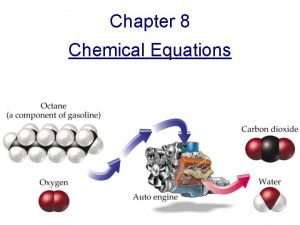
Chapter 12 Chemical Reactions 12 1 Understanding Chemical
















- Slides: 35

Chapter 12 Chemical Reactions

12. 1: Understanding Chemical Reactions O Forming New Substances O Chemical Reaction a process in which one or more substances change to make one or more new substances O The chemical and physical properties of the new substance differ from those of the original substances. O Examples of chemical changes: O Changing colors of leaves O baking muffins 2 Mg + O 2 2 Mg. O

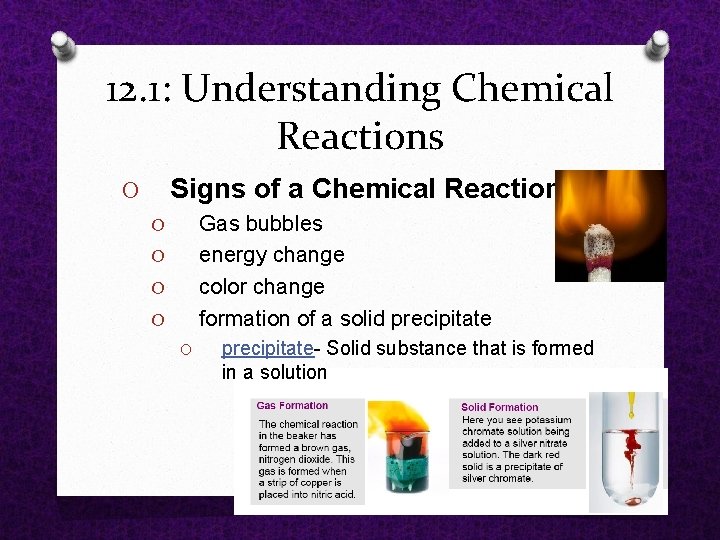
12. 1: Understanding Chemical Reactions Signs of a Chemical Reaction O Gas bubbles energy change color change formation of a solid precipitate O O O precipitate Solid substance that is formed in a solution

12. 1: Understanding Chemical Reactions O A change of properties: O The signs can help you identify a chemical reaction, but they do not guarantee a reaction took place. O Ex. Boiling water gives off gas, but this is a physical change. O The most important sign that a chemical change took place is the formation of new substances that have different properties.

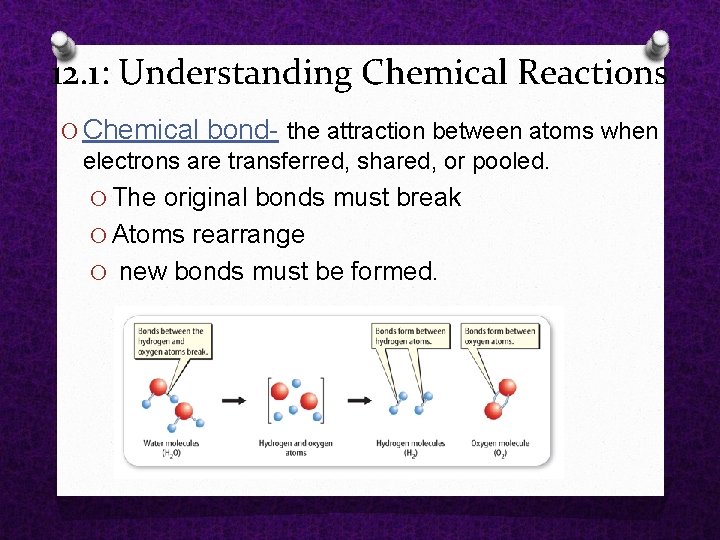
12. 1: Understanding Chemical Reactions O Chemical bond the attraction between atoms when electrons are transferred, shared, or pooled. O The original bonds must break O Atoms rearrange O new bonds must be formed.

