About Formulas Types of Bonding Ionic Covalent Metallic

About Formulas
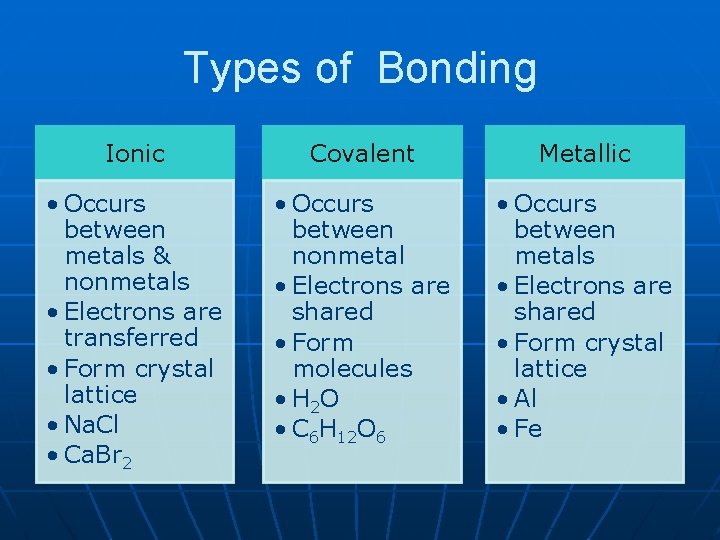
Types of Bonding Ionic Covalent Metallic • Occurs between metals & nonmetals • Electrons are transferred • Form crystal lattice • Na. Cl • Ca. Br 2 • Occurs between nonmetal • Electrons are shared • Form molecules • H 2 O • C 6 H 12 O 6 • Occurs between metals • Electrons are shared • Form crystal lattice • Al • Fe
Metals vs. Nonmetals n Most of the elements in the periodic table are metals. • Except for H, everything to the left of the staircase is a metal n Nonmetals are located to the right of the staircase.

Crystal Lattice vs. Molecule n n Molecule: Discrete unit, definite number of atoms. All molecules of a given compound are identical. Exact formula. Crystal Lattice: Regular, repetitive, 3 -D arrangement of atoms, ions, or molecules. No set size. Not perfect. No two exactly alike. • Formula gives smallest whole number ratios. (Formula unit). “Exact” formulas NOT useful.

2 -D repetitive patterns
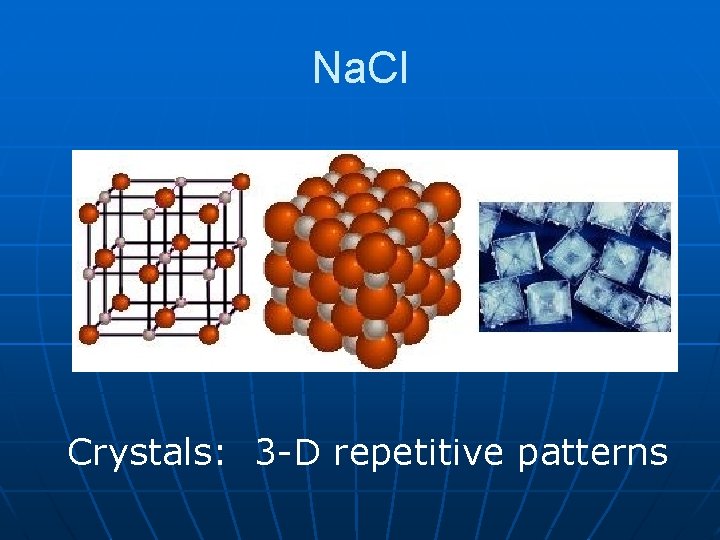
Na. Cl Crystals: 3 -D repetitive patterns

Formulas n n Tell the type & number of atoms in a compound. Microscopic level: imagine 1 atom, molecule, or formula unit. • Formula gives atom ratios. n Macroscopic level: imagine working in the lab • Formula gives mole ratio

Formulas n Ex: 2 H 2 O can mean • 2 molecules of water • 2 moles of water. 2 H 2 O molecules have 4 hydrogen atoms & 2 oxygen atoms. 2 Moles of H 2 O molecules have 4 moles of hydrogen atoms & 2 moles of oxygen atoms.

