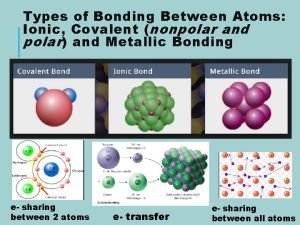
Bonding Types of Bonds Ionic Covalent Metallic Metallic

Bonding

Types of Bonds • Ionic • Covalent • Metallic

Metallic Bonds • Electrons are shared by many atoms • Electrons free to move • Two or more metals

Metallic Compounds • Generally high MP • Hard & lusterous • Less brittle • Conductors

Metallic Bonds • No debate about metallic bonds • Easy to identify

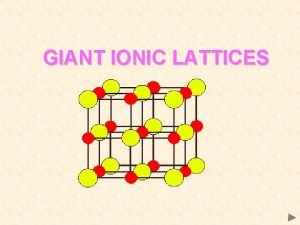
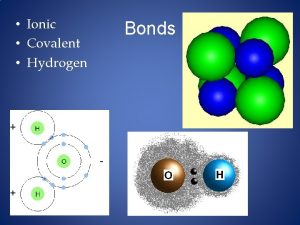
Ionic Bonds • Electrons are transferred from one atom to another creating (+) & (-) ions • Metal & nonmetal

Ionic Compounds • Held together by electrostatic charge • Very high MP • Brittle

Covalent Bonds • Electrons are shared by two atoms • Two nonmetals • Weaker than ionic

Covalent Compounds • Molecules • Low MP • Two nonmetals • Flexible

Molecule • Any compound that can exist as an entity by itself

Distinguishing Bonds • Distinguishing ionic & covalent bonds can be difficult, but generally determined by differenceelectronegativity

Bonds Types • Ionic • Polar covalent • Non polar covalent

Bond Types • Ionic: DEN > 1. 5 -1. 8 • Covalent: DEN < 1. 5 -1. 8 • Polar Covalent: 0. 5<DEN<1. 5 • Non polar covalent : DEN< 0. 5 • Not absolute

Coordinate Covalent Bonds • A covalent bond in which the two electrons are donated by one atom
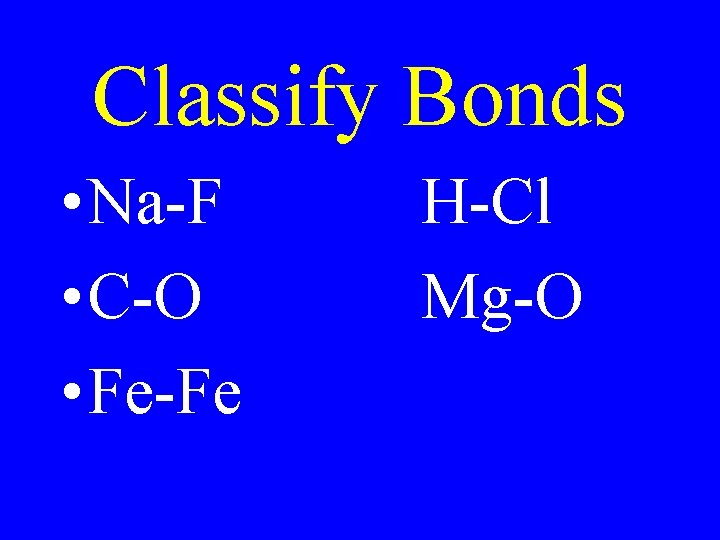
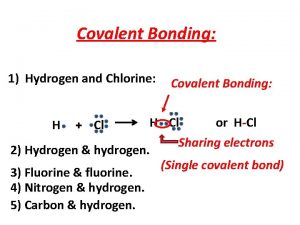
Classify Bonds • Na-F • C-O • Fe-Fe H-Cl Mg-O

Dipole • Polar bonds • Polar molecules
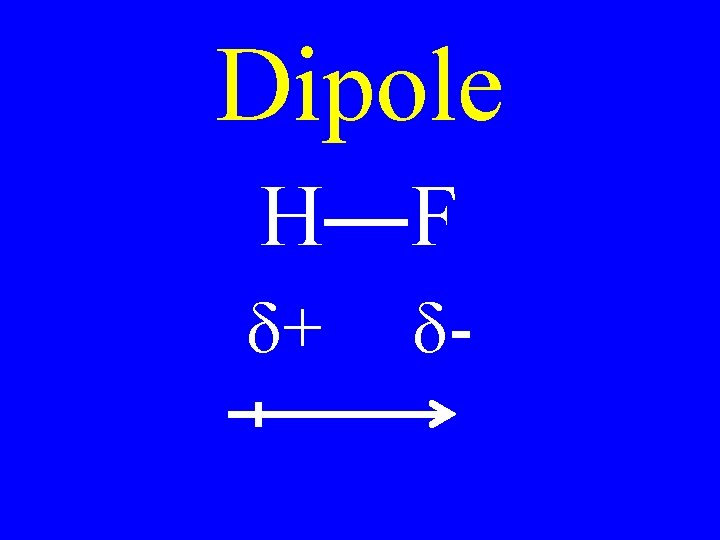
Dipole H F d+ d-
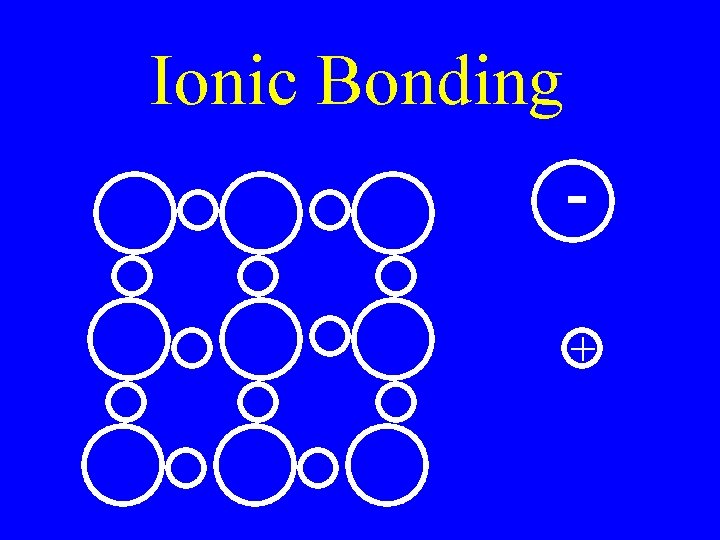
Ionic Bonding +

