Day 2 quiz questions Draw the Lewis structure


































- Slides: 67

Day 2 quiz questions • Draw the Lewis structure for C 2 Cl 3 N • Draw the Lewis structure for CH 2 O 2 Copyright 2012 John Wiley & Sons, Inc. 1 -1 Klein, Organic Chemistry 1 e

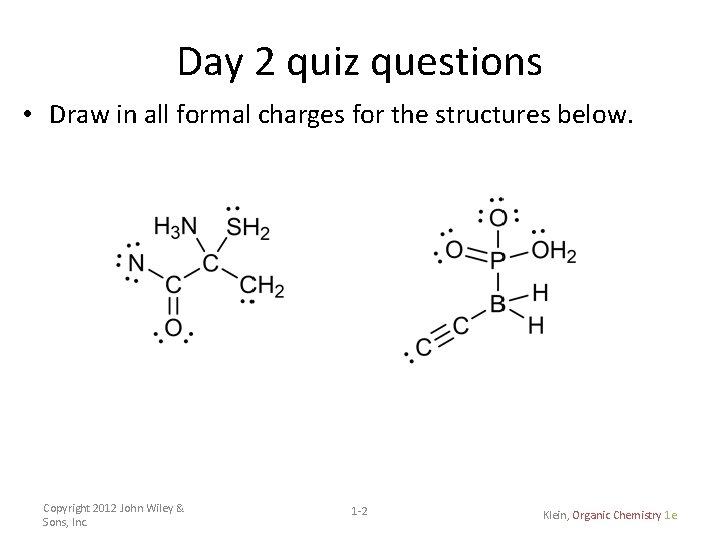
Day 2 quiz questions • Draw in all formal charges for the structures below. Copyright 2012 John Wiley & Sons, Inc. 1 -2 Klein, Organic Chemistry 1 e

Day 2 quiz questions • How many nodes are in the 3 s subshell? • How many nodes are in a typical sigma antibonding MO? • How many nodes are in a typical sp 3 orbital? • How many nodes are in a typical pi bonding MO? • How many nodes are in a typical pi antibonding MO Copyright 2012 John Wiley & Sons, Inc. 1 -3 Klein, Organic Chemistry 1 e

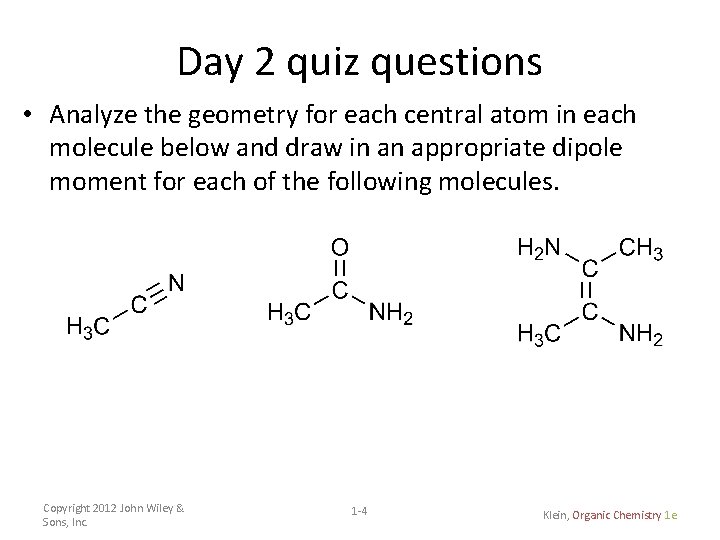
Day 2 quiz questions • Analyze the geometry for each central atom in each molecule below and draw in an appropriate dipole moment for each of the following molecules. Copyright 2012 John Wiley & Sons, Inc. 1 -4 Klein, Organic Chemistry 1 e

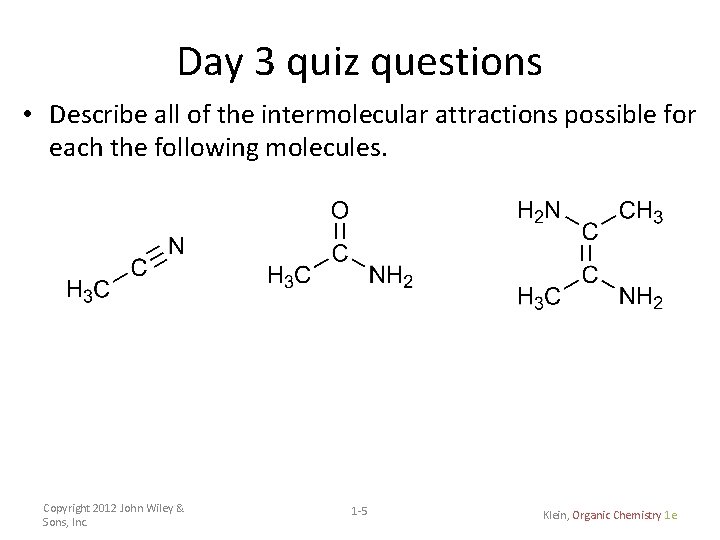
Day 3 quiz questions • Describe all of the intermolecular attractions possible for each the following molecules. Copyright 2012 John Wiley & Sons, Inc. 1 -5 Klein, Organic Chemistry 1 e

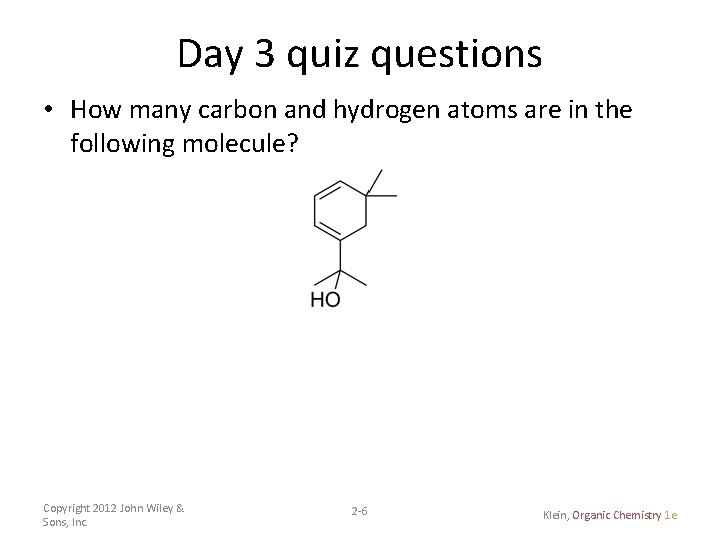
Day 3 quiz questions • How many carbon and hydrogen atoms are in the following molecule? Copyright 2012 John Wiley & Sons, Inc. 2 -6 Klein, Organic Chemistry 1 e

