2 4 Chemical Reactions and Enzymes Chemical Reactions



- Slides: 13

2. 4 Chemical Reactions and Enzymes

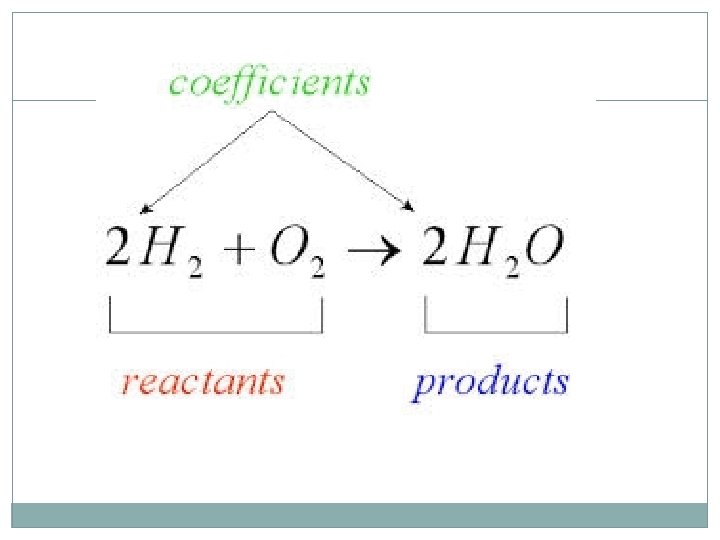
Chemical Reactions A chemical reaction is a process that changes, or transforms, one set of chemicals into another by changing the chemical bonds that join atoms in compounds. @Mass and energy are conserved during chemical transformations. @ The elements or compounds that enter into a chemical reaction are known as reactants. The elements or compounds produced by a chemical reaction are known as products.


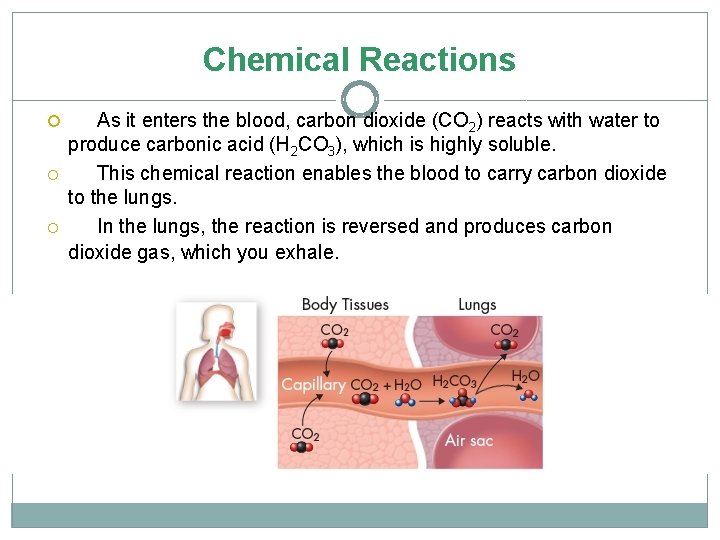
Chemical Reactions As it enters the blood, carbon dioxide (CO 2) reacts with water to produce carbonic acid (H 2 CO 3), which is highly soluble. This chemical reaction enables the blood to carry carbon dioxide to the lungs. In the lungs, the reaction is reversed and produces carbon dioxide gas, which you exhale.

What is released or absorbed whenever chemical bonds form or are broken?

Energy Changes @Energy is released or absorbed whenever chemical bonds are formed or broken during chemical reactions. @ Chemical reactions that release energy often occur on their own, or spontaneously. Chemical reactions that absorb energy will not occur without a source of energy.

