Chapter 7 Ionic and Metallic Bonding Section 7

Chapter 7 “Ionic and Metallic Bonding”

Section 7. 1 - Ions l OBJECTIVES: –Determine the number of valence electrons in an atom of a representative element.

Section 7. 1 - Ions l OBJECTIVES: –Explain how the octet rule applies to atoms of metallic and nonmetallic elements.

Section 7. 1 - Ions l OBJECTIVES: –Describe how cations form.

Section 7. 1 - Ions l OBJECTIVES: –Explain how anions form.

Valence Electrons are…? l The electrons responsible for the chemical properties of atoms, and are those in the outer energy level. l Valence electrons - The s and p electrons in the outer energy level – the highest occupied energy level l Core electrons – are those in the energy levels below.

Keeping Track of Electrons l l l Atoms in the same column. . . 1) Have the same outer electron configuration. 2) Have the same valence electrons. The number of valence electrons are easily determined. It is the group number for a representative element Group 2 A: Be, Mg, Ca, etc. – have 2 valence electrons
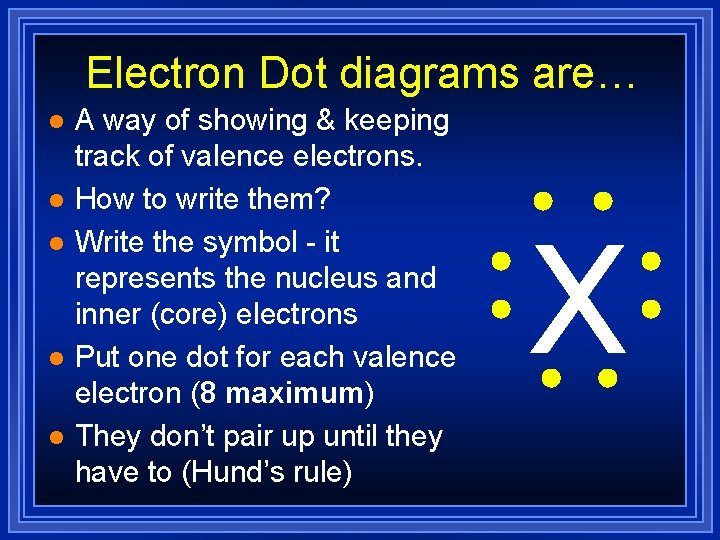
Electron Dot diagrams are… l l l A way of showing & keeping track of valence electrons. How to write them? Write the symbol - it represents the nucleus and inner (core) electrons Put one dot for each valence electron (8 maximum) They don’t pair up until they have to (Hund’s rule) X

The Electron Dot diagram for Nitrogen has 5 valence electrons to show. l First we write the symbol. l. Then add 1 electron at a time to each side. l. Now they are forced to pair up. l. We have now written the electron dot diagram for Nitrogen. l N

The Octet Rule In Chapter 6, we learned that noble gases are unreactive in chemical reactions l In 1916, Gilbert Lewis used this fact to explain why atoms form certain kinds of ions and molecules l The Octet Rule: in forming compounds, atoms tend to achieve a noble gas configuration; 8 in the outer level is stable l Each noble gas (except He, which has 2) has 8 electrons in the outer level l

Formation of Cations l Metals lose electrons to attain a noble gas configuration. l They make positive ions (cations) l If we look at the electron configuration, it makes sense to lose electrons: l Na 1 s 22 p 63 s 1 1 valence electron l Na 1+ 1 s 22 p 6 This is a noble gas configuration with 8 electrons in the outer level.

Electron Dots For Cations l Metals will have few valence electrons (usually 3 or less); calcium has only 2 valence electrons Ca

Electron Dots For Cations Metals will have few valence electrons l Metals will lose the valence electrons l Ca

Electron Dots For Cations Metals will have few valence electrons l Metals will lose the valence electrons l Forming positive ions l 2+ Ca This is named the “calcium ion”. NO DOTS are now shown for the cation.

Electron Dots For Cations l Let’s do Scandium, #21 l The electron configuration is: 2 2 6 2 1 1 s 2 s 2 p 3 s 3 p 4 s 3 d l Thus, it can lose 2 e- (making it 2+), or lose 3 e (making 3+) Sc = 2+ Sc Scandium (II) ion Sc = 3+ Sc Scandium (III) ion

Electron Dots For Cations l. Let’s do Silver, element #47 l. Predicted configuration is: 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 9 l. Actual configuration is: 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 14 d 10 Ag = 1+ Ag (can’t lose any more, charges of 3+ or greater are uncommon)

Electron Dots For Cations l Silver did the best job it could, but it did not achieve a true Noble Gas configuration l Instead, it is called a “pseudo-noble gas configuration”
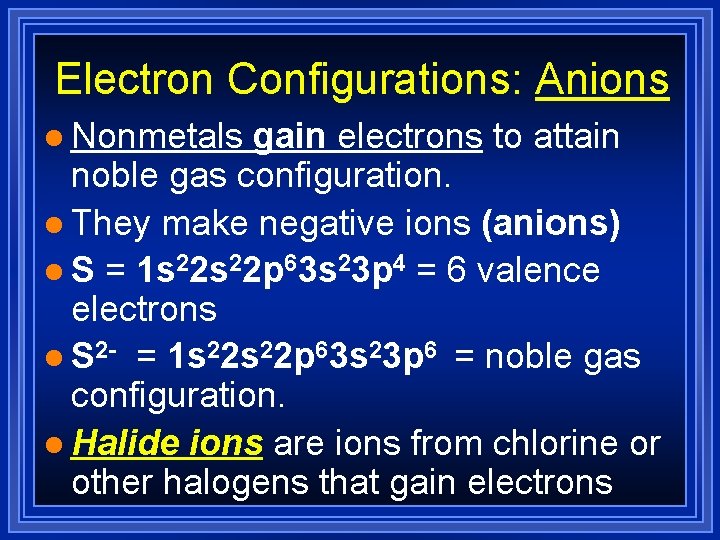
Electron Configurations: Anions l Nonmetals gain electrons to attain noble gas configuration. l They make negative ions (anions) l S = 1 s 22 p 63 s 23 p 4 = 6 valence electrons l S 2 - = 1 s 22 p 63 s 23 p 6 = noble gas configuration. l Halide ions are ions from chlorine or other halogens that gain electrons

