Chemical Quantities and Chemical Reactions Chemical Quantities Measuring







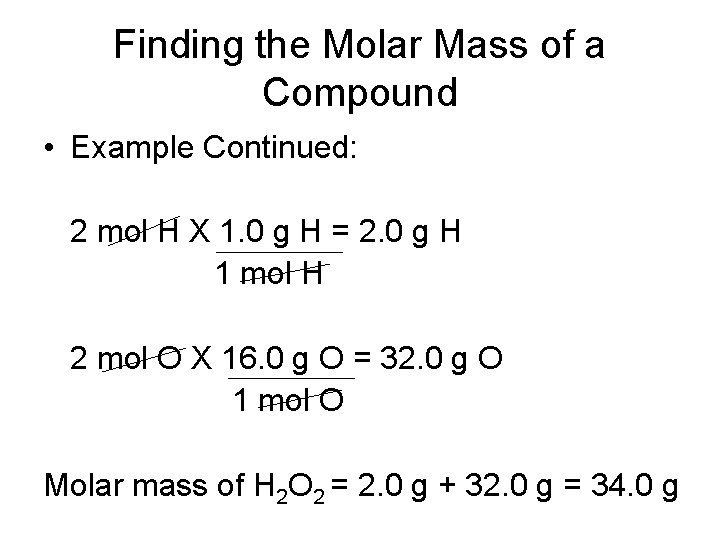


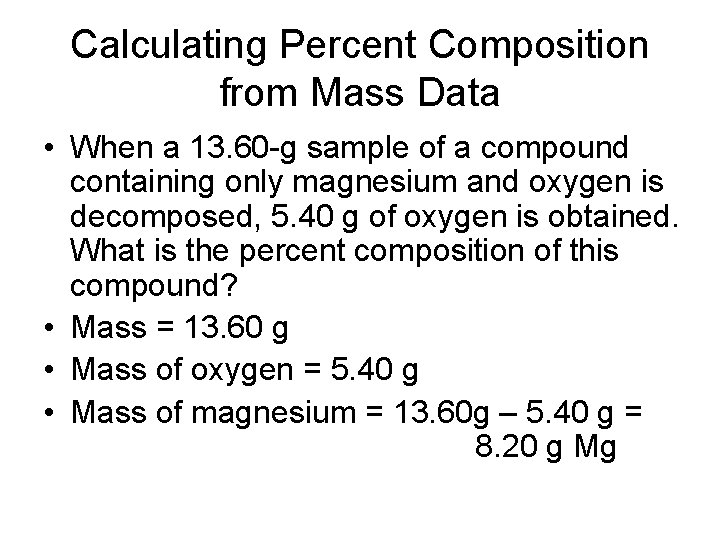
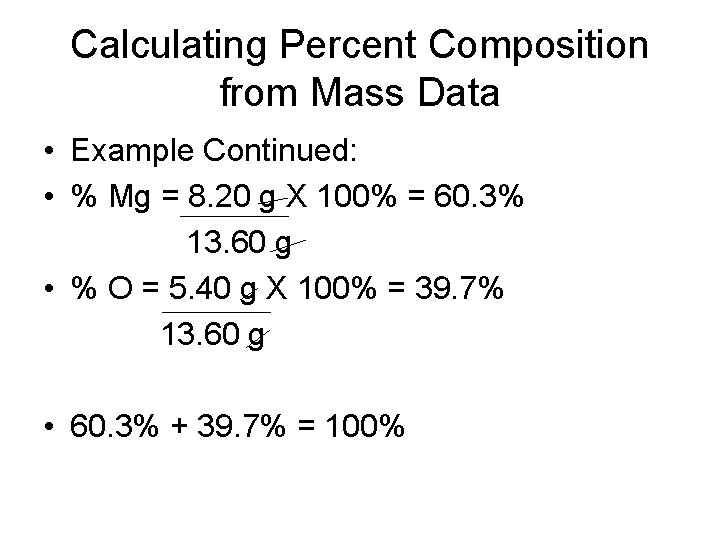











- Slides: 94

Chemical Quantities and Chemical Reactions

Chemical Quantities

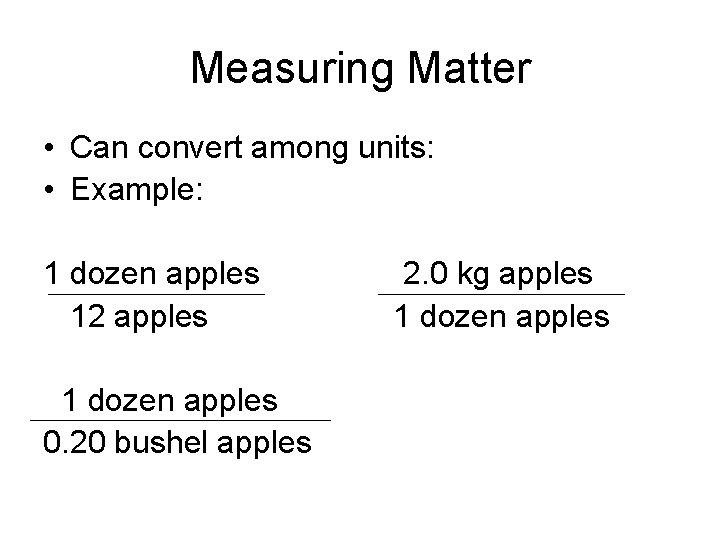
Measuring Matter • Often measure the amount of something by one of three different methods: –count –mass –volume

Measuring Matter • By count: - Example: 1 dozen apples = 12 apples • By mass: - Example: 1 dozen apples = 2. 0 kg apples • By volume: - Example: 1 dozen apples = 0. 20 bushel apples

Measuring Matter • Can convert among units: • Example: 1 dozen apples 12 apples 1 dozen apples 0. 20 bushel apples 2. 0 kg apples 1 dozen apples

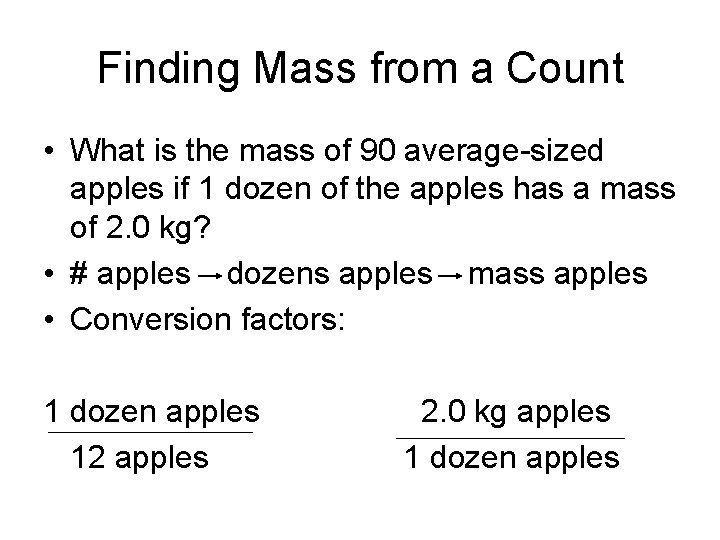
Finding Mass from a Count • What is the mass of 90 average-sized apples if 1 dozen of the apples has a mass of 2. 0 kg? • # apples dozens apples mass apples • Conversion factors: 1 dozen apples 12 apples 2. 0 kg apples 1 dozen apples

Finding Mass from a Count 90 apples X 1 dozen apples X 2. 0 kg apples 12 apples 1 dozen apples = 15 kg apples

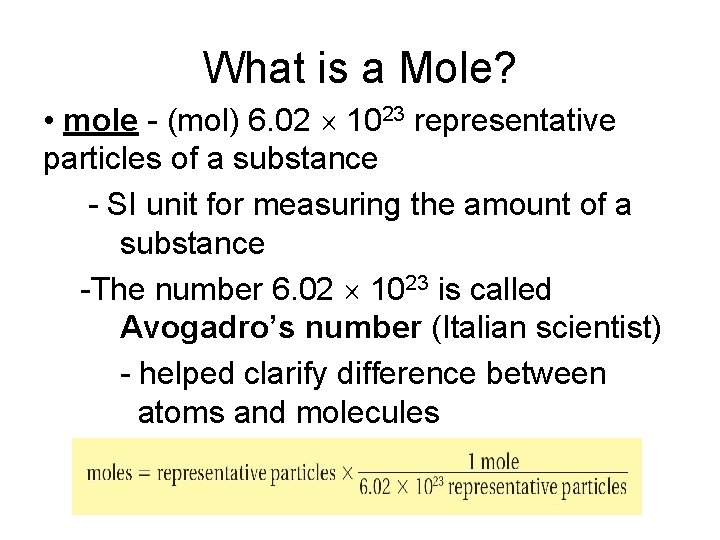
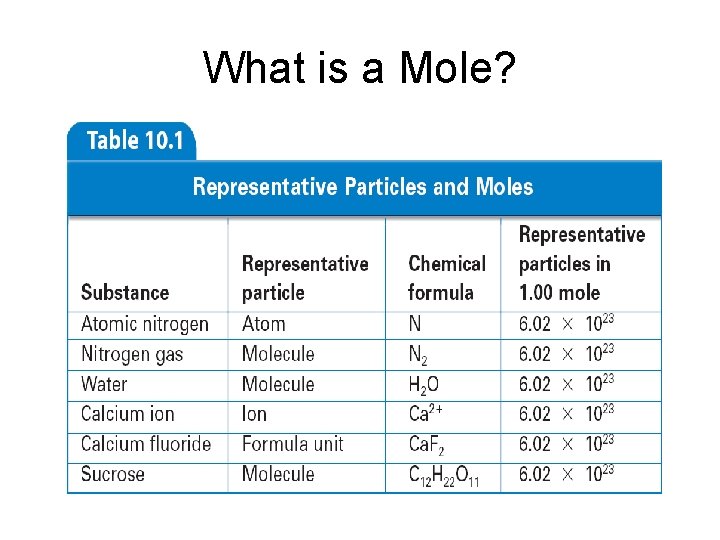
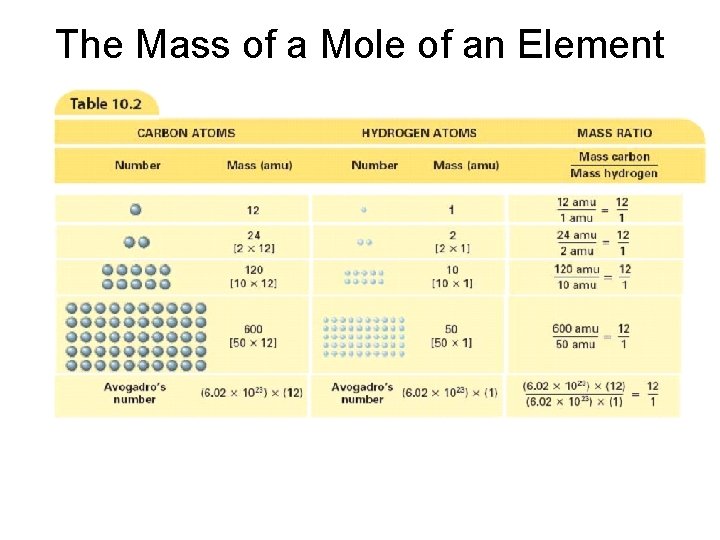
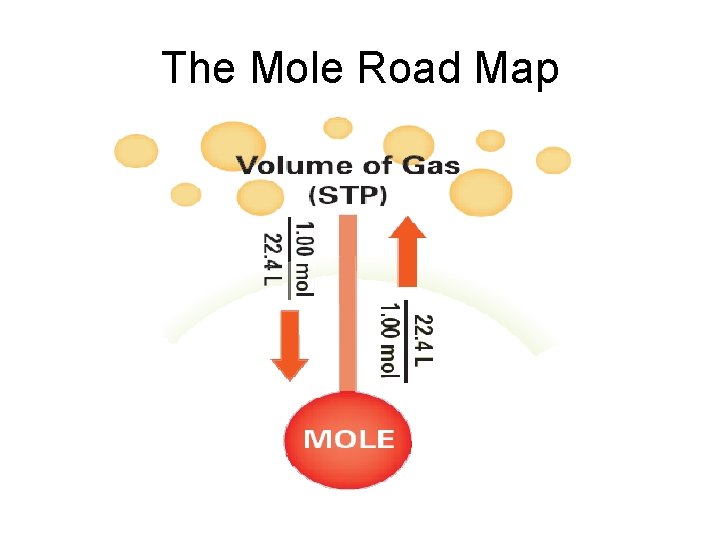
What is a Mole? • mole - (mol) 6. 02 1023 representative particles of a substance - SI unit for measuring the amount of a substance -The number 6. 02 1023 is called Avogadro’s number (Italian scientist) - helped clarify difference between atoms and molecules

What is a Mole? • Mole of any substance contains Avogadro’s number of representative particles, or 6. 02 1023 representative particles • Representative particle - refers to species present in a substance - usually atoms, molecules, or formula units

What is a Mole?

