Chemical reactions and chemical equations Chemical reactions are


- Slides: 8

Chemical reactions and chemical equations

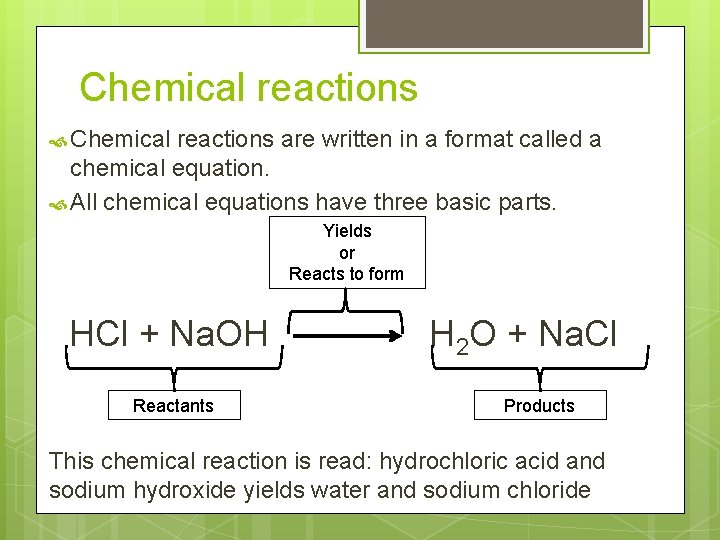
Chemical reactions are written in a format called a chemical equation. All chemical equations have three basic parts. Yields or Reacts to form HCl + Na. OH Reactants H 2 O + Na. Cl Products This chemical reaction is read: hydrochloric acid and sodium hydroxide yields water and sodium chloride

Chemical reactions Writing chemical equations helps to identify the different types of chemical reactions. There are four main types Synthesis Decomposition Single displacement Double displacement

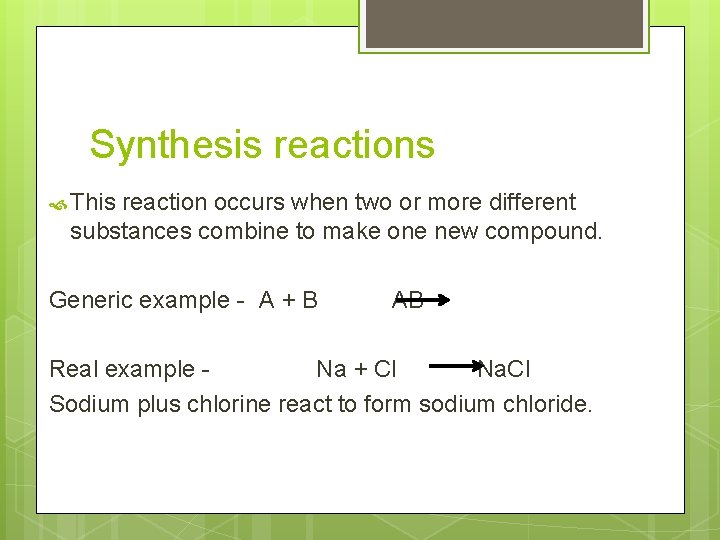
Synthesis reactions This reaction occurs when two or more different substances combine to make one new compound. Generic example - A + B AB Real example Na + Cl Na. Cl Sodium plus chlorine react to form sodium chloride.

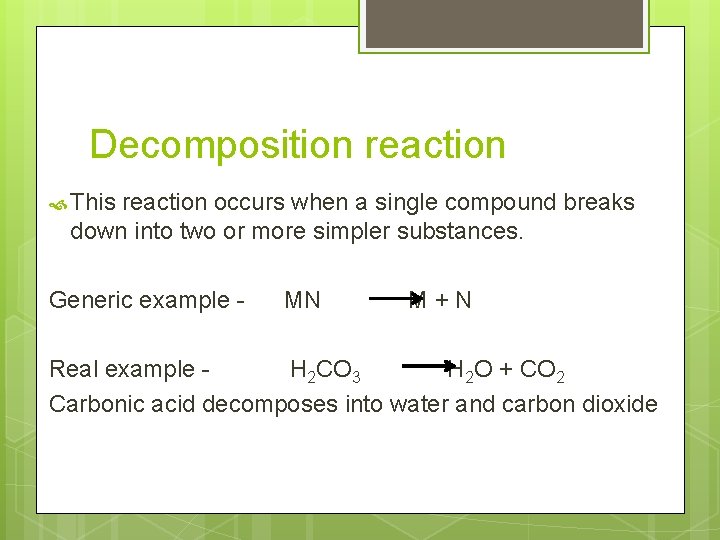
Decomposition reaction This reaction occurs when a single compound breaks down into two or more simpler substances. Generic example - MN M+N Real example H 2 CO 3 H 2 O + CO 2 Carbonic acid decomposes into water and carbon dioxide