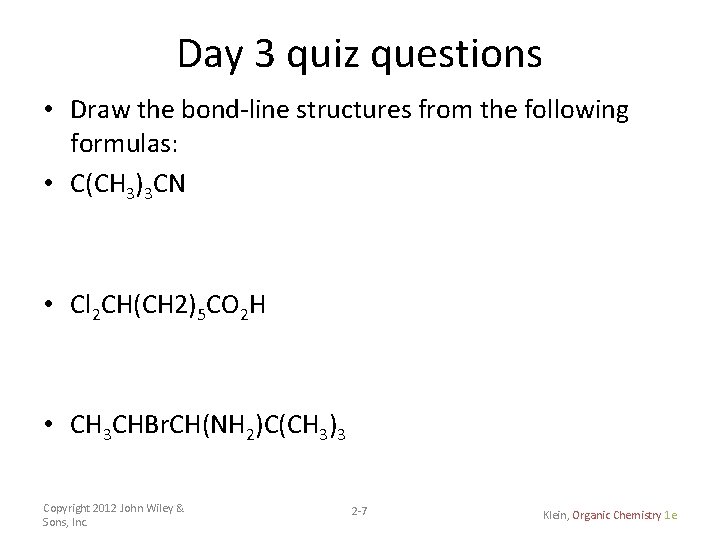
Day 3 quiz questions • Draw the bond-line structures from the following formulas: • C(CH 3)3 CN • Cl 2 CH(CH 2)5 CO 2 H • CH 3 CHBr. CH(NH 2)C(CH 3)3 Copyright 2012 John Wiley & Sons, Inc. 2 -7 Klein, Organic Chemistry 1 e

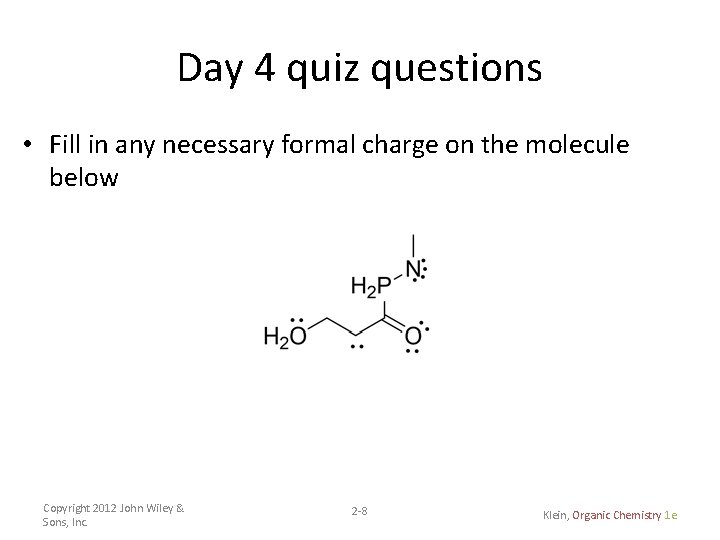
Day 4 quiz questions • Fill in any necessary formal charge on the molecule below Copyright 2012 John Wiley & Sons, Inc. 2 -8 Klein, Organic Chemistry 1 e

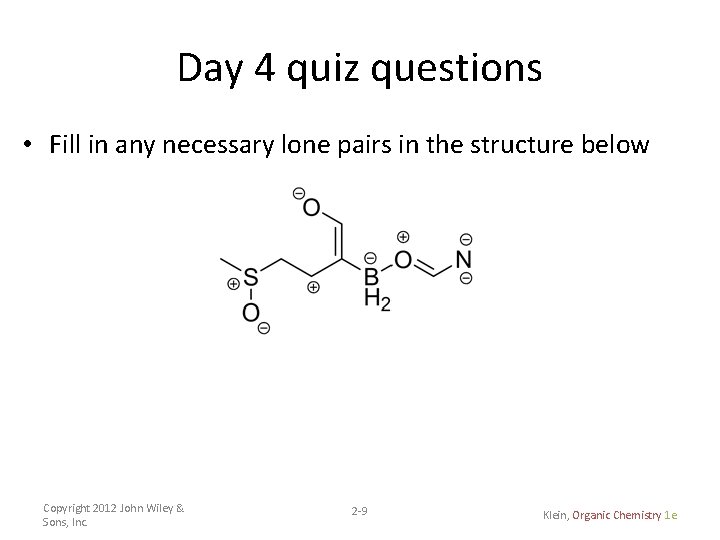
Day 4 quiz questions • Fill in any necessary lone pairs in the structure below Copyright 2012 John Wiley & Sons, Inc. 2 -9 Klein, Organic Chemistry 1 e

Day 4 quiz questions • Show all of the resonance contributors for the following molecule and show the curved arrows necessary to get from one contributor to the next. Copyright 2012 John Wiley & Sons, Inc. 2 -10 Klein, Organic Chemistry 1 e

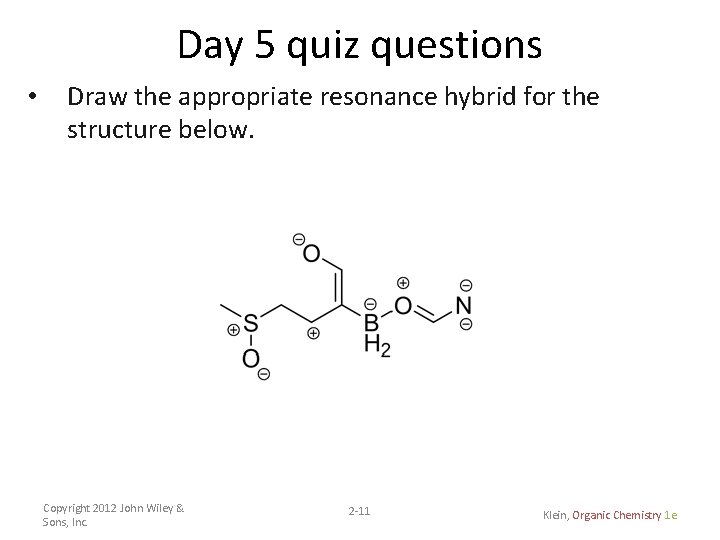
Day 5 quiz questions • Draw the appropriate resonance hybrid for the structure below. Copyright 2012 John Wiley & Sons, Inc. 2 -11 Klein, Organic Chemistry 1 e

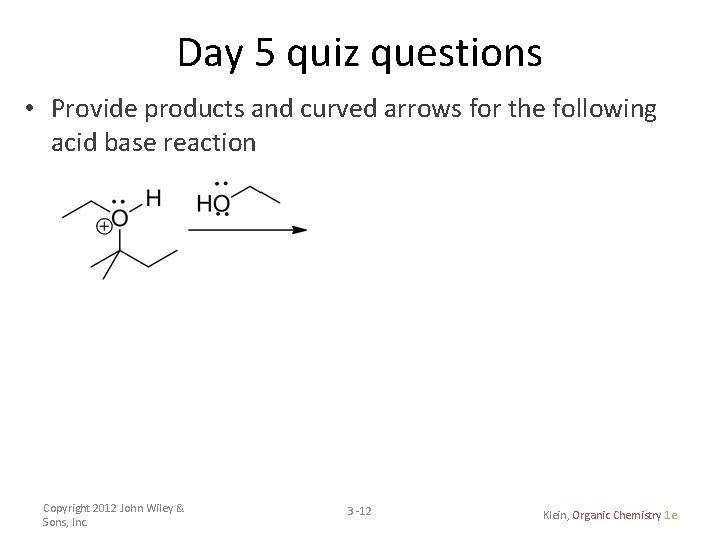
Day 5 quiz questions • Provide products and curved arrows for the following acid base reaction Copyright 2012 John Wiley & Sons, Inc. 3 -12 Klein, Organic Chemistry 1 e