Electron Dots For Anions Nonmetals will have many valence electrons (usually 5 or more) l They will gain electrons to fill outer shell. l P 3 - (This is called the “phosphide ion”, and should show dots)

Stable Electron Configurations All atoms react to try and achieve a noble gas configuration. l Noble gases have 2 s and 6 p electrons. l 8 valence electrons = already stable! l This is the octet rule (8 in the outer level is particularly stable). l Ar

Section 7. 2 Ionic Bonds and Ionic Compounds l OBJECTIVES: –Explain the electrical charge of an ionic compound.

Section 7. 2 Ionic Bonds and Ionic Compounds l OBJECTIVES: –Describe three properties of ionic compounds.
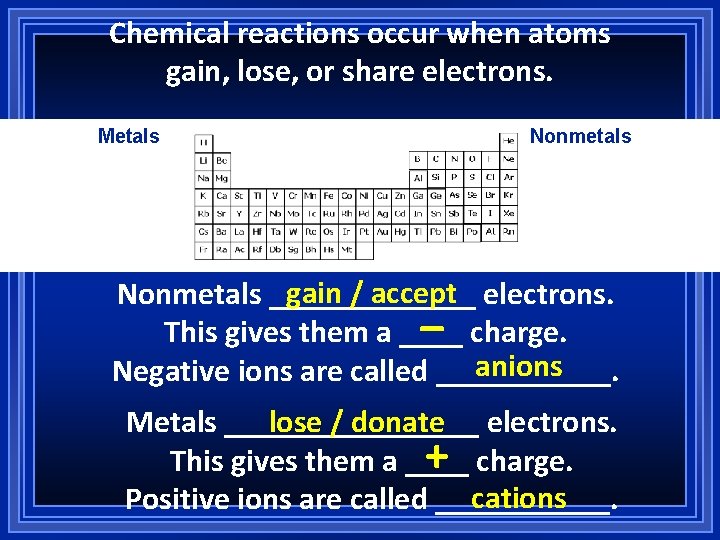
Chemical reactions occur when atoms gain, lose, or share electrons. Metals Nonmetals gain / accept electrons. Nonmetals _______ This gives them a ____ charge. anions Negative ions are called ______. _ Metals ________ lose / donate electrons. + charge. This gives them a ____ cations Positive ions are called ______.
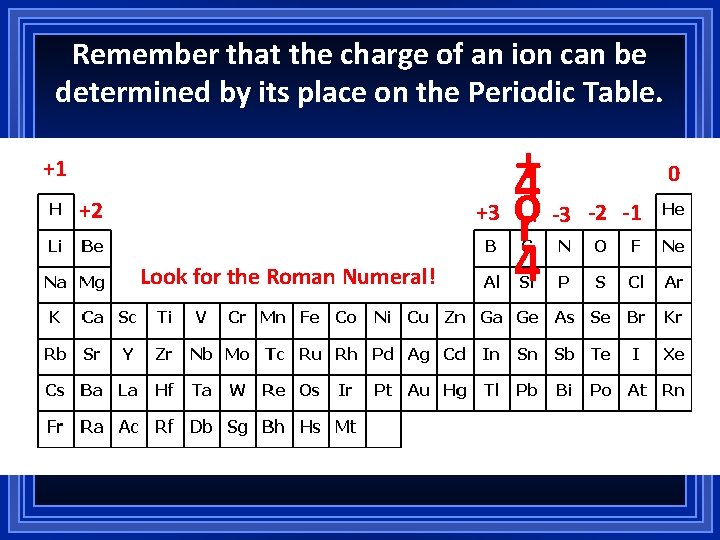
Remember that the charge of an ion can be determined by its place on the Periodic Table. +1 +2 Look for the Roman Numeral! + 4 +3 o -3 r 4 0 -2 -1
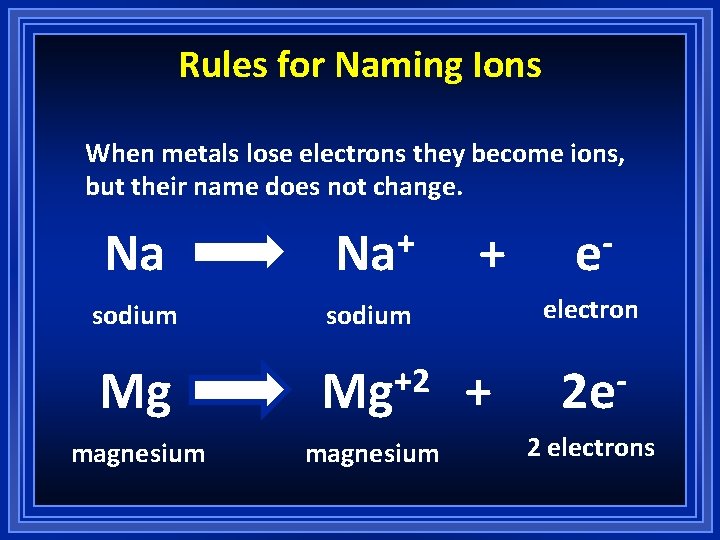
Rules for Naming Ions When metals lose electrons they become ions, but their name does not change. Na + sodium electron Mg +2 Mg 2 e magnesium + e 2 electrons
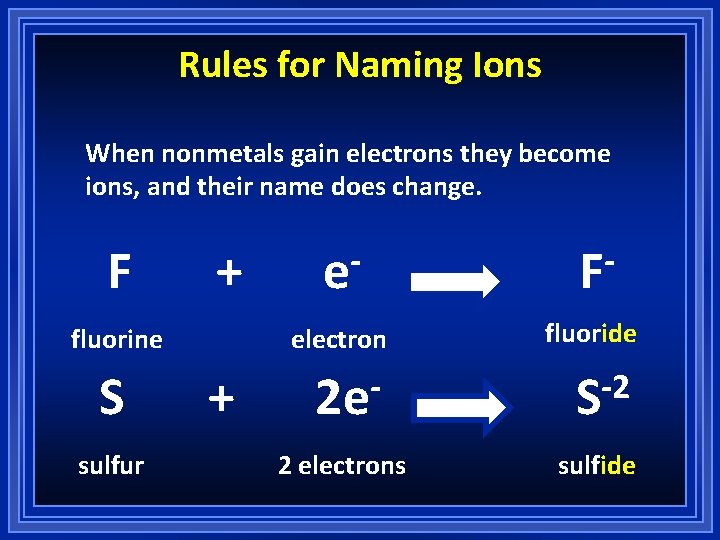
Rules for Naming Ions When nonmetals gain electrons they become ions, and their name does change. F + fluorine S sulfur + e F electron fluoride 2 e -2 S 2 electrons sulfide