Ionic vs. Covalent Formulas n By looking at the type of atoms, we can decide if the substance is an ionic compound or a molecular (covalent) compound. Molecular compounds usually have all nonmetals. Ionic compounds usually have metal + nonmetal.
Empirical Formula n n n Smallest whole number ratio of the elements in the compound. Ionic compounds have only empirical formulas. Covalent compounds have empirical and molecular formulas. They can be the same or different.
Molecular Formulas n n n For covalent (molecular) compounds. Give the exact composition of the molecule. Molecular compounds have both empirical and molecular formulas. They can be the same or different.
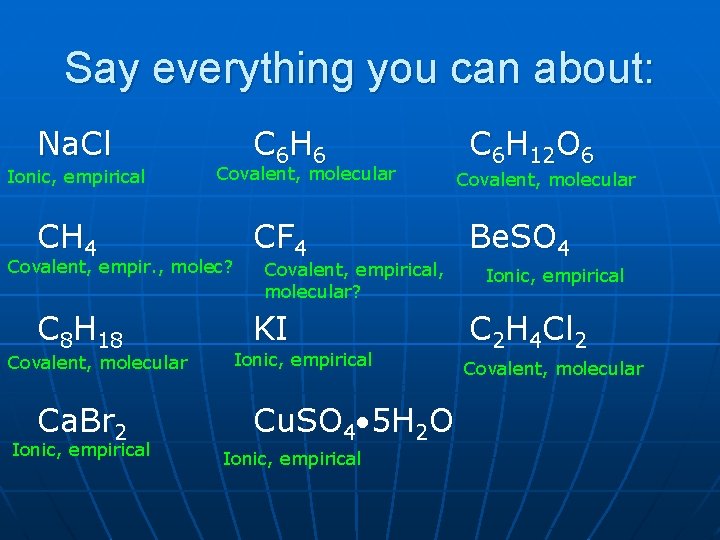
Say everything you can about: Na. Cl Ionic, empirical CH 4 C 6 H 6 Covalent, molecular Covalent, empir. , molec? C 8 H 18 Covalent, molecular Ca. Br 2 Ionic, empirical CF 4 Covalent, empirical, molecular? KI Ionic, empirical Cu. SO 4 5 H 2 O Ionic, empirical C 6 H 12 O 6 Covalent, molecular Be. SO 4 Ionic, empirical C 2 H 4 Cl 2 Covalent, molecular
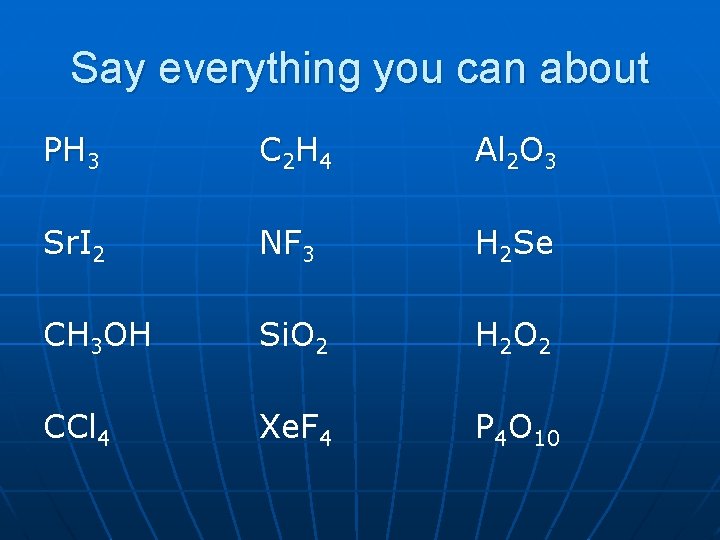
Say everything you can about PH 3 C 2 H 4 Al 2 O 3 Sr. I 2 NF 3 H 2 Se CH 3 OH Si. O 2 H 2 O 2 CCl 4 Xe. F 4 P 4 O 10