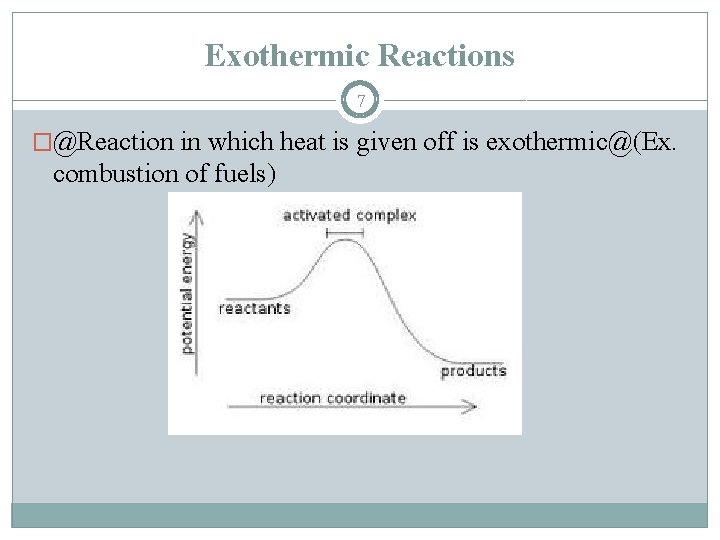
Exothermic Reactions 7 �@Reaction in which heat is given off is exothermic@(Ex. combustion of fuels)

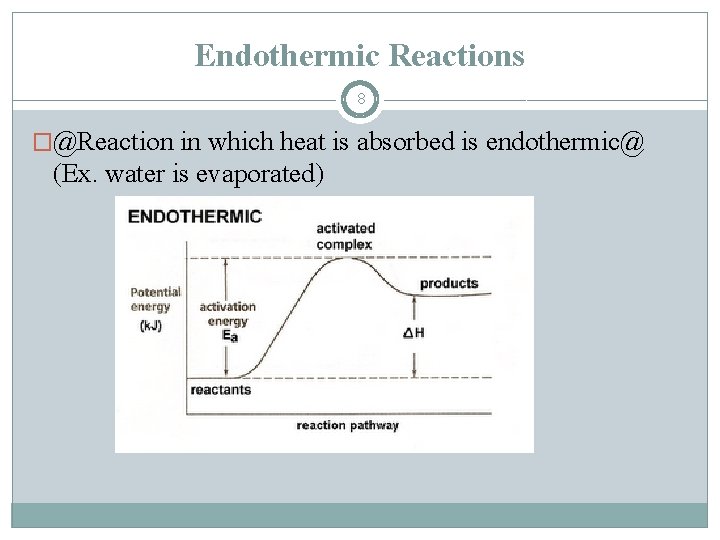
Endothermic Reactions 8 �@Reaction in which heat is absorbed is endothermic@ (Ex. water is evaporated)

Chemists call the energy needed to get a reaction started the.

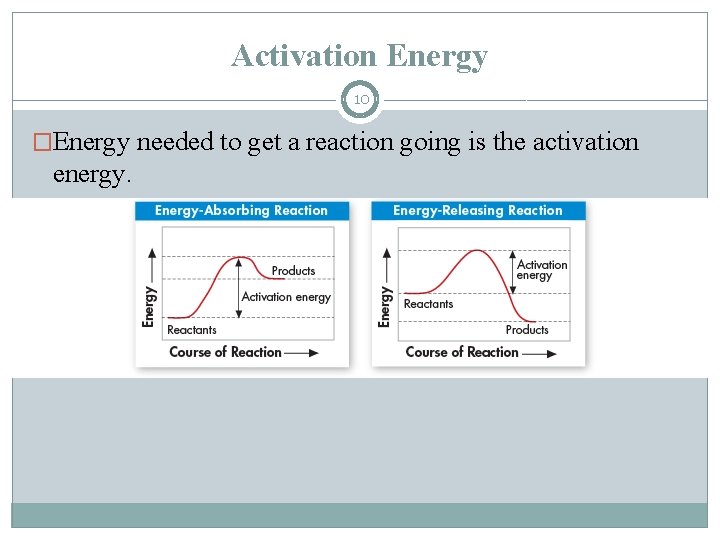
Activation Energy 10 �Energy needed to get a reaction going is the activation energy.