Day 5 quiz questions • For a base with an especially large value for Kb. Will its conjugate acid have a relatively low or high p. Ka? Explain why using the relative p. Ka values to illustrate. Copyright 2012 John Wiley & Sons, Inc. 3 -13 Klein, Organic Chemistry 1 e

Day 5 quiz questions • Which side of the following generic reaction will be favored, and what will the ratio of products/reactants be once equilibrium is reached? HA + B: HB + A: p. Ka = 5 8 Copyright 2012 John Wiley & Sons, Inc. 3 -14 Klein, Organic Chemistry 1 e

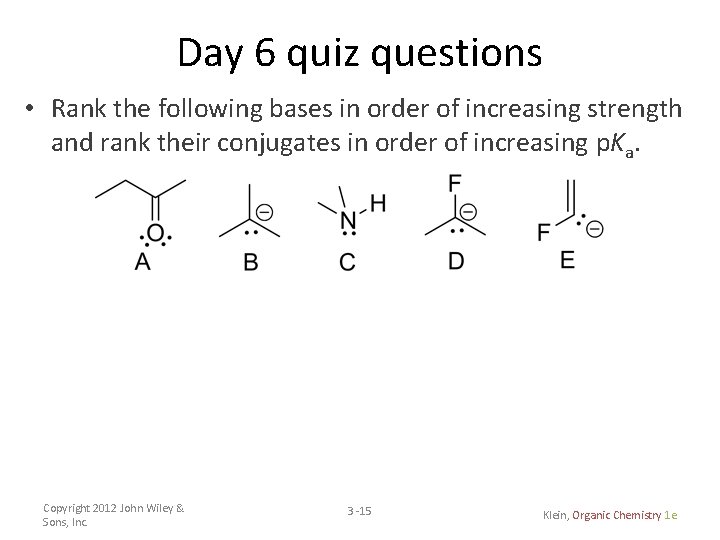
Day 6 quiz questions • Rank the following bases in order of increasing strength and rank their conjugates in order of increasing p. Ka. Copyright 2012 John Wiley & Sons, Inc. 3 -15 Klein, Organic Chemistry 1 e

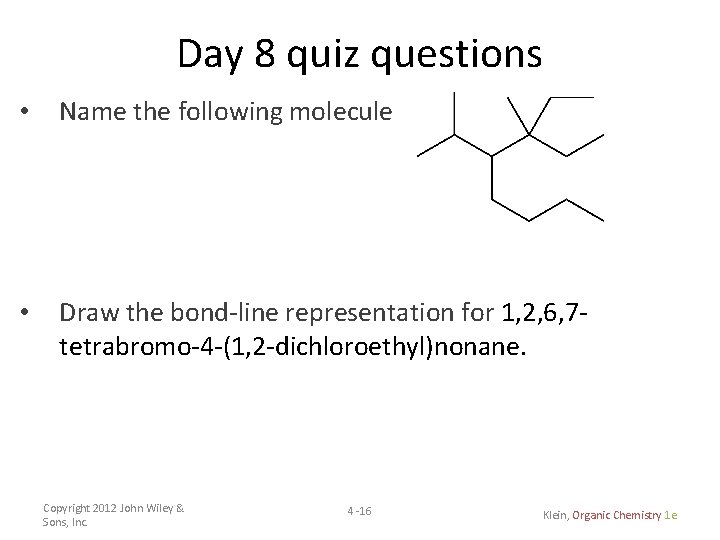
Day 8 quiz questions • Name the following molecule • Draw the bond-line representation for 1, 2, 6, 7 tetrabromo-4 -(1, 2 -dichloroethyl)nonane. Copyright 2012 John Wiley & Sons, Inc. 4 -16 Klein, Organic Chemistry 1 e

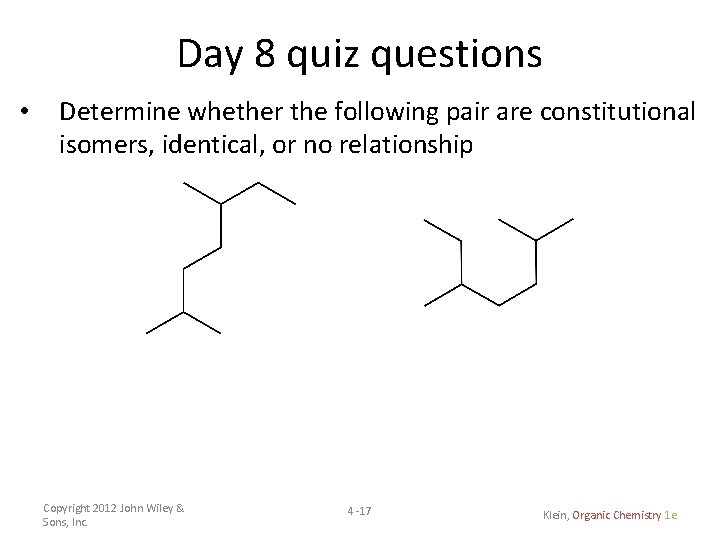
Day 8 quiz questions • Determine whether the following pair are constitutional isomers, identical, or no relationship Copyright 2012 John Wiley & Sons, Inc. 4 -17 Klein, Organic Chemistry 1 e

Day 9 quiz questions • Describe in how heat of combustion is used to determine the relative stabilities of hydrocarbons with the same formula. Copyright 2012 John Wiley & Sons, Inc. 4 -18 Klein, Organic Chemistry 1 e

Day 9 quiz questions • Given the following Newman Projection, name the molecule, draw its bond-line structure, and draw a Newman projection showing its highest energy conformation illustrating the torsional strain. Copyright 2012 John Wiley & Sons, Inc. 4 -19 Klein, Organic Chemistry 1 e