Empirical Formulas from % Composition n n n Convert to mass. Convert to moles. Divide by small. Multiply ‘til whole. Note 1: last step not always used. Note 2: sometimes you start at step 2, if they give analysis data in grams.
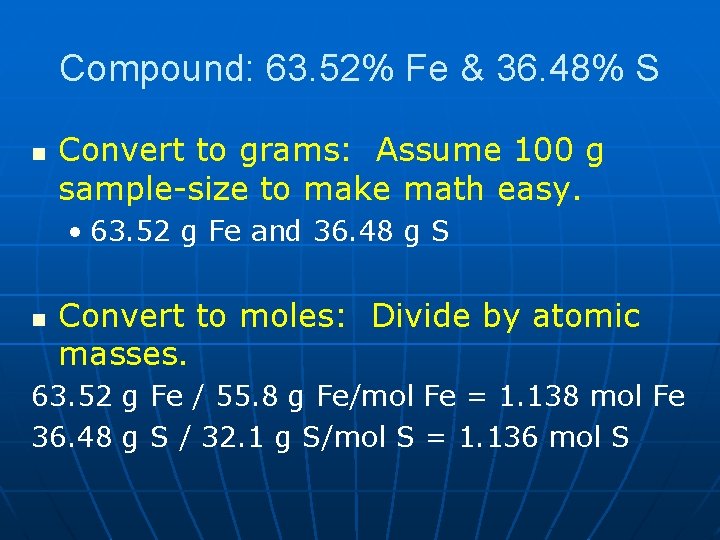
Compound: 63. 52% Fe & 36. 48% S n Convert to grams: Assume 100 g sample-size to make math easy. • 63. 52 g Fe and 36. 48 g S n Convert to moles: Divide by atomic masses. 63. 52 g Fe / 55. 8 g Fe/mol Fe = 1. 138 mol Fe 36. 48 g S / 32. 1 g S/mol S = 1. 136 mol S

Compound: 63. 52% Fe & 36. 48% S n Divide by small: This is where you take the ratio. • 1. 136 and 1. 138 are essentially the same. Divide both by 1. 136 and get 1 for each. • Empirical formula is Fe. S.

26. 56 % K, 35. 41% Cr, & remainder O n Assume 100 -g sample size. • 26. 56 g K • 35. 41 g Cr • 38. 03 g O n Convert to moles. 26. 56 g K / 39. 1 g K/mol K = 0. 679 mol K 35. 41 g Cr / 52. 0 g Cr/mol Cr = 0. 681 mol Cr 38. 03 g O / 16. 0 g O/mol O = 2. 38 mol O