Covalent Bonding Occurs when electron orbitals overlap

List & describe three types of bonds

Orbitals On the board Max 2 e per orbital

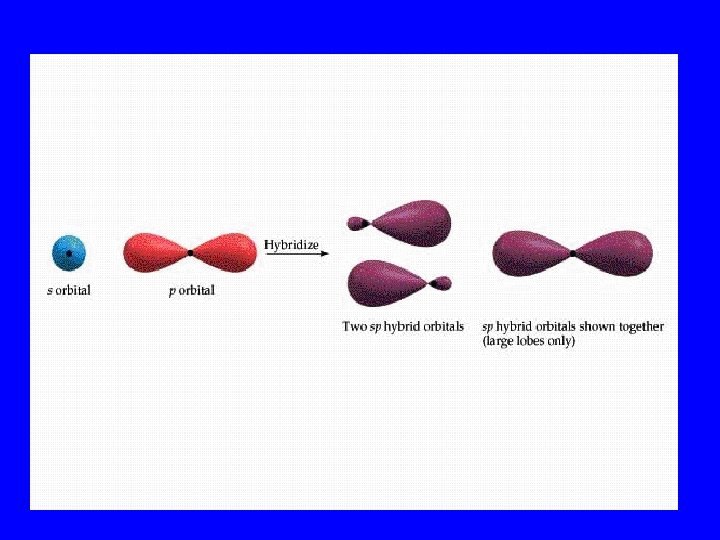
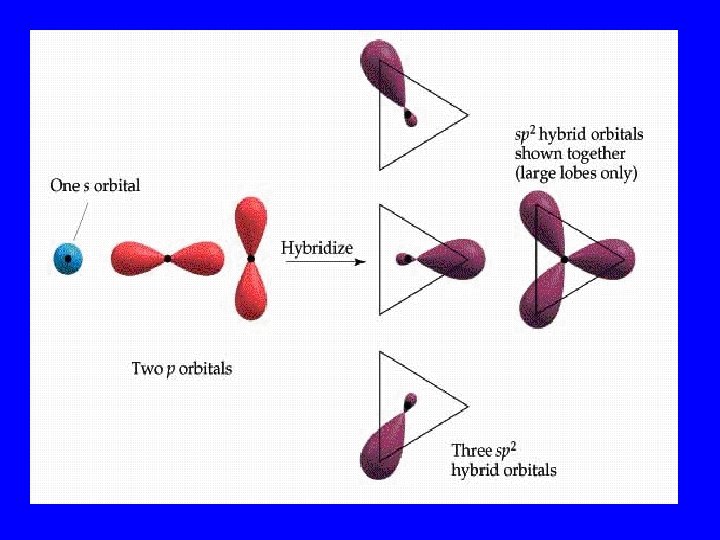
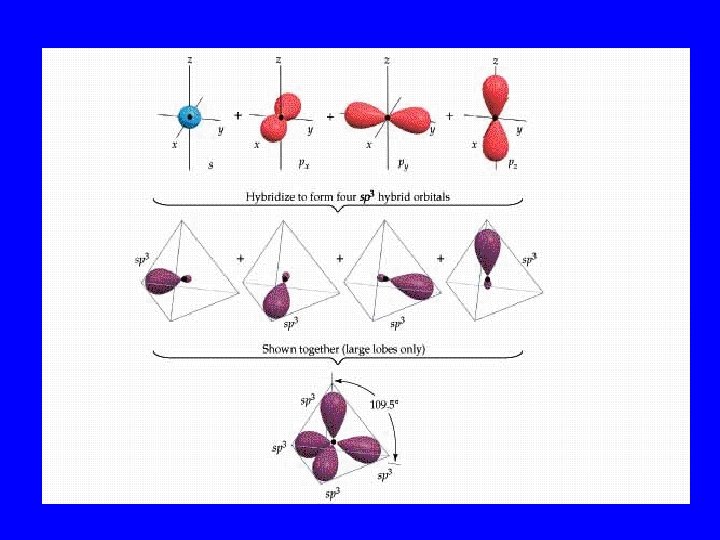
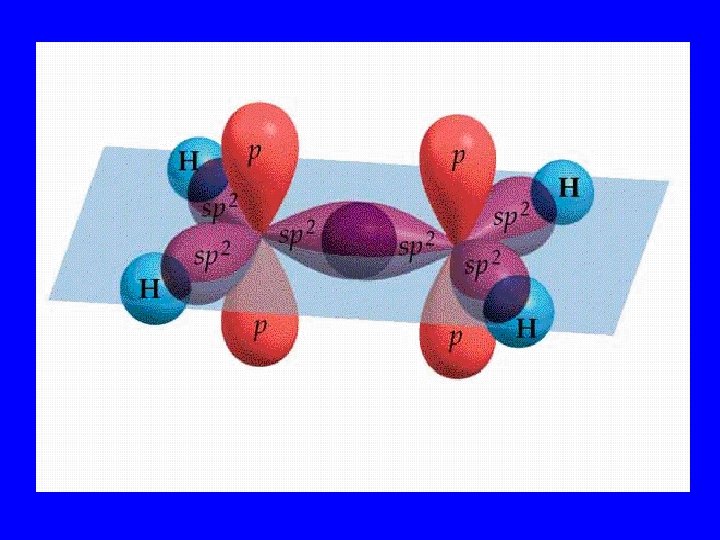
Hybridization • When s, p, and/or d orbitals (electron clouds) mix to make a new type of multilobed orbital

Hybrid Orbitals • sp 2 • sp 3 dsp 2 3 d sp


Electron Cloud Repulsion • In molecules each electron cloud repels other clouds enough to spread as far apart as possible

VSEPR • Valance Shell Electron Pair Repulsion • Electron pairs repel each other to spread out as much as possible
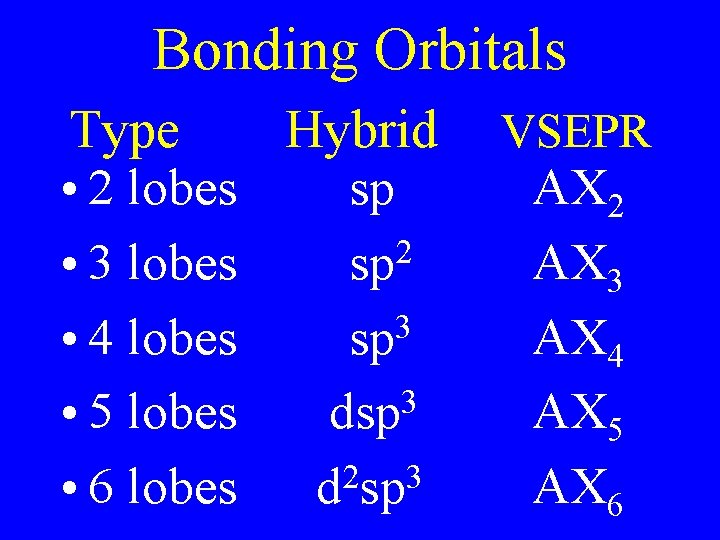
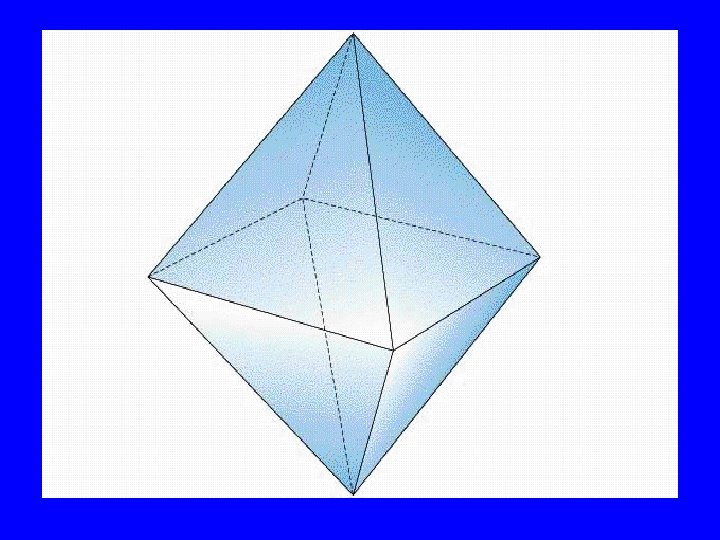
Bonding Orbitals Type Hybrid • 2 lobes sp 2 • 3 lobes sp 3 • 4 lobes sp 3 • 5 lobes dsp 2 3 • 6 lobes d sp VSEPR AX 2 AX 3 AX 4 AX 5 AX 6

VSEPR Orbitals
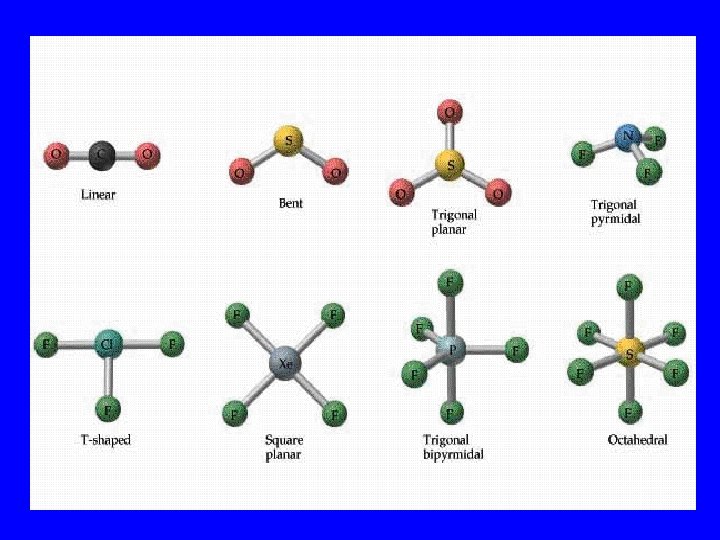

Bonding Orbital Shape • 2 lobes: Linear 180 o • 3 lobes: Trigonal planar 120 o o • 4 lobes: Tetrahedral 109. 5 • 5 lobes: Hexahedral 120&180 o • 6 lobes: Octahedral 90&180 o
Draw the Bonding Electron Dot Diagrams for Each Element

