the Myelodysplastic Syndromes what they are what they








































- Slides: 63

the Myelodysplastic Syndromes: what they are, what they mean and what are we doing about it Anthony Woods, MD September 30, 2006

outline • what is myelodysplasia? • diagnosis & classification • treatment strategies • specialty clinics

What is…? “clonal disorder affecting hematopoietic maturation, characterized by ineffective hematopoiesis and bone marrow failure with resultant cytopenias, often culminating in florid acute leukemia” huh?

historically • reports of cytopenic disorders began appearing in the early 20 th century 1942: “odo-leukemia” • odo = threshold 1949: “preleukemic anemia” (Chevalier et al) (Hamilton-Paterson) 1953: expanded definition to include all blood lines “clonal myeloid hemopathy” (Block et al)

historically • other terms used over the last 50 years: – – – herald state of leukemia refractory anemia sideroachrestic anemia idiopathic refractory sideroblastic anemia pancytopenia with hyperplastic marrow oligoblastic leukemia

soapbox no surprise that there is confusion and ignorance about this disorder: historical coupling of MDS to acute myeloid leukemia • there is a relationship • has hindered consideration of MDS as a distinct entity – biased investigational and therapeutic efforts towards the leukemia

historically • Paris, 1975: hemopoietic dysplasia – subsequently shortened to myelodysplasia • 1982: French-American-British classification scheme (FAB) • 1999 -2002: World Health Organization classification scheme

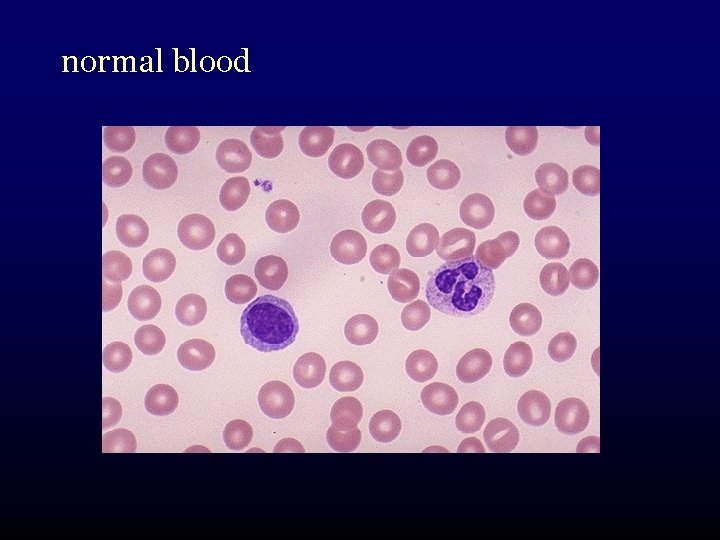
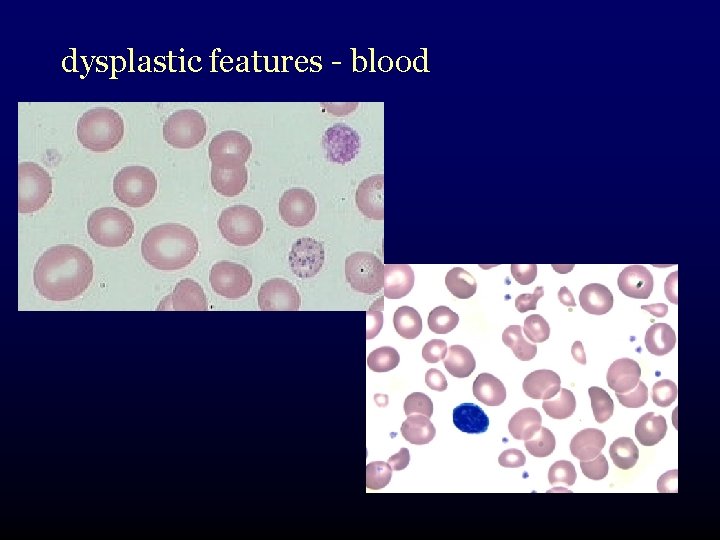
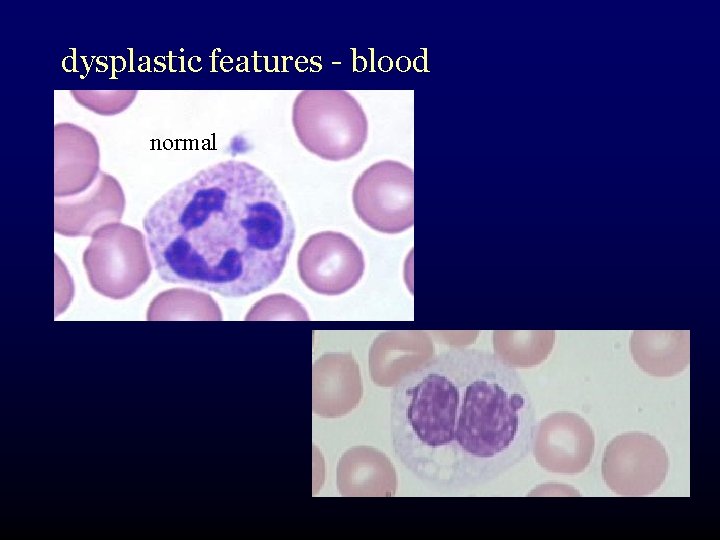
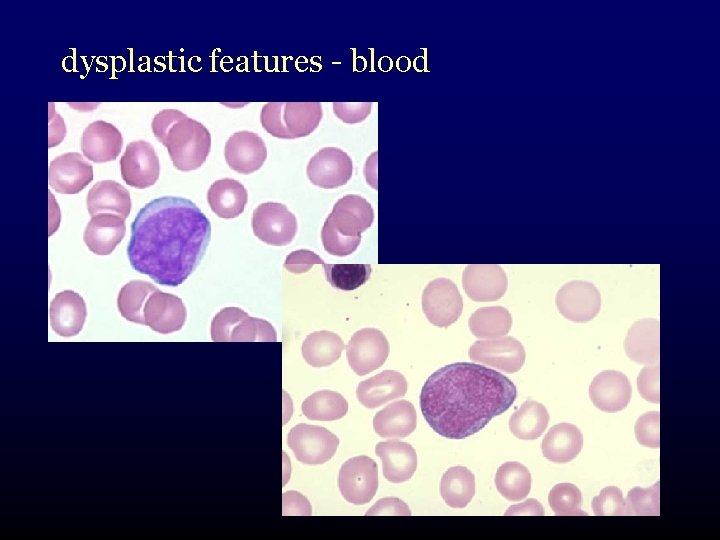
What is myelodysplasia? • disordered production of one or more cell lines “dysplasia” abnormal growth and differentiation of hematopoietic precursors • abnormal appearance under the microscope