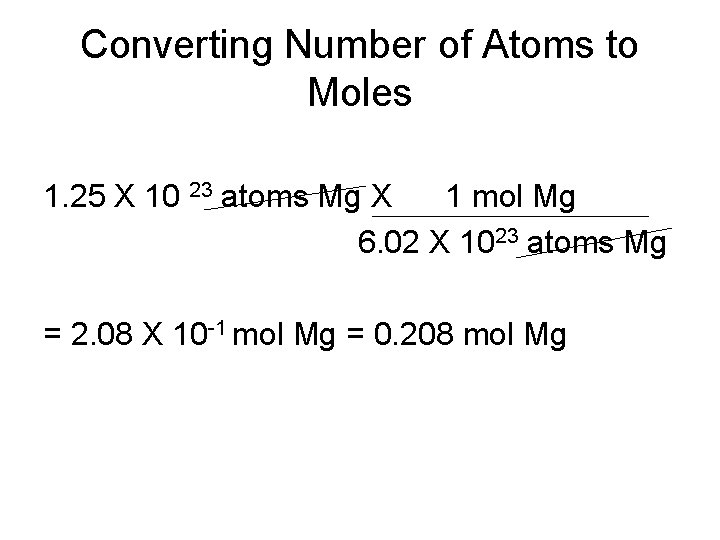
Converting Number of Atoms to Moles • Magnesium is a light metal used in the manufacture of aircraft, automobile wheels, tools, and garden furniture. How many moles of magnesium is 1. 25 X 1023 atoms of magnesium? • Conversion factors: 1 mol Mg 6. 02 X 10 23 atoms Mg

Converting Number of Atoms to Moles 1. 25 X 10 23 atoms Mg X 1 mol Mg 6. 02 X 1023 atoms Mg = 2. 08 X 10 -1 mol Mg = 0. 208 mol Mg

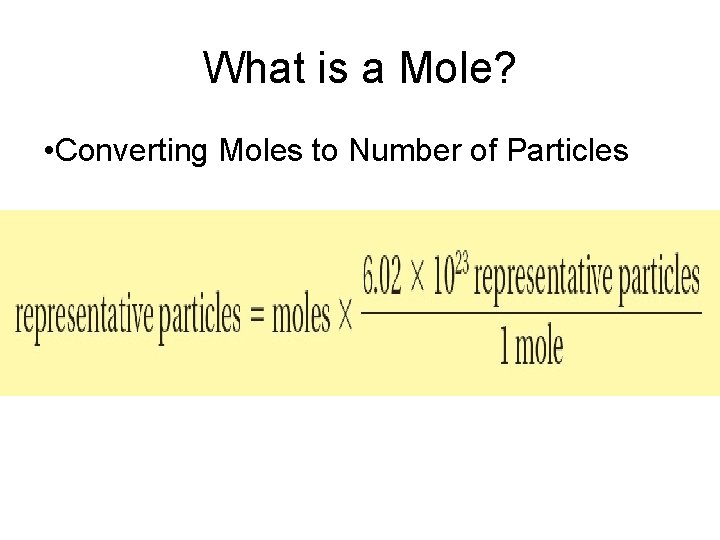
What is a Mole? • Converting Moles to Number of Particles

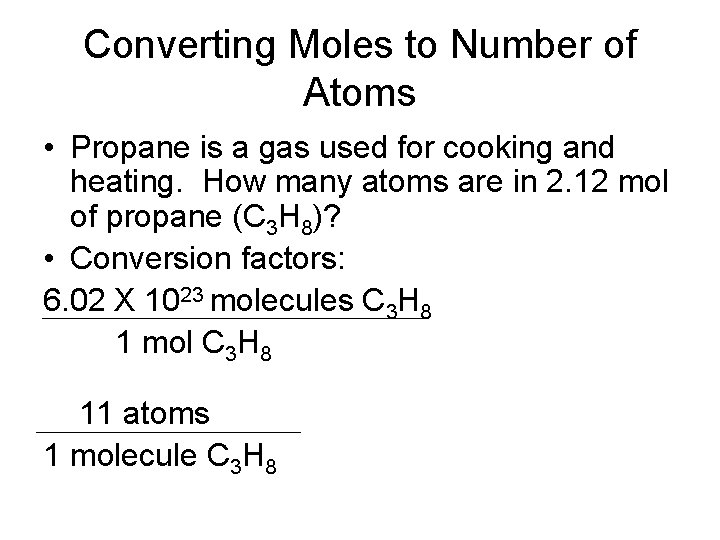
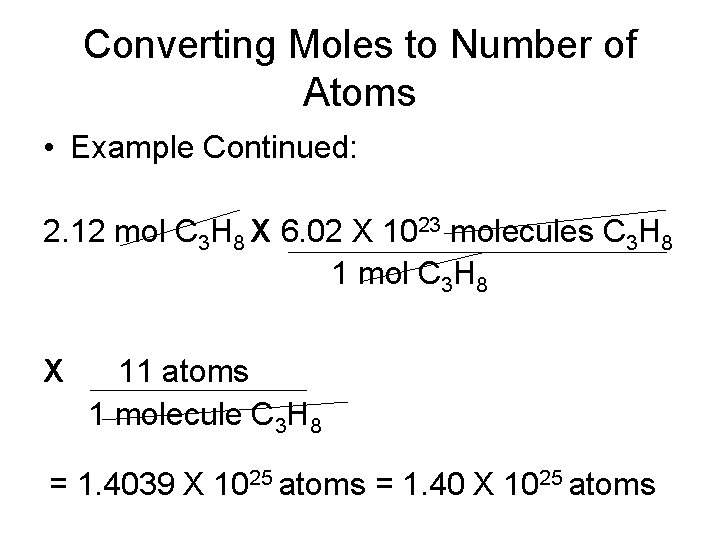
Converting Moles to Number of Atoms • Propane is a gas used for cooking and heating. How many atoms are in 2. 12 mol of propane (C 3 H 8)? • Conversion factors: 6. 02 X 1023 molecules C 3 H 8 1 mol C 3 H 8 11 atoms 1 molecule C 3 H 8

Converting Moles to Number of Atoms • Example Continued: 2. 12 mol C 3 H 8 X 6. 02 X 1023 molecules C 3 H 8 1 mol C 3 H 8 X 11 atoms 1 molecule C 3 H 8 = 1. 4039 X 1025 atoms = 1. 40 X 1025 atoms

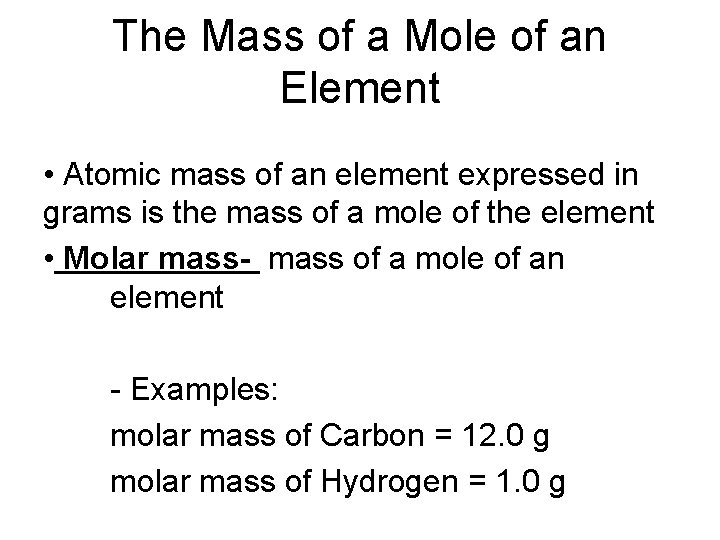
The Mass of a Mole of an Element • Atomic mass of an element expressed in grams is the mass of a mole of the element • Molar mass- mass of a mole of an element - Examples: molar mass of Carbon = 12. 0 g molar mass of Hydrogen = 1. 0 g

The Mass of a Mole of an Element

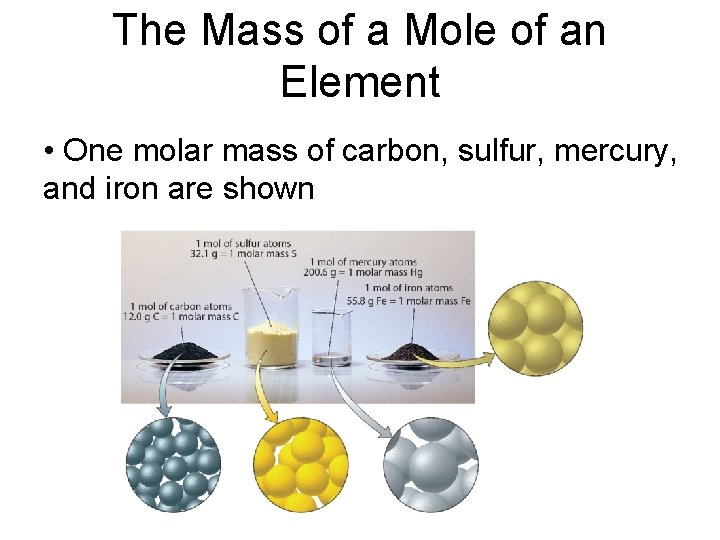
The Mass of a Mole of an Element • One molar mass of carbon, sulfur, mercury, and iron are shown

The Mass of a Mole of an Element • You must know formula of compound to calculate the mass of a mole of an element • To calculate the molar mass of a compound: - find the number of grams of each element in one mole of the compound - add the masses of the elements in the compound

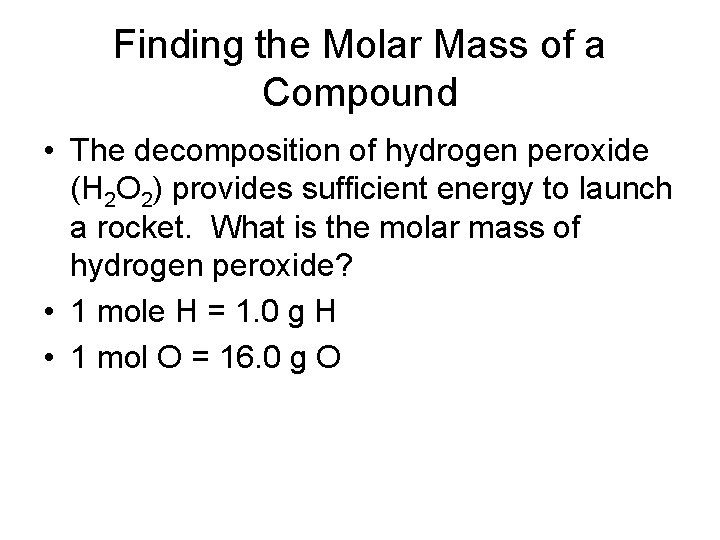
Finding the Molar Mass of a Compound • The decomposition of hydrogen peroxide (H 2 O 2) provides sufficient energy to launch a rocket. What is the molar mass of hydrogen peroxide? • 1 mole H = 1. 0 g H • 1 mol O = 16. 0 g O
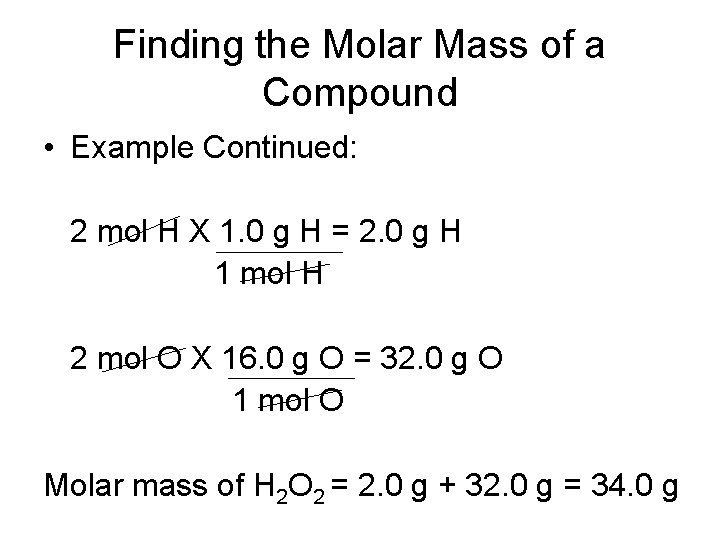
Finding the Molar Mass of a Compound • Example Continued: 2 mol H X 1. 0 g H = 2. 0 g H 1 mol H 2 mol O X 16. 0 g O = 32. 0 g O 1 mol O Molar mass of H 2 O 2 = 2. 0 g + 32. 0 g = 34. 0 g

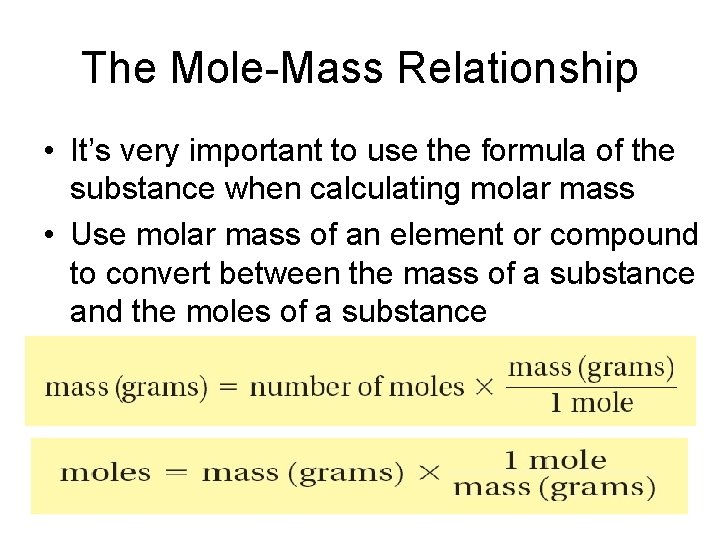
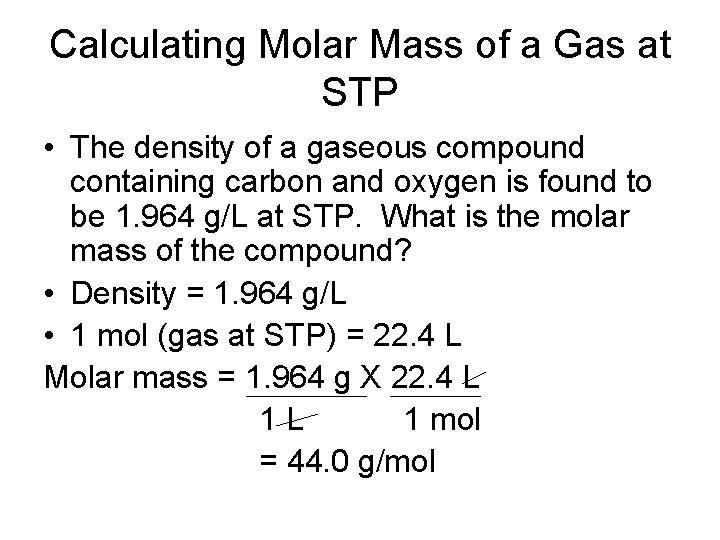
The Mole-Mass Relationship • It’s very important to use the formula of the substance when calculating molar mass • Use molar mass of an element or compound to convert between the mass of a substance and the moles of a substance

