Ionic and Covalent Bonding Including Naming Note We

Ionic and Covalent Bonding Including Naming Note: We likely won’t make it to covalent nomenclature, this is the one students find FAR easier than ionic. Please refer to the videos and naming hand out for help with this, and as always office hours, discussions, and facebook for extra help. If you don’t remember it from high school/1 P.
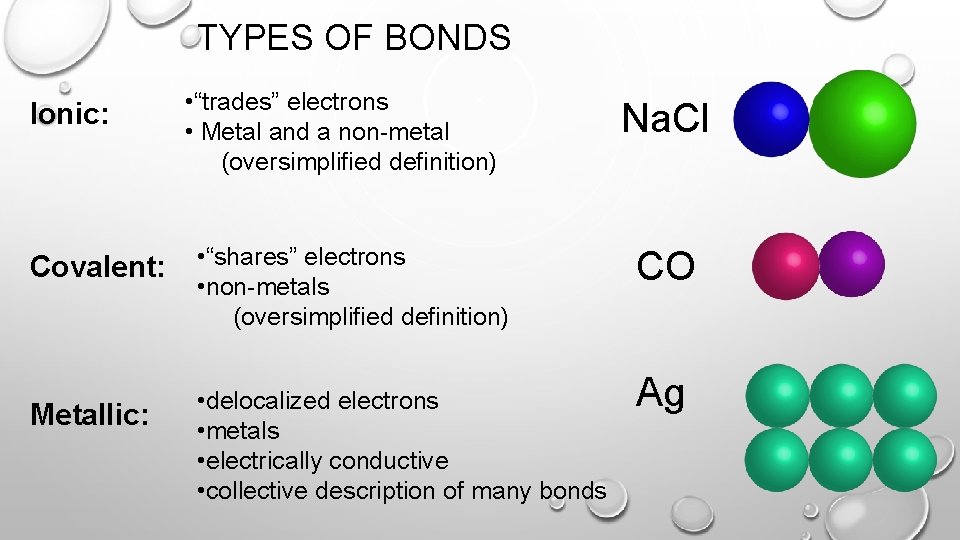
TYPES OF BONDS Ionic: • “trades” electrons • Metal and a non-metal (oversimplified definition) Covalent: • “shares” electrons • non-metals (oversimplified definition) Metallic: • delocalized electrons • metals • electrically conductive • collective description of many bonds Na. Cl CO Ag

For the Following Choose the Ionic Compounds (based on previous definition) • Na. Cl • CH 3 Cl • Mg. Cl 2 • SO 2 • Na 2 SO 3
For the binary ionic compounds in the last slide, decide which has more covalent character. Reminder note from videos (see slides included FYI): Na. Cl Mg. Cl 2 Summary of slides: The more polarizing power and polarizability lead to a bond with more covalent character. Small highly charged cations have more polarizing power. Large highly negatively charged cations have more polarizability. For cations which have more polarizability?
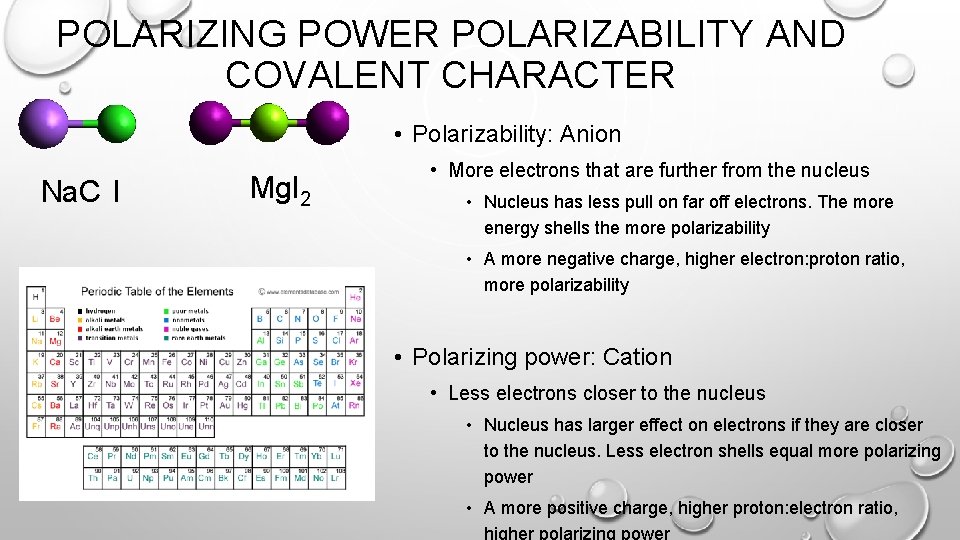
POLARIZING POWER POLARIZABILITY AND COVALENT CHARACTER • Polarizability: Anion Na. C l Mg. I 2 • More electrons that are further from the nucleus • Nucleus has less pull on far off electrons. The more energy shells the more polarizability • A more negative charge, higher electron: proton ratio, more polarizability • Polarizing power: Cation • Less electrons closer to the nucleus • Nucleus has larger effect on electrons if they are closer to the nucleus. Less electron shells equal more polarizing power • A more positive charge, higher proton: electron ratio,
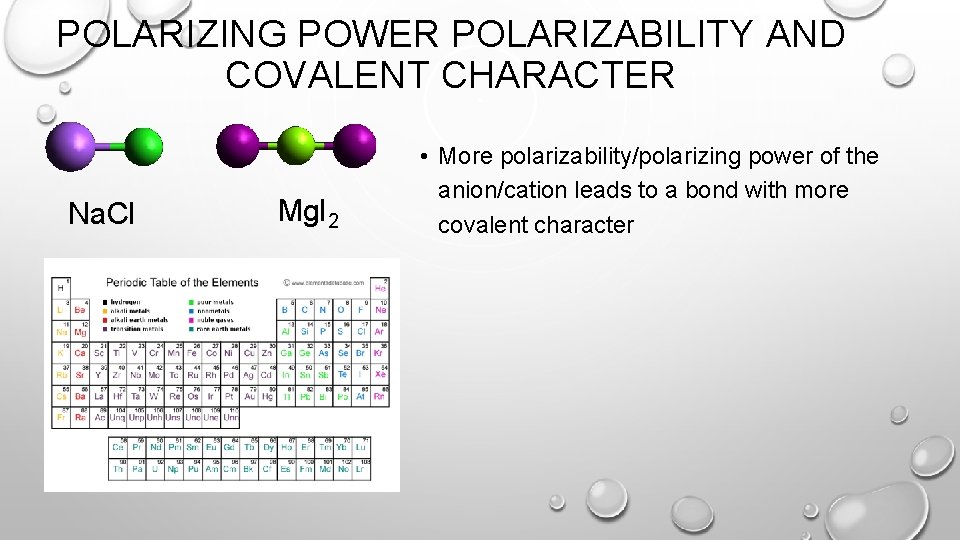
POLARIZING POWER POLARIZABILITY AND COVALENT CHARACTER Na. Cl Mg. I 2 • More polarizability/polarizing power of the anion/cation leads to a bond with more covalent character

