Lectures Balancing chemical reactions Chemical Quantities and reactions

Lectures: Balancing chemical reactions Chemical Quantities and reactions Course lecturer : Dr. Altijana Hromić-Jahjefendić
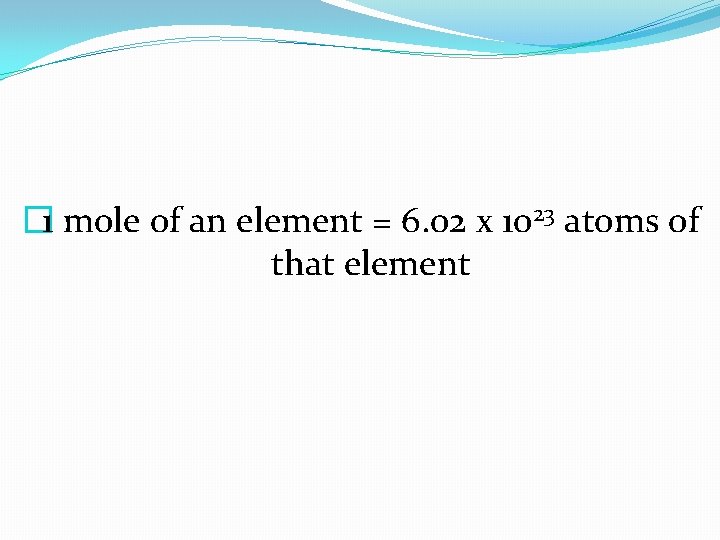
� 1 mole of an element = 6. 02 x 1023 atoms of that element
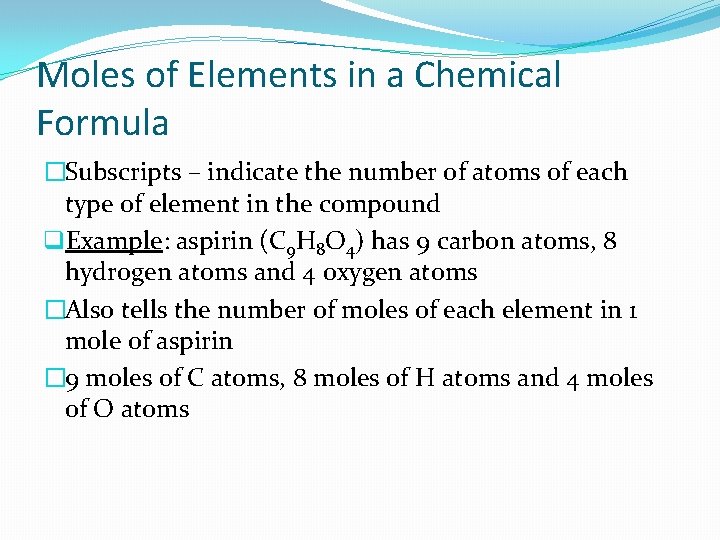
Moles of Elements in a Chemical Formula �Subscripts – indicate the number of atoms of each type of element in the compound q. Example: aspirin (C 9 H 8 O 4) has 9 carbon atoms, 8 hydrogen atoms and 4 oxygen atoms �Also tells the number of moles of each element in 1 mole of aspirin � 9 moles of C atoms, 8 moles of H atoms and 4 moles of O atoms

