Covalent Bonds Why do atoms bond Atoms want

Covalent Bonds
Why do atoms bond? • Atoms want noble gas configuration (octet) • For ionic bonds there is a transfer of electrons to get an octet of electrons • For covalent bonds there is a sharing of electrons to get an octet
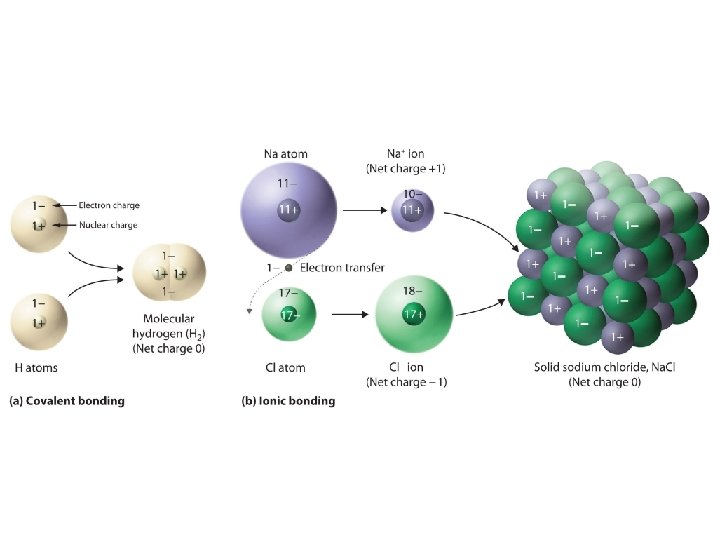
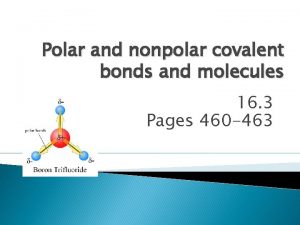
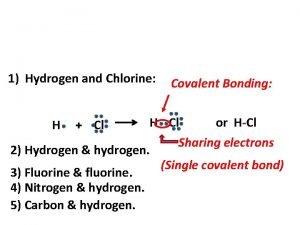
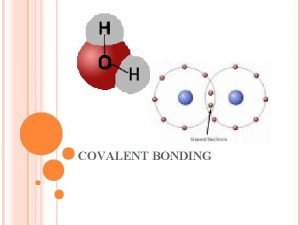
What is a covalent bond? • Covalent bond - is the chemical bond that results from sharing of valence electrons – Occurs with elements close to each other on the periodic table – Ex: H 2 O – Between a nonmetal and a nonmetal – Molecule is two or more atoms bonded covalently
Examples of Molecules • • • F 2 H 2 O NH 3 (ammonia) CH 4 (methane) Notice there are no metals, only non-metals
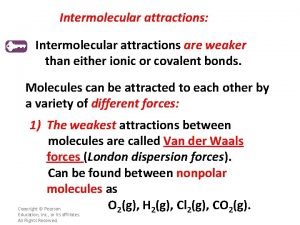
Diatomic molecules • Some atoms do not exist as a single atom • Atoms that exist as two H 2, O 2, N 2, Cl 2, Br 2, I 2, F 2 • HONCl. Br. IF • Magnificent 7 -don’t forget H

