgent Intra.agfanet
Intra.agfanet Parenteral route advantages and disadvantages
Parenteral route advantages and disadvantages Modello intra 13
Modello intra 13 Lshp intra
Lshp intra Kpb intra s.r.o.
Kpb intra s.r.o. Myslo.intra
Myslo.intra Major intra and extracellular electrolytes
Major intra and extracellular electrolytes Inter and intra personal skills
Inter and intra personal skills Pk 21
Pk 21 Intrauems
Intrauems Intas intra web
Intas intra web Como se alimentan los parasitos intra y extracelulares
Como se alimentan los parasitos intra y extracelulares Parenteral route definition
Parenteral route definition Intra alveolar pressure
Intra alveolar pressure Intra call
Intra call Trasferimenti intra-societari
Trasferimenti intra-societari Intra lase
Intra lase Intra country vs inter country
Intra country vs inter country Introduction to role play
Introduction to role play Introduction of role play
Introduction of role play History also history physical
History also history physical Career portfolio table of contents
Career portfolio table of contents Deep perineal pouch contents
Deep perineal pouch contents Trali symptoms
Trali symptoms Ffp
Ffp Regio thorax
Regio thorax Branches of common femoral artery
Branches of common femoral artery Horizontal
Horizontal The immortal life of henrietta lacks table of contents
The immortal life of henrietta lacks table of contents Contents of internal capsule
Contents of internal capsule Triangular space contents
Triangular space contents Ark of the covenant lampstand
Ark of the covenant lampstand Anatomy of a comic
Anatomy of a comic Hepatorenal fossa
Hepatorenal fossa