Moles and Elements in a given formula
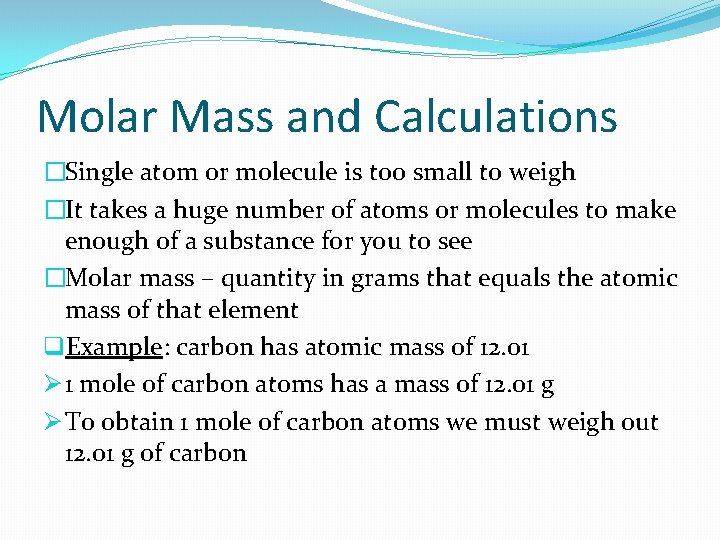
Molar Mass and Calculations �Single atom or molecule is too small to weigh �It takes a huge number of atoms or molecules to make enough of a substance for you to see �Molar mass – quantity in grams that equals the atomic mass of that element q. Example: carbon has atomic mass of 12. 01 Ø 1 mole of carbon atoms has a mass of 12. 01 g Ø To obtain 1 mole of carbon atoms we must weigh out 12. 01 g of carbon
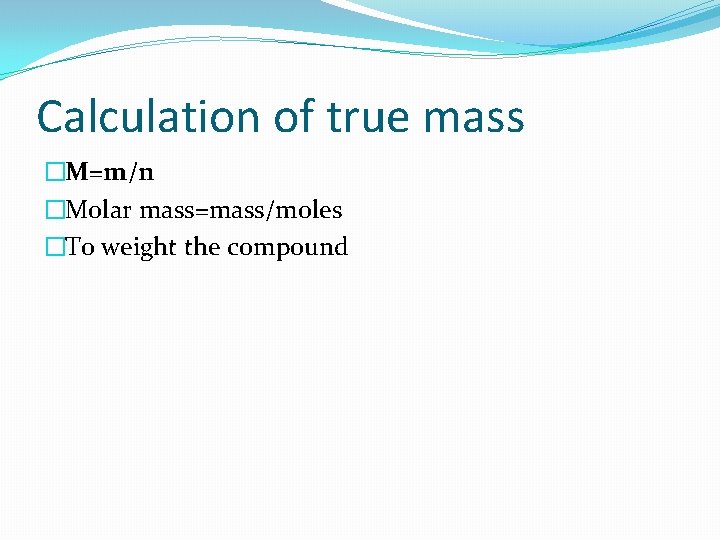
Calculation of true mass �M=m/n �Molar mass=mass/moles �To weight the compound
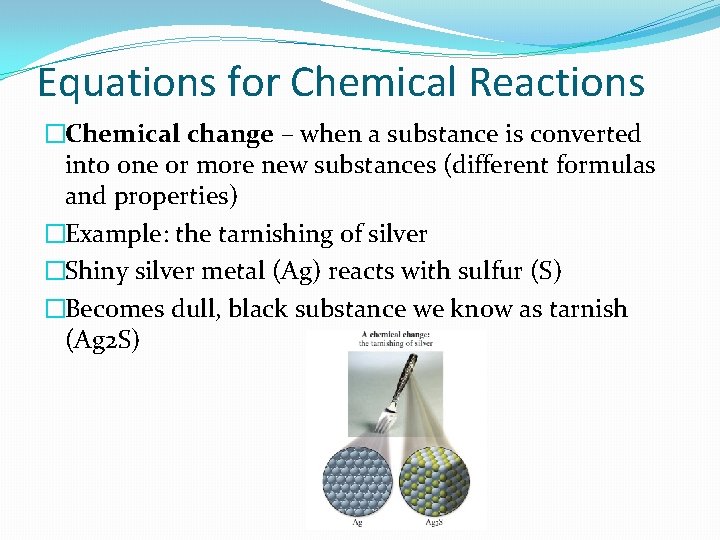
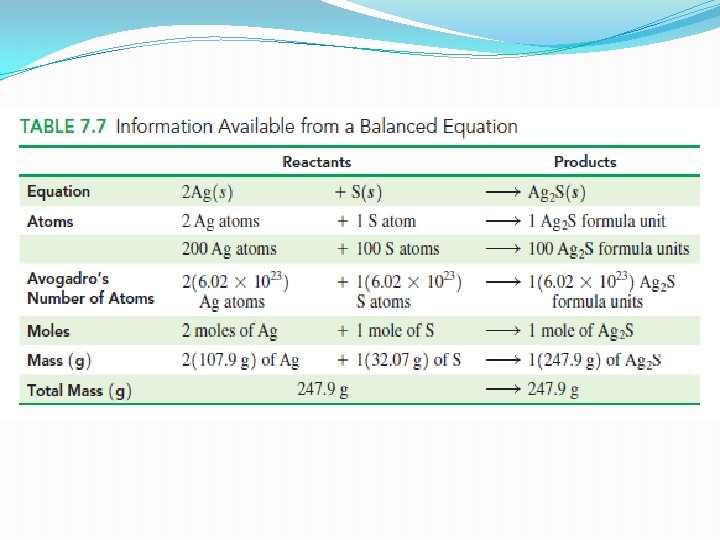
Equations for Chemical Reactions �Chemical change – when a substance is converted into one or more new substances (different formulas and properties) �Example: the tarnishing of silver �Shiny silver metal (Ag) reacts with sulfur (S) �Becomes dull, black substance we know as tarnish (Ag 2 S)
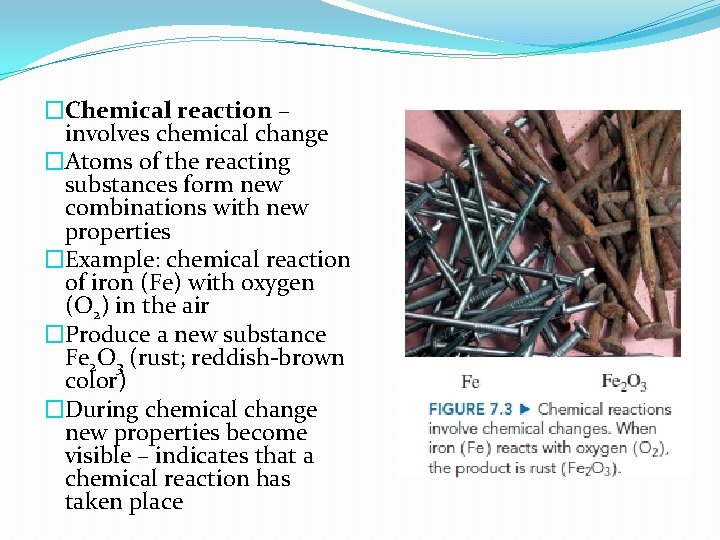
�Chemical reaction – involves chemical change �Atoms of the reacting substances form new combinations with new properties �Example: chemical reaction of iron (Fe) with oxygen (O 2) in the air �Produce a new substance Fe 2 O 3 (rust; reddish-brown color) �During chemical change new properties become visible – indicates that a chemical reaction has taken place
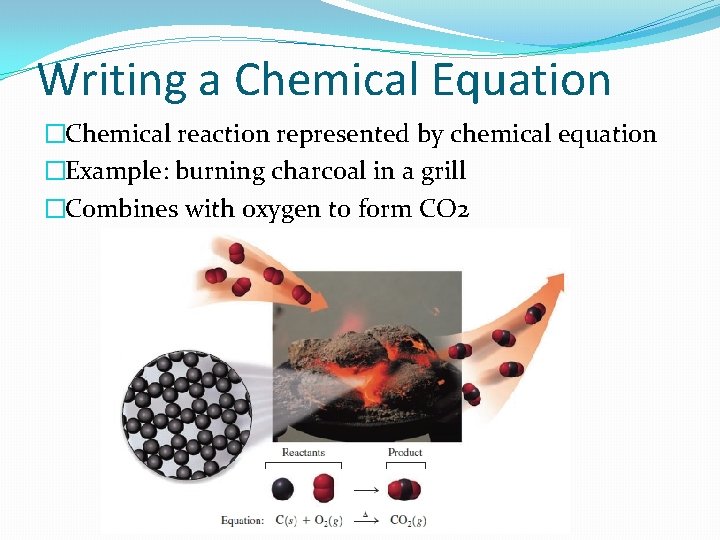
Writing a Chemical Equation �Chemical reaction represented by chemical equation �Example: burning charcoal in a grill �Combines with oxygen to form CO 2