normal blood

dysplastic features - blood

dysplastic features - blood normal

dysplastic features - blood

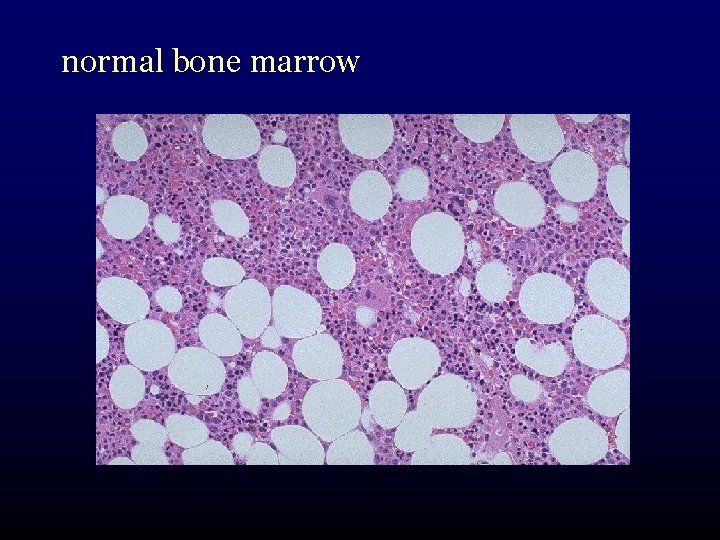
normal bone marrow

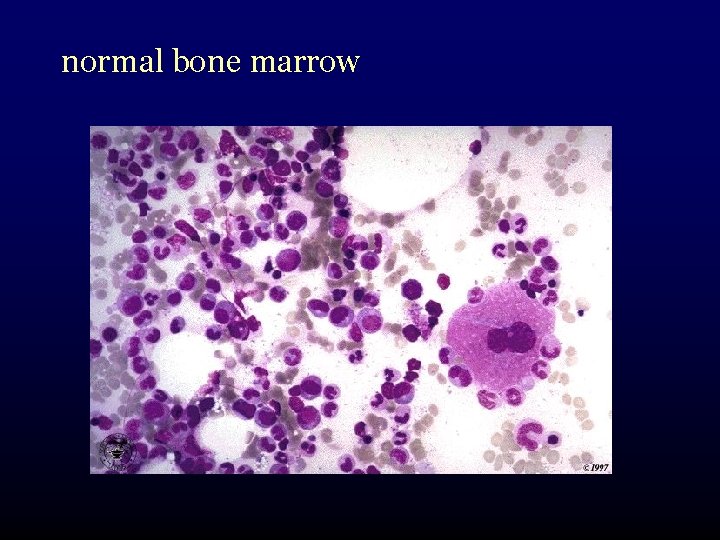
normal bone marrow

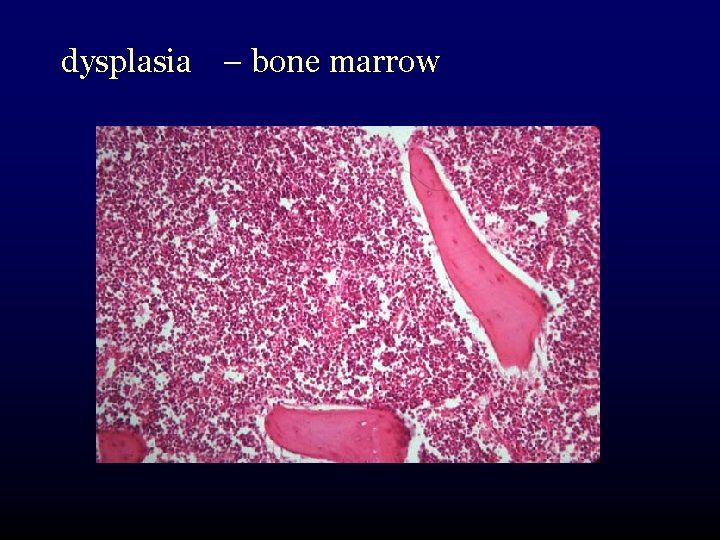
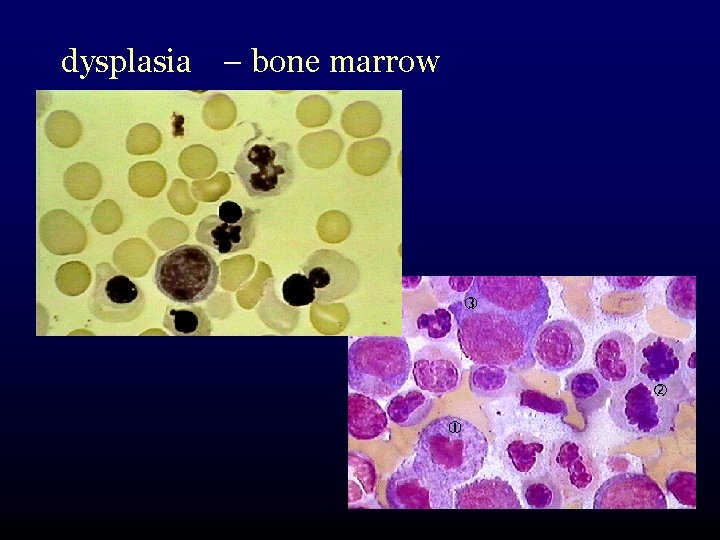
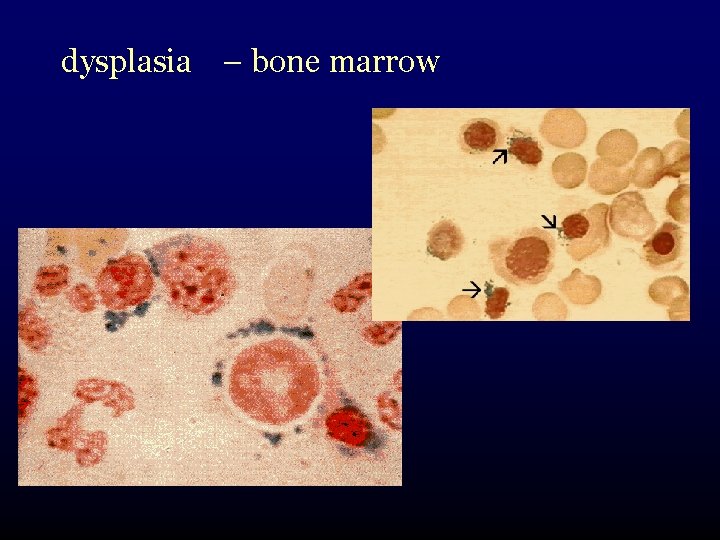
dysplasia – bone marrow