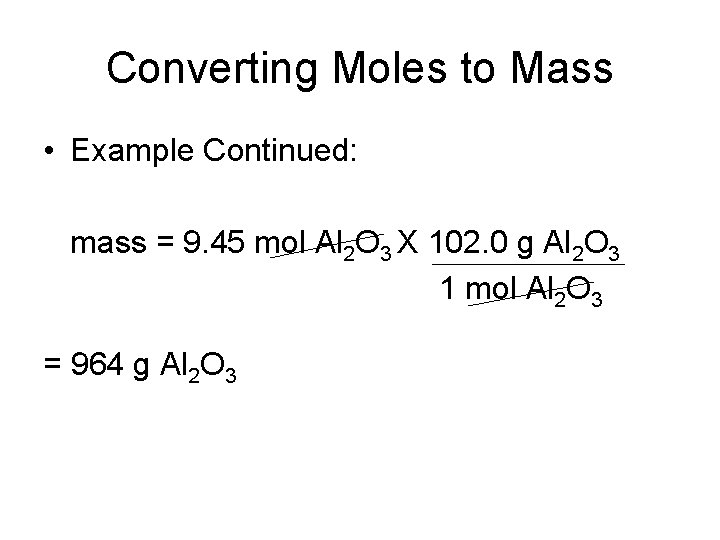
Converting Moles to Mass • The aluminum satellite dishes are resistant to corrosion because aluminum reacts with oxygen in the air to form a coating of aluminum oxide (Al 2 O 3). This tough, resistant coating prevents any further corrosion. What is the mass of 9. 45 mol of aluminum oxide? • Moles = 9. 45 mol Al 2 O 3 • 1 mol Al 2 O 3 = 102. 0 g Al 2 O 3

Converting Moles to Mass • Example Continued: mass = 9. 45 mol Al 2 O 3 X 102. 0 g Al 2 O 3 1 mol Al 2 O 3 = 964 g Al 2 O 3

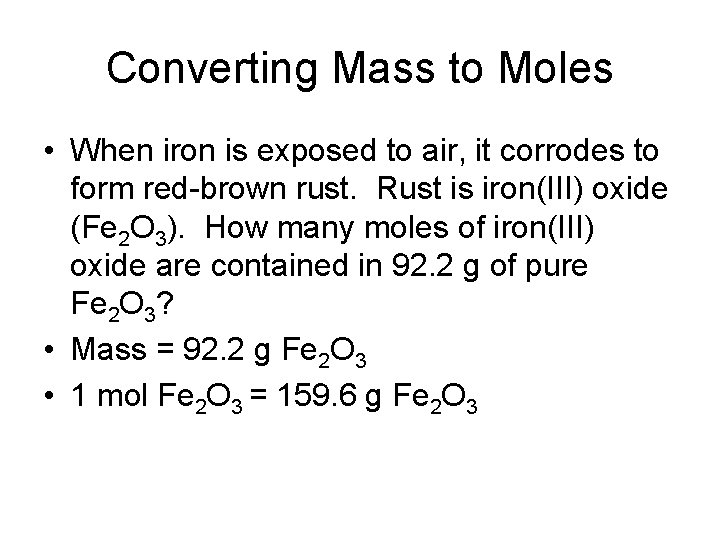
Converting Mass to Moles • When iron is exposed to air, it corrodes to form red-brown rust. Rust is iron(III) oxide (Fe 2 O 3). How many moles of iron(III) oxide are contained in 92. 2 g of pure Fe 2 O 3? • Mass = 92. 2 g Fe 2 O 3 • 1 mol Fe 2 O 3 = 159. 6 g Fe 2 O 3

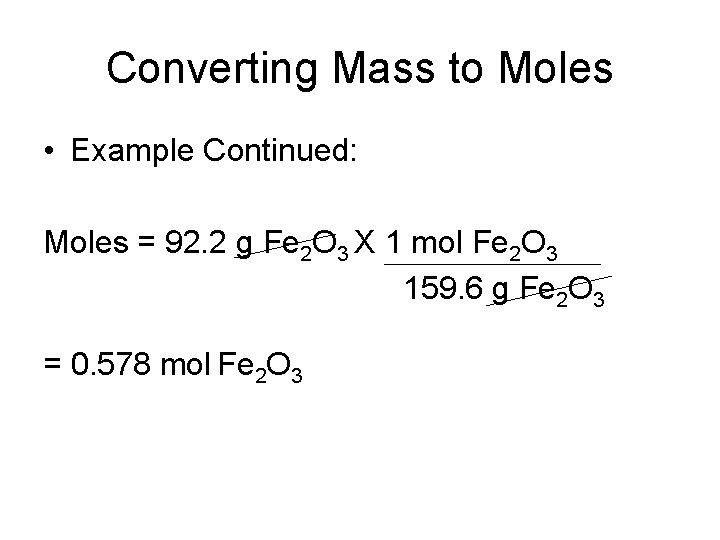
Converting Mass to Moles • Example Continued: Moles = 92. 2 g Fe 2 O 3 X 1 mol Fe 2 O 3 159. 6 g Fe 2 O 3 = 0. 578 mol Fe 2 O 3

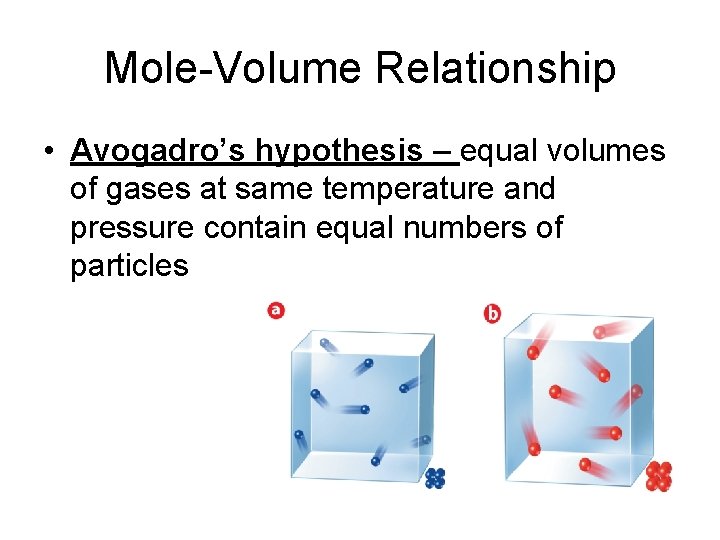
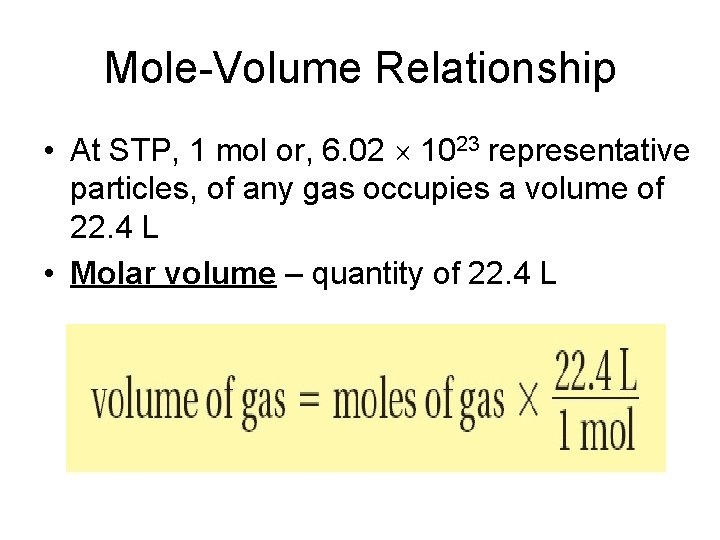
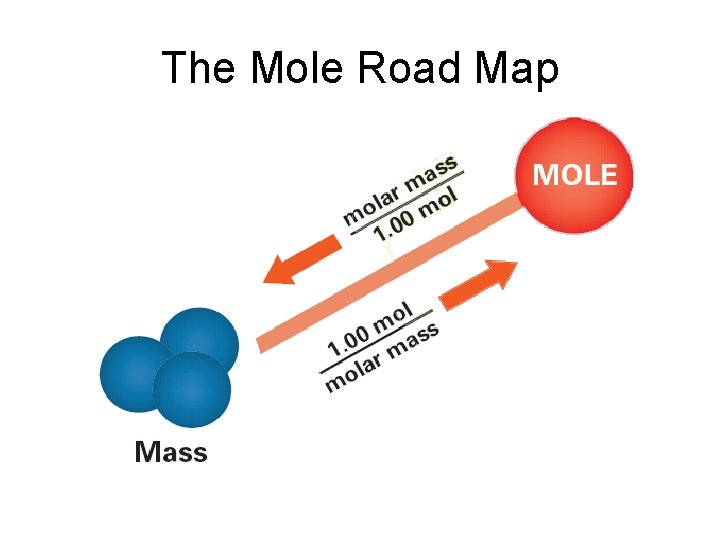
Mole-Volume Relationship • Avogadro’s hypothesis – equal volumes of gases at same temperature and pressure contain equal numbers of particles

Mole–Volume Relationship • Volume of a gas varies with temperature and pressure - Example: balloon filled with helium shrinks when outside on a cold day - The volume of a gas is usually measured at a standard temperature and pressure • Standard temperature and pressure (STP) - means a temperature of 0°C and a pressure of 101. 3 k. Pa, or 1 atmosphere (atm)

Mole-Volume Relationship • At STP, 1 mol or, 6. 02 1023 representative particles, of any gas occupies a volume of 22. 4 L • Molar volume – quantity of 22. 4 L

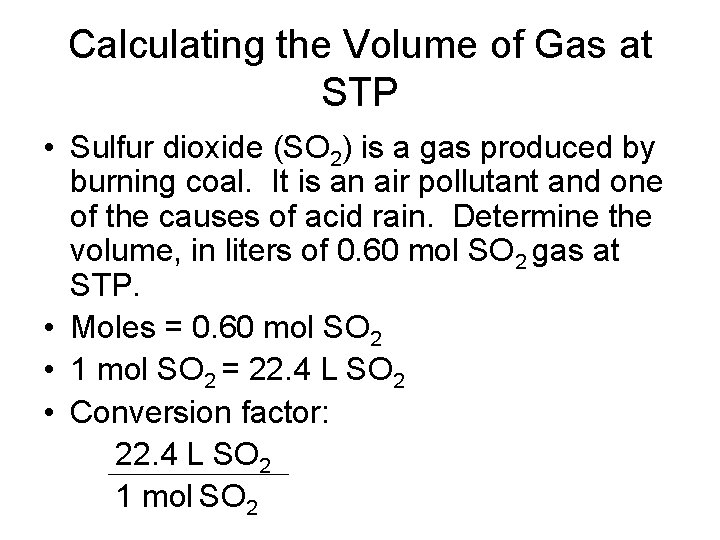
Calculating the Volume of Gas at STP • Sulfur dioxide (SO 2) is a gas produced by burning coal. It is an air pollutant and one of the causes of acid rain. Determine the volume, in liters of 0. 60 mol SO 2 gas at STP. • Moles = 0. 60 mol SO 2 • 1 mol SO 2 = 22. 4 L SO 2 • Conversion factor: 22. 4 L SO 2 1 mol SO 2

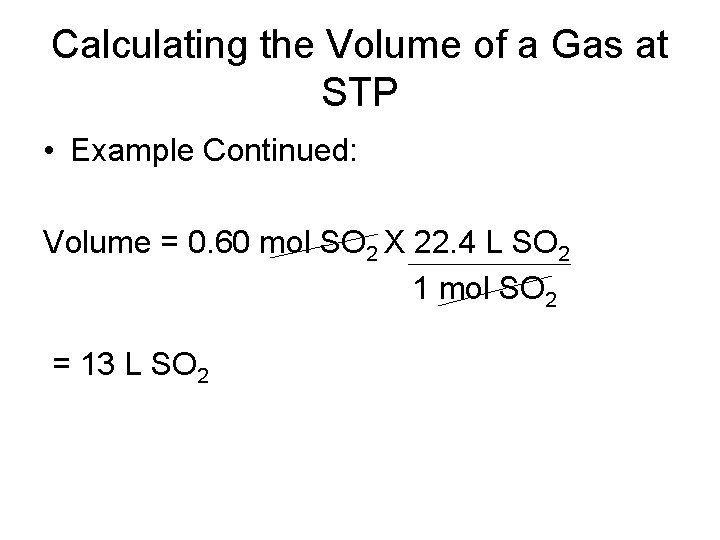
Calculating the Volume of a Gas at STP • Example Continued: Volume = 0. 60 mol SO 2 X 22. 4 L SO 2 1 mol SO 2 = 13 L SO 2