Rules for Naming Ions 1. The names of metals do not change. 2. Changing the name of nonmetals: root of element name + -ide = name of ion Examples: The name of chlorine’s ion: chlor- + -ide = chloride The name of nitrogen’s ion: nitr- + -ide = nitride

Examples of naming ions: The name of calcium’s ion: calcium (The names of metals don’t change!) The name of oxygen’s ion: ox- + -ide = oxide The name of aluminum’s ion: aluminum (The names of metals don’t change!)

Write the name of each of the ions on your notes. sulfide nitride potassium oxide lithium bromide chloride hydrogen (+), hydride (-)

Steps for Naming Ionic Compounds Ca. Br 2 calcium bromide Step 1: Write the name of the metal ion. Step 2: Write the name of the nonmetal ion. Step 3: YOU ARE DONE! It is that easy.
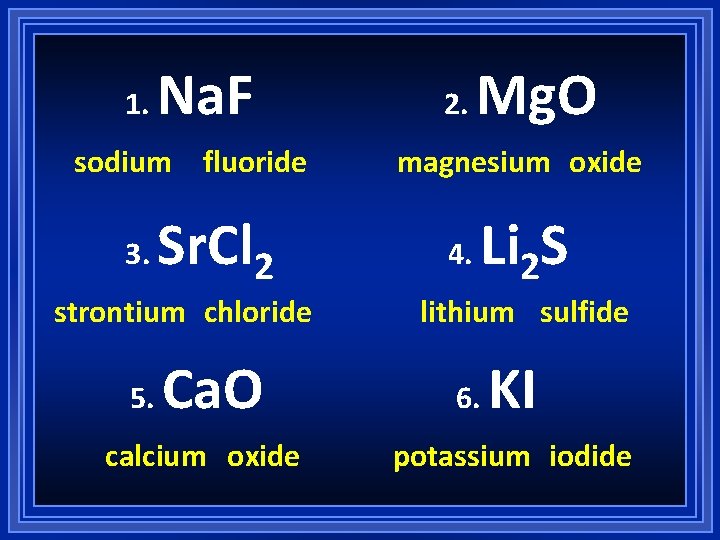
1. Na. F sodium fluoride 3. Sr. Cl 2 strontium chloride 5. Ca. O calcium oxide 2. Mg. O magnesium oxide 4. Li 2 S lithium sulfide 6. KI potassium iodide

You can also determine the formula of an ionic compound from its name. To do this, you will need to use what you already know about the Periodic Table. magnesium iodide +2 Mg I Mg. I 2 - Step 1: Write the symbol of the metal ion. Step 2: Write the symbol of the nonmetal ion. Step 3: Determine the charges using the periodic table. Step 4: Determine the formula from the ions.