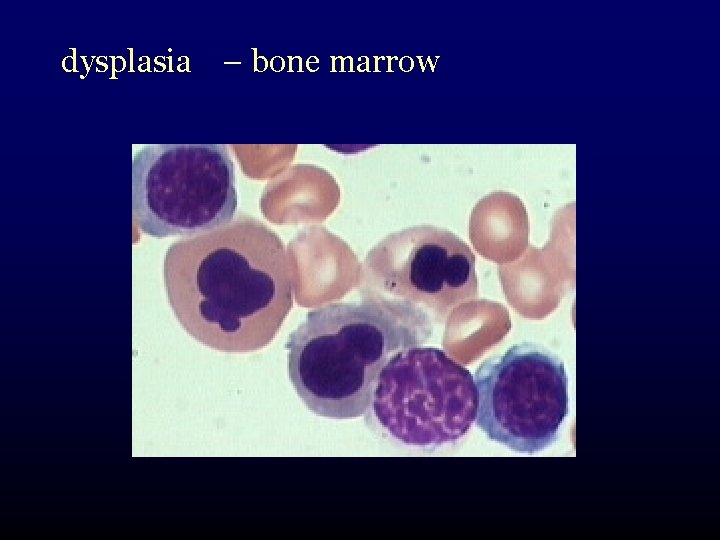
dysplasia – bone marrow

dysplasia – bone marrow

dysplasia – bone marrow

what goes wrong with marrow cells? • multistep process • sequence of successive DNA mutations in an early blood cell precursor cell • evolution of this cell into a “clone” – self-reproducing abnormal cell – growth advantage over normal marrow cells

what goes wrong with marrow cells? complex multistep process: • DNA mutations in an early blood cell precursor cell • emergence as an abnormal clone – self-reproducing, abnormal cell – growth advantage over normal marrow cells BUT at the same time incapable of producing normal blood cells

what goes wrong with marrow cells? expansion of the abnormal clone results in ineffective hematopoiesis – marrow looks full – no useful blood cell production actually occurring – suppression and inhibition of normal marrow growth “weeds growing in the garden” Dr. R. Wells

what goes wrong with marrow cells? • worse, over time the clone becomes more and more unstable • ~ 25% of persons with MDS develop acute myeloid leukemia

what goes wrong with marrow cells? MDS AML C. Willman, ASH Education Program, 2000

causes • may follow exposures to bone marrow toxins – chemotherapy – radiation – organic compounds • some follow inherited tendencies • Fanconi anemia, disorders of DNA repair • > 80% have no identifiable exposure or cause

in whom and how often • can affect people of any age – including children • more common in advancing age – North America: mid-late 60’s – China: 50 • 10000 -15000 new diagnoses per year in USA – Canada 10% ?

in whom and how often MDS Foundation estimates that in people older than 70 there are 15 - 50 new diagnoses / 100, 000 persons per year extrapolating USA estimates, perhaps 3000 - 6000 Canadians have an MDS diagnosis at any given time

diagnosis • requires suspicion • typically 2 settings where MDS should be suspected: 1. signs or symptoms of a blood disorder – fatigue, exercise intolerance, pale – serious or recurrent infections – inappropriate bleeding and bruising

diagnosis 2. unexpected finding in blood suggesting MDS: – low blood count of any kind • > 80% have anemia ± others • 30 – 45% have low platelets – macrocytosis (large red cells) – high monocyte count – abnormal appearing blood cells

diagnosis required evaluation: • complete history and examination • complete blood counts and diff • iron, B 12 and folate levels • bone marrow aspirate & biopsy – chromosome analysis: “cytogenetics” • serum erythropoietin levels – prior to transfusions

diagnosis • tests that are useful in some clinical circumstances – HLA tissue typing (if BMT a consideration) – HIV testing – other specific tests • PNH • other HLA determinations

diagnosis • no perfect diagnostic test • no absolute diagnostic criteria • combination of findings: – appearance of dysplasia in blood and marrow – abnormal cytogenetic testing

complications related to low blood counts • fatigue, decreased exercise tolerance • serious, recurrent infection • bleeding • the latter two are responsible for the majority of severe and life-threatening complications of MDS

complications • approximately 25% of patients undergo a transformation to acute myeloid leukemia • arbitrary distinction of having 20% blast cells in bone marrow • represents an increase in the aggressiveness of their MDS • associated with a worse prognosis

complications suspected if: • drop in baseline blood counts • higher blast cell numbers showing up in blood • higher transfusion requirements • non-specific symptoms – weight loss

classification 2 classification systems still in use • FAB System • newer WHO classification