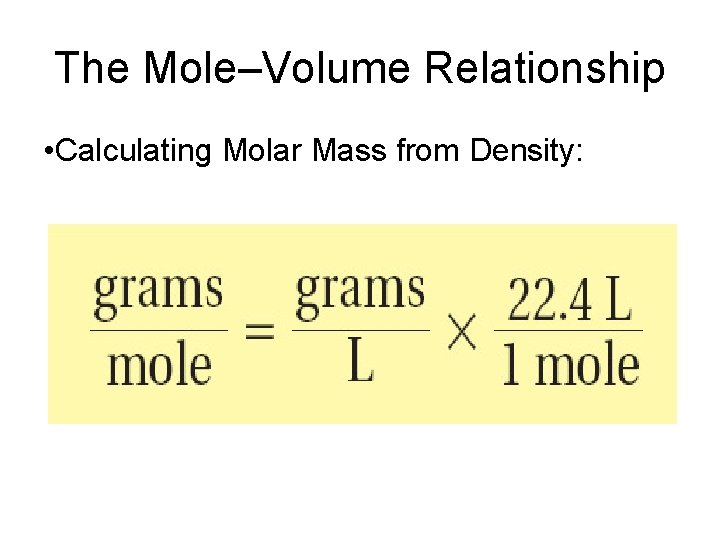
The Mole–Volume Relationship • Calculating Molar Mass from Density:

Calculating Molar Mass of a Gas at STP • The density of a gaseous compound containing carbon and oxygen is found to be 1. 964 g/L at STP. What is the molar mass of the compound? • Density = 1. 964 g/L • 1 mol (gas at STP) = 22. 4 L Molar mass = 1. 964 g X 22. 4 L 1 L 1 mol = 44. 0 g/mol

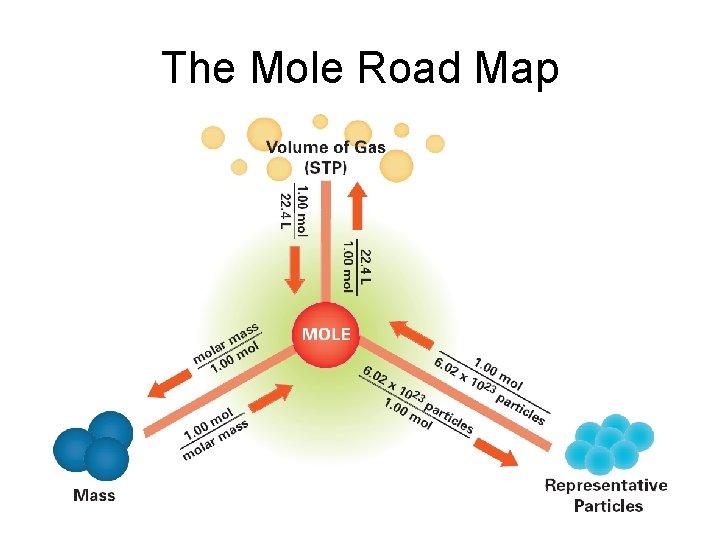
The Mole Road Map

The Mole Road Map

The Mole Road Map

The Mole Road Map

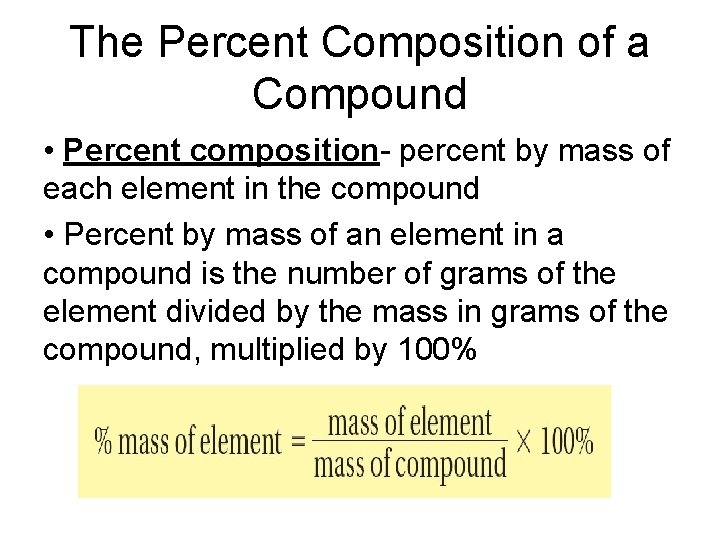
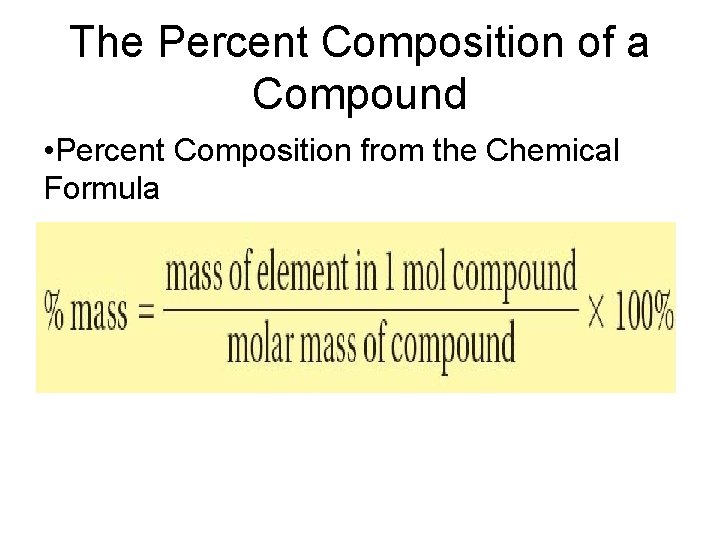
The Percent Composition of a Compound • Percent composition- percent by mass of each element in the compound • Percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100%

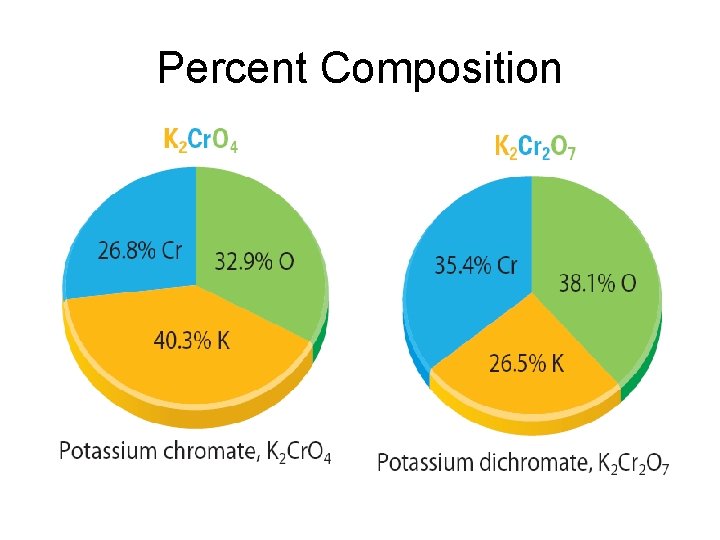
Percent Composition
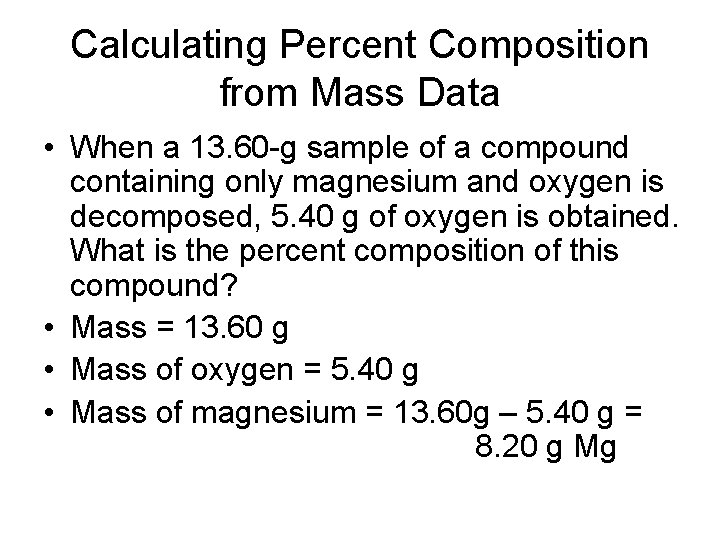
Calculating Percent Composition from Mass Data • When a 13. 60 -g sample of a compound containing only magnesium and oxygen is decomposed, 5. 40 g of oxygen is obtained. What is the percent composition of this compound? • Mass = 13. 60 g • Mass of oxygen = 5. 40 g • Mass of magnesium = 13. 60 g – 5. 40 g = 8. 20 g Mg
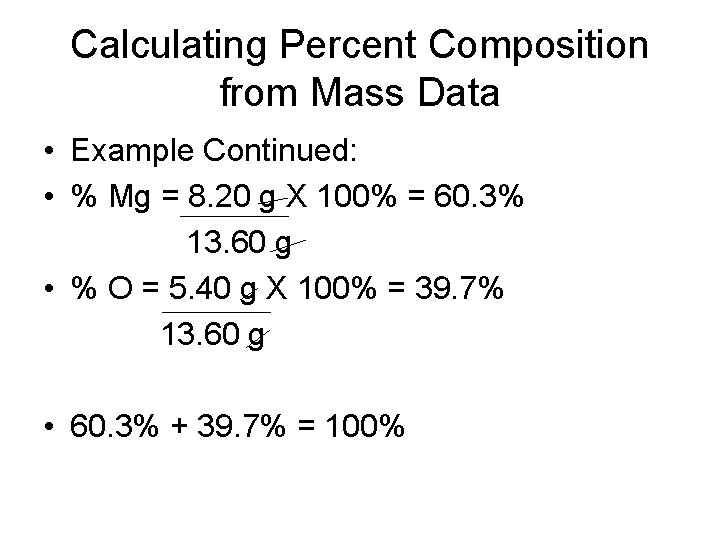
Calculating Percent Composition from Mass Data • Example Continued: • % Mg = 8. 20 g X 100% = 60. 3% 13. 60 g • % O = 5. 40 g X 100% = 39. 7% 13. 60 g • 60. 3% + 39. 7% = 100%

The Percent Composition of a Compound • Percent Composition from the Chemical Formula

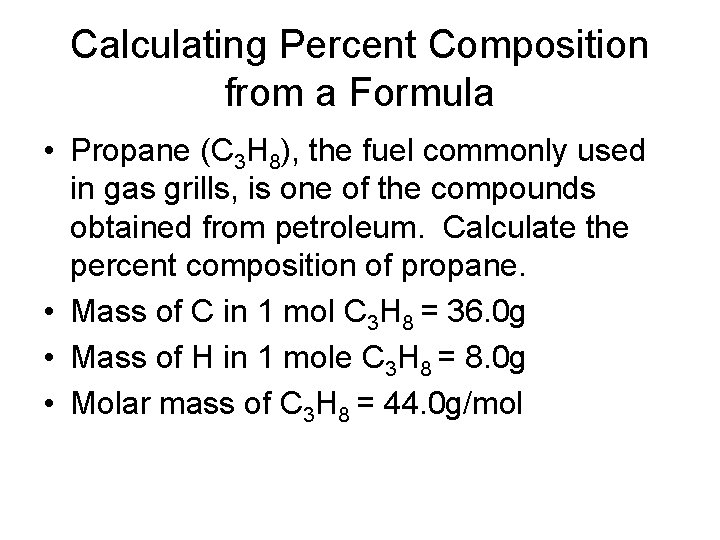
Calculating Percent Composition from a Formula • Propane (C 3 H 8), the fuel commonly used in gas grills, is one of the compounds obtained from petroleum. Calculate the percent composition of propane. • Mass of C in 1 mol C 3 H 8 = 36. 0 g • Mass of H in 1 mole C 3 H 8 = 8. 0 g • Molar mass of C 3 H 8 = 44. 0 g/mol

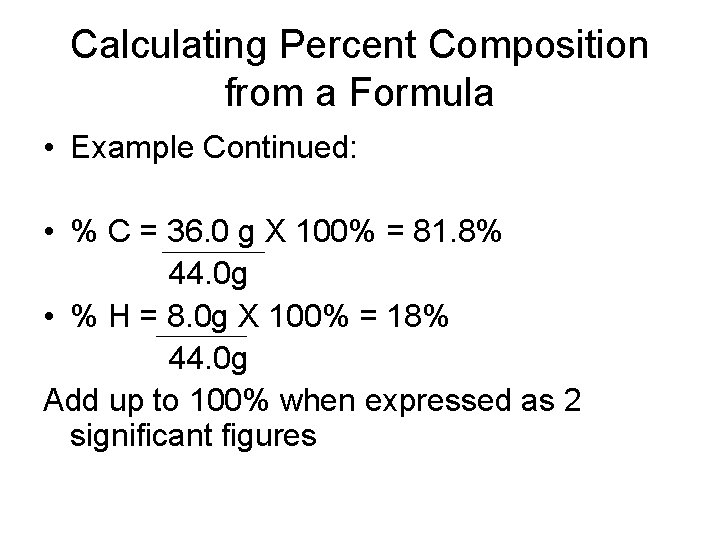
Calculating Percent Composition from a Formula • Example Continued: • % C = 36. 0 g X 100% = 81. 8% 44. 0 g • % H = 8. 0 g X 100% = 18% 44. 0 g Add up to 100% when expressed as 2 significant figures

The Percent Composition of a Compound • Percent Composition as a Conversion Factor –You can use percent composition to calculate the number of grams of any element in a specific mass of a compound.