Bonding Electron Dot Diagrams • Electron dot diagrams that go through 4 singles before any electrons are paired up
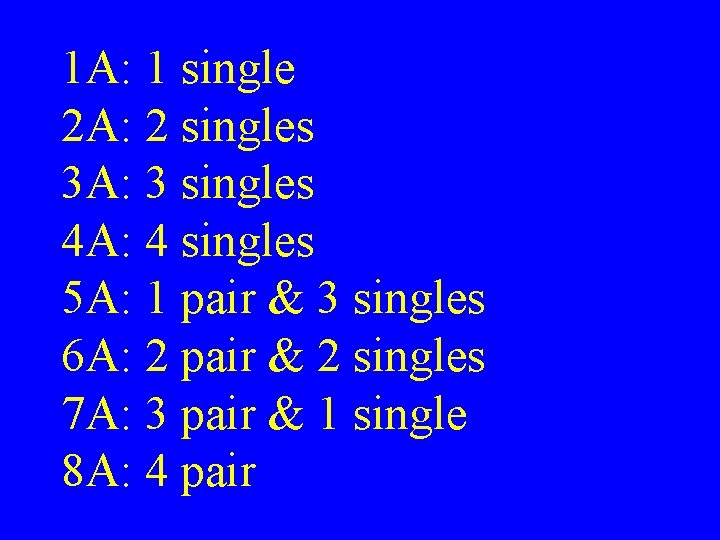
1 A: 1 single 2 A: 2 singles 3 A: 3 singles 4 A: 4 singles 5 A: 1 pair & 3 singles 6 A: 2 pair & 2 singles 7 A: 3 pair & 1 single 8 A: 4 pair

Calculate the density SO 2 at o 47 C under 83. 1 k. Pa Pressure:
Lewis Dot Diagrams • Representation of valence electrons and bonds in a molecule or polyatomic ion

Drawing LDDs Draw the bonding electron dot diagram for each element in the molecule with the element with the most unpaired e near the center

Drawing LDDs • If there is more than one carbon, connect the carbons by connecting single dots between one carbon & another
Drawing LDDs • Connect a single dot on one atom to a single dot on another (never two on the same atom)(never connect one dot to more than one other dot)
Drawing LDDs • Repeat connecting the dots until all singled dots are connected making sure to obey the octet rule if possible
Drawing LDDs • Recognize polyatomic ions -2 • H 2 CO 3: CO 3 is a polyatomic ion; thus, the three Os must connect to the C

Drawing LDDs • Redraw the molecule neatly making sure to include all dots
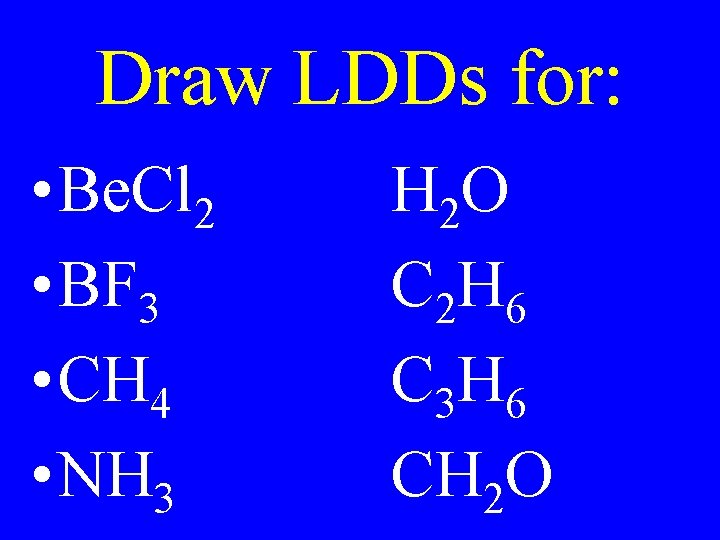
Draw LDDs for: • Be. Cl 2 • BF 3 • CH 4 • NH 3 H 2 O C 2 H 6 C 3 H 6 CH 2 O
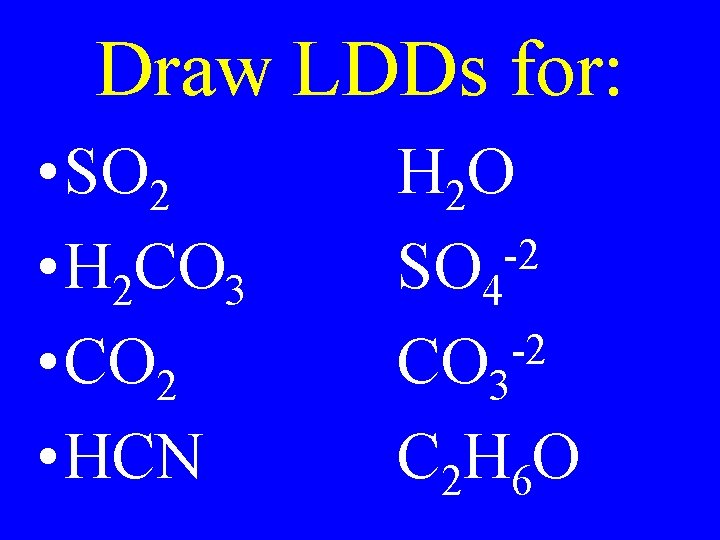
Draw LDDs for: • SO 2 • H 2 CO 3 • CO 2 • HCN H 2 O -2 SO 4 -2 CO 3 C 2 H 6 O
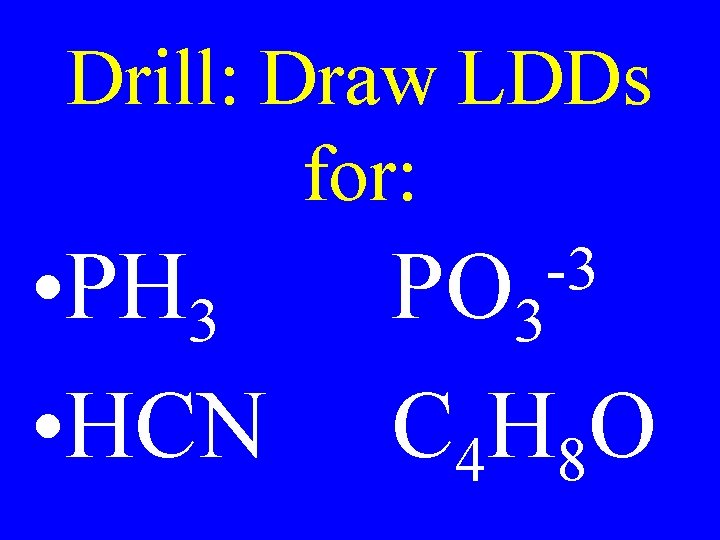
Drill: Draw LDDs for: • PH 3 • HCN -3 PO 3 C 4 H 8 O