12. 1: Understanding Chemical Reactions reaction? How do new substances form in a chemical O 1. Original bonds must break O Molecules are always moving O If molecules bump into each other with enough energy, the chemical bonds in the molecules break. O 2. Atoms rearrange O 3. New bonds must form

12. 1: Understanding Chemical Reactions O New Bonds, New Substances O What happens with Na + Cl 2? O Sodium—silver metal that reacts violently with water O Chlorine—green yellow gas that is poisonous O Na bonds and Cl Cl bonds break O Na+ Cl bonds form O Sodium chloride—harmless ionic compound that is white and dissolves in water 7

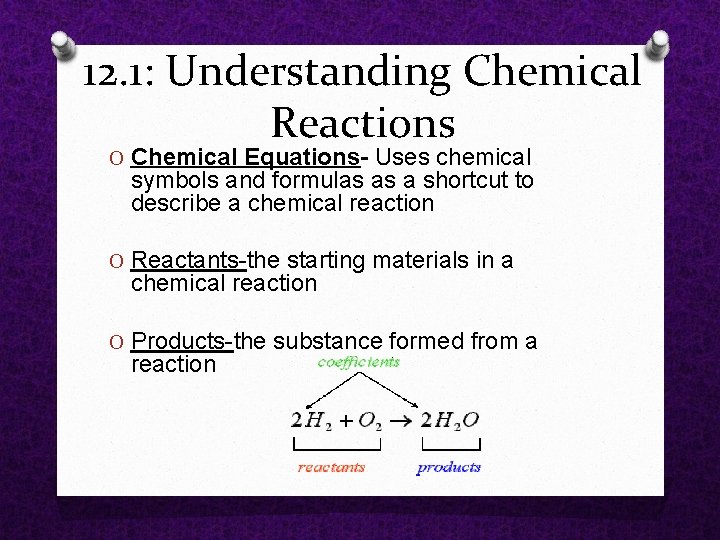
12. 1: Understanding Chemical Reactions O Chemical Formulas a shorthand way to use chemical symbols and numbers to represent a substance. It shows how many atoms of each kind ore present in a molecule.

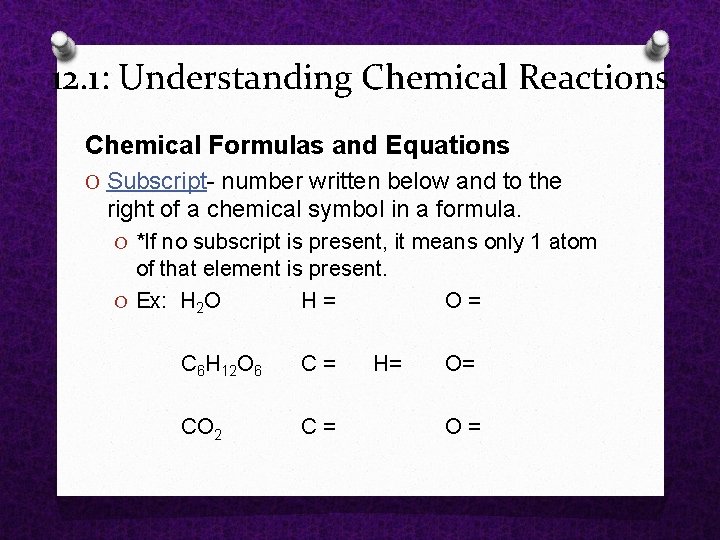
12. 1: Understanding Chemical Reactions Chemical Formulas and Equations O Subscript number written below and to the right of a chemical symbol in a formula. O *If no subscript is present, it means only 1 atom of that element is present. O Ex: H 2 O H = O = C 6 H 12 O 6 C = O= CO 2 C = H= O =

12. 1: Understanding Chemical Reactions O Chemical Equations- Uses chemical symbols and formulas as a shortcut to describe a chemical reaction O Reactants the starting materials in a chemical reaction O Products the substance formed from a reaction

12. 1: Understanding Chemical Reactions The Reason Equations Must be Balanced: O ·Atoms are never lost or gained in a chemical reaction O ·They are just rearranged O ·The # of atoms in the reactants must = # of atoms in the products O This is called balancing the reaction

12. 1: Understanding Chemical Reactions O Atoms are NEVER lost or gained in a chemical reaction O Lavoisier’s law of conservation of mass states that the total mass of the reactants before a chemical reaction is the same as the total mass of the products after the chemical reaction