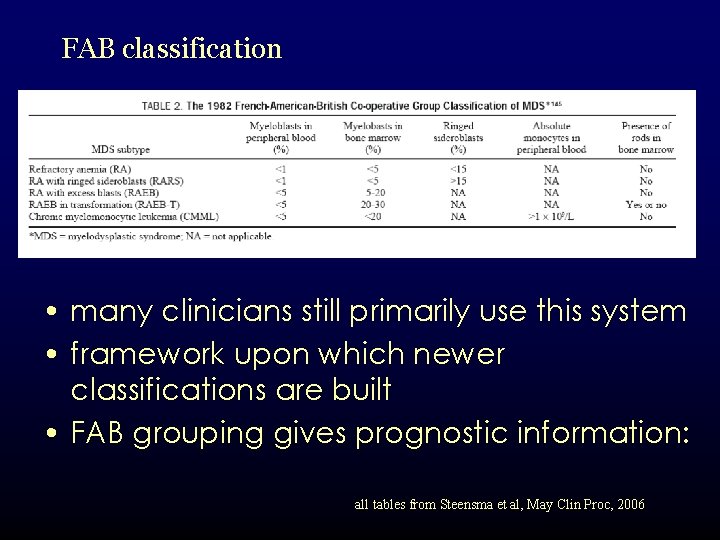
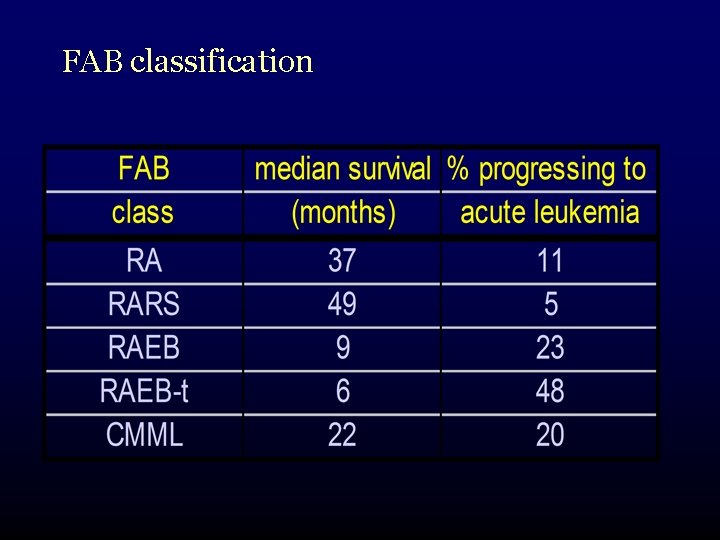
FAB classification • many clinicians still primarily use this system • framework upon which newer classifications are built • FAB grouping gives prognostic information: all tables from Steensma et al, May Clin Proc, 2006

FAB classification

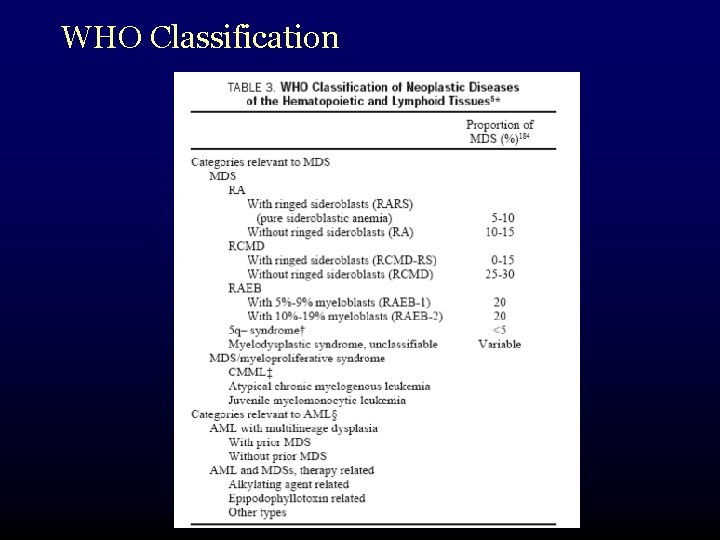
WHO Classification meant to refine the FAB system: • incorporated new information – cytogenetics • added subcategories for recognized specific sub-entities – 5 q- syndrome • also takes into account changes in AML diagnostic criteria – 20% vs 30%

