Naming Covalent Compounds Chapter 5 3 Naming Covalent



























- Slides: 32

Naming Covalent Compounds Chapter 5. 3

Naming Covalent Compounds • Covalent bonds are formed between nonmetals • For covalent compounds, specific prefixes are used to describe how many atoms of each element are present • Example: – N 2 = Dinitrogen • “Di” is the prefix for 2 • Therefore, dinitrogen = 2 nitrogen atoms

Prefixes • • • 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta- 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca-

Rules for Naming Covalent Compounds • 1 st element: Do not change the ending – Add necessary prefix to indicate number of atoms (DO NOT include “mono” for one atom here) • 2 nd element: Change the ending to “ide” – Add necessary prefix to indicate the number of atoms (including “mono” for one atom)

Examples • Name the following: – PF 5 – Phosphorus Pentafluoride - N 2 O 3 - Dinitrogen trioxide

Formulas • Molecular formula—a chemical formula that shows the atoms and number of atoms in a molecule • Empirical formula—the simplest formula for the ratio of the amount of atoms in a chemical formula • Ex: Molecular formula: N 2 H 4 – Empirical formula: NH 2 • Ex: Molecular formula: C 2 H 4 O 2 – Empirical formula = CH 2 O

Practice Naming Covalent Compounds • What is the correct molecular formula for… diphosphorus pentoxide

P 2 O 5

Practice Naming Covalent Compounds • What is the molecular formula for… Arsenic pentafluoride

As. F 5

Practice Naming Covalent Compounds • What is the correct name for… Tetraphosphorus trisulfide

P 4 S 3

Practice Naming Covalent Compounds • What is the correct name for… Boron trifluoride

BF 3

Practice Naming Covalent Compounds • What is the correct name for… P 4 S 3

tetraphosphorus trisulfide

Practice Naming Covalent Compounds • What is the correct name for… CO 2

carbon dioxide

Practice Naming Covalent Compounds • What is the correct name for… SF 6

Sulfur hexafluoride

Practice Naming Covalent Compounds • What is the correct name for… Se. F 6

Selenium hexafluoride

Practice Naming Covalent Compounds • What is the correct name for… PBr 3

phosphorus tribromide

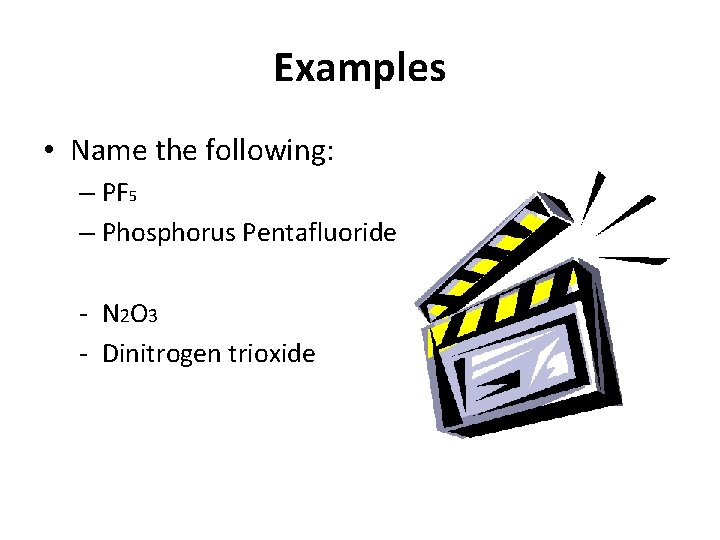
Practicing Empirical Formulas… Molecule formula = C 6 H 12 O 6 What is the empirical (simplified) formula?