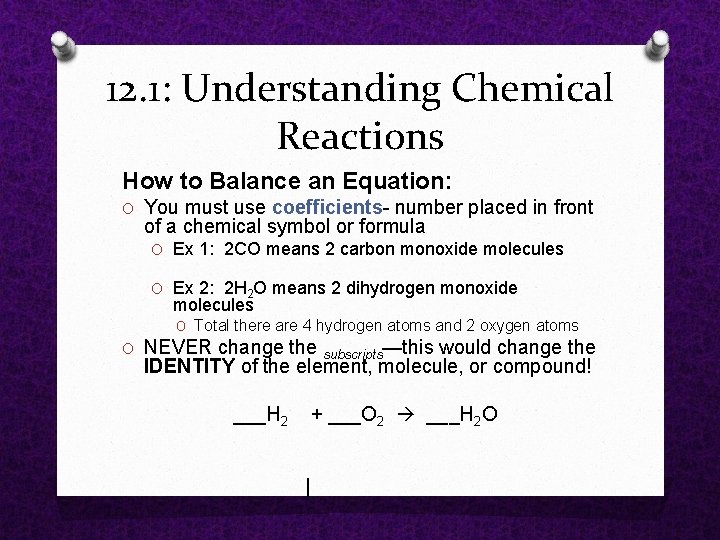
12. 1: Understanding Chemical Reactions How to Balance an Equation: O You must use coefficients number placed in front of a chemical symbol or formula O Ex 1: 2 CO means 2 carbon monoxide molecules O Ex 2: 2 H 2 O means 2 dihydrogen monoxide molecules O Total there are 4 hydrogen atoms and 2 oxygen atoms O NEVER change the subscripts—this would change the IDENTITY of the element, molecule, or compound! ___H 2 + ___O 2 ___H 2 O |

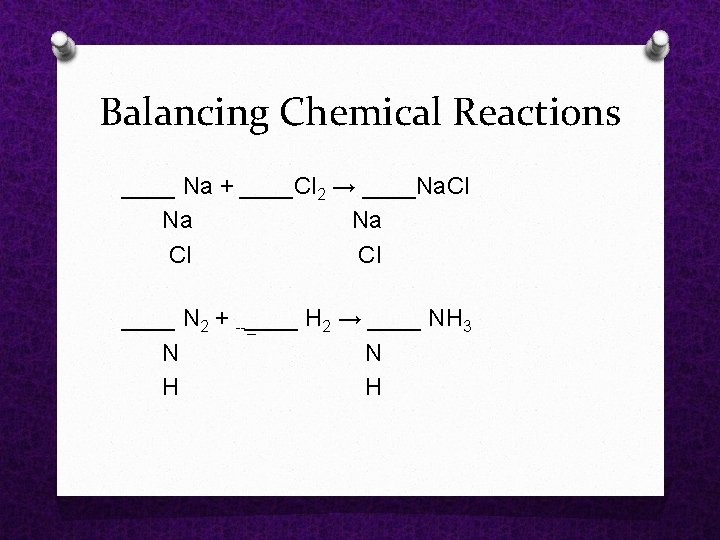
Balancing Chemical Reactions ____ Na + ____Cl 2 → ____Na. Cl Na Cl ____ N 2 + _____ H 2 → ____ NH 3 N N H H

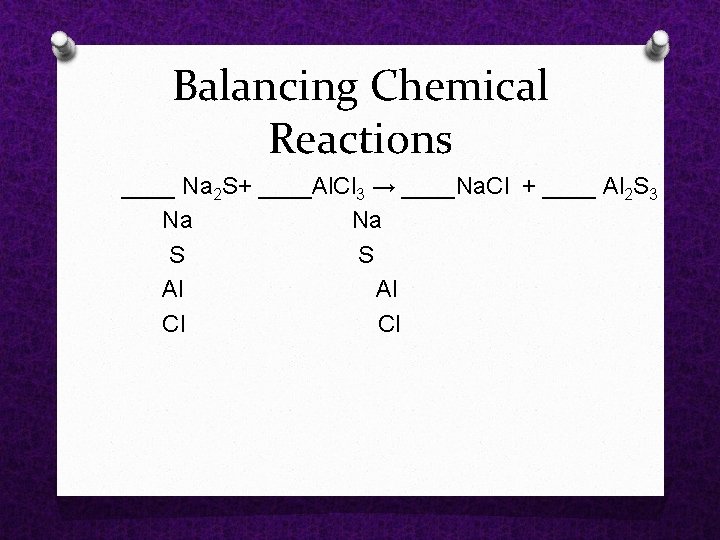
Balancing Chemical Reactions ____ Na 2 S+ ____Al. Cl 3 → ____Na. Cl + ____ Al 2 S 3 Na S S Al Cl