26. 56 % K, 35. 41% Cr, & remainder O n Divide by small: • 0. 679 and 0. 681 are essentially the same. • Much smaller than 2. 38. • Divide each by 0. 68 • K: 1, Cr: 1, O: 3. 5 KCr. O 3. 5? No dec’s n n Multiply ‘til whole: K 2 Cr 2 O 7. Multiply all three by 2, doesn’t change relationship.
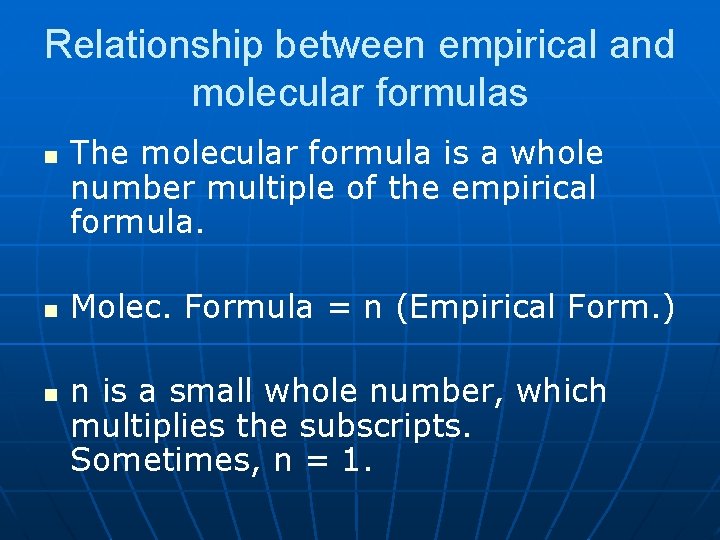
Relationship between empirical and molecular formulas n n n The molecular formula is a whole number multiple of the empirical formula. Molec. Formula = n (Empirical Form. ) n is a small whole number, which multiplies the subscripts. Sometimes, n = 1.
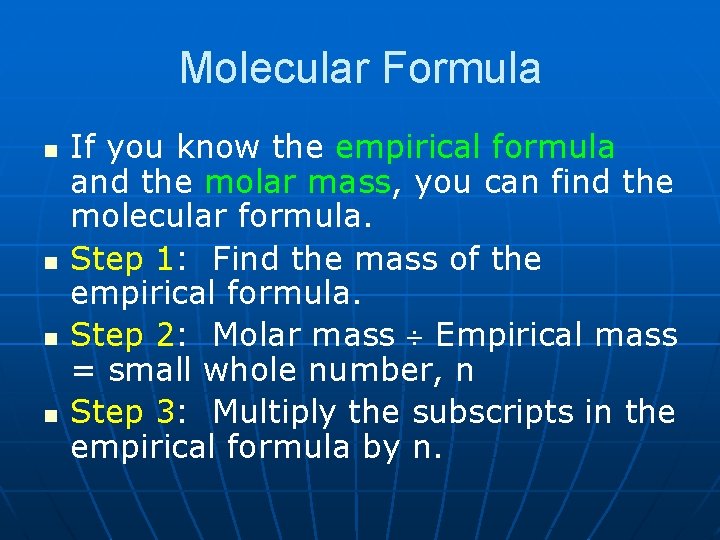
Molecular Formula n n If you know the empirical formula and the molar mass, you can find the molecular formula. Step 1: Find the mass of the empirical formula. Step 2: Molar mass Empirical mass = small whole number, n Step 3: Multiply the subscripts in the empirical formula by n.
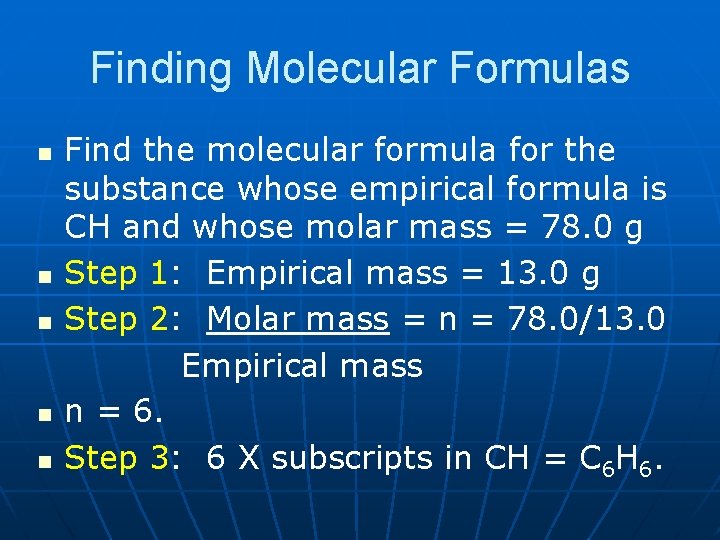
Finding Molecular Formulas n n n Find the molecular formula for the substance whose empirical formula is CH and whose molar mass = 78. 0 g Step 1: Empirical mass = 13. 0 g Step 2: Molar mass = n = 78. 0/13. 0 Empirical mass n = 6. Step 3: 6 X subscripts in CH = C 6 H 6.

What can you say about Cu. SO 4 5 H 2 O? n n n It’s a hydrated salt. For every mole of Cu. SO 4, there are 5 moles of water. If it’s heated, it dries out. The water goes into the air and you’re left with the anhydrous salt, Cu. SO 4 5 H 2 O Cu. SO 4 + 5 H 2 O