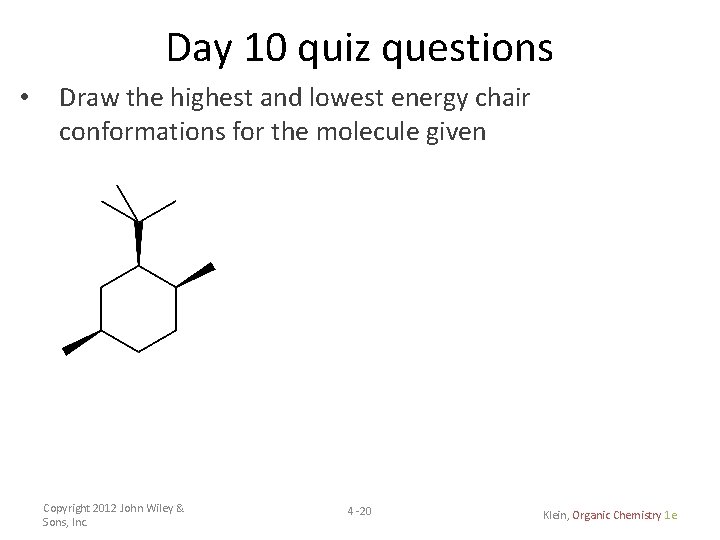
Day 10 quiz questions • Draw the highest and lowest energy chair conformations for the molecule given Copyright 2012 John Wiley & Sons, Inc. 4 -20 Klein, Organic Chemistry 1 e

Day 10 quiz questions • Draw a pair of constitutional isomers. EXPLAIN Copyright 2012 John Wiley & Sons, Inc. 5 -21 Klein, Organic Chemistry 1 e

Day 10 quiz questions • Draw a pair of cis-trans isomers. EXPLAIN Copyright 2012 John Wiley & Sons, Inc. 5 -22 Klein, Organic Chemistry 1 e

Day 10 quiz questions • Draw a pair of enantiomers. Explain Copyright 2012 John Wiley & Sons, Inc. 5 -23 Klein, Organic Chemistry 1 e

Day 10 quiz questions • Draw a molecule with 3 chiral centers. Copyright 2012 John Wiley & Sons, Inc. 5 -24 Klein, Organic Chemistry 1 e

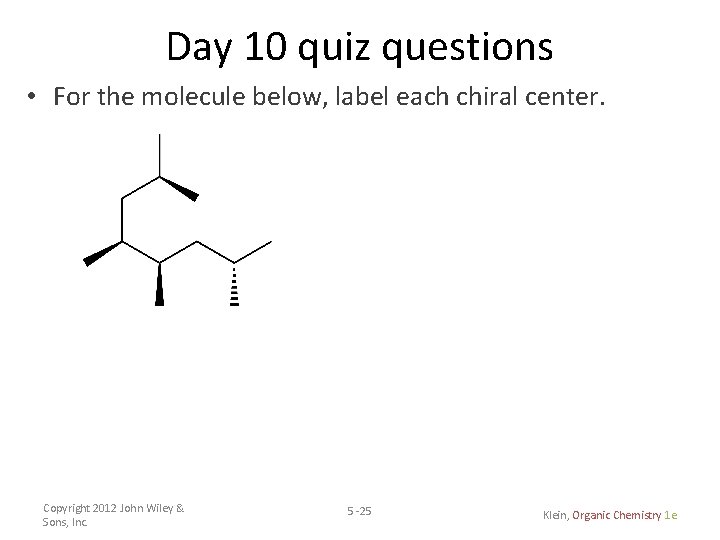
Day 10 quiz questions • For the molecule below, label each chiral center. Copyright 2012 John Wiley & Sons, Inc. 5 -25 Klein, Organic Chemistry 1 e

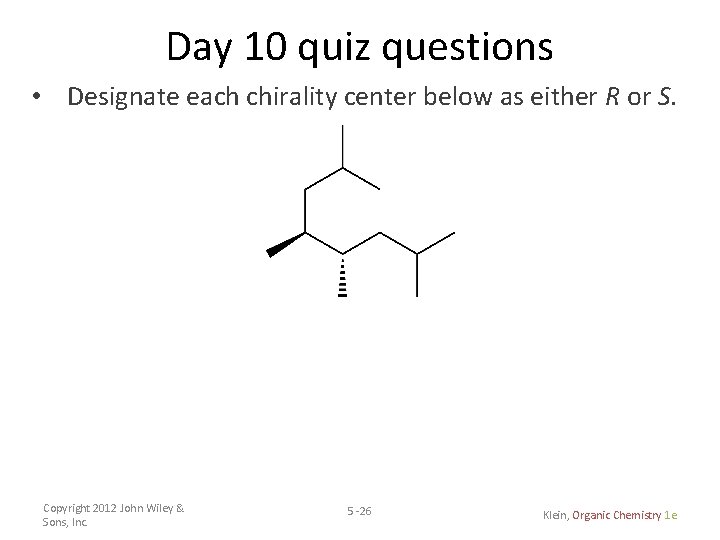
Day 10 quiz questions • Designate each chirality center below as either R or S. Copyright 2012 John Wiley & Sons, Inc. 5 -26 Klein, Organic Chemistry 1 e

Day 11 quiz questions • Draw a pair of diastereomers that are not cis-trans isomers. EXPLAIN Copyright 2012 John Wiley & Sons, Inc. 5 -27 Klein, Organic Chemistry 1 e

Day 11 quiz questions • If pure R enantiomer has a specific rotation of +33 degrees, what will the rotation be when you have a mixture with a R/S ratio = 44/56? Copyright 2012 John Wiley & Sons, Inc. 5 -28 Klein, Organic Chemistry 1 e

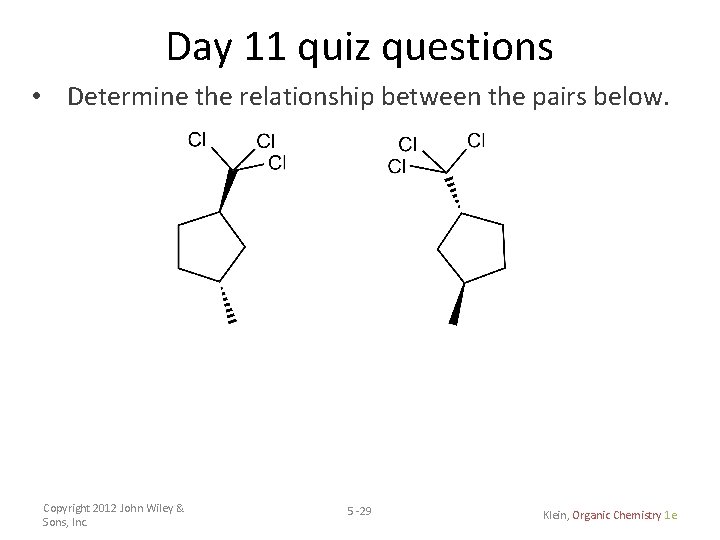
Day 11 quiz questions • Determine the relationship between the pairs below. Copyright 2012 John Wiley & Sons, Inc. 5 -29 Klein, Organic Chemistry 1 e