Balancing Chemical Reactions ____ Ca+ ____O 2 → ____Ca. O Ca O O ____ Ca. Cl 2+ ____Li 2 S → ____Ca. S + ____ Li. Cl Ca Cl Li S S

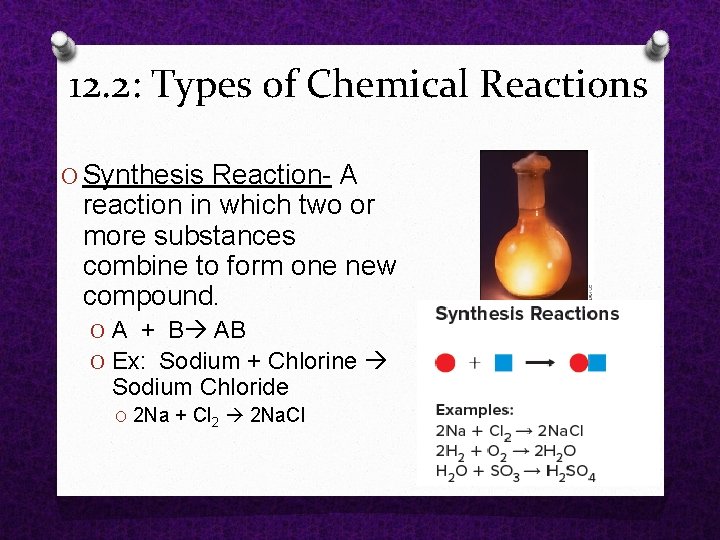
12. 2: Types of Chemical Reactions O Synthesis Reaction A reaction in which two or more substances combine to form one new compound. O A + B AB O Ex: Sodium + Chlorine Sodium Chloride O 2 Na + Cl 2 2 Na. Cl

12. 2: Types of Chemical Reactions Synthesis Reaction

12. 2: Types of Chemical Reactions O Decomposition Reaction A reaction in which a single compound breaks down to form two or more simpler substances. O AB A + B O Ex: Carbonic Acid = Water and carbon Dioxide O H 2 CO 3 H 2 O + CO 2

12. 2: Types of Chemical Reactions Decomposition Reaction

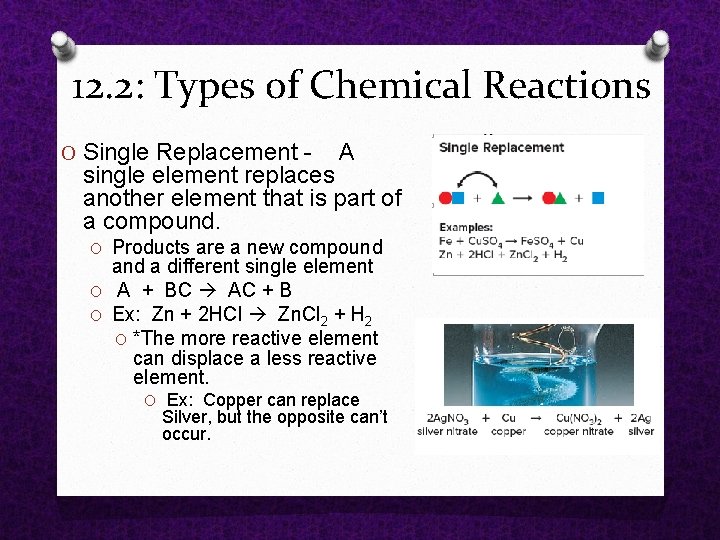
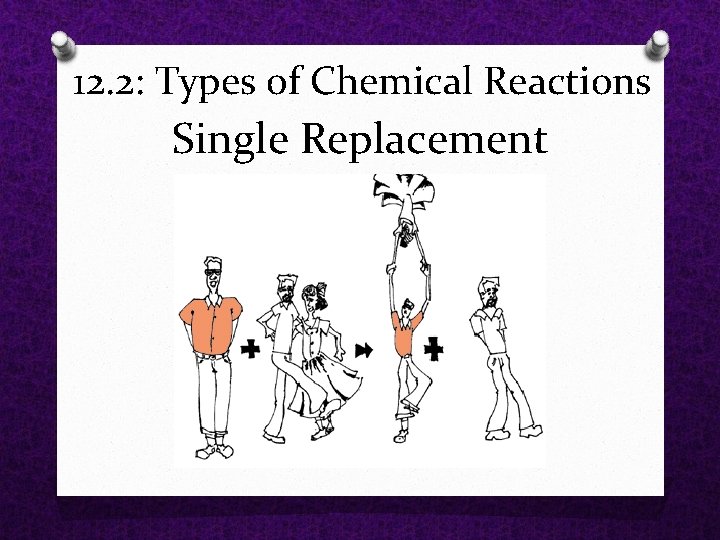
O Single Replacement A single element replaces another element that is part of a compound. O Products are a new compound a different single element O A + BC AC + B O Ex: Zn + 2 HCl Zn. Cl 2 + H 2 O *The more reactive element can displace a less reactive element. O Ex: Copper can replace Silver, but the opposite can’t occur. 12. 2: Types of Chemical Reactions