Check HW • Problem 1 • Page 202
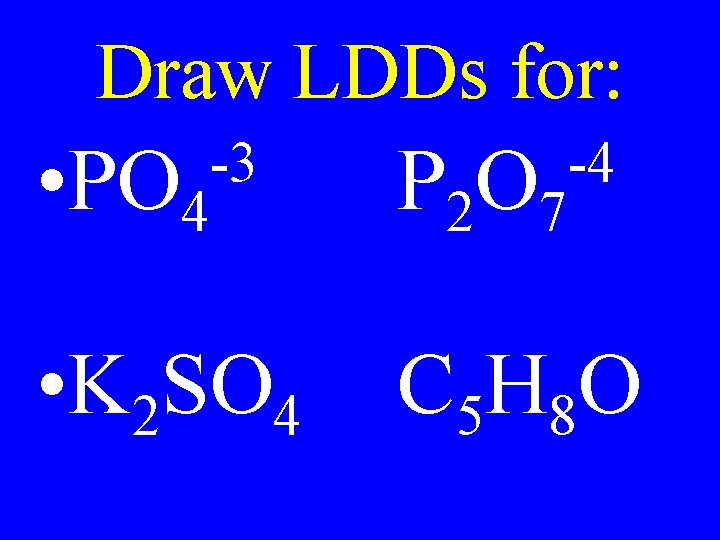
Draw LDDs for: -3 • PO 4 P 2 O 7 • K 2 SO 4 C 5 H 8 O -4

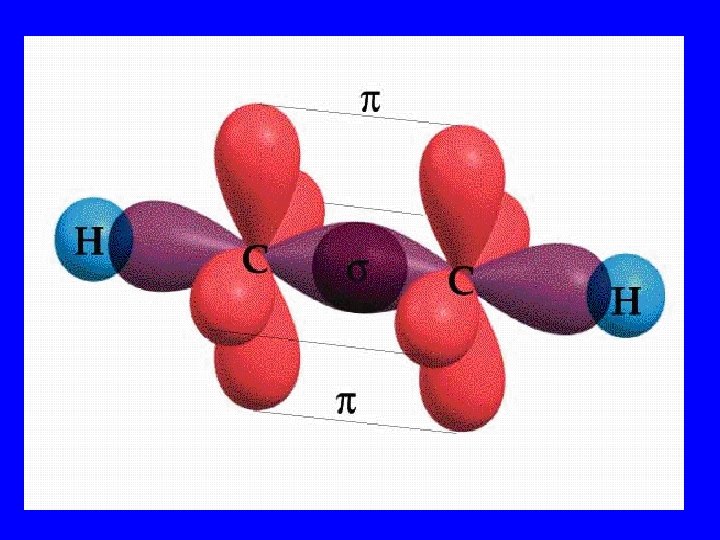
Sigma Bonds(s) • End to end orbital overlap • All single bonds are sigma bonds • All multiple bonds contain one sigma bond
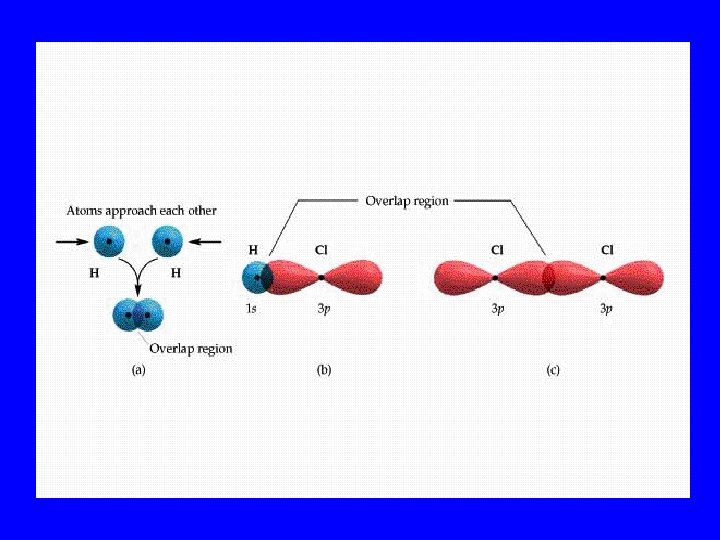
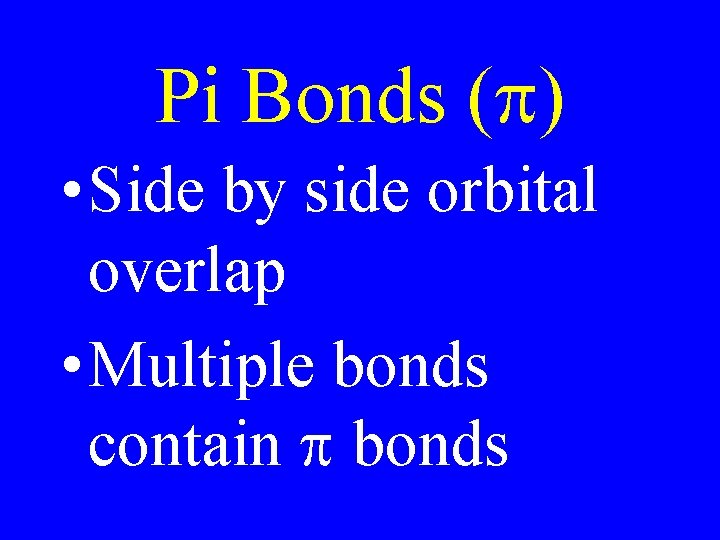
Pi Bonds (p) • Side by side orbital overlap • Multiple bonds contain p bonds
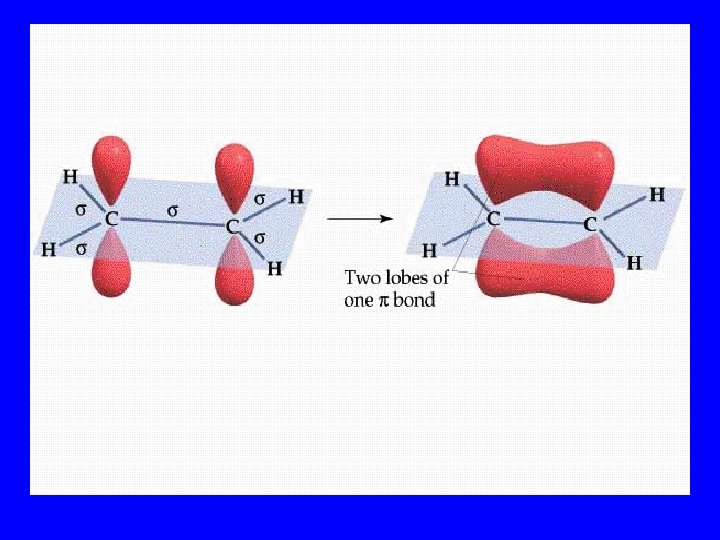

Multiple Bonds • Double: 1 s & 1 p • Triple: 1 s & 2 p

Expanded Octets • Sometimes atoms can be surrounded by more than 8 electrons • Columns 5 A-8 A
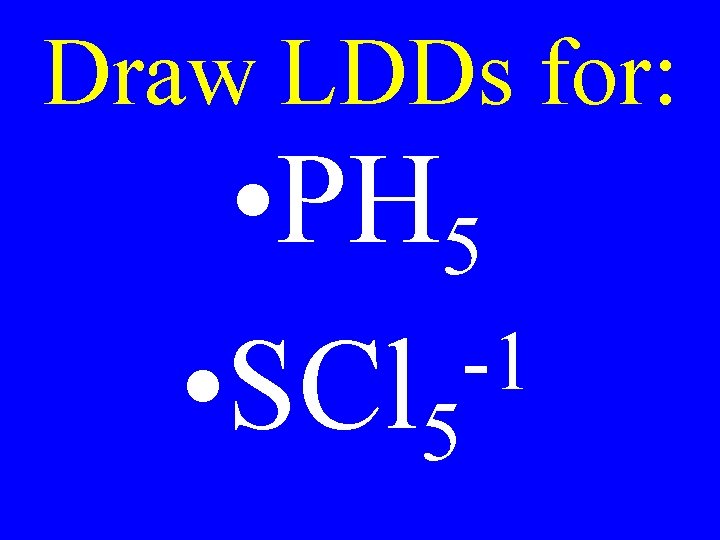
Draw LDDs for: • PH 5 -1 • SCl 5