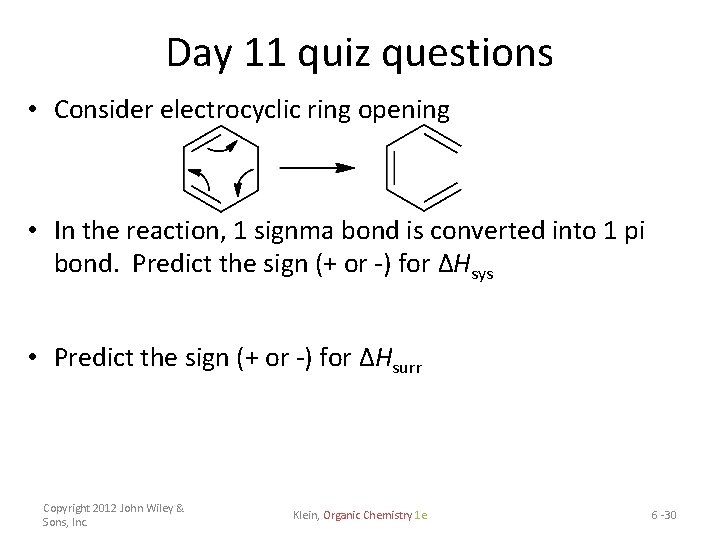
Day 11 quiz questions • Consider electrocyclic ring opening • In the reaction, 1 signma bond is converted into 1 pi bond. Predict the sign (+ or -) for ΔHsys • Predict the sign (+ or -) for ΔHsurr Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 6 -30

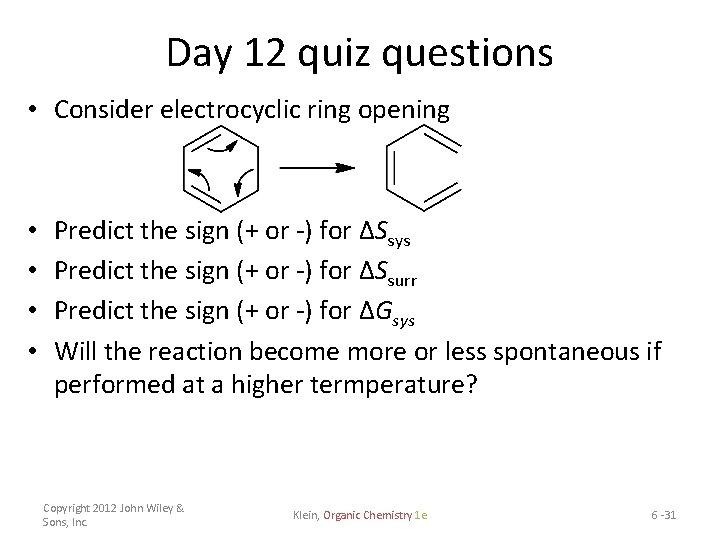
Day 12 quiz questions • Consider electrocyclic ring opening • • Predict the sign (+ or -) for ΔSsys Predict the sign (+ or -) for ΔSsurr Predict the sign (+ or -) for ΔGsys Will the reaction become more or less spontaneous if performed at a higher termperature? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 6 -31

Day 12 quiz questions • Explain why an equilibrium mixture is always lower in free energy than pure products or pure reactants. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 6 -32

Day 12 quiz questions • Reactants A and B can react by two different pathways. Pathway 1 is thermodynamically favored, and pathway 2 is kinetically favored. Draw a reaction coordinate diagraph to illustrate the relative energies and explain which pathway will be favored at low temperatures versus higher temperatures. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 6 -33

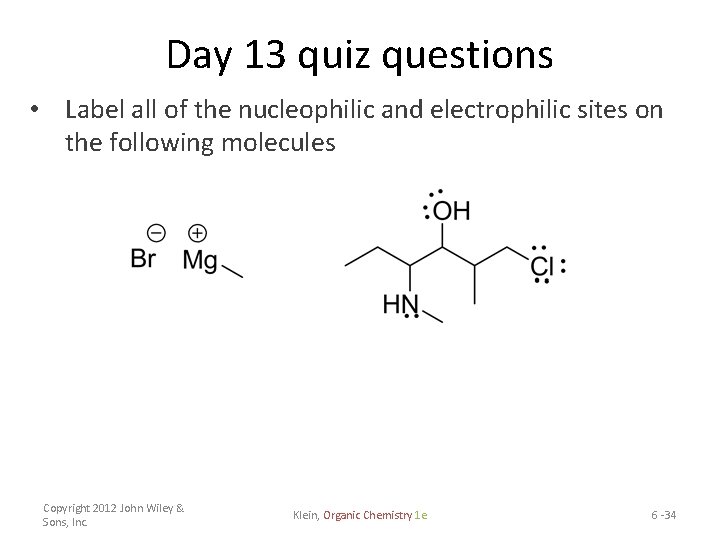
Day 13 quiz questions • Label all of the nucleophilic and electrophilic sites on the following molecules Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 6 -34

Day 13 quiz questions • Draw a mechanism for a generic nucleophilic attack followed by a proton transfer. • Draw a mechanism for a generic loss of leaving group followed by a carbocation rearrangement. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 6 -35

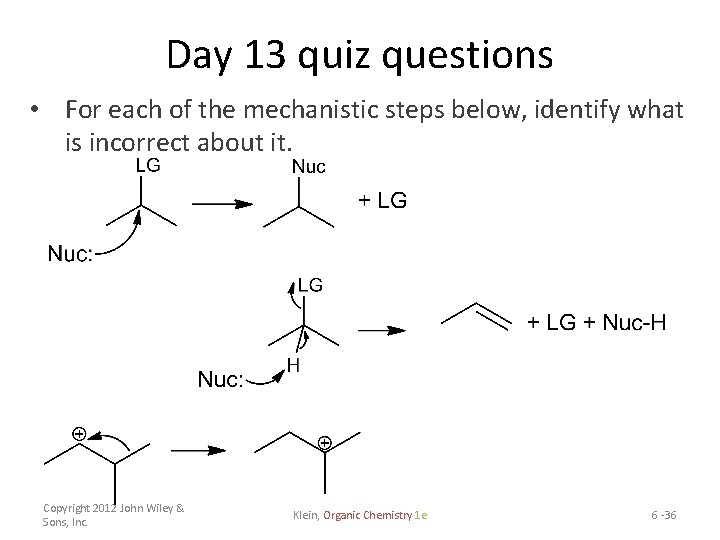
Day 13 quiz questions • For each of the mechanistic steps below, identify what is incorrect about it. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 6 -36