�In chemical equation – reactants and products �Reactants are written on the left of the arrow and products on the right �If there are two or more formulas on the same side they are separated by plus (+) signs �Each formula is followed by physical state of the substance: solid (s), liquid (l) or gas (g). �If the substance is dissolved in water it is aqueous (aq) solution
Identifying a Balanced Chemical Equation �In chemical reaction bonds between atoms of the reactants are broken �New bonds are formed to give the products �All atoms are conserved – atoms cannot be gained, lost or changed into other types of atoms during a chemical reaction �Every chemical reaction must be written as a balanced equation �Same number of atoms for each element in the reactants as well as in products!!!!
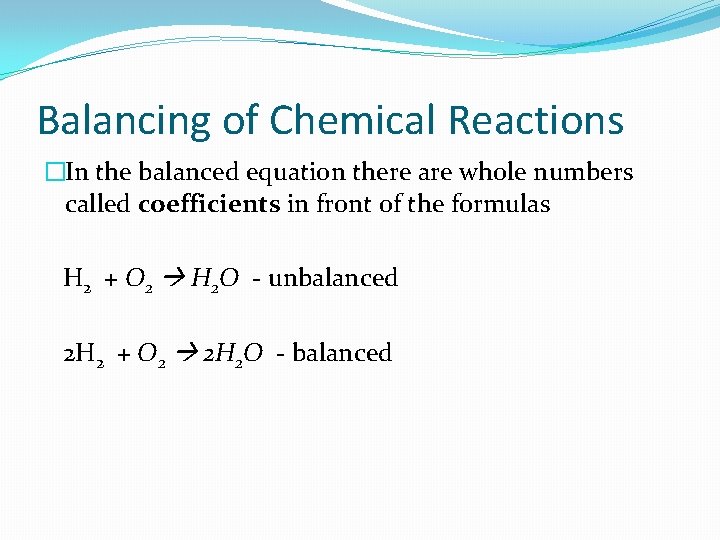
Balancing of Chemical Reactions �In the balanced equation there are whole numbers called coefficients in front of the formulas H 2 + O 2 H 2 O - unbalanced 2 H 2 + O 2 2 H 2 O - balanced

This illustrates the Law of Conservation of Matter, which states that matter cannot be created or destroyed during a chemical reaction.