Note: This isn’t and won’t be covered in the videos. You won’t need to do lattice energy problems any more complex than shown in the next couple of slides. LATTICE ENERGIES We will not be calculating the numbers, this equation will be for illustration purposes only. Charge Ion 1 Charge Ion 2 If charge increases what happens to energy? If internuclear distance increases what happens to energy? Internuclear radius
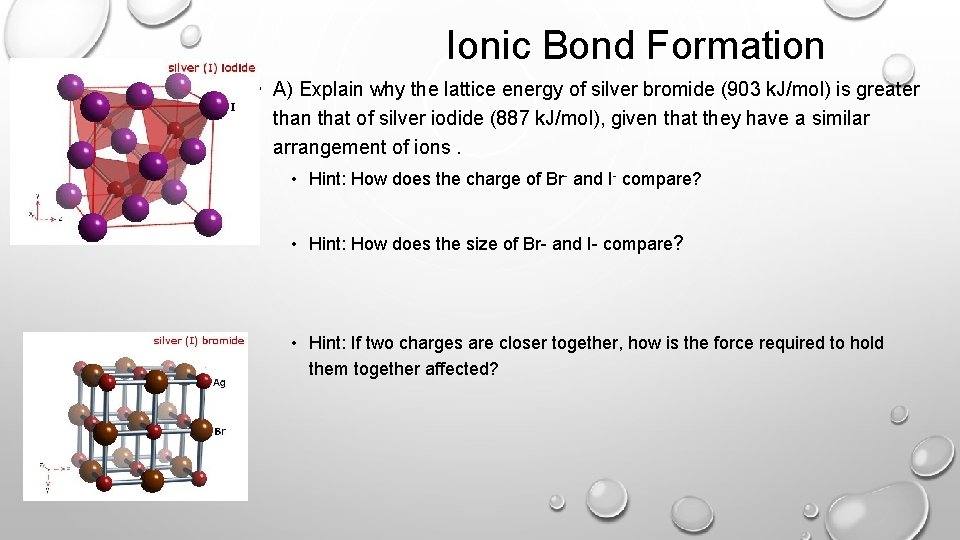
Ionic Bond Formation • A) Explain why the lattice energy of silver bromide (903 k. J/mol) is greater than that of silver iodide (887 k. J/mol), given that they have a similar arrangement of ions. • Hint: How does the charge of Br- and I- compare? • Hint: How does the size of Br- and I- compare? • Hint: If two charges are closer together, how is the force required to hold them together affected?
NAMING: IONIC • Name the Compounds we identified as ionic on the first problem. • Na. Cl • Mg. Cl 2 • Na 2 SO 3
NAMING: COVALENT • Name the Compounds below. • SO 2 • NO • PCl 5
- Slides: 10