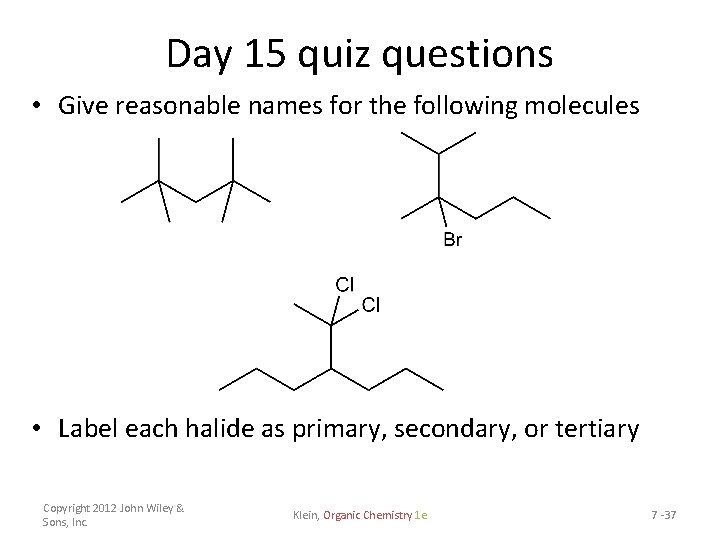
Day 15 quiz questions • Give reasonable names for the following molecules • Label each halide as primary, secondary, or tertiary Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 7 -37

Day 15 quiz questions • Give the best set of reaction conditions to promote SN 2 for the following substrate. • Describe experiments that could be done to support the proposed mechanism Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 7 -38

Day 15 quiz questions • Give the best set of reaction conditions to promote SN 1 for the following substrate. • Describe experiments that could be done to support the proposed mechanism Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 7 -39

Day 16 quiz questions • Propose reaction conditions and give a complete mechanism for the following substitution reaction Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 7 -40

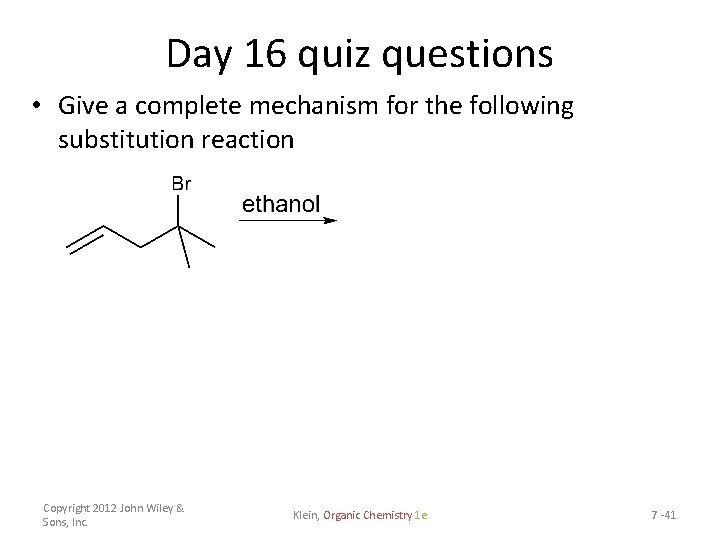
Day 16 quiz questions • Give a complete mechanism for the following substitution reaction Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 7 -41

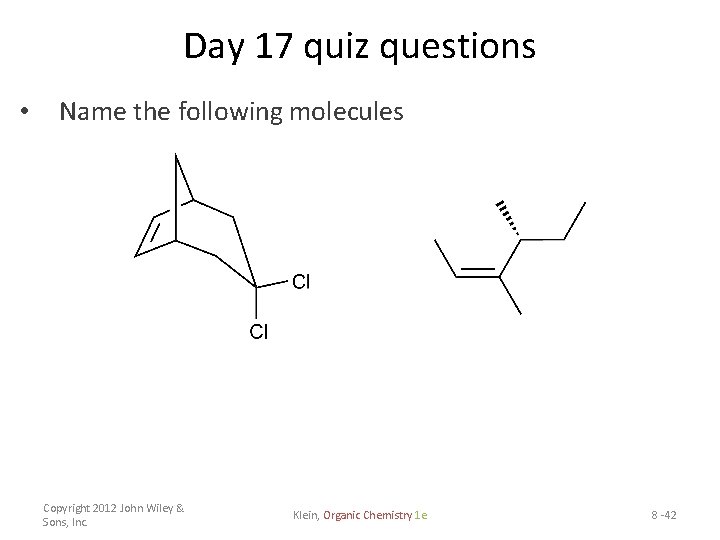
Day 17 quiz questions • Name the following molecules Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 8 -42

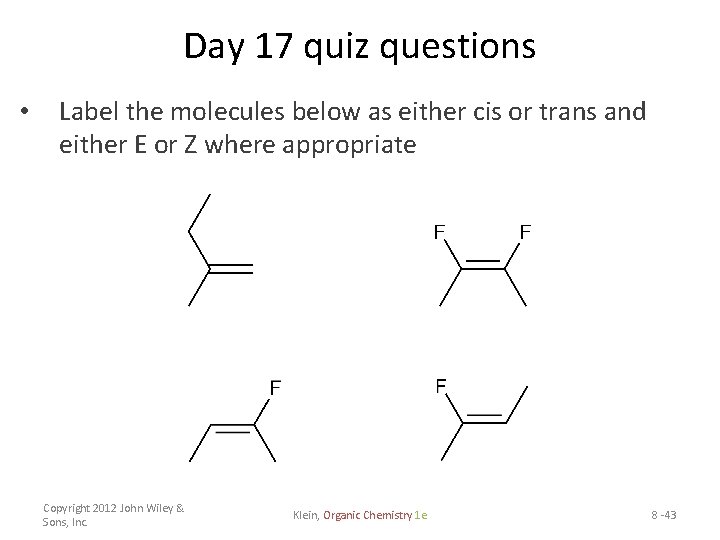
Day 17 quiz questions • Label the molecules below as either cis or trans and either E or Z where appropriate Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 8 -43

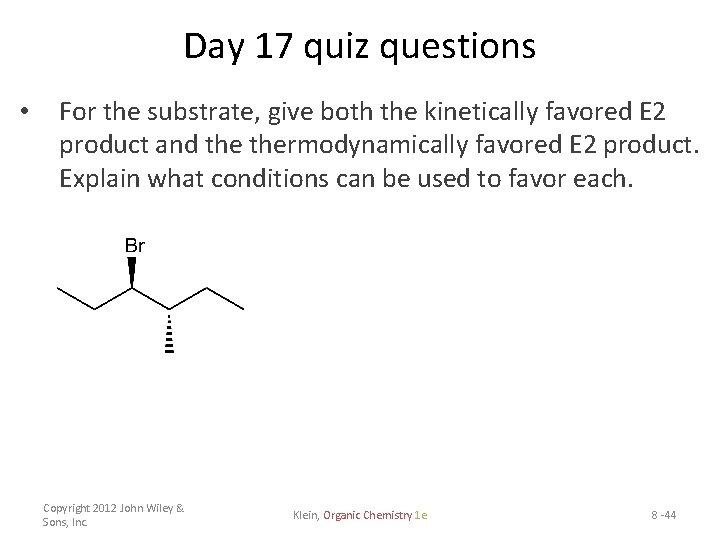
Day 17 quiz questions • For the substrate, give both the kinetically favored E 2 product and thermodynamically favored E 2 product. Explain what conditions can be used to favor each. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 8 -44