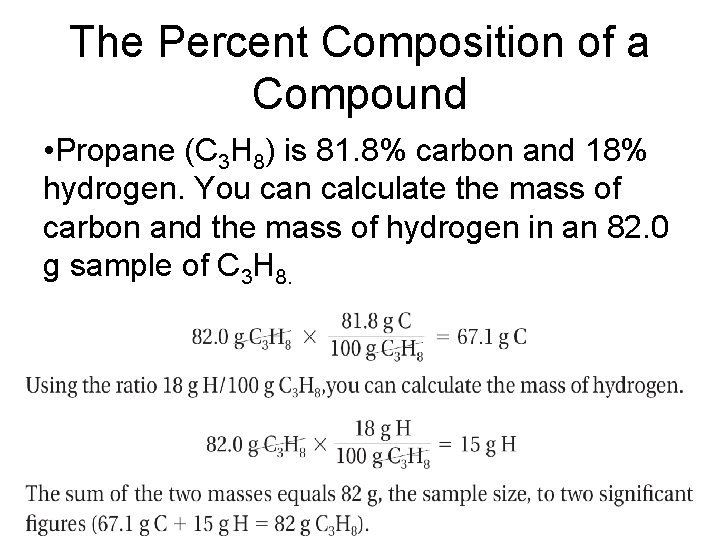
The Percent Composition of a Compound • Propane (C 3 H 8) is 81. 8% carbon and 18% hydrogen. You can calculate the mass of carbon and the mass of hydrogen in an 82. 0 g sample of C 3 H 8.

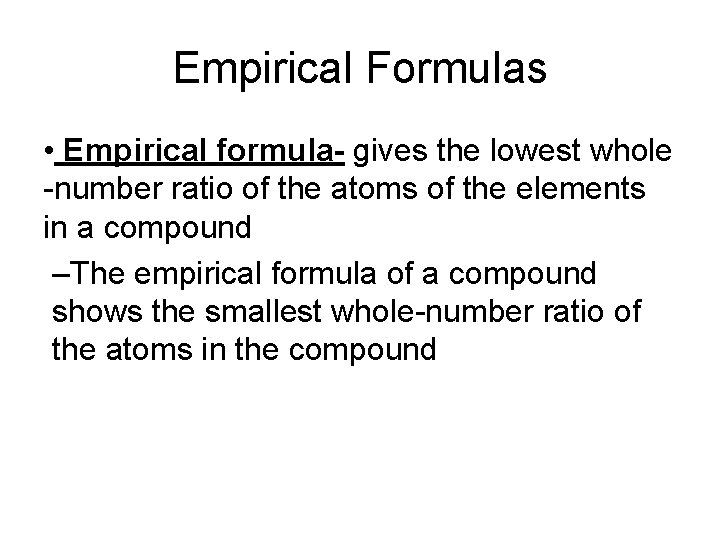
Empirical Formulas • Empirical formula- gives the lowest whole -number ratio of the atoms of the elements in a compound –The empirical formula of a compound shows the smallest whole-number ratio of the atoms in the compound

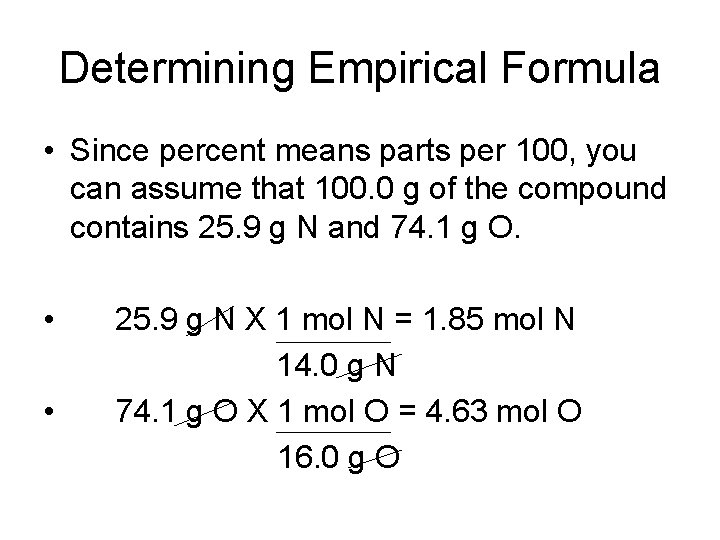
Determining Empirical Formula • A compound is analyzed and found to contain 25. 9% nitrogen and 74. 1% oxygen. What is the empirical formula of the compound? • Percent of nitrogen = 25. 9% N • Percent of oxygen = 74. 1% O

Determining Empirical Formula • Since percent means parts per 100, you can assume that 100. 0 g of the compound contains 25. 9 g N and 74. 1 g O. • • 25. 9 g N X 1 mol N = 1. 85 mol N 14. 0 g N 74. 1 g O X 1 mol O = 4. 63 mol O 16. 0 g O

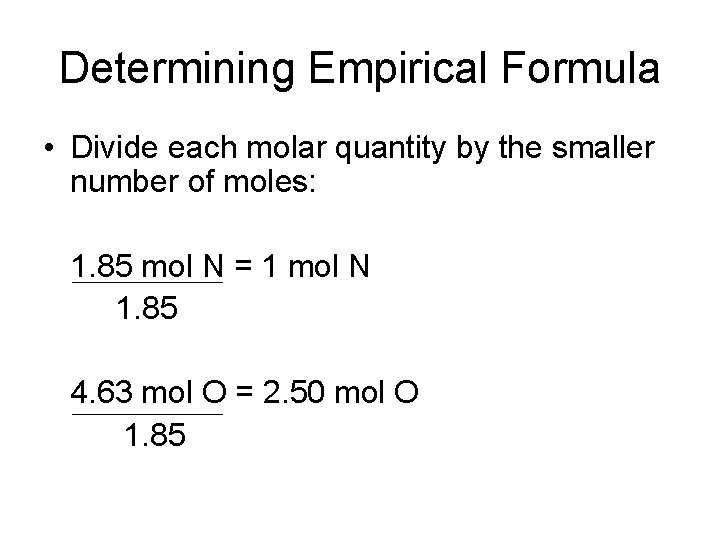
Determining Empirical Formula • Divide each molar quantity by the smaller number of moles: 1. 85 mol N = 1 mol N 1. 85 4. 63 mol O = 2. 50 mol O 1. 85

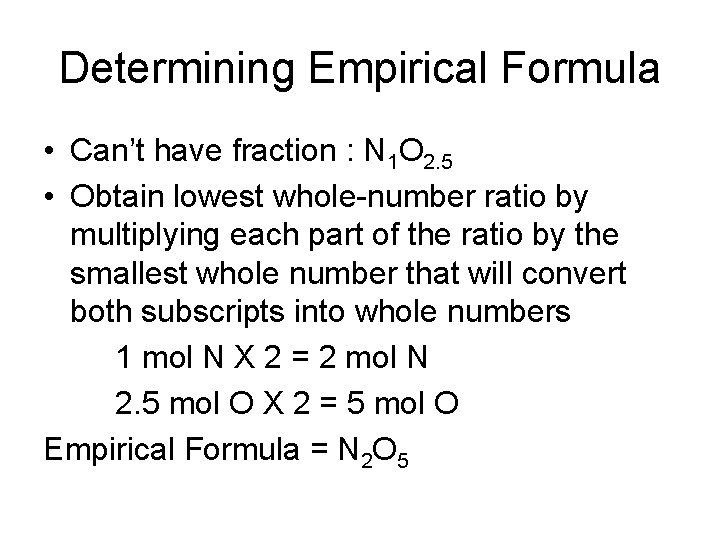
Determining Empirical Formula • Can’t have fraction : N 1 O 2. 5 • Obtain lowest whole-number ratio by multiplying each part of the ratio by the smallest whole number that will convert both subscripts into whole numbers 1 mol N X 2 = 2 mol N 2. 5 mol O X 2 = 5 mol O Empirical Formula = N 2 O 5

Empirical Formulas • Ethyne (C 2 H 2) is a gas used in welder’s torches. Styrene (C 8 H 8) is used in making polystyrene • These two compounds of carbon have the same empirical formula (CH) but different molecular formulas

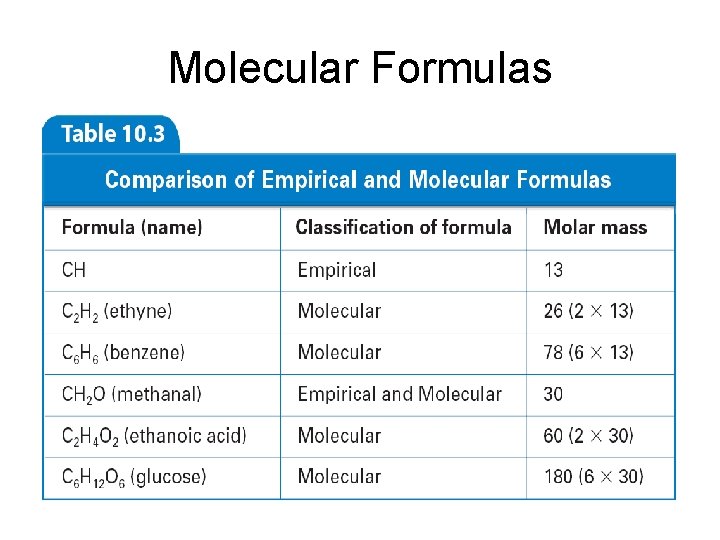
Molecular Formulas • Molecular formula of a compound is either the same as its experimentally determined empirical formula, or it is a simple wholenumber multiple of its empirical formula - must know compound’s molar mass to determine molecular formulas

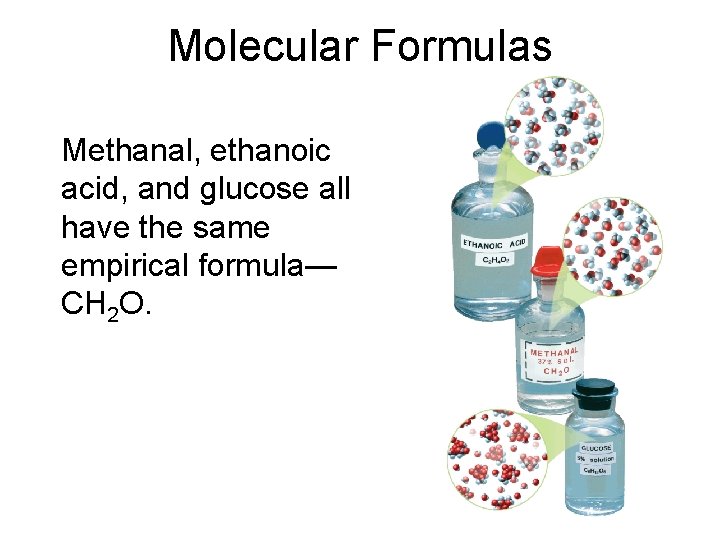
Molecular Formulas Methanal, ethanoic acid, and glucose all have the same empirical formula— CH 2 O.

Molecular Formulas

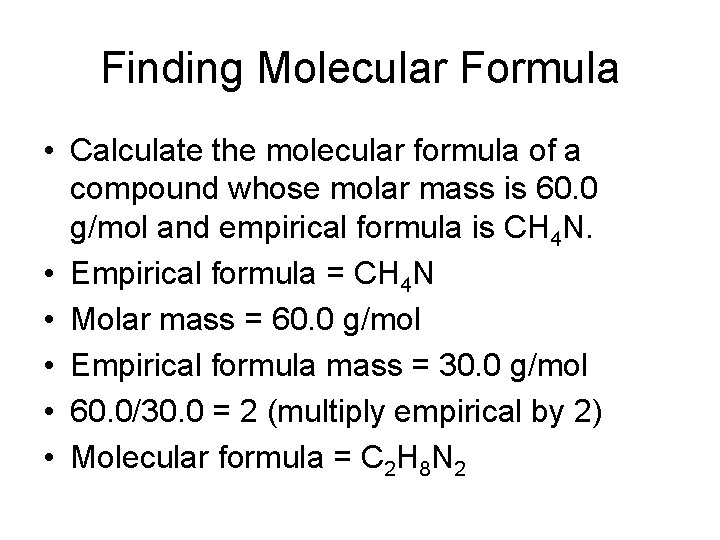
Finding Molecular Formula • Calculate the molecular formula of a compound whose molar mass is 60. 0 g/mol and empirical formula is CH 4 N. • Empirical formula = CH 4 N • Molar mass = 60. 0 g/mol • Empirical formula mass = 30. 0 g/mol • 60. 0/30. 0 = 2 (multiply empirical by 2) • Molecular formula = C 2 H 8 N 2

Chemical Reactions

Writing Chemical Equations • Word Equations –To write a word equation: • write the names of the reactants to the left of the arrow separated by plus signs • write the names of the products to the right of the arrow, also separated by plus signs • Reactant + Reactant Product + Product

Writing Chemical Equations • Methane + Oxygen Carbon dioxide + Water

Writing Chemical Equations • iron + oxygen iron(III) oxide

Writing Chemical Equations • Hydrogen Peroxide Water and Oxygen

Writing Chemical Equations • Chemical Equations – Chemical equation- is a representation of a chemical reaction • the formulas of the reactants (on the left) are connected by an arrow with the formulas of the products (on the right)

Writing Chemical Equations • Skeleton equation- a chemical equation that does not indicate the relative amounts of the reactants and products • Here is the equation for rusting: Fe + O 2 Fe 2 O 3