Remember that the names of transition metals include their charge because their charges are less predictable. What are the charges of the transition metals below: Iron (II) _______ +2 +2 Copper (II) _______ Tin (IV) _______ +4 +2 Lead (II) _______ Iron (III) _______ +3 +1 Copper (I) _______ Tin (II) _______ +2 +4 Lead (IV) _______ We know they are positive because metals are always positive.
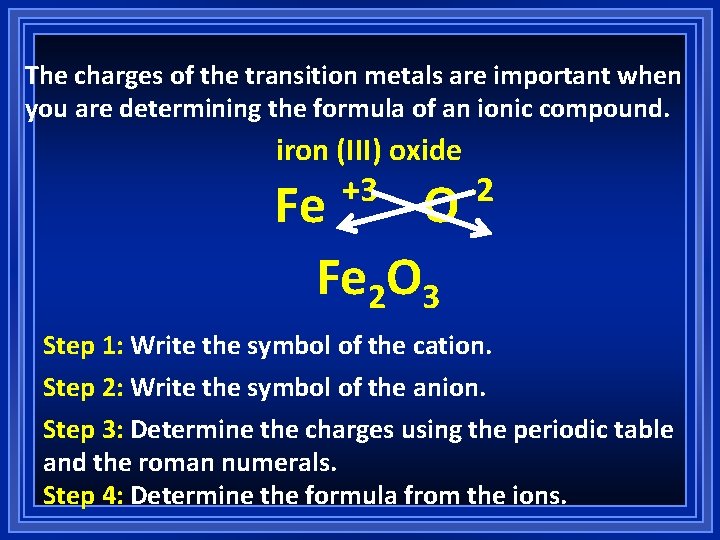
The charges of the transition metals are important when you are determining the formula of an ionic compound. iron (III) oxide +3 Fe O Fe 2 O 3 -2 Step 1: Write the symbol of the cation. Step 2: Write the symbol of the anion. Step 3: Determine the charges using the periodic table and the roman numerals. Step 4: Determine the formula from the ions.

l Anions Ionic Bonding and cations are held together by opposite charges (+ and -) compounds are called salts. l Simplest ratio of elements in an ionic compound is called the formula unit. l The bond is formed through the transfer of electrons (lose and gain) l Electrons are transferred to achieve noble gas configuration. l Ionic

Ionic Compounds Also called SALTS 2) Made from: a CATION with an ANION (or literally from a metal combining with a nonmetal) 1)

Ionic Bonding Na Cl The metal (sodium) tends to lose its one electron from the outer level. The nonmetal (chlorine) needs to gain one more to fill its outer level, and will accept the one electron that sodium is going to lose.

Ionic Bonding + Na Cl - Note: Remember that NO DOTS are now shown for the cation!