Types of Reaction �A great number of reactions occur in nature �There are some general patterns of classification
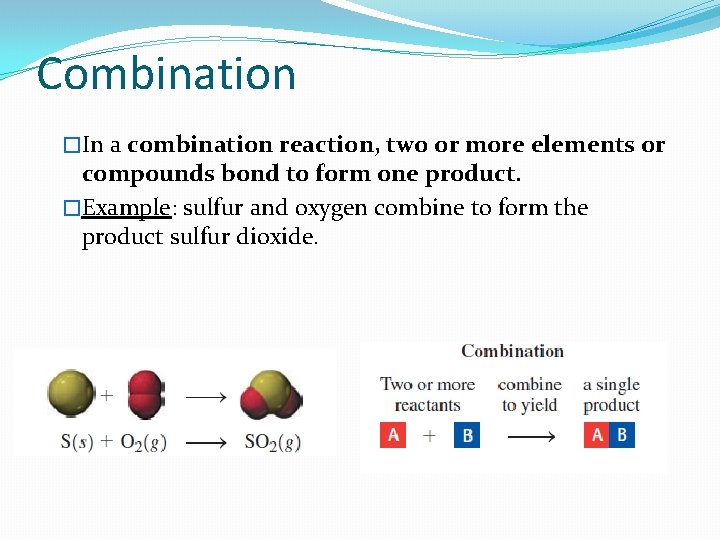
Combination �In a combination reaction, two or more elements or compounds bond to form one product. �Example: sulfur and oxygen combine to form the product sulfur dioxide.
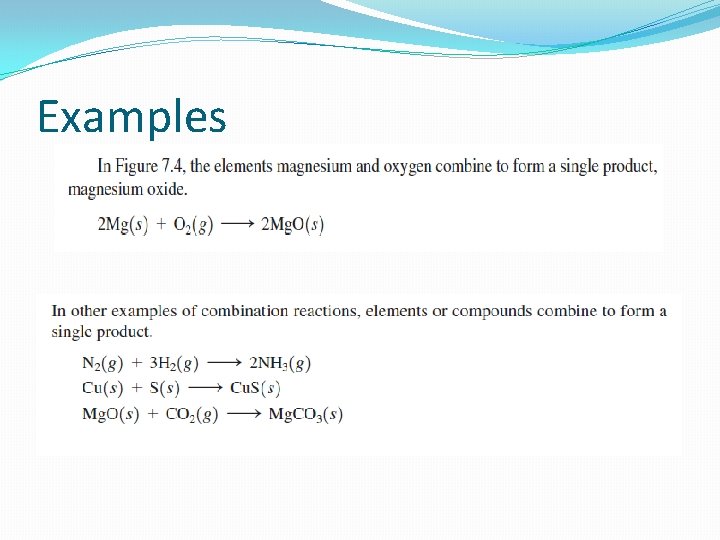
Examples
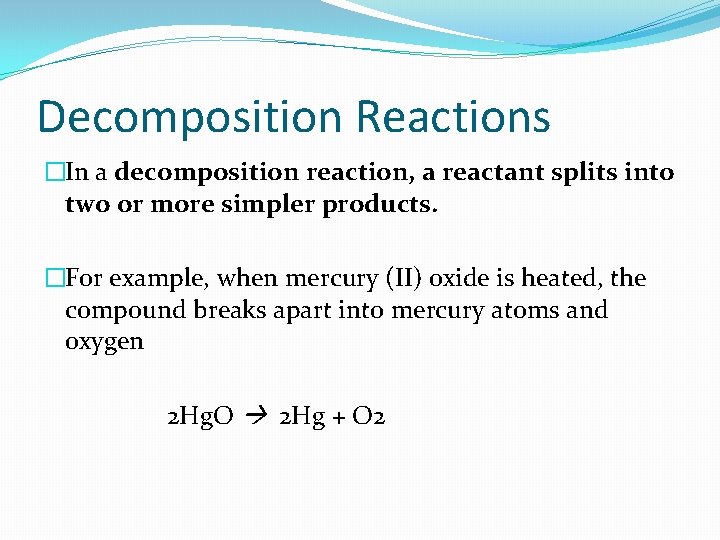
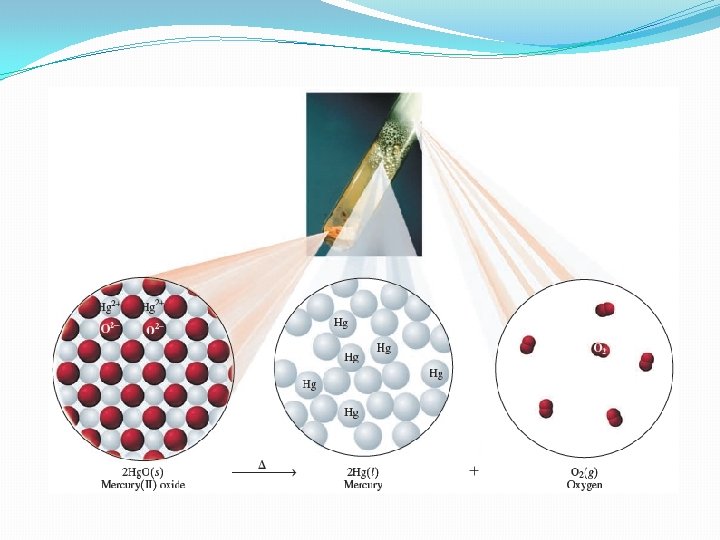
Decomposition Reactions �In a decomposition reaction, a reactant splits into two or more simpler products. �For example, when mercury (II) oxide is heated, the compound breaks apart into mercury atoms and oxygen 2 Hg. O 2 Hg + O 2
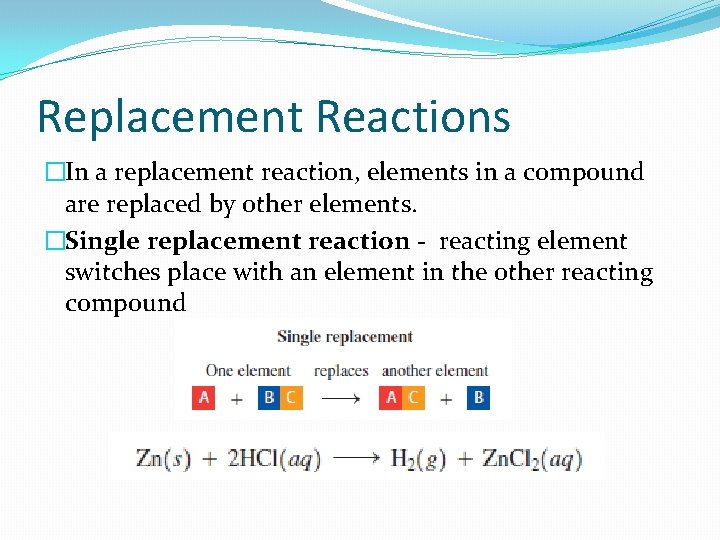
Replacement Reactions �In a replacement reaction, elements in a compound are replaced by other elements. �Single replacement reaction - reacting element switches place with an element in the other reacting compound
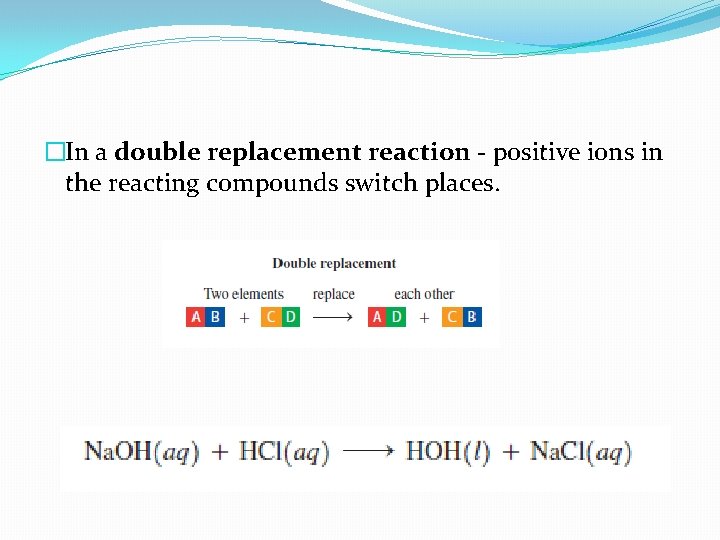
�In a double replacement reaction - positive ions in the reacting compounds switch places.