Day 17 quiz questions • Since tertiary substrates react more readily than secondary or primary in both E 1 and E 2 mechanisms, what factor(s) usually controls which mechanism will dominate and why? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 8 -45

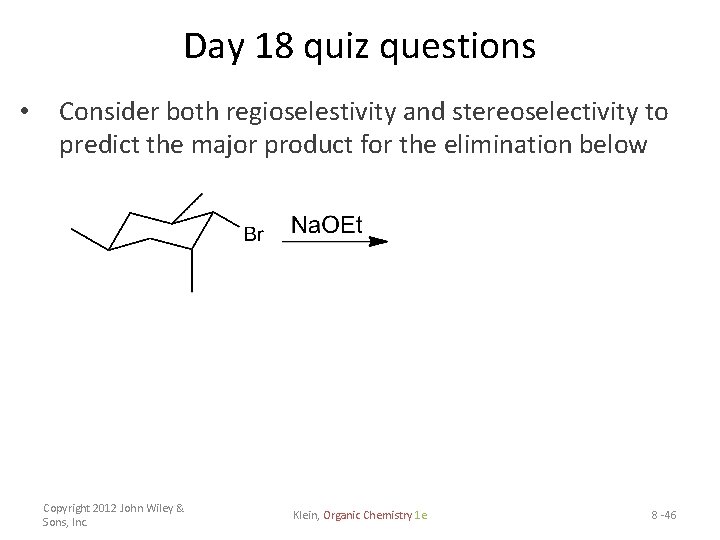
Day 18 quiz questions • Consider both regioselestivity and stereoselectivity to predict the major product for the elimination below Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 8 -46

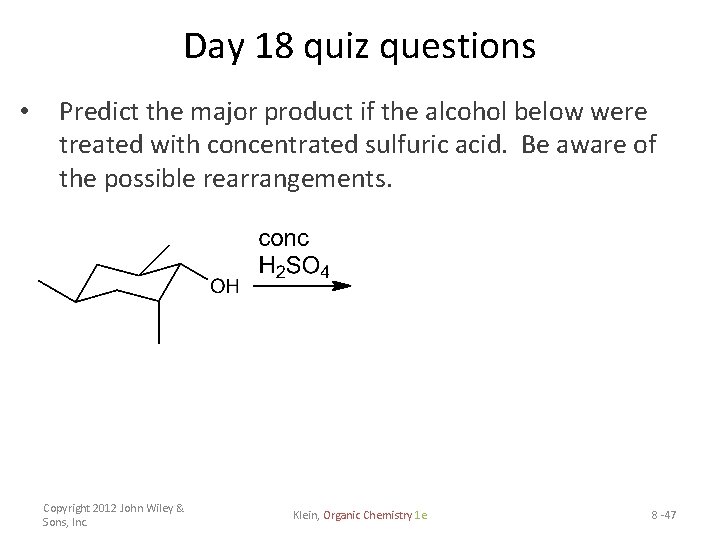
Day 18 quiz questions • Predict the major product if the alcohol below were treated with concentrated sulfuric acid. Be aware of the possible rearrangements. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 8 -47

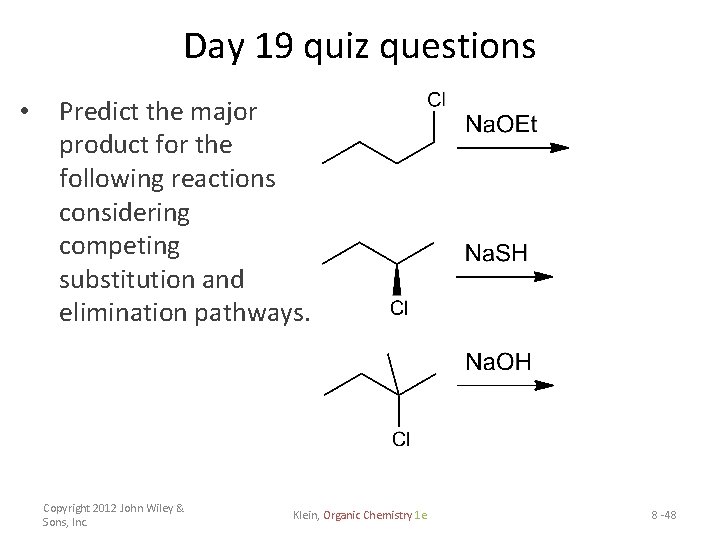
Day 19 quiz questions • Predict the major product for the following reactions considering competing substitution and elimination pathways. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 8 -48

Day 19 quiz questions • Explain why a completely nonpolar bond will not give a stretching signal in the IR spectra. Would you expect to see a signal for C-H stretching for a nonpolar molecule? Why or why not? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 15 -49

Day 20 quiz questions • Explain how IR might be used to qualitatively determine the degree of substitution when ammonia is treated with excess bromoethane. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 15 -50

Day 20 quiz questions • How might you use EI GCMS to distinguish between constitutional isomers? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 15 -51

Day 20 quiz questions • Explain how an experiment involving isotopic labeling might be used to explore the type of fragmentation that occurs in the MS analysis of organic compounds. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 15 -52

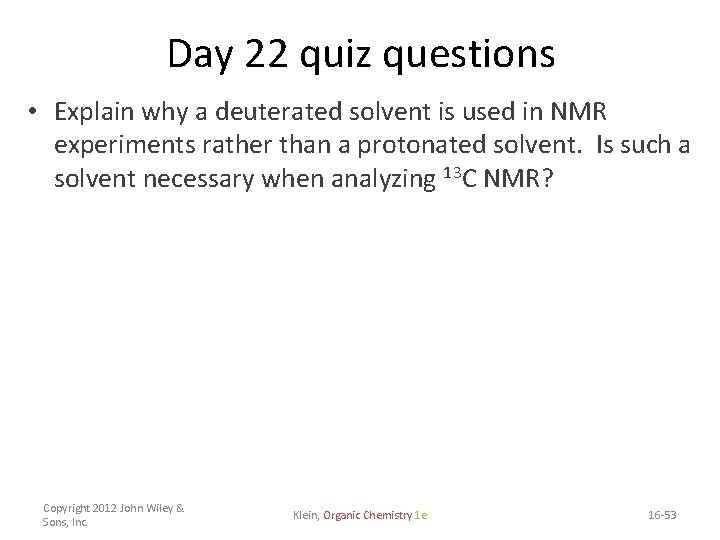
Day 22 quiz questions • Explain why a deuterated solvent is used in NMR experiments rather than a protonated solvent. Is such a solvent necessary when analyzing 13 C NMR? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 16 -53

Day 22 quiz questions • Identify all the groups of equivalent protons in the molecule below and explain WHY enantiotopic protons are equivalent while diastereotopic protons are not. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 16 -54

Day 23 quiz questions • Predict the chemical shift, integration, and splitting patterns for all of the protons in the following molecule Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 16 -55

Day 24 quiz questions • Predict the number of signals and chemical shifts in the 13 C NMR spectrum and DEPT spectrum for the molecule below Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 16 -56