Drill: Draw LDDs: • Si. F 6 • Xe. F 4 -2

Check HW • Problem 17 • Page 203
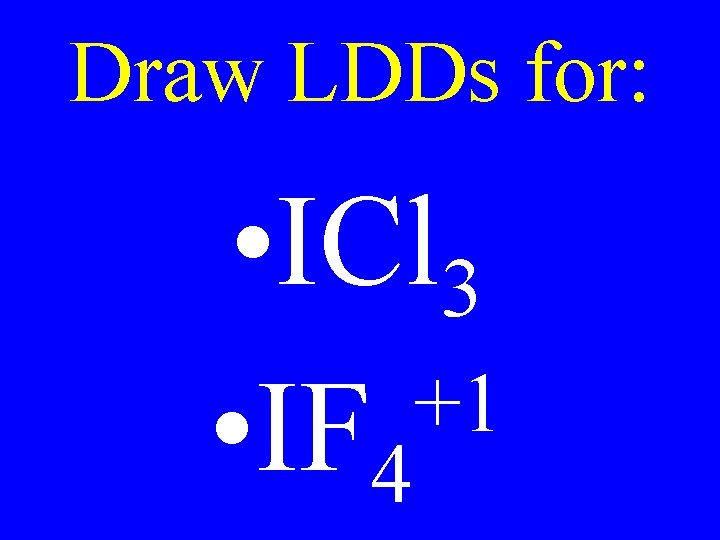
Draw LDDs for: • ICl 3 +1 • IF 4
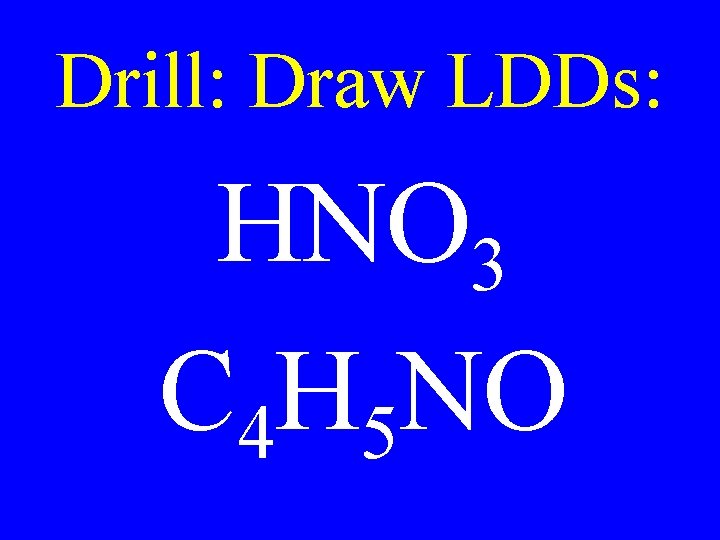
Drill: Draw LDDs: HNO 3 C 4 H 5 NO
Drill: Draw LDDs: • P 3 O 10 -5 • K 2 SO 4 -1 SCl 5 C 5 H 8 O

Drill: Predict the type hybridization & VSEPR type for central atoms containing 2, 3, 4, 5, or 6 lobes of electron clouds

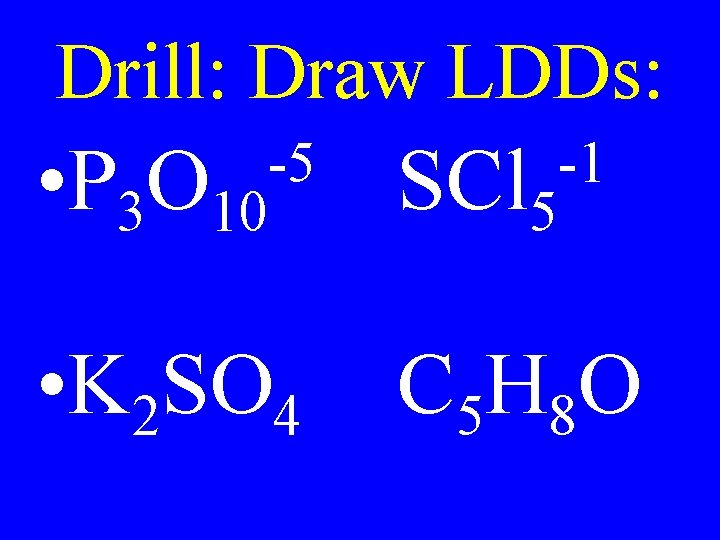
Intermolecular Forces • Weak temporary attractions between atoms from one molecule to another or another part of a larger molecule

Intermolecular Forces • Hydrogen-bond • Dipole-dipole • Dipole-induced dipole • London dispersion forces

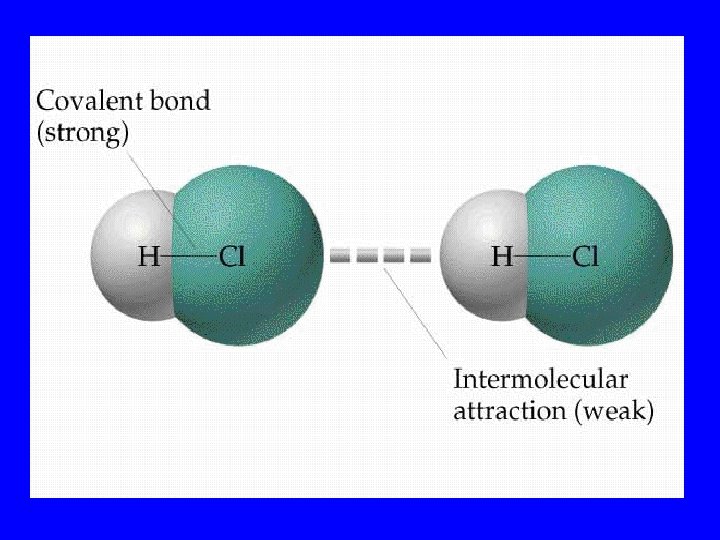
Hydrogen Bond • Strongest of the intermolecular forces • Occurs when H is bound to one highly EN element & connects to another
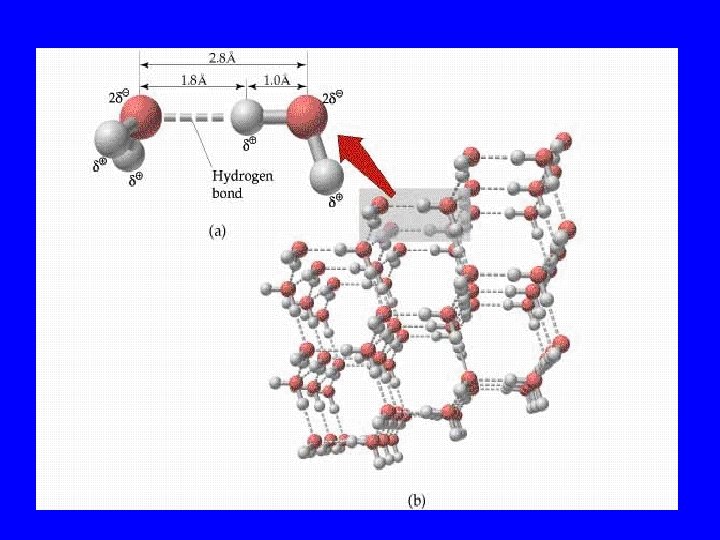

Dipole-Dipole • When two polar molecules connect

Dipole-Induced Dipole • When a polar molecule gets near a non-polar one, it induces the non-polar one to become polar; thus, they connect

London Dispersion • Instantaneous attraction for fractions of seconds in which non-polar molecules connect • Very weak force

Draw Lewis Dot Diagram for: ICl 5 Determine: bond s, hybridization, VSEPR, & shape
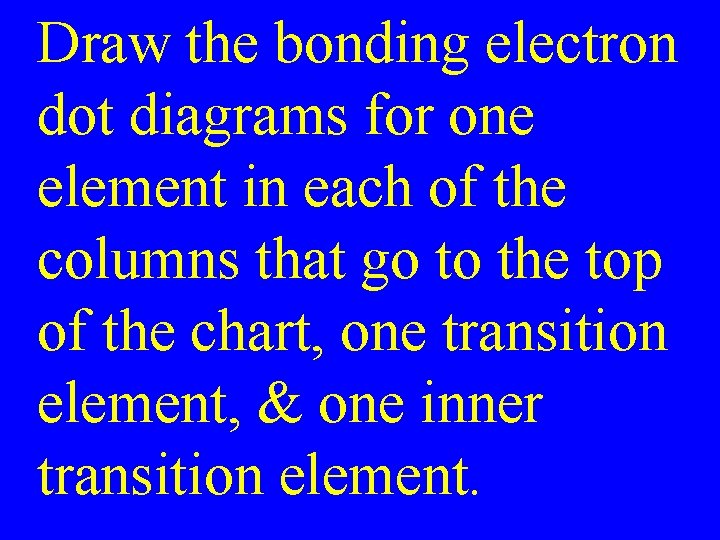
Draw the bonding electron dot diagrams for one element in each of the columns that go to the top of the chart, one transition element, & one inner transition element.