Combustion reactions �A carbon-containing compound (fuel) burns in oxygen from the air to produce CO 2, water or energy in the form of heat or flame q. Examples: Burning of a candle or fuel
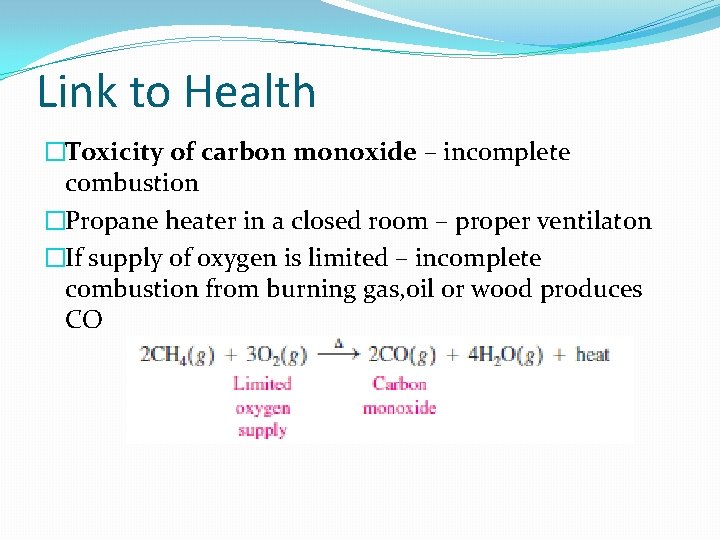
Link to Health �Toxicity of carbon monoxide – incomplete combustion �Propane heater in a closed room – proper ventilaton �If supply of oxygen is limited – incomplete combustion from burning gas, oil or wood produces CO

Link to Health �CO (carbon monoxide) is a colorless, odorless and poisonous gas �If inhaled, passes into the bloodstream �Attaches to hemoglobin which reduces the amount of oxygen (O 2) reaching the cells �Result: person can experience a reduction in exercise capability, visual perception and other disorders

Link to Health �Hemoglobin is the protein that transports O 2 in the blood �If amount of hemoglobin bound to CO is about 10% shortness of breath, mild headache and drowsiness �Heavy smokers can have up to 9% �If the amount is 30% - dizziness, mental confusion, severe headache �If amount is 50% or more – person could become uncounscious and die if not treated immediatelly with oxygen
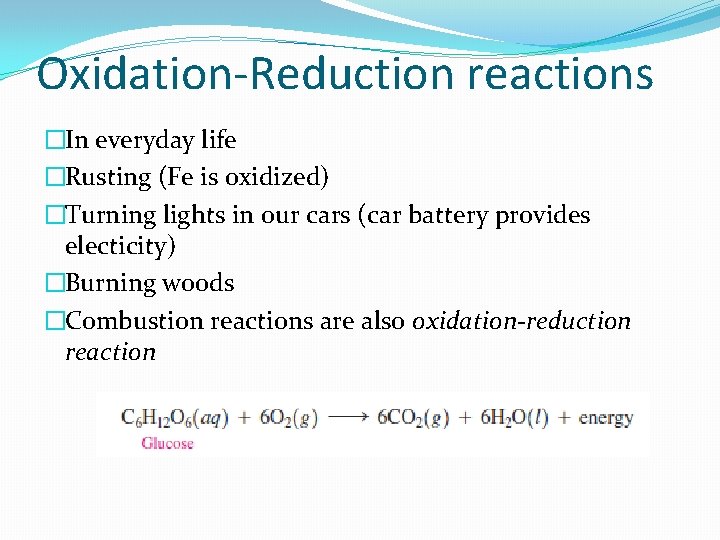
Oxidation-Reduction reactions �In everyday life �Rusting (Fe is oxidized) �Turning lights in our cars (car battery provides electicity) �Burning woods �Combustion reactions are also oxidation-reduction reaction