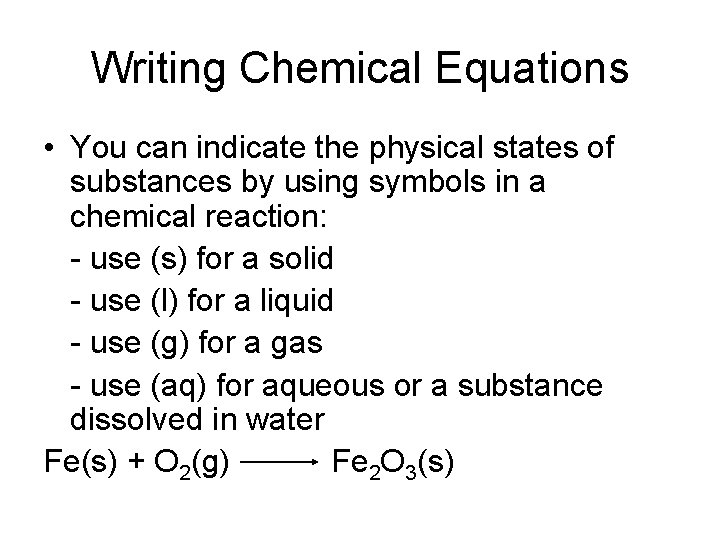
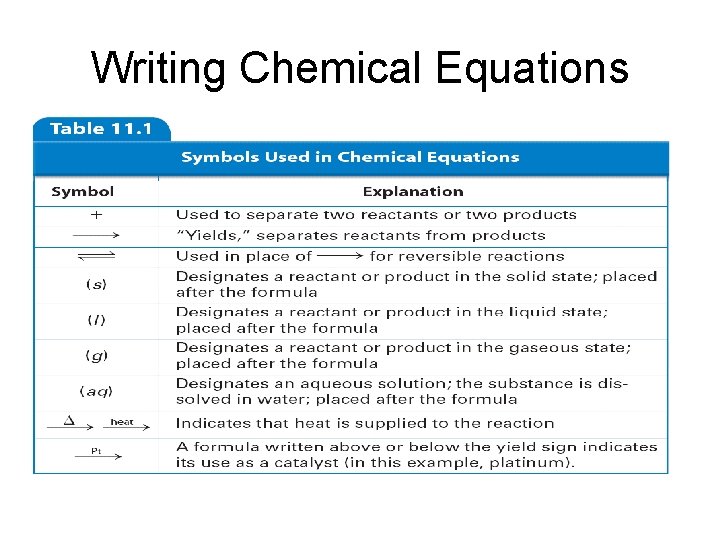
Writing Chemical Equations • You can indicate the physical states of substances by using symbols in a chemical reaction: - use (s) for a solid - use (l) for a liquid - use (g) for a gas - use (aq) for aqueous or a substance dissolved in water Fe(s) + O 2(g) Fe 2 O 3(s)

Writing Chemical Equations

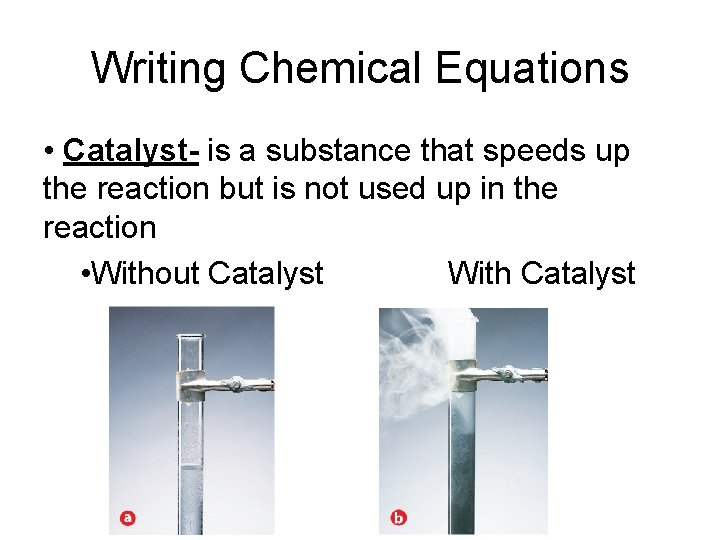
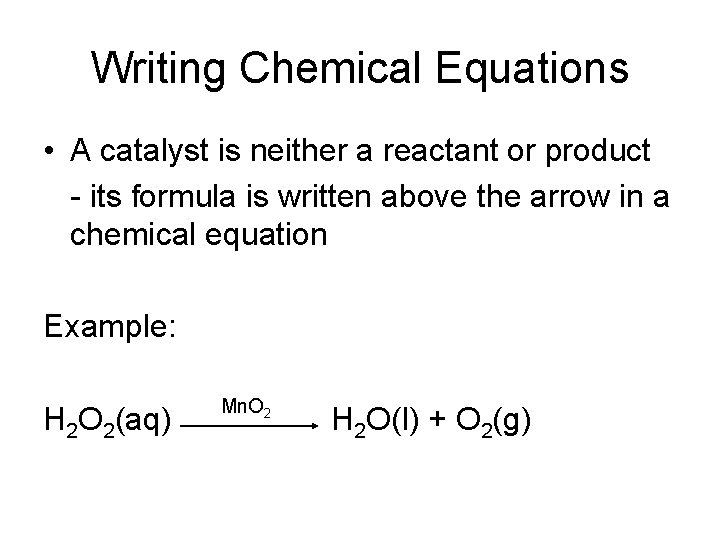
Writing Chemical Equations • Catalyst- is a substance that speeds up the reaction but is not used up in the reaction • Without Catalyst With Catalyst

Writing Chemical Equations • A catalyst is neither a reactant or product - its formula is written above the arrow in a chemical equation Example: H 2 O 2(aq) Mn. O 2 H 2 O(l) + O 2(g)

Writing a Skeleton Equation • Hydrochloric acid and solid sodium hydrogen carbonate are reacted together. The products formed are aqueous sodium chloride, water, and carbon dioxide gas. Write a skeleton equation for this reaction. • Solid sodium hydrogen carbonate: Na. HCO 3(s) • Hydrochloric acid: HCl(aq) • Water: H 2 O(l) • Carbon dioxide gas: CO 2(g)

Writing a Skeleton Equation • Example Continued: Na. HCO 3(s) + HCl(aq) Na. Cl(aq) + H 2 O(l) + CO 2(g)

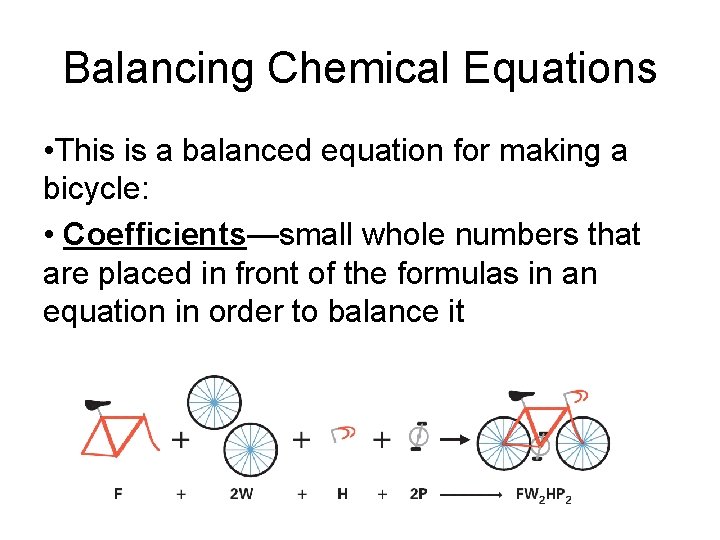
Balancing Chemical Equations • This is a balanced equation for making a bicycle: • Coefficients—small whole numbers that are placed in front of the formulas in an equation in order to balance it

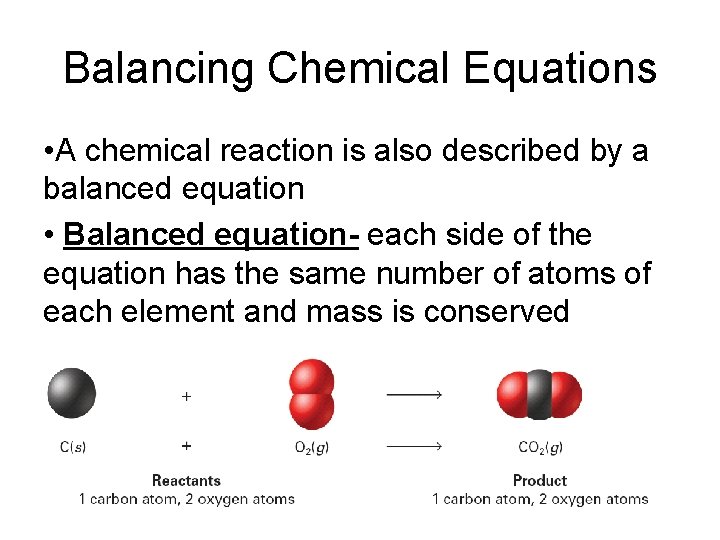
Balancing Chemical Equations • A chemical reaction is also described by a balanced equation • Balanced equation- each side of the equation has the same number of atoms of each element and mass is conserved

Balancing Chemical Equations • To write a balanced chemical equation: - write the skeleton equation - use coefficients to balance the equation so that it obeys the law of conservation of mass

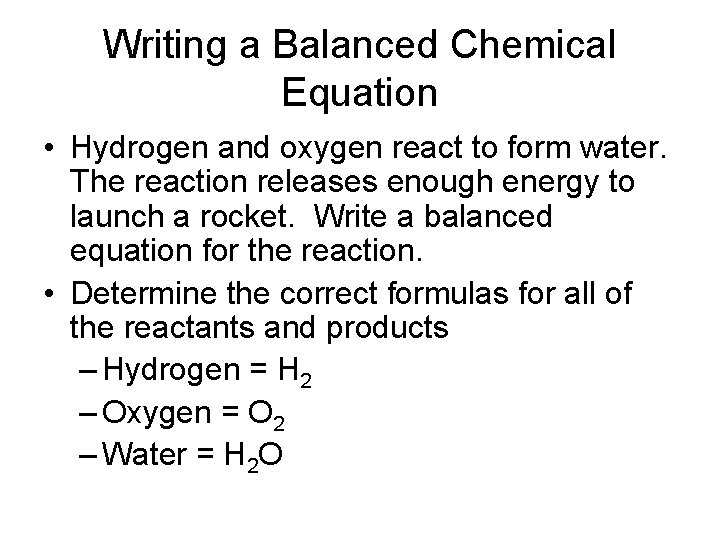
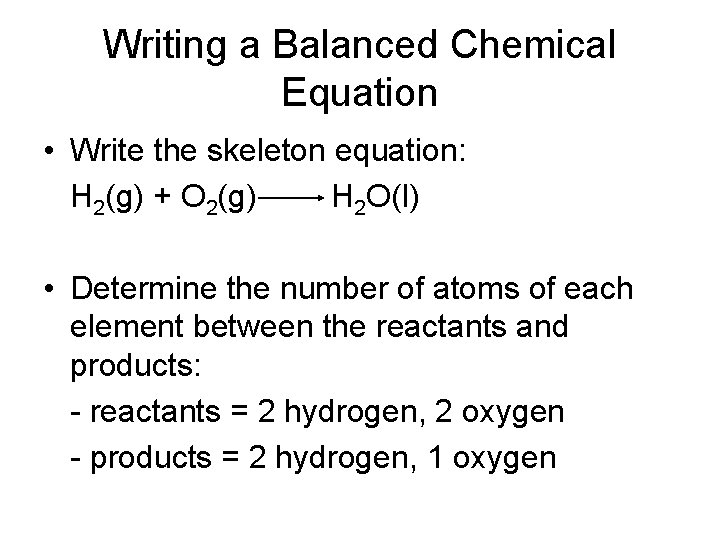
Writing a Balanced Chemical Equation • Hydrogen and oxygen react to form water. The reaction releases enough energy to launch a rocket. Write a balanced equation for the reaction. • Determine the correct formulas for all of the reactants and products – Hydrogen = H 2 – Oxygen = O 2 – Water = H 2 O

Writing a Balanced Chemical Equation • Write the skeleton equation: H 2(g) + O 2(g) H 2 O(l) • Determine the number of atoms of each element between the reactants and products: - reactants = 2 hydrogen, 2 oxygen - products = 2 hydrogen, 1 oxygen

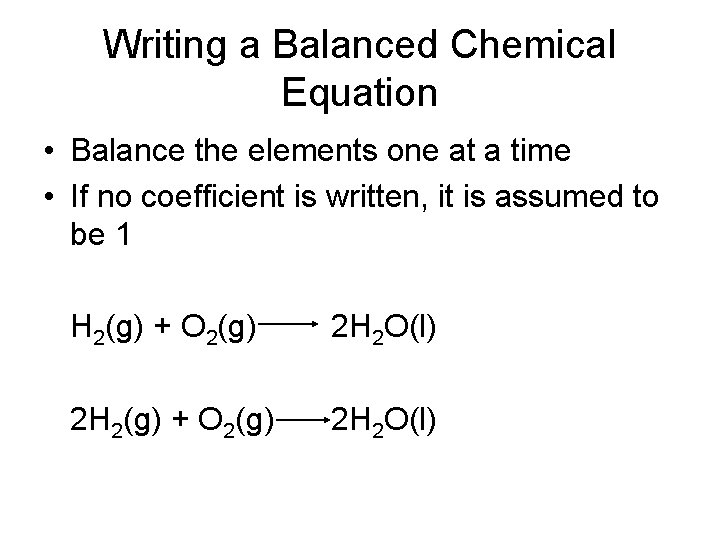
Writing a Balanced Chemical Equation • Balance the elements one at a time • If no coefficient is written, it is assumed to be 1 H 2(g) + O 2(g) 2 H 2 O(l) 2 H 2(g) + O 2(g) 2 H 2 O(l)