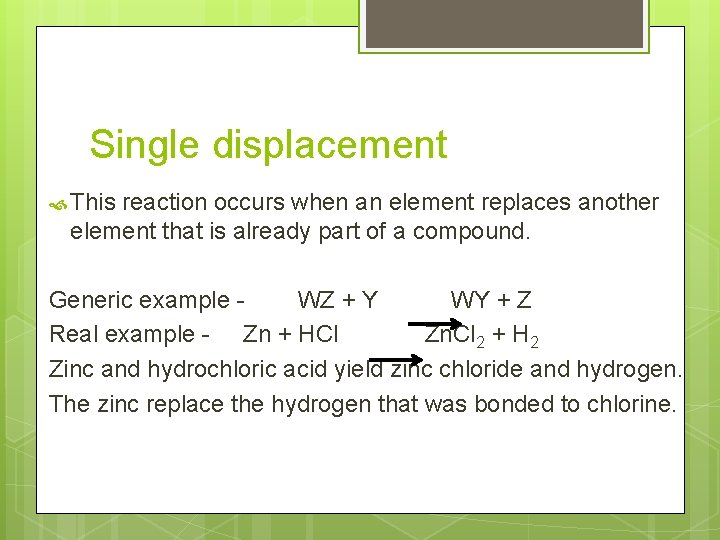
Single displacement This reaction occurs when an element replaces another element that is already part of a compound. Generic example WZ + Y WY + Z Real example - Zn + HCl Zn. Cl 2 + H 2 Zinc and hydrochloric acid yield zinc chloride and hydrogen. The zinc replace the hydrogen that was bonded to chlorine.

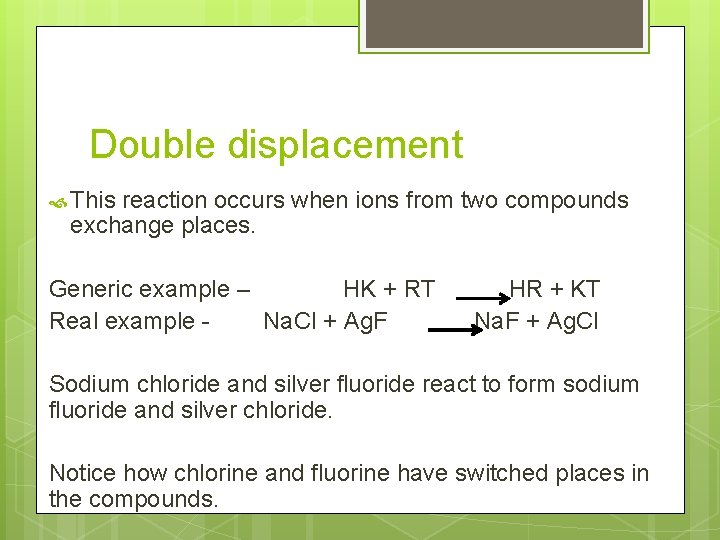
Double displacement This reaction occurs when ions from two compounds exchange places. Generic example – HK + RT Real example Na. Cl + Ag. F HR + KT Na. F + Ag. Cl Sodium chloride and silver fluoride react to form sodium fluoride and silver chloride. Notice how chlorine and fluorine have switched places in the compounds.

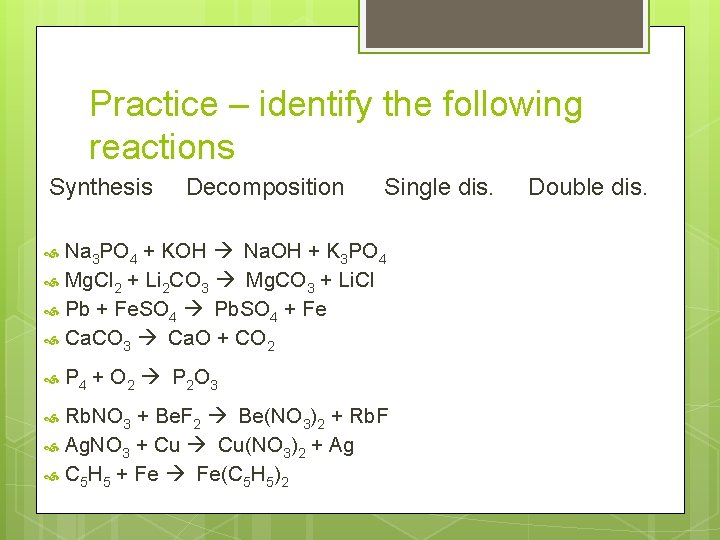
Practice – identify the following reactions Synthesis Decomposition Single dis. Na 3 PO 4 + KOH à Na. OH + K 3 PO 4 Mg. Cl 2 + Li 2 CO 3 à Mg. CO 3 + Li. Cl Pb + Fe. SO 4 à Pb. SO 4 + Fe Ca. CO 3 à Ca. O + CO 2 P 4 + O 2 à P 2 O 3 Rb. NO 3 + Be. F 2 à Be(NO 3)2 + Rb. F Ag. NO 3 + Cu à Cu(NO 3)2 + Ag C 5 H 5 + Fe à Fe(C 5 H 5)2 Double dis.
 Antigentest åre
Antigentest åre Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Are kc and kp equal
Are kc and kp equal Types of reactions
Types of reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 I intro
I intro