�Also called redox reactions �Electrons are transferred from one substance to another �One substance loses electrons, another one gains electrons �Oxidation – loss of electrons �Reduction – gain of electrons
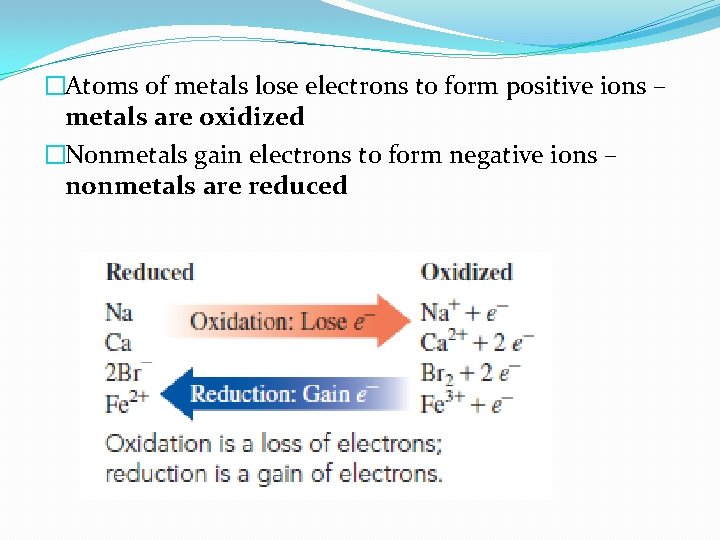
�Atoms of metals lose electrons to form positive ions – metals are oxidized �Nonmetals gain electrons to form negative ions – nonmetals are reduced
Oxidation and Reduction in Biological Systems �In the body cells �Oxidation of organic carbon compounds involves the transfer of hydrogen atoms (protons and electrons) �Necessary for the production of energy in the cells �However, redox reactions depend on the process that occurs �But oxidation is always loss of electrons �Reduction is always gain of electrons

Balancing of chemical reactions �H 2 + O 2 → H 2 O �S + O 2 → SO 2 �Mg + O 2 → Mg. O �Mg. O + CO 2 → Mg. CO 3 �N 2 + H 2 → NH 3 �Hg. O → Hg + O 2 �Zn + HCl → H 2 + Zn. Cl 2 �Na. OH + HCl → H 2 O + Na. Cl �C 6 H 12 O 6 + O 2 → CO 2 + H 2 O + energy �Fe 2 S 3 + HCl → Fe. Cl 3 + H 2 S �CH 4 + O 2 → CO 2 + H 2 O
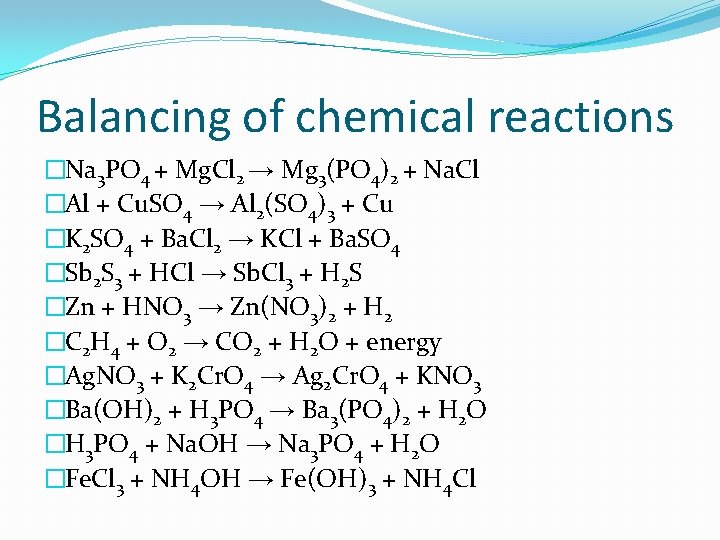
Balancing of chemical reactions �Na 3 PO 4 + Mg. Cl 2 → Mg 3(PO 4)2 + Na. Cl �Al + Cu. SO 4 → Al 2(SO 4)3 + Cu �K 2 SO 4 + Ba. Cl 2 → KCl + Ba. SO 4 �Sb 2 S 3 + HCl → Sb. Cl 3 + H 2 S �Zn + HNO 3 → Zn(NO 3)2 + H 2 �C 2 H 4 + O 2 → CO 2 + H 2 O + energy �Ag. NO 3 + K 2 Cr. O 4 → Ag 2 Cr. O 4 + KNO 3 �Ba(OH)2 + H 3 PO 4 → Ba 3(PO 4)2 + H 2 O �H 3 PO 4 + Na. OH → Na 3 PO 4 + H 2 O �Fe. Cl 3 + NH 4 OH → Fe(OH)3 + NH 4 Cl
Summary �Molar mass and calculations �Chemical reactions �Balancing of chemical reactions
Mole relationships in Chemical Equations �In any chemical reaction, the total amount of matter in the reactants is equal to the total amount of matter in the products. �Thus, the total mass of all the reactants must be equal to the total mass of all the products �Known as the law of conservation of mass, which states that there is no change in the total mass of the substances reacting in a chemical reaction.
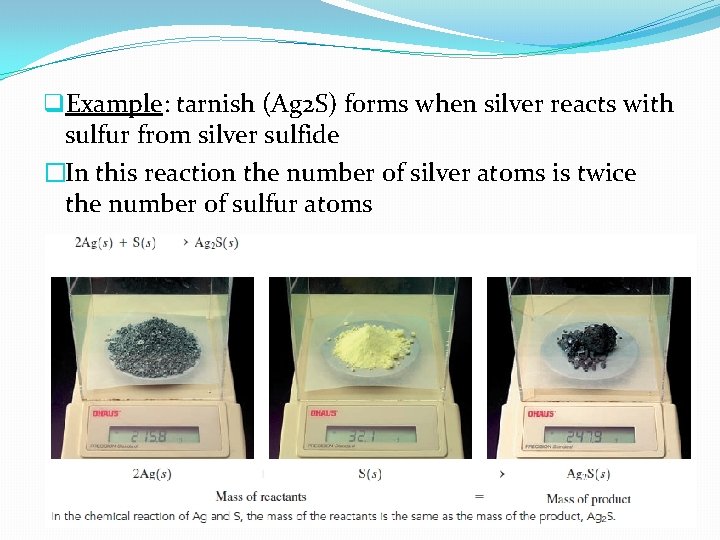
q. Example: tarnish (Ag 2 S) forms when silver reacts with sulfur from silver sulfide �In this reaction the number of silver atoms is twice the number of sulfur atoms