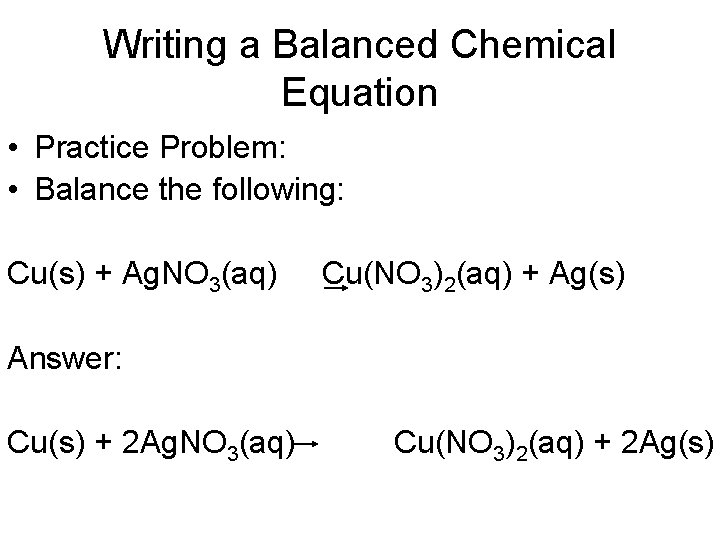
Writing a Balanced Chemical Equation • Practice Problem: • Balance the following: Cu(s) + Ag. NO 3(aq) Cu(NO 3)2(aq) + Ag(s) Answer: Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s)

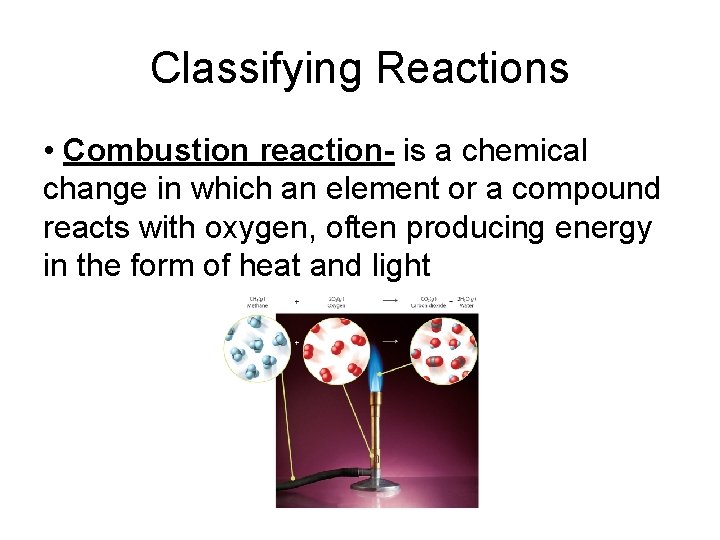
Classifying Reactions • The five general types of reaction are: -combination -decomposition -single-replacement -double-replacement -combustion

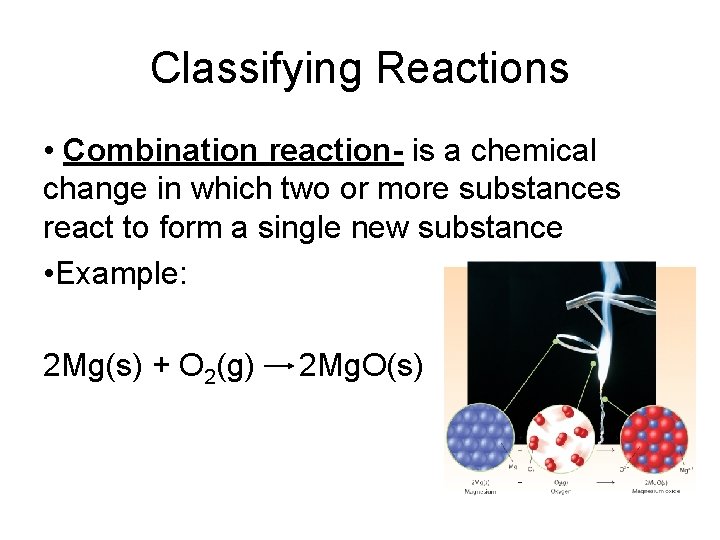
Classifying Reactions • Combination reaction- is a chemical change in which two or more substances react to form a single new substance • Example: 2 Mg(s) + O 2(g) 2 Mg. O(s)

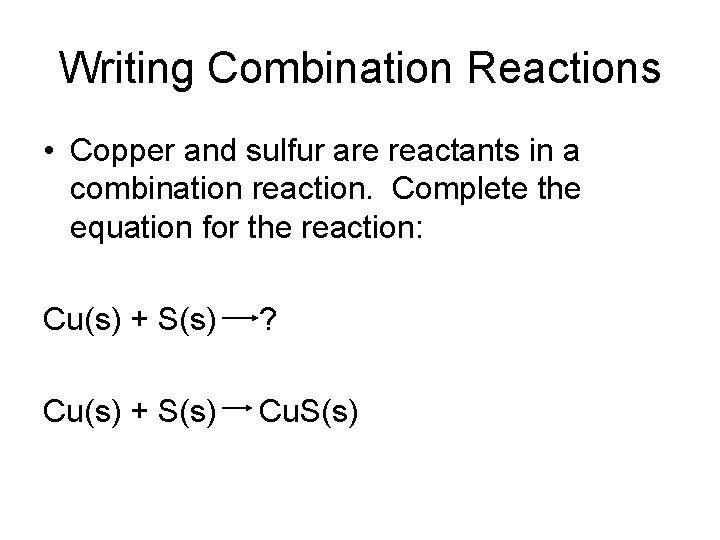
Writing Combination Reactions • Copper and sulfur are reactants in a combination reaction. Complete the equation for the reaction: Cu(s) + S(s) ? Cu(s) + S(s) Cu. S(s)

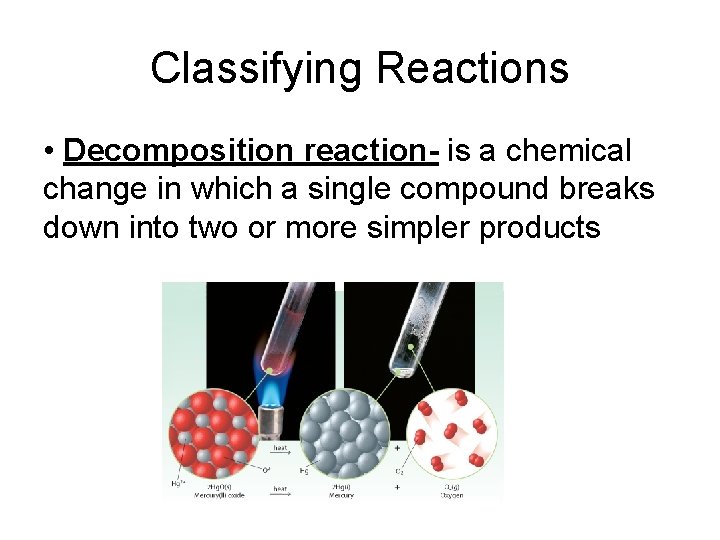
Classifying Reactions • Decomposition reaction- is a chemical change in which a single compound breaks down into two or more simpler products

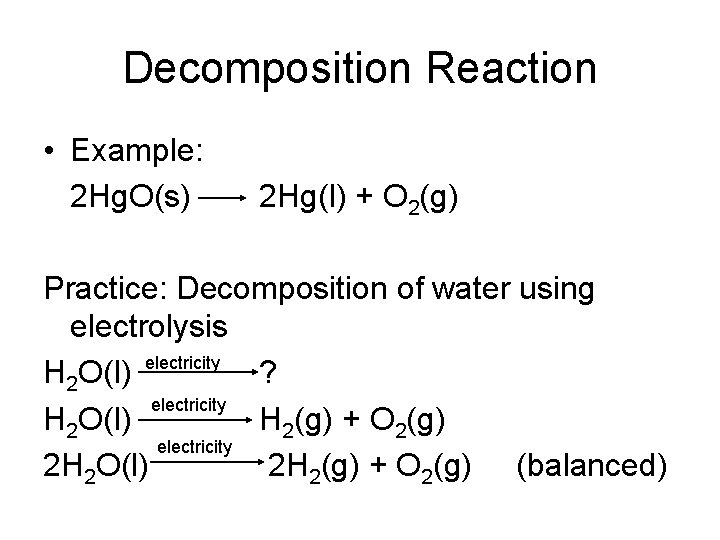
Decomposition Reaction • Example: 2 Hg. O(s) 2 Hg(l) + O 2(g) Practice: Decomposition of water using electrolysis H 2 O(l) electricity ? electricity H 2 O(l) H 2(g) + O 2(g) electricity 2 H 2 O(l) 2 H 2(g) + O 2(g) (balanced)

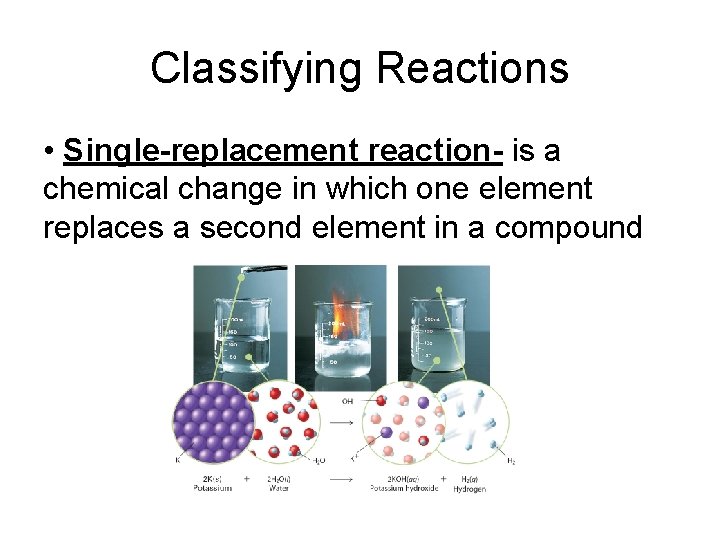
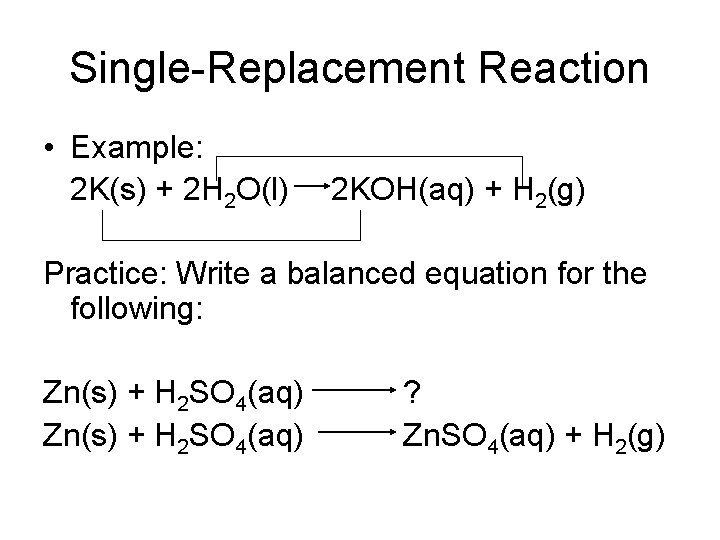
Classifying Reactions • Single-replacement reaction- is a chemical change in which one element replaces a second element in a compound

Single-Replacement Reaction • Example: 2 K(s) + 2 H 2 O(l) 2 KOH(aq) + H 2(g) Practice: Write a balanced equation for the following: Zn(s) + H 2 SO 4(aq) ? Zn. SO 4(aq) + H 2(g)

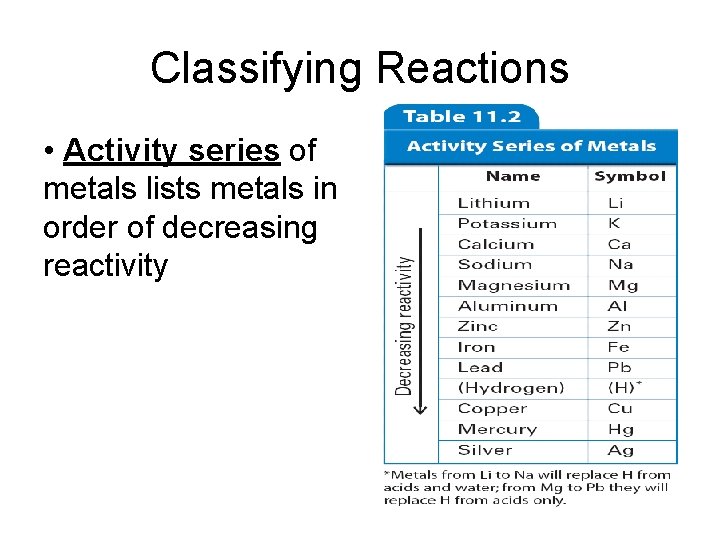
Classifying Reactions • Activity series of metals lists metals in order of decreasing reactivity