12. 2: Types of Chemical Reactions Single Replacement

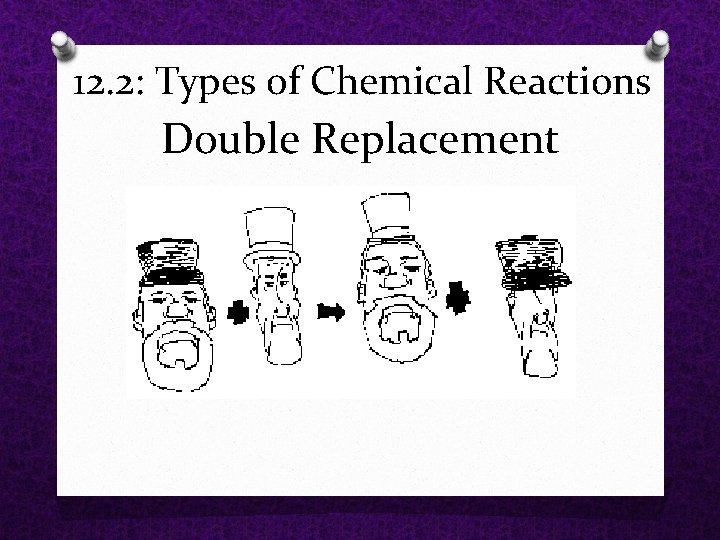
12. 2: Types of Chemical Reactions O Double Replacement Reaction A reaction in which ions from two compounds switch places. O AB + CD AD + CB

12. 2: Types of Chemical Reactions Double Replacement

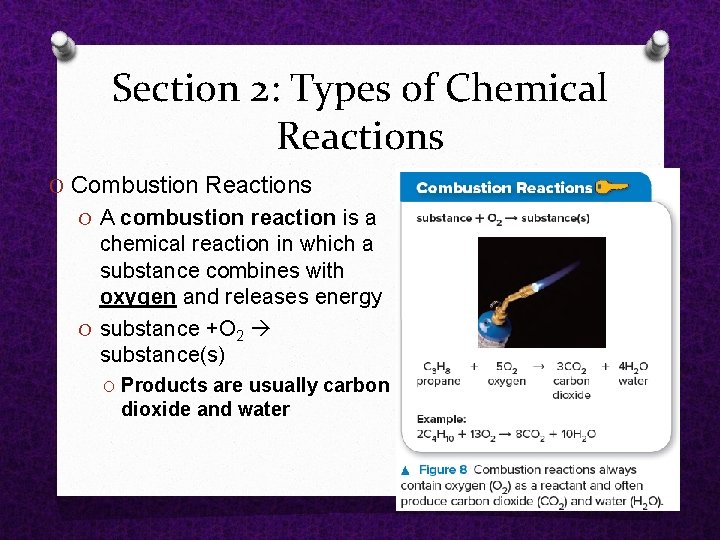
Section 2: Types of Chemical Reactions O Combustion Reactions O A combustion reaction is a chemical reaction in which a substance combines with oxygen and releases energy O substance +O 2 substance(s) O Products are usually carbon dioxide and water