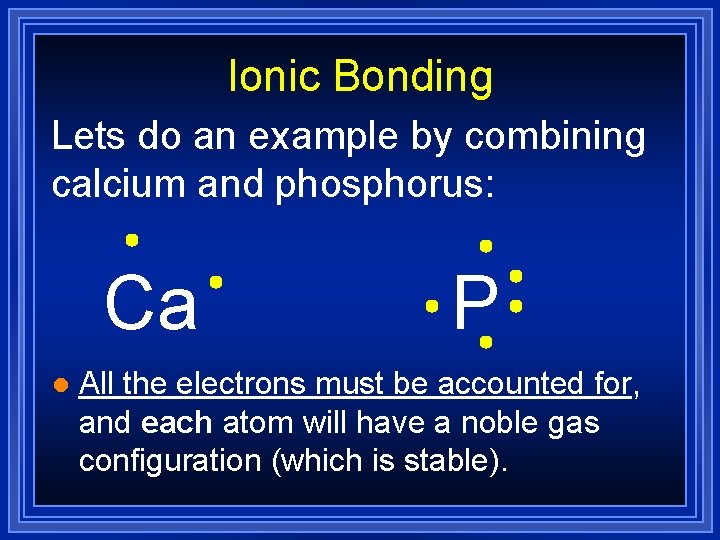
Ionic Bonding Lets do an example by combining calcium and phosphorus: Ca l P All the electrons must be accounted for, and each atom will have a noble gas configuration (which is stable).
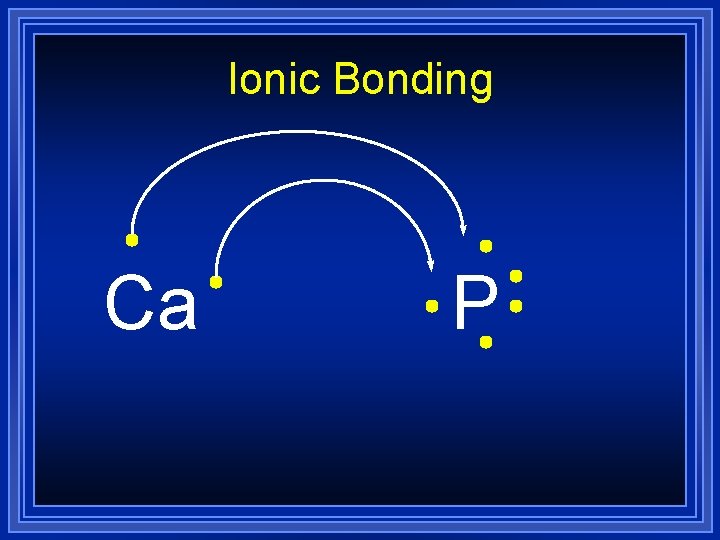
Ionic Bonding Ca P
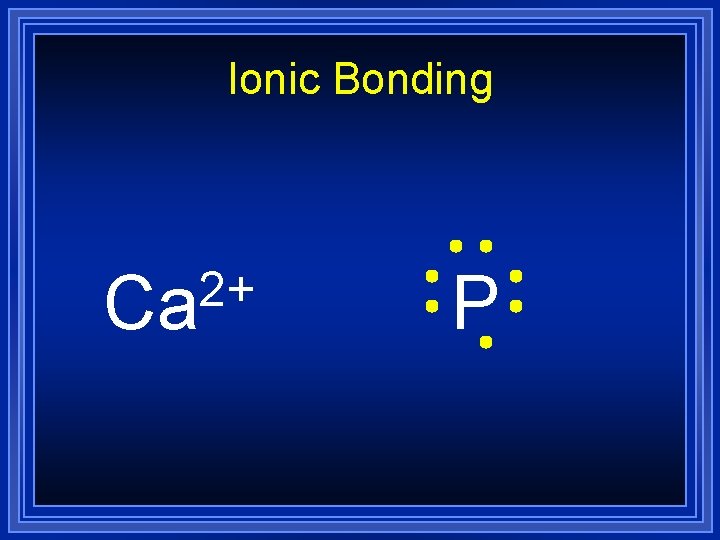
Ionic Bonding 2+ Ca P
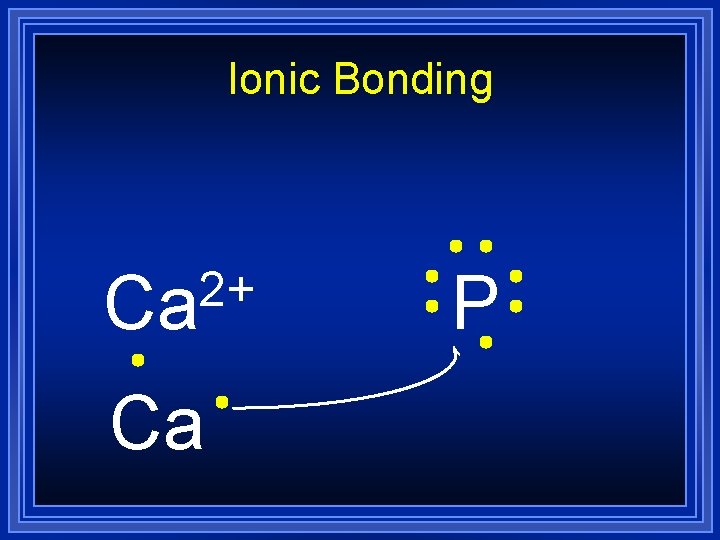
Ionic Bonding 2+ Ca Ca P