CH 2 O

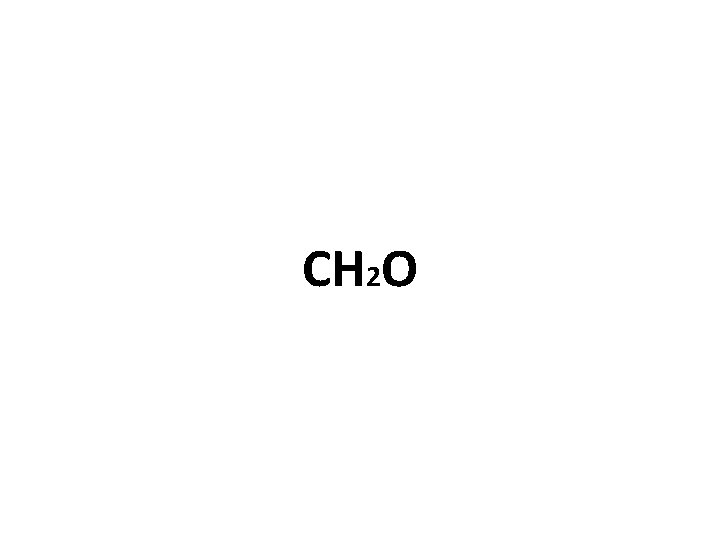
Practicing Empirical Formulas… Molecule formula = C 6 H 6 What is the empirical (simplified) formula?

CH

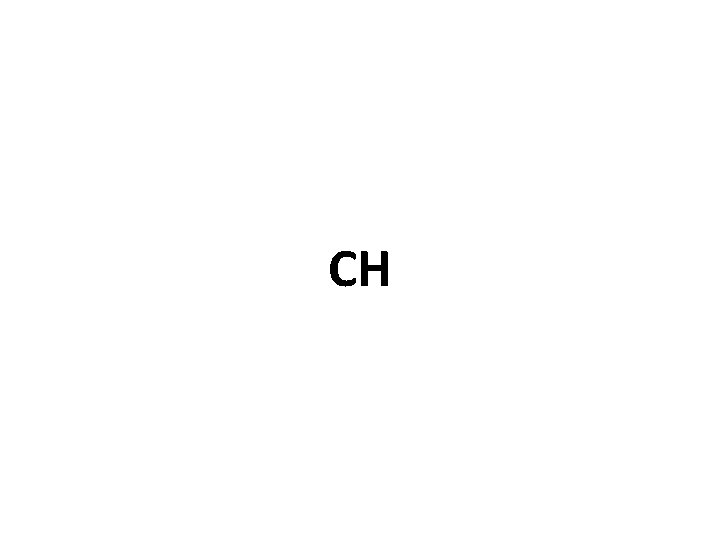
Practicing Empirical Formulas… Molecule formula = C 9 H 18 N 6 What is the empirical (simplified) formula?

C 3 H 6 N 2

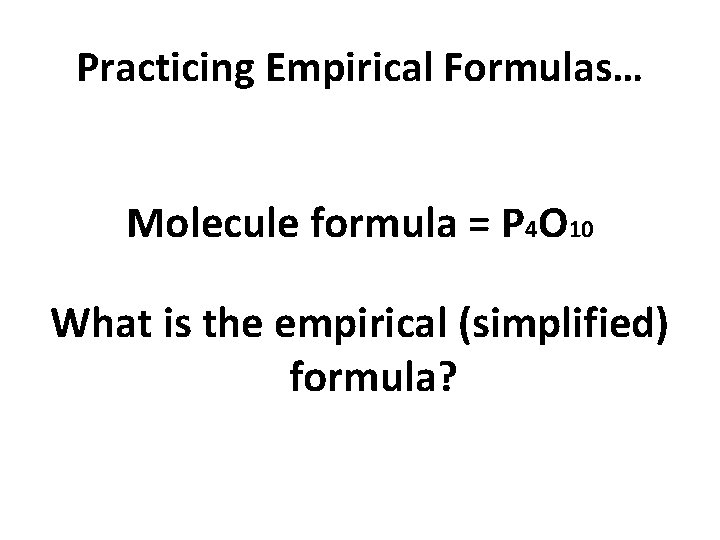
Practicing Empirical Formulas… Molecule formula = P 4 O 10 What is the empirical (simplified) formula?

P 2 O 5