12. 3: Energy Changes and Chemical Reactions O The Law of Conservation of Energy O Similar to the law of conservation of mass O The law of conservation of energy states that ENERGY cannot be created or destroyed O Instead, energy changes forms O Stored in chemical bonds

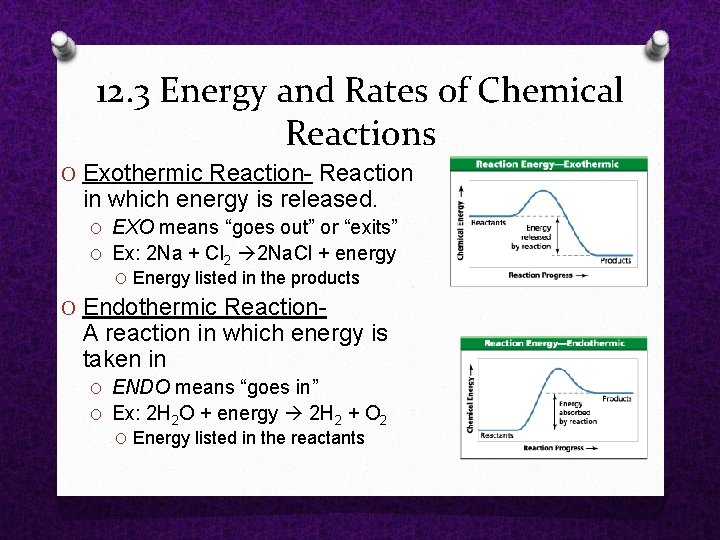
O Exothermic Reaction in which energy is released. O EXO means “goes out” or “exits” O Ex: 2 Na + Cl 2 2 Na. Cl + energy O Energy listed in the products O Endothermic Reaction A reaction in which energy is taken in O ENDO means “goes in” O Ex: 2 H 2 O + energy 2 H 2 + O 2 O Energy listed in the reactants 12. 3 Energy and Rates of Chemical Reactions

12. 3: Energy Changes and Chemical Reactions O Activation Energy is the minimum amount of energy required to start a chemical reaction O Sources of Activation Energy O Friction O Electric spark O Light O Heat 28

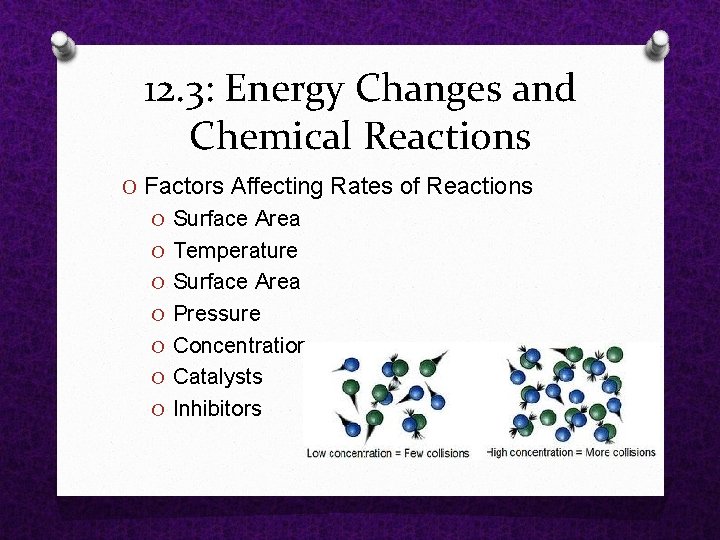
12. 3: Energy Changes and Chemical Reactions O Factors Affecting Rates of Reactions O Surface Area O Temperature O Surface Area O Pressure O Concentration O Catalysts O Inhibitors

12. 3: Energy Changes and Chemical Reactions O Surface Area O Surface area is the amount of exposed, outer surface area of a substance O Increasing the surface area of solid reactants increases the rate of a reaction O More reactants exposed O More collisions

12. 3: Energy Changes and Chemical Reactions O Temperature O A higher temperature causes a faster rate of reaction O Particles move faster O More collisions O More energy O More have activation energy to react

12. 3: Energy Changes and Chemical Reactions O Concentration and pressure O A higher concentration of reactants causes a faster rate of reaction O More particles present O Smaller distance to travel O More collisions O A higher pressure pushes particles closer together and causes a faster rate of reaction O Smaller distance to travel O More collisions