Identify as ionic, covalent, or metallic bonds Na-Cl S-Cl Mg-S N-O Fe-Cr H-Cl C-C Fe-Fe
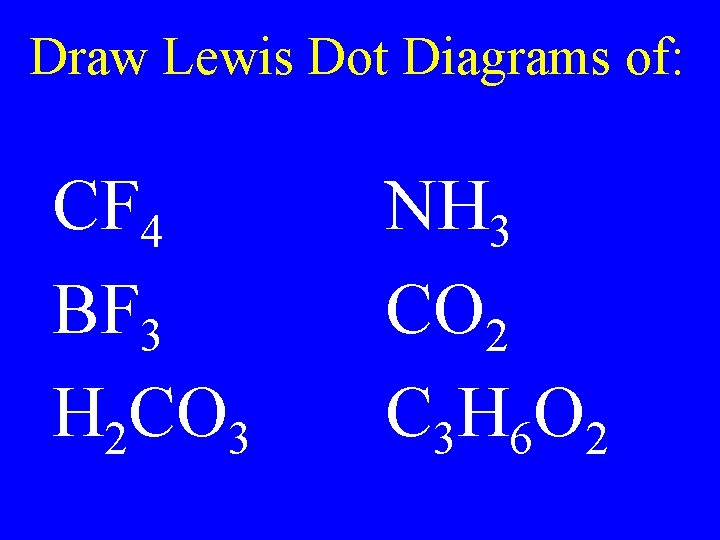
Draw Lewis Dot Diagrams of: CF 4 BF 3 H 2 CO 3 NH 3 CO 2 C 3 H 6 O 2
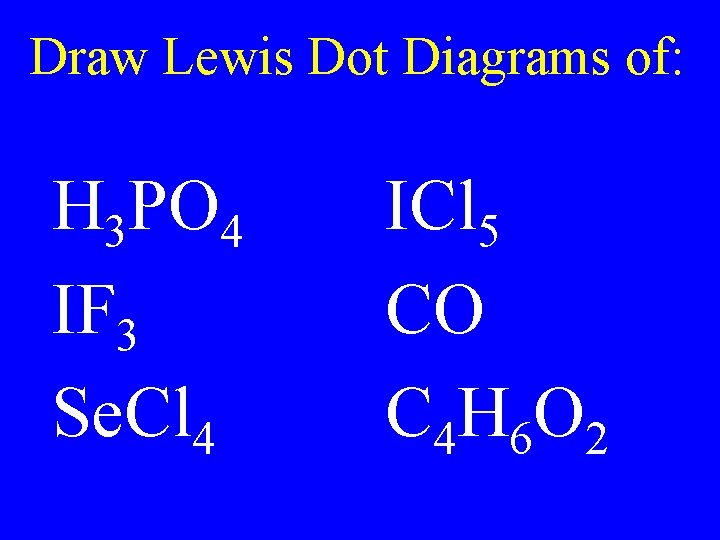
Draw Lewis Dot Diagrams of: H 3 PO 4 IF 3 Se. Cl 4 ICl 5 CO C 4 H 6 O 2
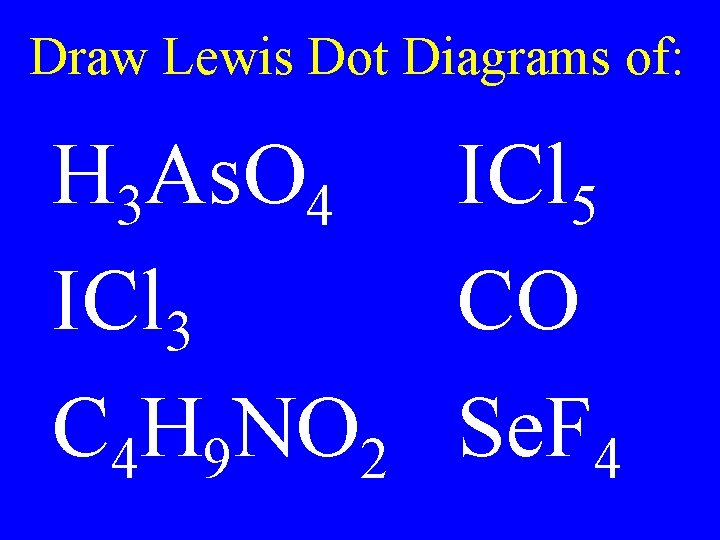
Draw Lewis Dot Diagrams of: H 3 As. O 4 ICl 5 ICl 3 CO C 4 H 9 NO 2 Se. F 4
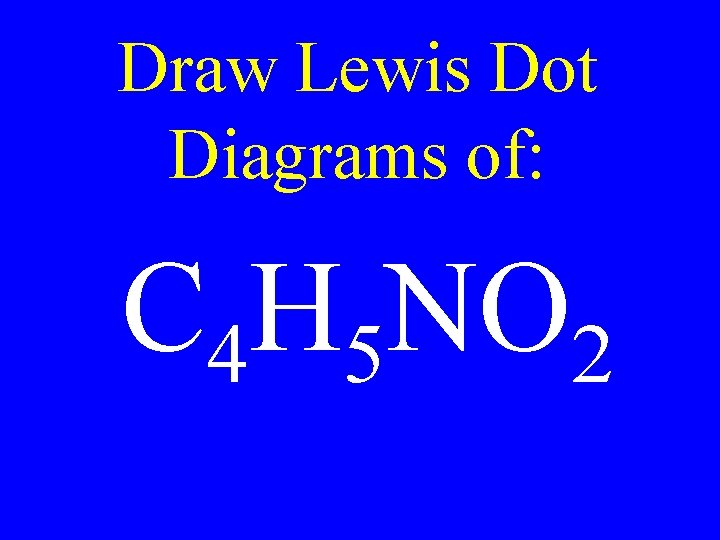
Draw Lewis Dot Diagrams of: C 4 H 5 NO 2

List & describe the four types of intermolecular forces
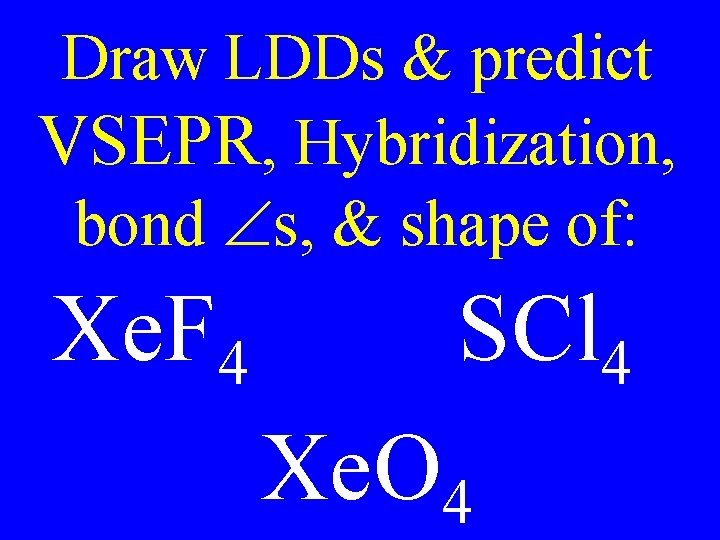
Draw LDDs & predict VSEPR, Hybridization, bond s, & shape of: Xe. F 4 SCl 4 Xe. O 4
Molecular Orbital Theory • Count valence e-s • Draw orbital diagram • Fill in the chart • Add the total bonding & antibonding orbital to get bond order(strength of bond-single double-triple-etc)