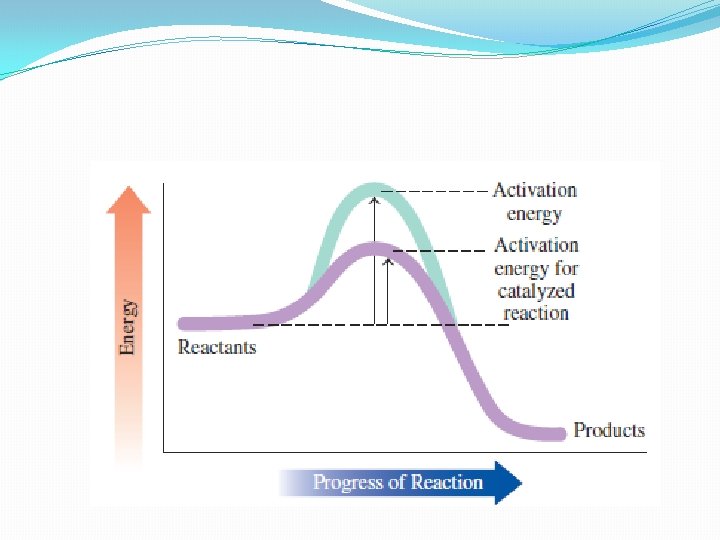
Energy in Chemical Reactions �Molecules of the reactants must collide with each other, have proper orientation and energy �Sufficient energy to break the bonds of the reactants �Activation energy – amount of energy required to break the bonds between atoms