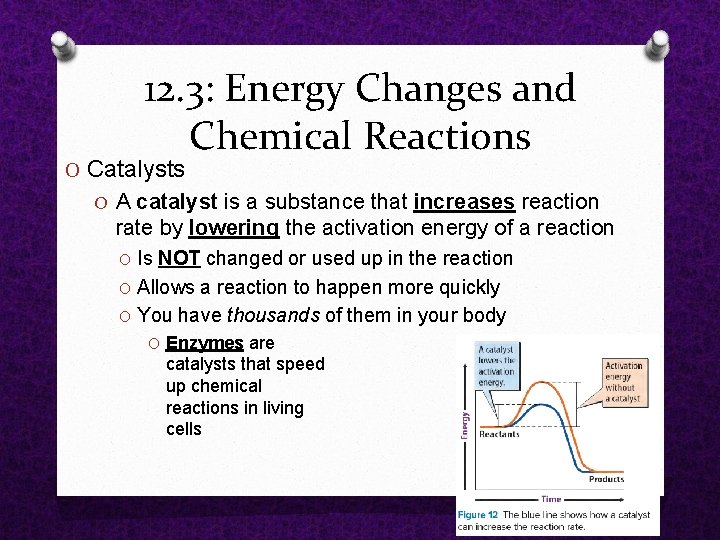
12. 3: Energy Changes and Chemical Reactions O Catalysts O A catalyst is a substance that increases reaction rate by lowering the activation energy of a reaction O Is NOT changed or used up in the reaction O Allows a reaction to happen more quickly O You have thousands of them in your body O Enzymes are catalysts that speed up chemical reactions in living cells

12. 3: Energy Changes and Chemical Reactions O Inhibitors O An inhibitor is a substance that slows down, or even stops, a chemical reaction O A greater amount of inhibitor will slow down a chemical reaction

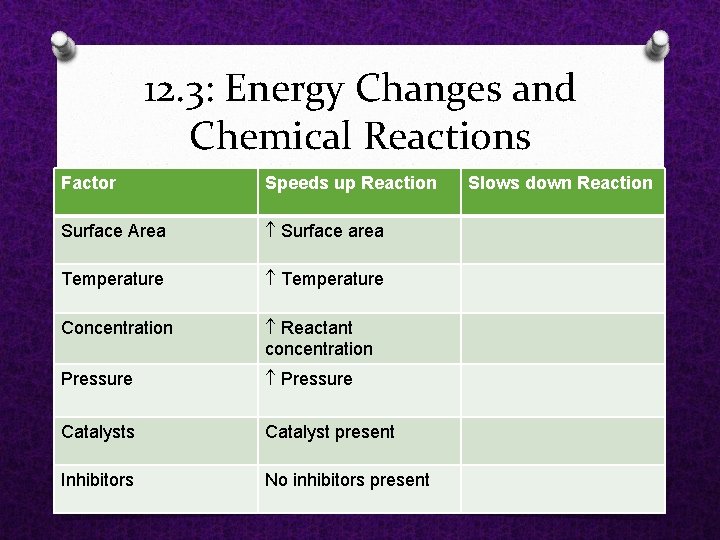
12. 3: Energy Changes and Chemical Reactions Factor Speeds up Reaction Surface Area Surface area Temperature Concentration Reactant concentration Pressure Catalysts Catalyst present Inhibitors No inhibitors present Slows down Reaction
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key Are kc and kp equal
Are kc and kp equal Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chapter assessment chemical reactions
Chapter 9 chapter assessment chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Reduction half reaction
Reduction half reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations Chapter 11 chemical reactions answers
Chapter 11 chemical reactions answers What is an active metal
What is an active metal Stoichiometry island diagram
Stoichiometry island diagram Unit 5 chemical reactions
Unit 5 chemical reactions Types of chemical reactions redox
Types of chemical reactions redox Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Chemical reaction types
Chemical reaction types Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immunochemical reaction
Principles of immunochemical reaction Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Combination reaction equation
Combination reaction equation Unit 11 chemical reactions
Unit 11 chemical reactions Toxic reactions chemical equations worksheet answers
Toxic reactions chemical equations worksheet answers