Determine the Bond Order of N 2 • Each N has 5 valence • The total valence e s = 10 es
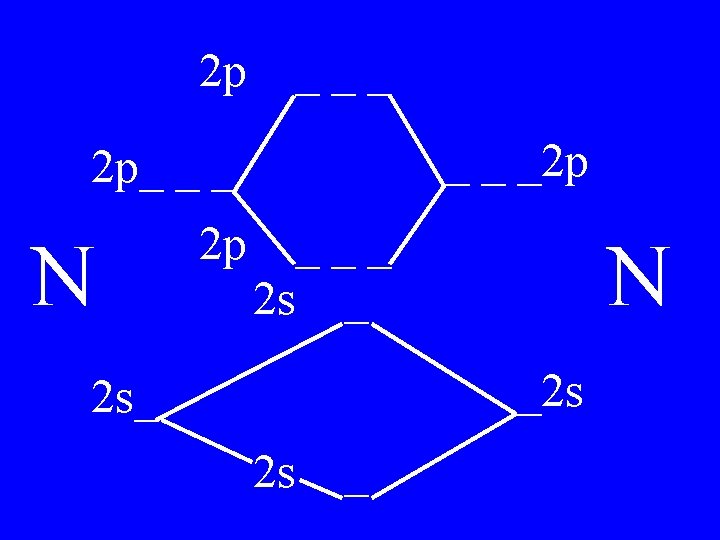
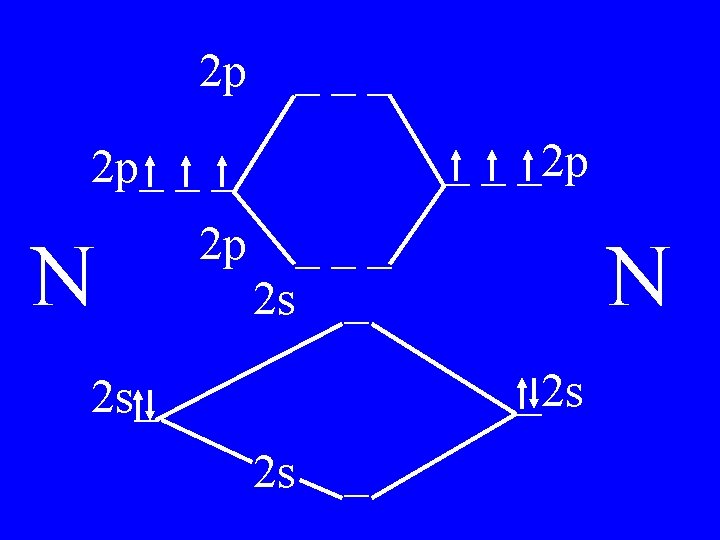
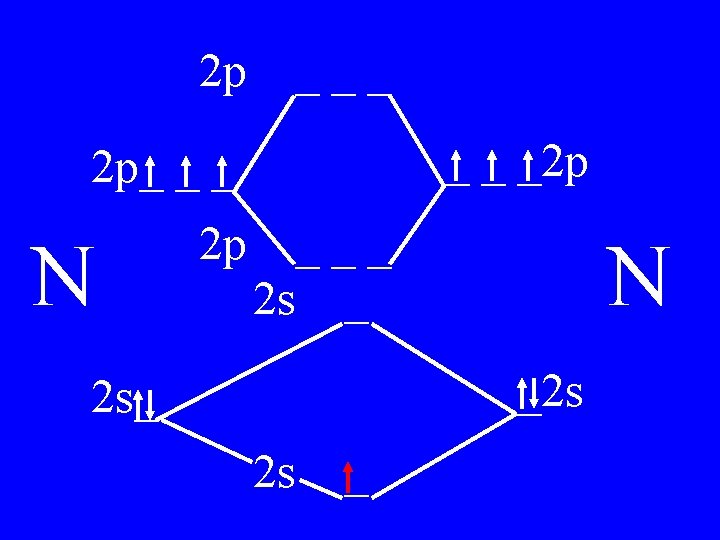
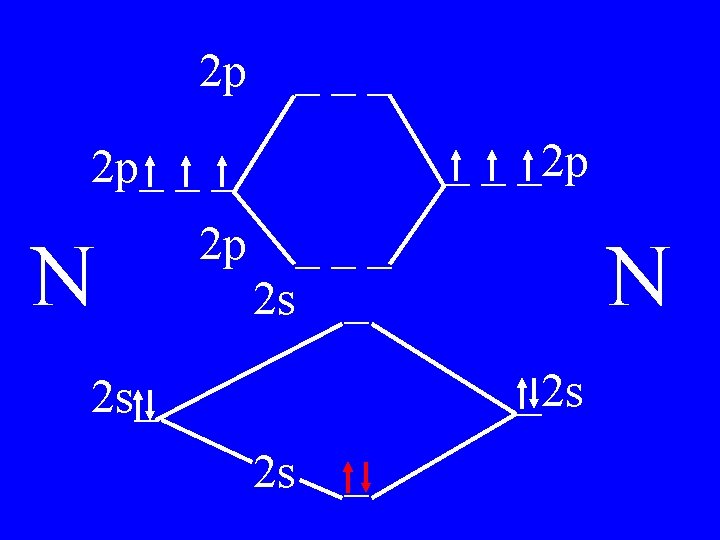
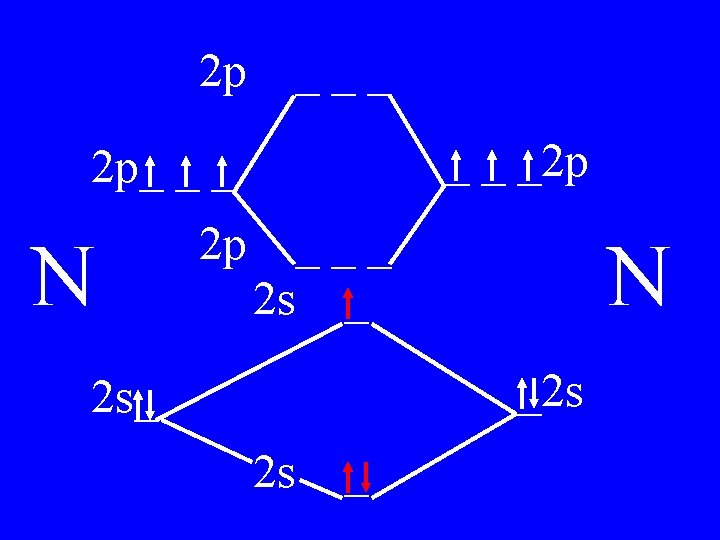
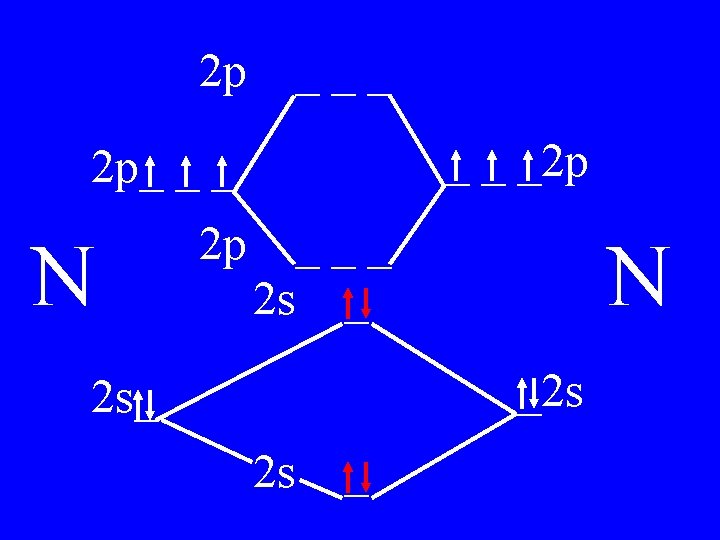
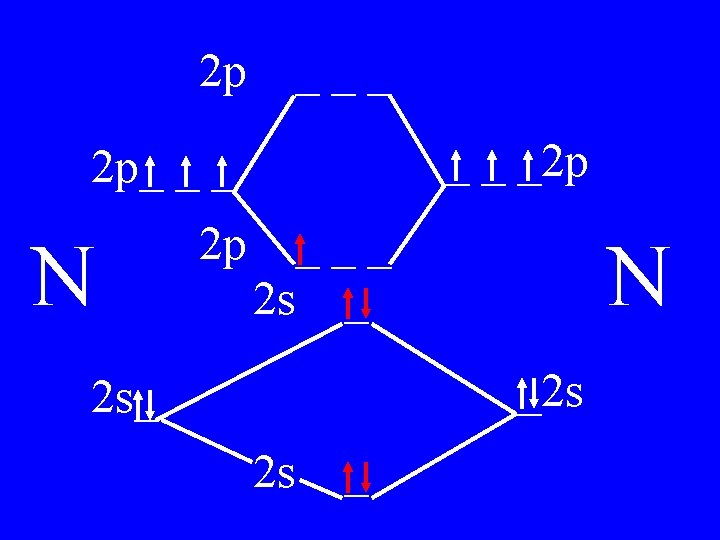