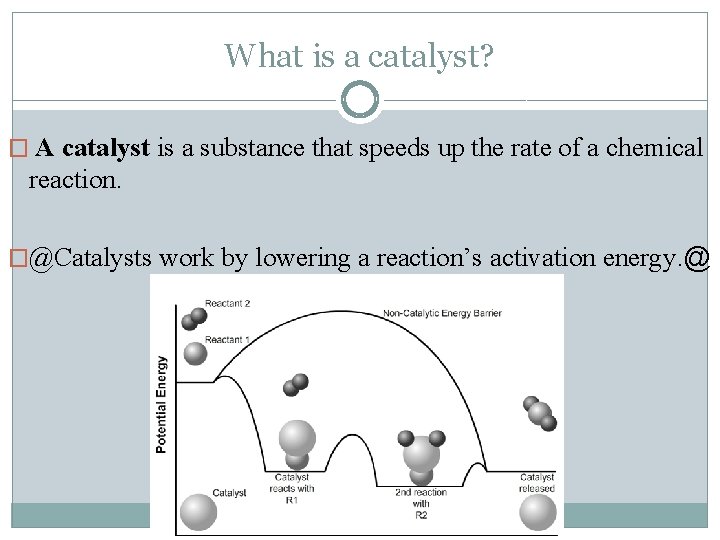
What is a catalyst? � A catalyst is a substance that speeds up the rate of a chemical reaction. �@Catalysts work by lowering a reaction’s activation energy. @

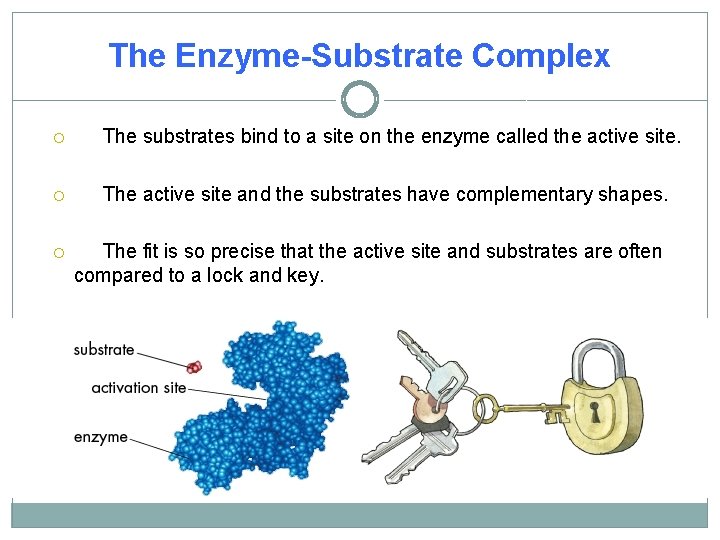
The Enzyme-Substrate Complex The substrates bind to a site on the enzyme called the active site. The active site and the substrates have complementary shapes. The fit is so precise that the active site and substrates are often compared to a lock and key.

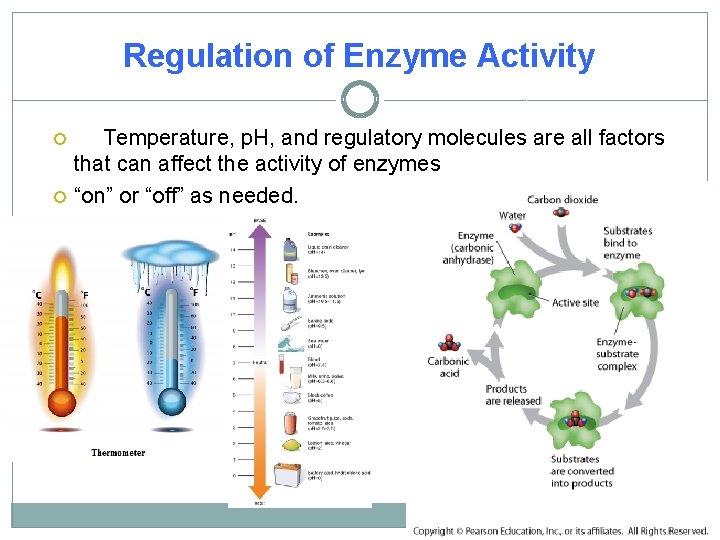
Regulation of Enzyme Activity Temperature, p. H, and regulatory molecules are all factors that can affect the activity of enzymes “on” or “off” as needed.
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Enzymes affect reactions in living cells by changing the
Enzymes affect reactions in living cells by changing the Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Chemical nature of enzymes
Chemical nature of enzymes Examples of redox reaction
Examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions reactants and products
Chemical reactions reactants and products