WHO Classification

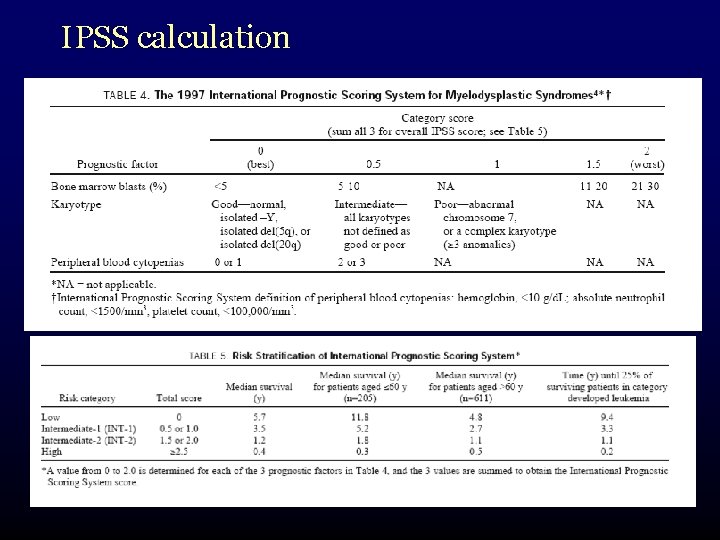
International Prognostic Scoring System • need for a better system to predict prognosis in certain patient groups • IPSS: a tool specifically designed for prognostic purposes • arose out of a 1997 international workshop on MDS Risk Analysis • analyzed factors in 816 patients

IPSS calculation

therapy • many potential therapies available to & tried in MDS patients • most show some benefit • most benefits are – small – only in a minority subset of patients • hard to know who should receive them and what to expect

transfusion and general supportive measures • most persons with MDS require transfusion support at some time during their course • most people do not tolerate hemoglobin < 80 g/L – at least 80 or for comfort/symptoms – CMV negative products in potential BMT recipients

therapy • platelet transfusion is individualized based on baseline counts and bleeding symptoms – alloimmunization to platelets – local blood bank guidelines • adjunctive agents can be used – tranexamic acid

therapy • management of iron overload – Dr. Wells

therapy • prompt attention given to infectious symptoms • primary care physicians should be aware of increased importance of infections in persons with MDS • trials of growth factors with infections – inpatient setting – selected outpatients

therapy growth factors: erythropoietin and G-CSF • rationale is to encourage hematopoiesis • responses are variable • expensive, not readily obtainable to everyone – 3 rd party insurance – “sneak” past renal guidelines

therapy • EPO compounds (Eprex) have generally shown response rates of 15 -20% • better responses seen in: – RCMD-RS – low EPO levels – lighter transfusion needs • responses generally last 1 -2 years • doses: 40 -60, 000 units SC weekly • newer compounds appear equally effective – darbepoietin (Aranesp)

therapy • response rates may increase up to twofold if recombinant granulocyte colony stimulating factor (G-CSF) is added • generally done in a stepwise fashion • doses of 1 µcg/kg day typical starting point • similar difficulties in obtaining drug

therapy chemotherapy • high-risk MDS patients with high blast counts or those who have transformed are candidates for treatment with traditional chemotherapy regimens • similar to AML therapy

therapy epigenetic therapy • DNA transcription (and normal cell growth and development) in MDS can be altered by factors not directly related to DNA sequences – chemical alterations of DNA itself – chemical changes in support proteins • histones

therapy • demethylation agents: allow transcription of key tumor suppressor genes • histone deacetylation agents: alter binding of DNA to histones and increase “accessibility” of DNA to transcription factors

therapy 5 -azacytidine • methyltransferase inhibitor • delays progression of MDS • improvements in blood counts in ~ 20% patients • improvements in quality of life • neutropenia observed as side effect (!)