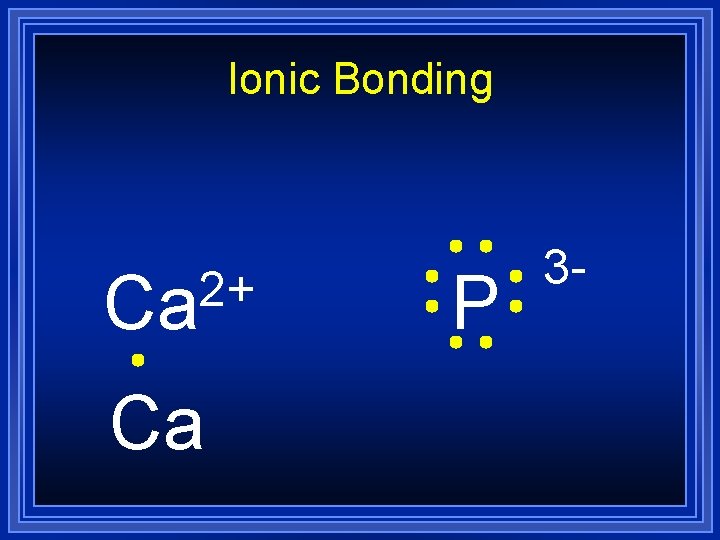
Ionic Bonding 2+ Ca Ca P 3 -
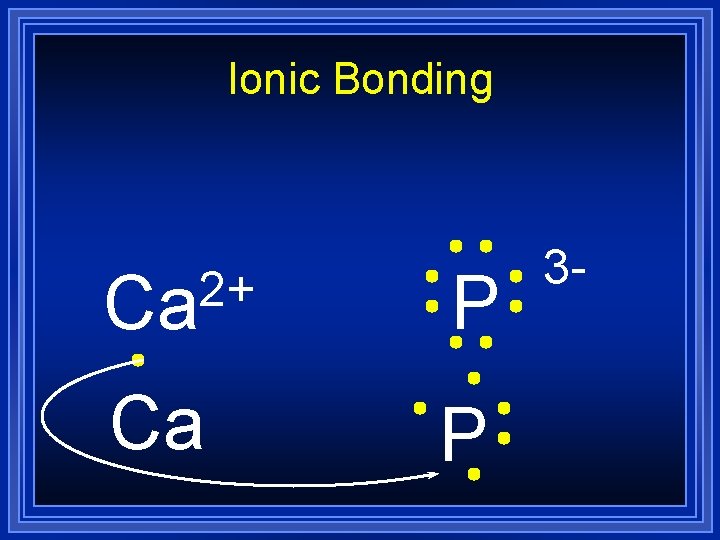
Ionic Bonding 2+ Ca P 3 -
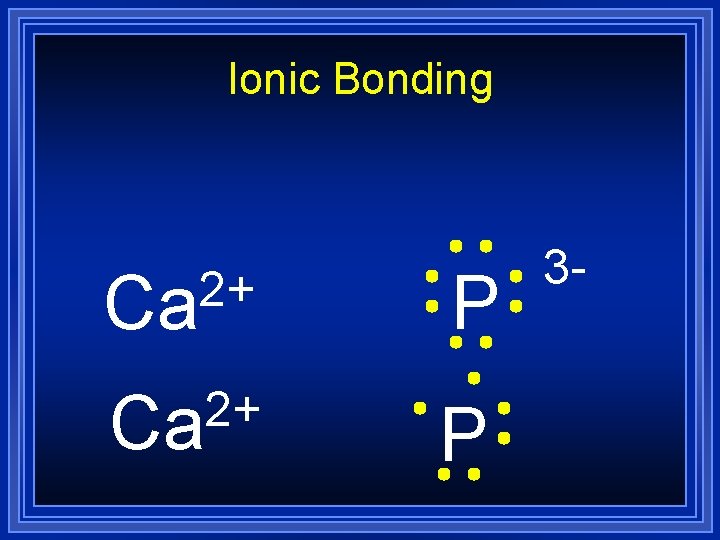
Ionic Bonding 2+ Ca P 3 -
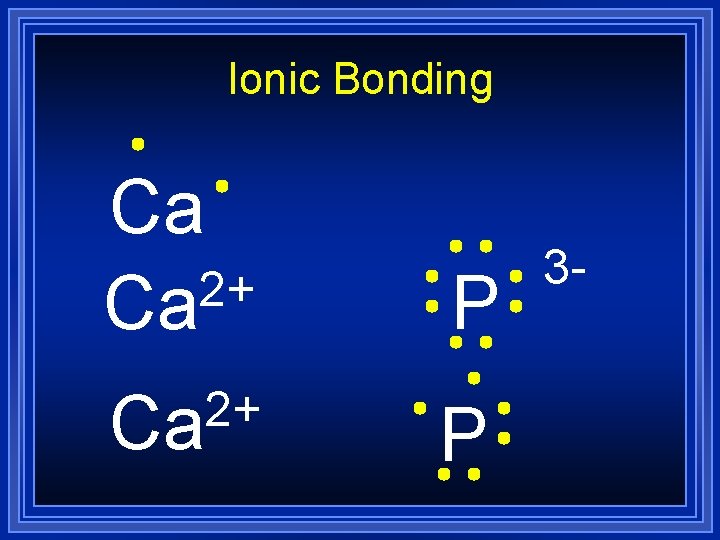
Ionic Bonding Ca 2+ Ca P 3 -
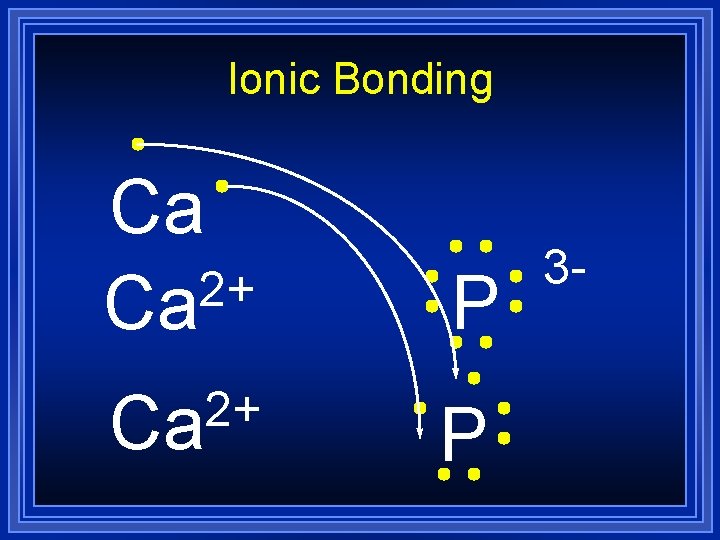
Ionic Bonding Ca 2+ Ca P 3 -
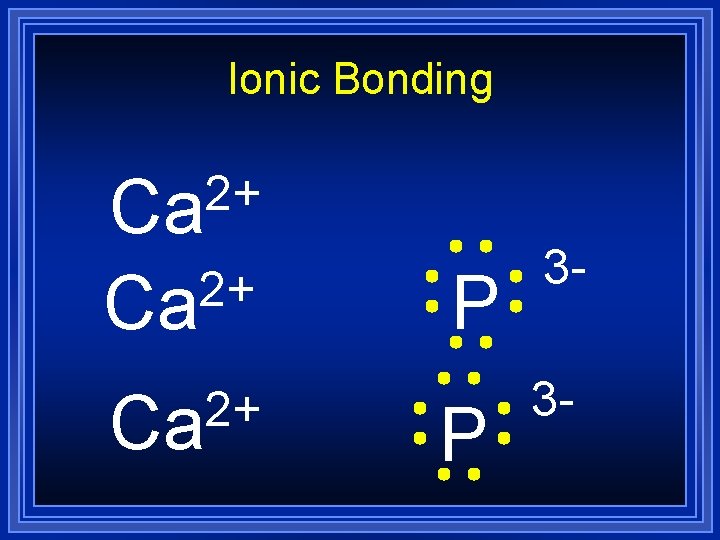
Ionic Bonding 2+ Ca P 3 -