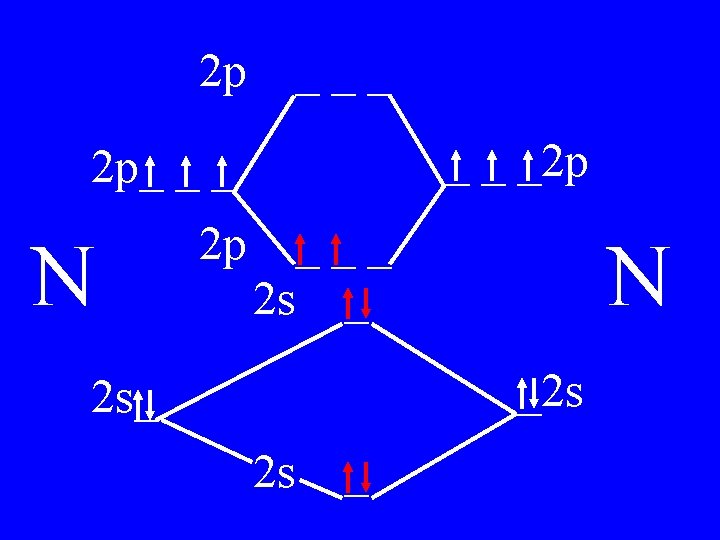
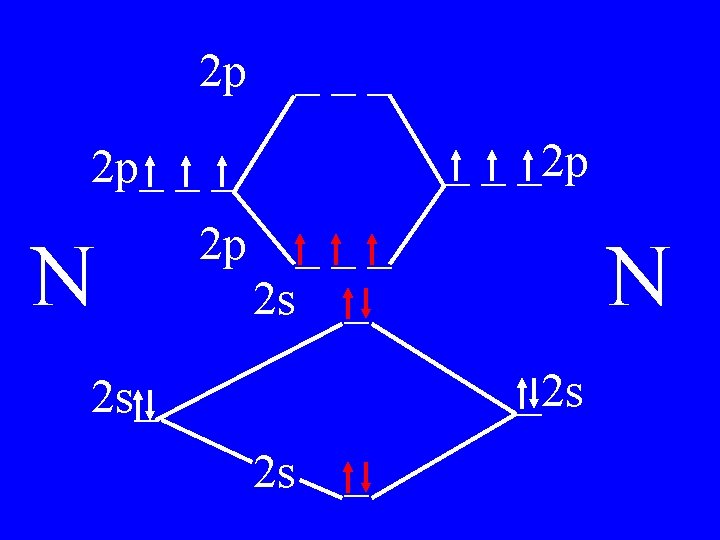
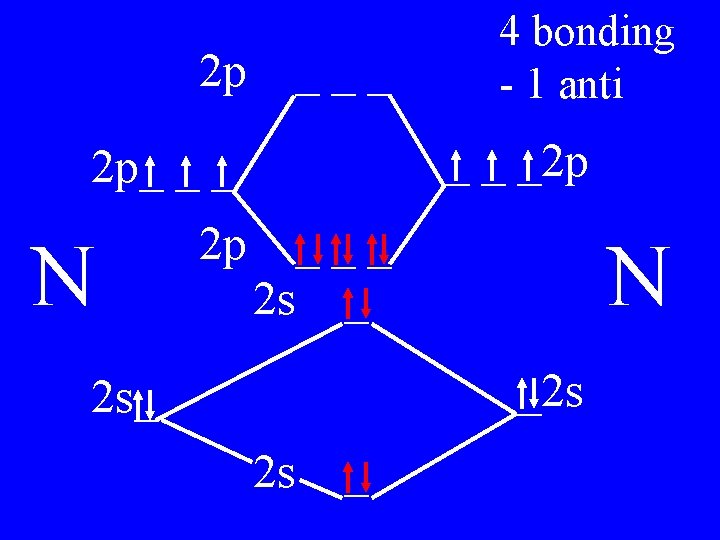
2 p ___ _ _ _2 p 2 p_ _ _ N 2 p 4 bonding - 1 anti ___ 2 s _ N _2 s 2 s_ 2 s _
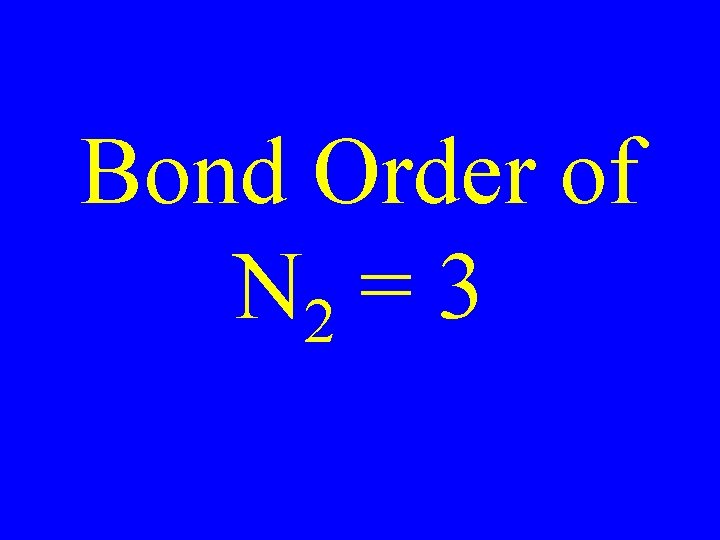
Bond Order of N 2 = 3
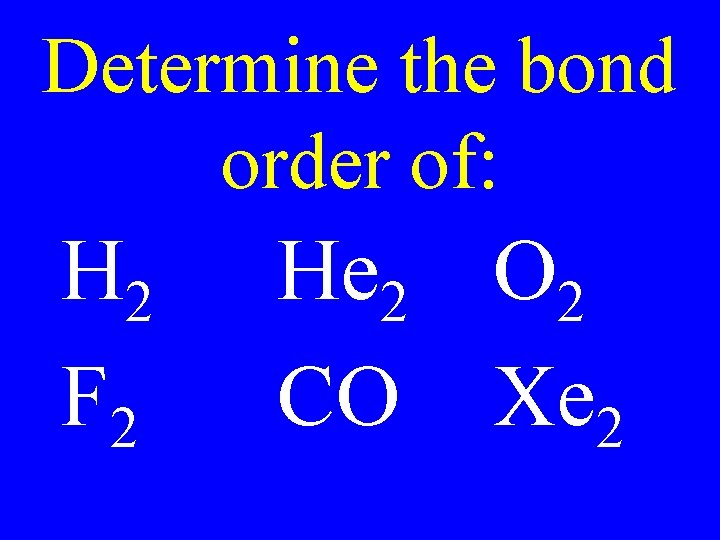
Determine the bond order of: H 2 F 2 He 2 O 2 CO Xe 2
- Slides: 97