3 Types of Covalent Bonds • Single Covalent Bond • Double Covalent Bond • Triple Covalent Bond
3 Pencil Demo for Single, Double and Triple Covent bonds • Make as many observations as you can i. e. facial expressions, body position, muscles used, noises made, force or energy exerted, etc.
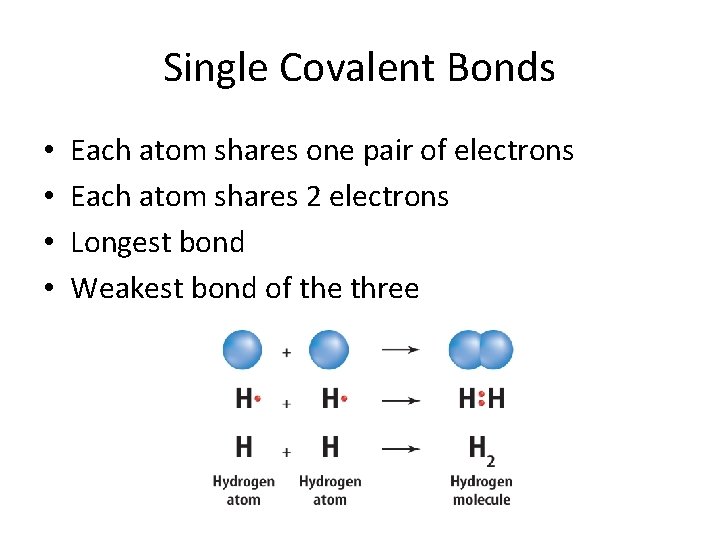
Single Covalent Bonds • • Each atom shares one pair of electrons Each atom shares 2 electrons Longest bond Weakest bond of the three
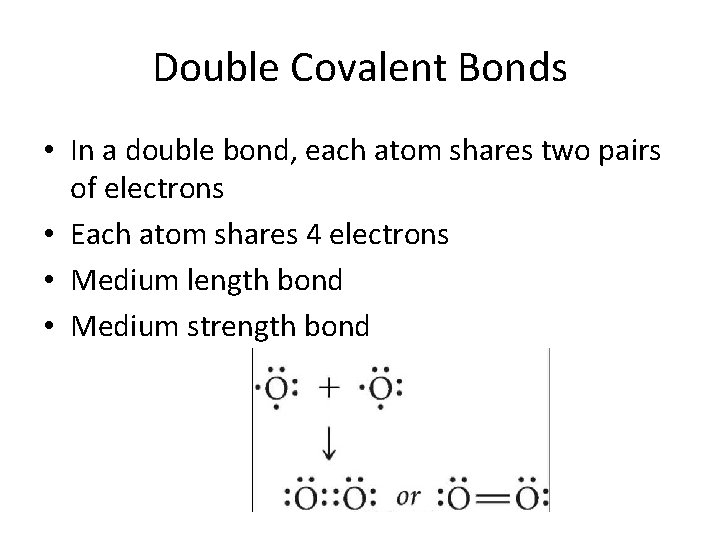
Double Covalent Bonds • In a double bond, each atom shares two pairs of electrons • Each atom shares 4 electrons • Medium length bond • Medium strength bond
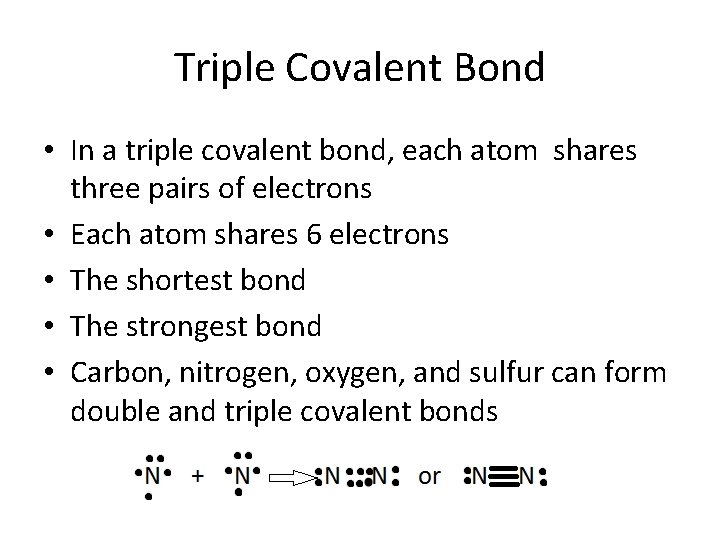
Triple Covalent Bond • In a triple covalent bond, each atom shares three pairs of electrons • Each atom shares 6 electrons • The shortest bond • The strongest bond • Carbon, nitrogen, oxygen, and sulfur can form double and triple covalent bonds

Covalent Molecule Properties • Covalent molecular solids tend to be soft solids, liquids, or gases at room temperature • Low melting and boiling points • Poor conductors of heat and electricity • Non-electrolytes – do not conduct electricity in water

Strength of Covalent Bond • Several factors control bond strength —Number of shared electrons-the more electrons shared, greater the bond —Size of the atom —Bond length – the greater the bond length, the weaker the bond
- Slides: 13