Ionic Bonding = Ca 3 P 2 Formula Unit This is a chemical formula, which shows the kinds and numbers of atoms in the smallest representative particle of the substance. For an ionic compound, the smallest representative particle is called a: Formula Unit

Properties of Ionic Compounds 1. 2. l Crystalline solids - a regular repeating arrangement of ions in the solid: Fig. 7. 9, page 197 – Ions are strongly bonded together. – Structure is rigid. High melting points Coordination number- number of ions of opposite charge surrounding it
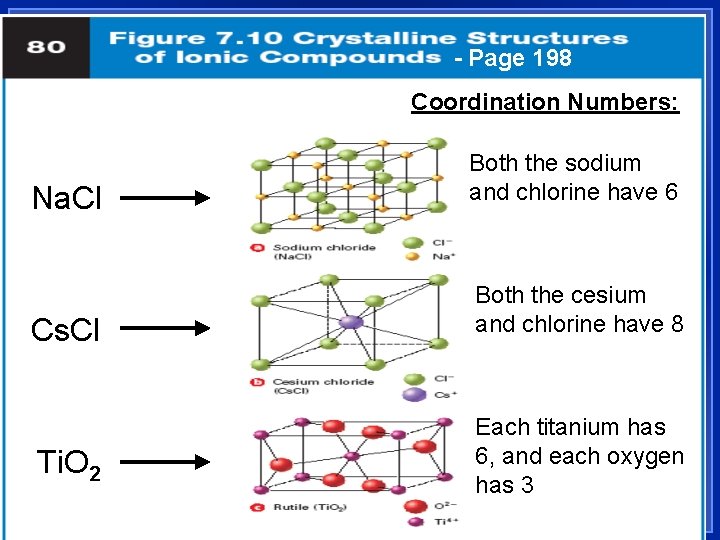
- Page 198 Coordination Numbers: Na. Cl Both the sodium and chlorine have 6 Cs. Cl Both the cesium and chlorine have 8 Ti. O 2 Each titanium has 6, and each oxygen has 3

Do they Conduct? l l 3. Conducting electricity means allowing charges to move. In a solid, the ions are locked in place. Ionic solids are insulators. When melted, the ions can move around. Melted ionic compounds conduct. – Na. Cl: must get to about 800 ºC. – Dissolved in water, they also conduct (free to move in aqueous solutions)
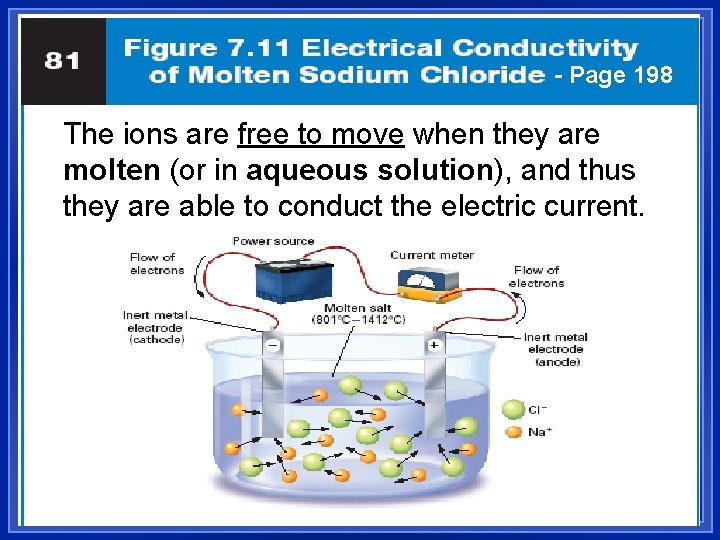
- Page 198 The ions are free to move when they are molten (or in aqueous solution), and thus they are able to conduct the electric current.
Section 7. 3 Bonding in Metals l OBJECTIVES: –Model the valence electrons of metal atoms.

Section 7. 3 Bonding in Metals l OBJECTIVES: –Describe the arrangement of atoms in a metal.
Section 7. 3 Bonding in Metals l OBJECTIVES: –Explain the importance of alloys.

Metallic Bonds are… l How metal atoms are held together in the solid. l Metals hold on to their valence electrons very weakly. l Think of them as positive ions (cations) floating in a sea of electrons: Fig. 7. 12, p. 201
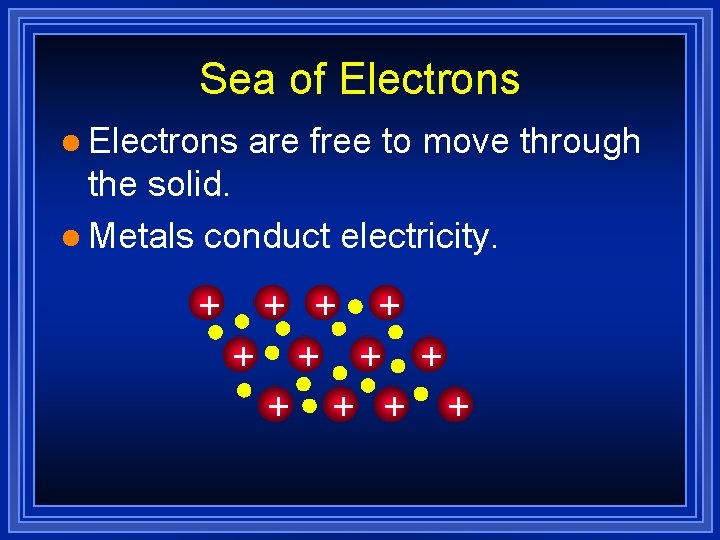
Sea of Electrons l Electrons are free to move through the solid. l Metals conduct electricity. + + +