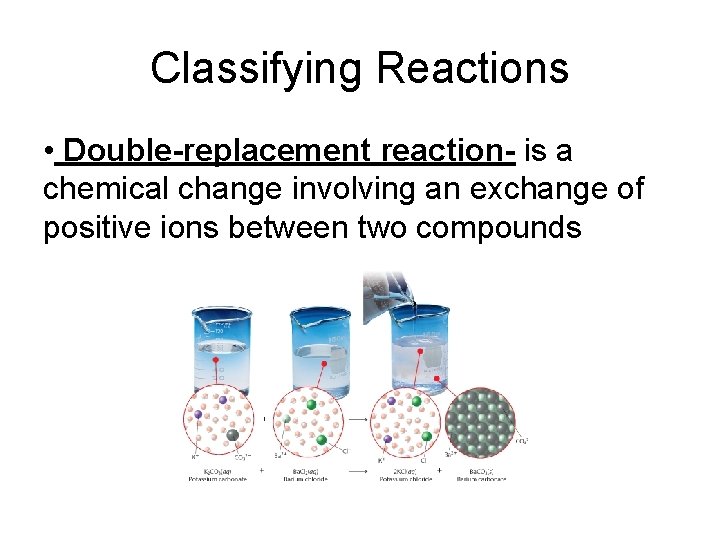
Classifying Reactions • Double-replacement reaction- is a chemical change involving an exchange of positive ions between two compounds

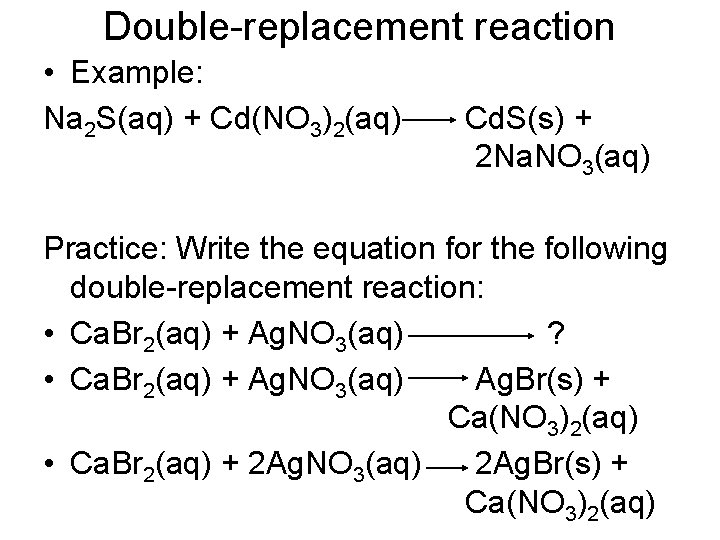
Double-replacement reaction • Example: Na 2 S(aq) + Cd(NO 3)2(aq) Cd. S(s) + 2 Na. NO 3(aq) Practice: Write the equation for the following double-replacement reaction: • Ca. Br 2(aq) + Ag. NO 3(aq) ? • Ca. Br 2(aq) + Ag. NO 3(aq) Ag. Br(s) + Ca(NO 3)2(aq) • Ca. Br 2(aq) + 2 Ag. NO 3(aq) 2 Ag. Br(s) + Ca(NO 3)2(aq)

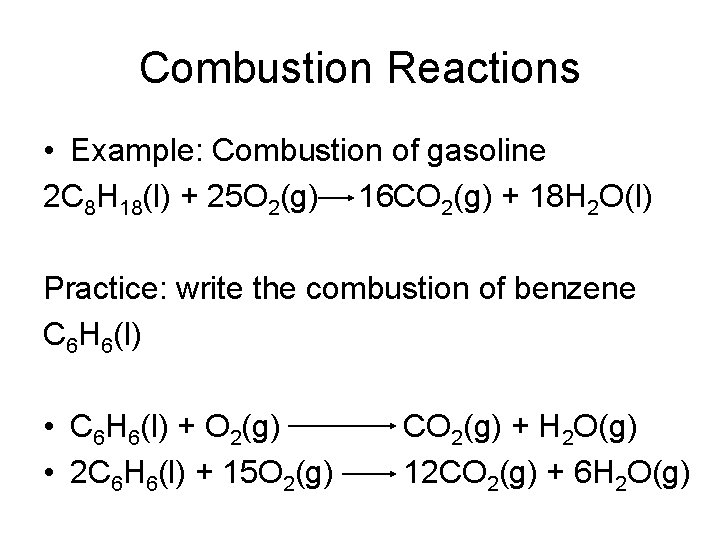
Classifying Reactions • Combustion reaction- is a chemical change in which an element or a compound reacts with oxygen, often producing energy in the form of heat and light

Combustion Reactions • Example: Combustion of gasoline 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(l) Practice: write the combustion of benzene C 6 H 6(l) • C 6 H 6(l) + O 2(g) • 2 C 6 H 6(l) + 15 O 2(g) CO 2(g) + H 2 O(g) 12 CO 2(g) + 6 H 2 O(g)

Predicting the Products of a Chemical Reaction • The number of elements and/or compounds reacting is a good indicator of possible reaction type and thus possible products

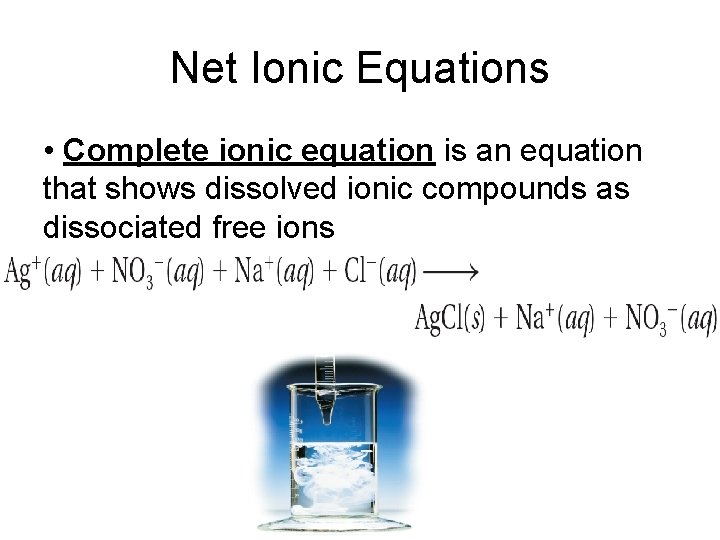
Net Ionic Equations • Complete ionic equation is an equation that shows dissolved ionic compounds as dissociated free ions

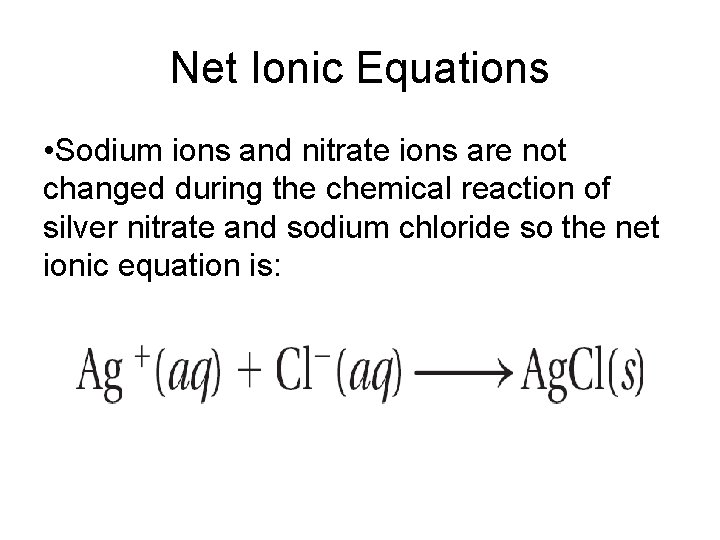
Net Ionic Equations • Spectator ion - ion that appears on both sides of an equation and is not directly involved in the reaction • Net ionic equation- is an equation for a reaction in solution that shows only those particles that are directly involved in the chemical change - balanced with respect to both mass and charge

Net Ionic Equations • Sodium ions and nitrate ions are not changed during the chemical reaction of silver nitrate and sodium chloride so the net ionic equation is:

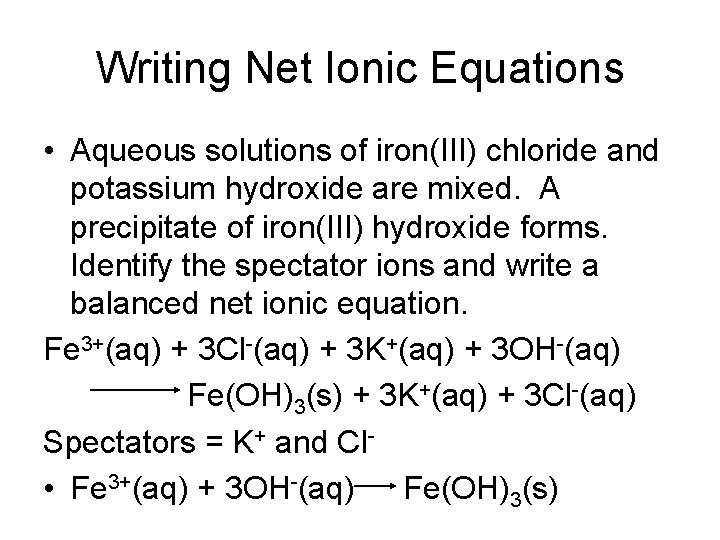
Writing Net Ionic Equations • Aqueous solutions of iron(III) chloride and potassium hydroxide are mixed. A precipitate of iron(III) hydroxide forms. Identify the spectator ions and write a balanced net ionic equation. Fe 3+(aq) + 3 Cl-(aq) + 3 K+(aq) + 3 OH-(aq) Fe(OH)3(s) + 3 K+(aq) + 3 Cl-(aq) Spectators = K+ and Cl • Fe 3+(aq) + 3 OH-(aq) Fe(OH)3(s)

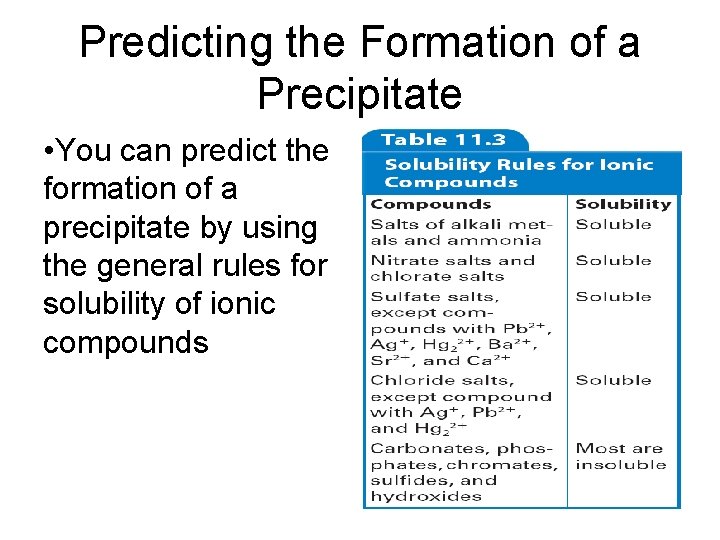
Predicting the Formation of a Precipitate • You can predict the formation of a precipitate by using the general rules for solubility of ionic compounds
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Types of reactions
Types of reactions Linear quantity
Linear quantity Example of redox reaction
Example of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Tools for measuring kinematic quantities
Tools for measuring kinematic quantities Tools for measuring kinematic quantities
Tools for measuring kinematic quantities What is measuring quantities
What is measuring quantities What is measuring quantities
What is measuring quantities Chemical reactions reactants and products
Chemical reactions reactants and products Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Percent yield of copper lab
Percent yield of copper lab What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Chapter 7 chemical quantities answer key
Chapter 7 chemical quantities answer key Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 7 chemical quantities answer key
Chapter 7 chemical quantities answer key Stoichiometry review answers
Stoichiometry review answers Chemical quantities calculator
Chemical quantities calculator Molar mass map
Molar mass map Chapter 10 chemical quantities practice problems answer key
Chapter 10 chemical quantities practice problems answer key Unit chemical quantities the mole 1 step
Unit chemical quantities the mole 1 step Chapter 9 chemical quantities
Chapter 9 chemical quantities Stoichiometry island diagram
Stoichiometry island diagram Different types of redox reactions
Different types of redox reactions Types of reaction
Types of reaction Types of reactions chemistry
Types of reactions chemistry Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Unit 4: toxins lesson 73 worksheet answers
Unit 4: toxins lesson 73 worksheet answers Four types of chemical reactions
Four types of chemical reactions Www.biology-roots.com
Www.biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Chemical reactions in everyday life
Chemical reactions in everyday life Combustion chemical reaction definition
Combustion chemical reaction definition 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions study guide
Chemical reactions study guide Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Equilibrium occurs when
Equilibrium occurs when Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide 5 chemical reactions
5 chemical reactions Chapter 11 chemical reactions answers
Chapter 11 chemical reactions answers Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Iupac nomenclature of esters
Iupac nomenclature of esters Chemical reactions summary
Chemical reactions summary Chemical reaction of bread
Chemical reaction of bread Solvent in chemical reactions
Solvent in chemical reactions Three types of chemical reactions
Three types of chemical reactions Mass relationships in chemical reactions
Mass relationships in chemical reactions Indications of chemical reactions
Indications of chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reaction rules
Chemical reaction rules Rearrangement of atoms in a chemical reaction
Rearrangement of atoms in a chemical reaction Synthesis reaction
Synthesis reaction Classification of chemical reactions worksheet
Classification of chemical reactions worksheet Combustion chemical reaction
Combustion chemical reaction Laser beam welding (lbw)
Laser beam welding (lbw) Types of reactions grade 11
Types of reactions grade 11 Describing chemical reactions
Describing chemical reactions Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Macromolecule
Macromolecule Mnvii
Mnvii Are all chemical reactions reversible
Are all chemical reactions reversible Chemical reactions in water
Chemical reactions in water Solvent in chemical reactions
Solvent in chemical reactions Chemical reactions in soil
Chemical reactions in soil