Energy in Chemical Reactions Three Conditions Required for a Reaction to Occur � 1. Collision - The reactants must collide. � 2. Orientation - The reactants must align properly to break and form bonds. � 3. Energy - The collision must provide the energy of activation.
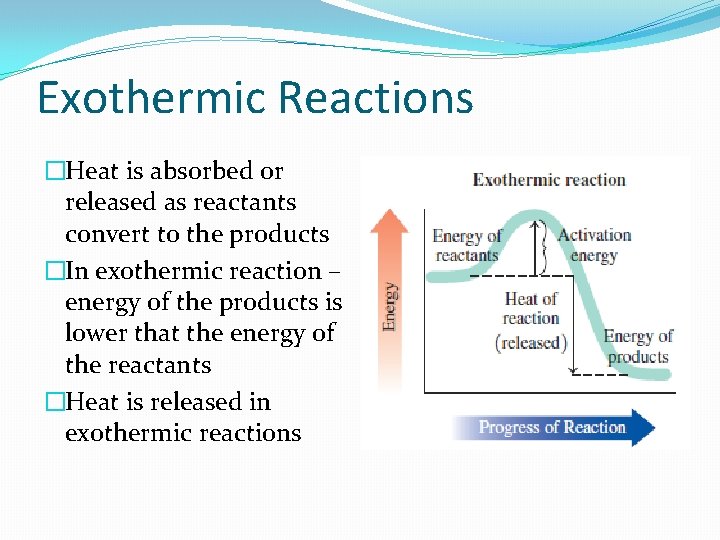
Exothermic Reactions �Heat is absorbed or released as reactants convert to the products �In exothermic reaction – energy of the products is lower that the energy of the reactants �Heat is released in exothermic reactions
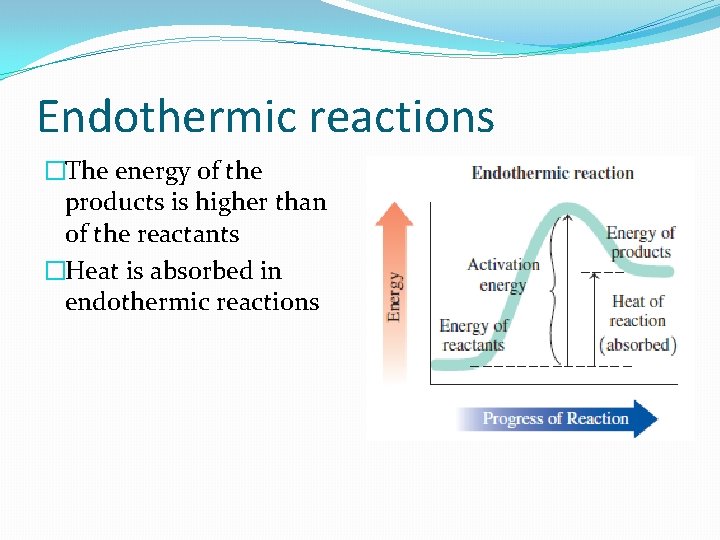
Endothermic reactions �The energy of the products is higher than of the reactants �Heat is absorbed in endothermic reactions

Chemistry Link to Health �First-aid station: cold pack �Reduces swelling from an injury, removes heat from inflammation �Inside of cold pack is solid ammonium nitrate separated from the compartment containig water �Activation: hiting or squeezing to break the walls and mix nitrate with water
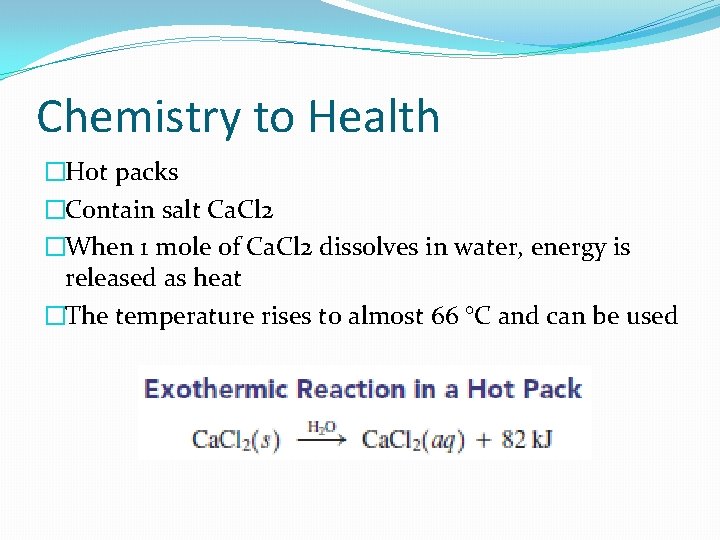
Chemistry to Health �Hot packs �Contain salt Ca. Cl 2 �When 1 mole of Ca. Cl 2 dissolves in water, energy is released as heat �The temperature rises to almost 66 °C and can be used
Rate of Reaction �The rate (speed) is measured by: - the amount of reactant used up - the amount of product formed �In a certain period of time �The rate is affected by: - Changes in temperature - Changes in the concentration of the reactants - Addition of catalysts

Temperature �Higher temperatures – increase in kinetic energy makes reactants move faster and collide often �Higher temperatures – faster reactions q. Example: cooking �Lower temperatures – slower reactions q. Example: in cardiac surgery, body temperature is lowered to 28 °C so the heart can be stopped and less oxygen is required by the brain �The reason why some people survived submersion in icy lakes for longer period
Concentrations of Reactants �Adding reactants – increasing the rate of reaction �More collisions between the reactants -> reaction faster q. Example: patient with difficulty breathing may be given a mixture with a higher oxygen contant �The increase in the number of oxygen molecules in the lungs – increases the rate at which oxygen combines with hemoglobin
Catalysts �To speed up a reaction – lower the energy of activation �Adding catalyst �Provides alternate pathway with a lower energy requirenment �Result: more collisions form product successfully �Many uses in industry (margarine production speed up with platinum) �In body – enzymes �Make most metabolic reactions go at the rates needed for proper cellular activity

Summary �Types of reactions �Redox reactions �Mole relationships in chemical equations �Mass calculations for reactions �Energy in chemical reactions �Rate of reactions
Assignment �Calculate the mass in grams for following compounds: Ø 1. 75 moles of C 19 H 20 FNO 3 Ø 4. 42 moles of C 4 H 8 O 4 Ø 2 moles of Ga 2(CO 3)2 Ø 5 moles of Al 2(SO 4)3 Ø 0. 5 moles of Mg(OH)2 Ø 3. 75 moles of Fe 2 O 3 Ø M=m/n
- Slides: 47