Metals are Malleable l Hammered into shape (bend). l Also ductile - drawn into wires. l Both malleability and ductility explained in terms of the mobility of the valence electrons
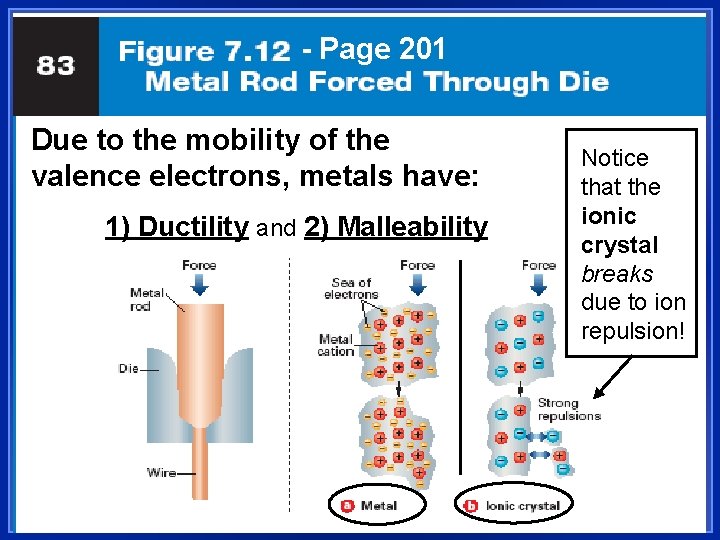
- Page 201 Due to the mobility of the valence electrons, metals have: 1) Ductility and 2) Malleability Notice that the ionic crystal breaks due to ion repulsion!
Malleable Force + + +
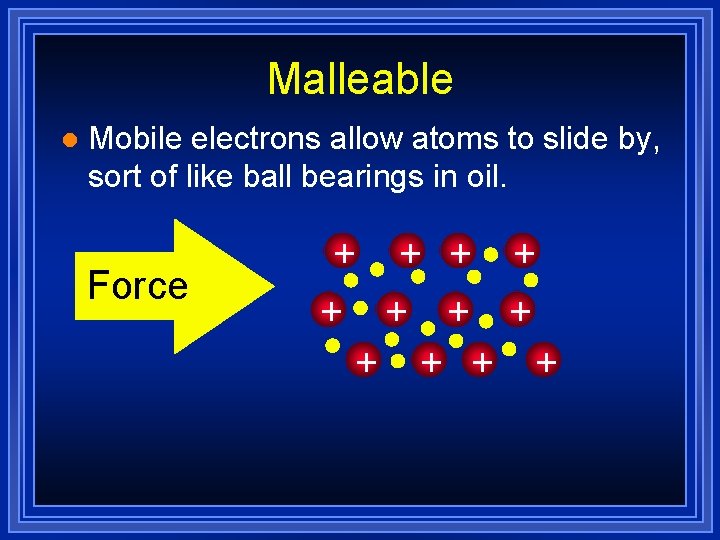
Malleable l Mobile electrons allow atoms to slide by, sort of like ball bearings in oil. Force + + +
Ionic solids are brittle Force + + - + +
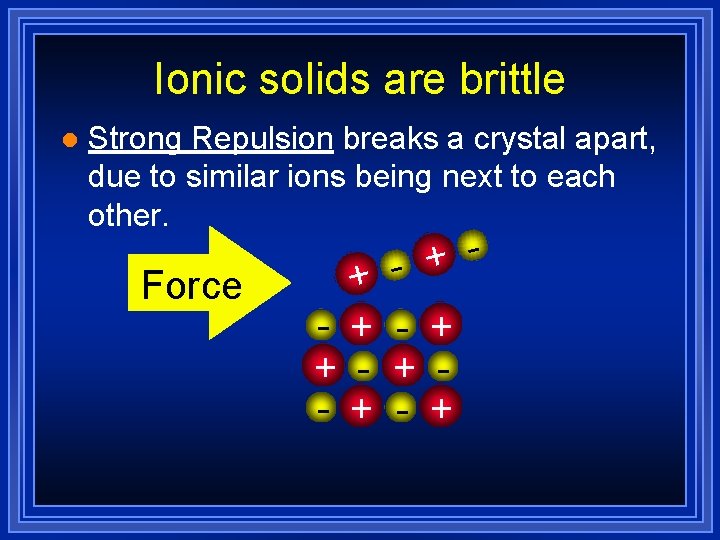
Ionic solids are brittle l Strong Repulsion breaks a crystal apart, due to similar ions being next to each other. Force + + - + - + - +

Crystalline structure of metal l If made of one kind of atom, metals are among the simplest crystals; very compact & orderly l Note Fig. 7. 14, p. 202 for types: 1. Body-centered cubic: –every atom (except those on the surface) has 8 neighbors –Na, K, Fe, Cr, W

Crystalline structure of metal 2. Face-centered cubic: – every atom has 12 neighbors – Cu, Ag, Au, Al, Pb 3. Hexagonal close-packed – every atom also has 12 neighbors – different pattern due to hexagonal – Mg, Zn, Cd

Alloys l We use lots of metals every day, but few are pure metals l Alloys are mixtures of 2 or more elements, at least 1 is a metal l made by melting a mixture of the ingredients, then cooling l Brass: an alloy of Cu and Zn l Bronze: Cu and Sn

Why use alloys? Properties are often superior to the pure element l Sterling silver (92. 5% Ag, 7. 5% Cu) is harder and more durable than pure Ag, but still soft enough to make jewelry and tableware l Steels are very important alloys – corrosion resistant, ductility, hardness, toughness, cost l

More about Alloys… l Table 7. 3, p. 203 – lists a few alloys l Types? a) substitutional alloy- the atoms in the components are about the same size l b) interstitial alloy- the atomic sizes quite different; smaller atoms fit into the spaces between larger l “Amalgam”- dental use, contains Hg

- Slides: 71