therapy 5 -aza-2’-deoxycytabine (decitabine) • similar mechanism • less neutropenia & similar response rates – perhaps substantially better in some European studies • availability and difficulties with awkward approved dosing schedules have been obstacles

therapy immune suppression • antithymocyte globulin, cyclosporine – responses of 20 -30% in trials – pts with relatively hypoplastic marrows appear to be better candidates immunomodulatory therapy • thalidomide and lenalidomide

therapy thalidomide has shown promise, but has an unfavorable toxicity profile in MDS • have had > 33% drop-out rates in studies

therapy lenalidomide (Revlimid): exciting! • large improvements seen in transfusion requirements – best in 5 q- patients, but also in other karyotypes – normalization of cytogenetics • appears much less toxic – reversible neutropenia and thrombocytopenia • availability!

therapy • vitamins: high-dose B complex • danazol: thrombocytopenia • monoclonal antibodies • splenectomy all may have roles in individual patients

therapy • stem cell transplantation – Dr. L. Savoie • definite role in select patients – higher-risk disease – younger patients

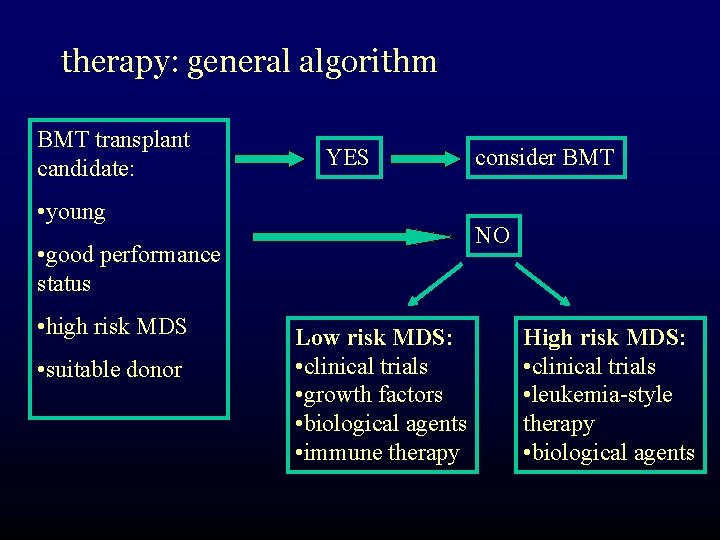
therapy: general algorithm BMT transplant candidate: YES • young NO • good performance status • high risk MDS • suitable donor consider BMT Low risk MDS: • clinical trials • growth factors • biological agents • immune therapy High risk MDS: • clinical trials • leukemia-style therapy • biological agents

The Role of the Specialty Clinic: 1. provide guidance for primary caregivers 2. maximize supportive care 3. optimize individualized management 5. dissemination of current state of knowledge 4. clinical trials & 5. data collection:


 Insidan region jh
Insidan region jh Best language nih
Best language nih Post castration syndrome gynecology
Post castration syndrome gynecology Cerebellar syndromes
Cerebellar syndromes What is geriatric syndromes
What is geriatric syndromes Cerebellar syndromes
Cerebellar syndromes Neuroendocrine syndrome in gynecology
Neuroendocrine syndrome in gynecology Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Tư thế ngồi viết
Tư thế ngồi viết Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là V. c c
V. c c Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Lp html
Lp html Phép trừ bù
Phép trừ bù Lời thề hippocrates
Lời thề hippocrates Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Cong thức tính động năng
Cong thức tính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ độ dài liên kết
độ dài liên kết Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Các số nguyên tố là gì
Các số nguyên tố là gì Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Fecboak
Fecboak đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp ưu thế lai là gì
ưu thế lai là gì Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Tư thế ngồi viết
Tư thế ngồi viết Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Complete the sentences with if or when
Complete the sentences with if or when Grammar rules frustrate me they're not logical they are so
Grammar rules frustrate me they're not logical they are so They seek him here they seek him there
They seek him here they seek him there For they not know what they do
For they not know what they do They have not rejected you but me
They have not rejected you but me Knowledge not shared is wasted
Knowledge not shared is wasted Rankings: what are they and do they matter?
Rankings: what are they and do they matter? Jordan 14
Jordan 14 First person singular
First person singular Who are they?
Who are they? Flowers die if they don't get any water
Flowers die if they don't get any water Omg they all join
Omg they all join Malcolm macbeth description
Malcolm macbeth description Esp they say
Esp they say Raccoon what do they eat
Raccoon what do they eat























































