Day 24 quiz questions • If you want to favor addition rather than elimination, do you generally want a high or low temperature, and why? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 9 -57

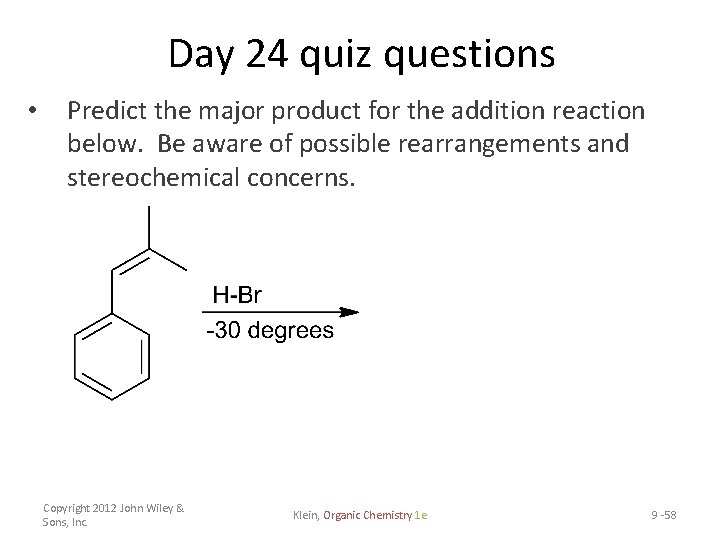
Day 24 quiz questions • Predict the major product for the addition reaction below. Be aware of possible rearrangements and stereochemical concerns. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 9 -58

Day 24 quiz questions • How and why will the concentration of acid affect whether an acid catalyzed hydration will favor products or reactants at equilibrium? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 9 -59

Day 25 quiz questions • Give an example reaction for Markovnikov hydration without the possibility of rearrangement. • Give an example reaction for syn anti. Markovnikov hydration. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 9 -60

Day 25 quiz questions • Should a halogenation reaction be overall first or second order kinetics? Also, Explain why it gives anti addition rather than syn. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 9 -61

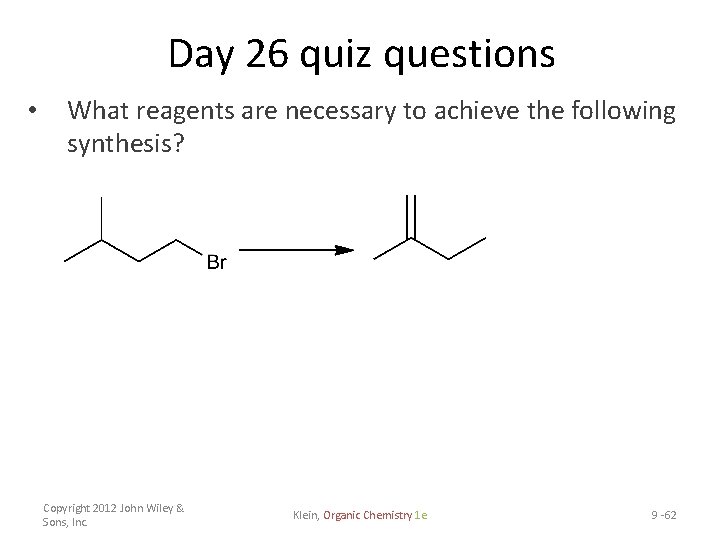
Day 26 quiz questions • What reagents are necessary to achieve the following synthesis? Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 9 -62

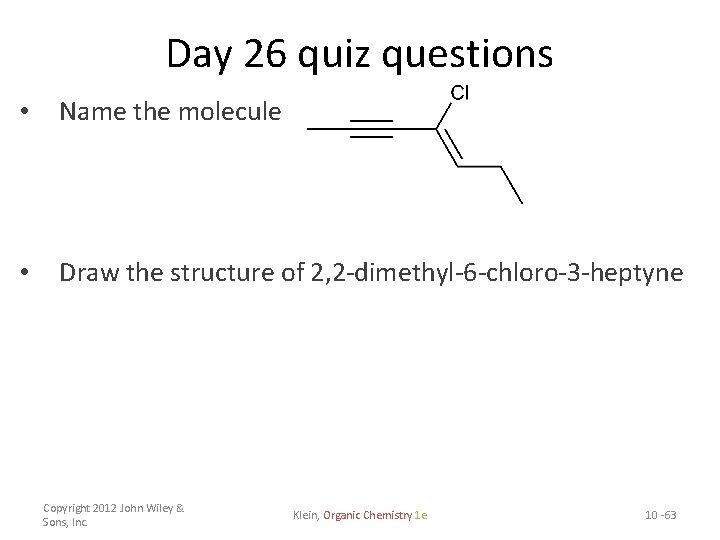
Day 26 quiz questions • Name the molecule • Draw the structure of 2, 2 -dimethyl-6 -chloro-3 -heptyne Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 10 -63

Day 27 quiz questions • Give 2 sets of reagents that could be used to synthesize 1 -pentyne through the elimination a dihalide. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 10 -64

Day 27 quiz questions • Give a set of reagents that could be used to synthesize cis-2 -pentene from an addition reaction. • Give a set of reagents that could be used to synthesize trans-2 -pentene from an addition reaction. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 10 -65

Day 27 quiz questions • Give a set of reagents that could be used to synthesize a ketone from an addition reaction. • Give a set of reagents that could be used to synthesize an aldehyde from an addition reaction. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 10 -66

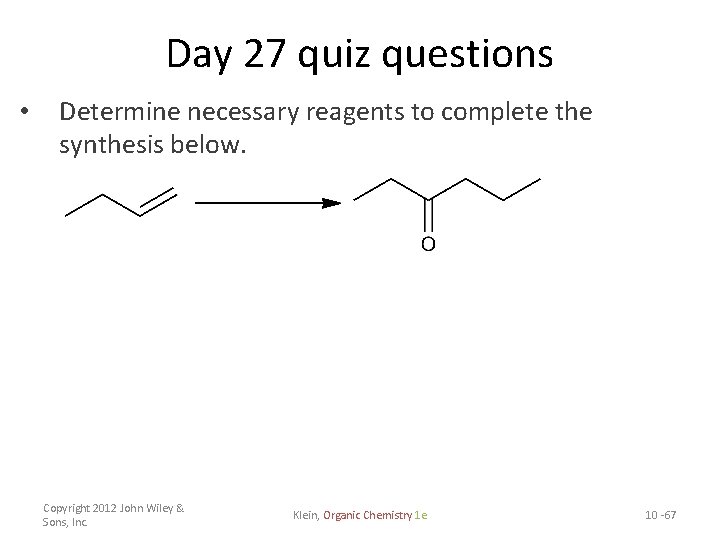
Day 27 quiz questions • Determine necessary reagents to complete the synthesis below. Copyright 2012 John Wiley & Sons, Inc. Klein, Organic Chemistry 1 e 10 -67