Cu. SO 4 5 H 2 O Cu. SO 4 + 5 H 2 O n The mole ratio in the formula can be used to predict how much water would be given off by any size sample. • If you had 2 moles of Cu. SO 4 5 H 2 O, how much water would you lose on heating? • If you had 5 moles of Cu. SO 4 5 H 2 O, how much water would you lose?
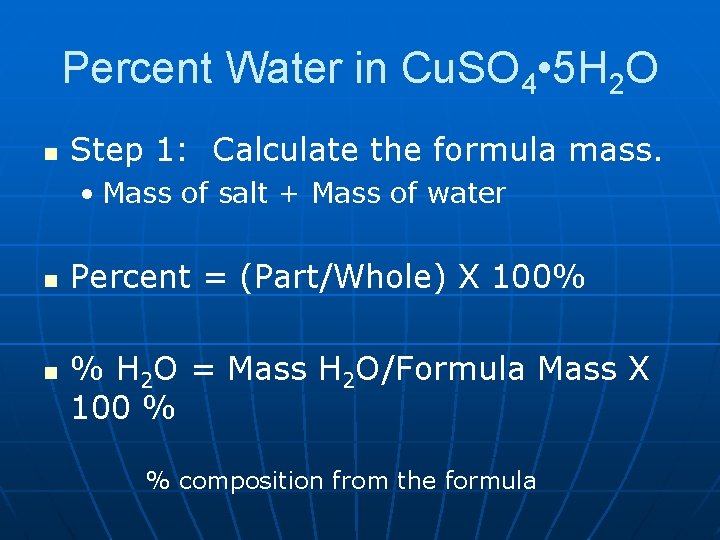
Percent Water in Cu. SO 4 • 5 H 2 O n Step 1: Calculate the formula mass. • Mass of salt + Mass of water n n Percent = (Part/Whole) X 100% % H 2 O = Mass H 2 O/Formula Mass X 100 % % composition from the formula
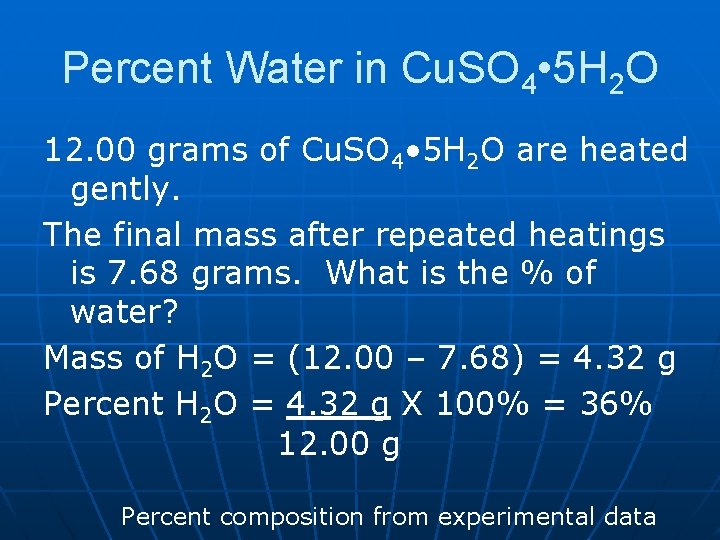
Percent Water in Cu. SO 4 • 5 H 2 O 12. 00 grams of Cu. SO 4 • 5 H 2 O are heated gently. The final mass after repeated heatings is 7. 68 grams. What is the % of water? Mass of H 2 O = (12. 00 – 7. 68) = 4. 32 g Percent H 2 O = 4. 32 g X 100% = 36% 12. 00 g Percent composition from experimental data

Cu. SO 4 x. H 2 O Cu. SO 4 + x. H 2 O n n Use experimental data to find x: 12. 00 grams of Cu. SO 4 • 5 H 2 O are heated gently. (Hydrated salt. ) 7. 68 g = mass anhydrous salt remaining = mass Cu. SO 4. Mass of H 2 O = (12. 00 – 7. 68) = 4. 32 g

Cu. SO 4 x. H 2 O Cu. SO 4 + x. H 2 O n n Note: moles of Cu. SO 4 & moles of H 2 O can be determined. Have grams already. • Convert to moles • Divide by small • Multiply ‘til whole.
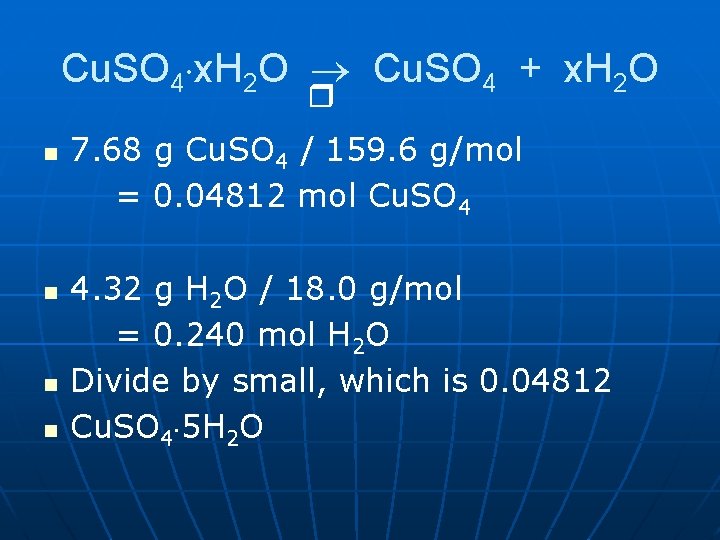
Cu. SO 4 x. H 2 O Cu. SO 4 + x. H 2 O n n 7. 68 g Cu. SO 4 / 159. 6 g/mol = 0. 04812 mol Cu. SO 4 4. 32 g H 2 O / 18. 0 g/mol = 0. 240 mol H 2 O Divide by small, which is 0. 04812 Cu. SO 4 5 H 2 O
- Slides: 29