Evolving Treatments for Acute Leukemia and Myelodysplastic Syndromes















- Slides: 42

Evolving Treatments for Acute Leukemia and Myelodysplastic Syndromes Mark B Juckett MD University of Wisconsin



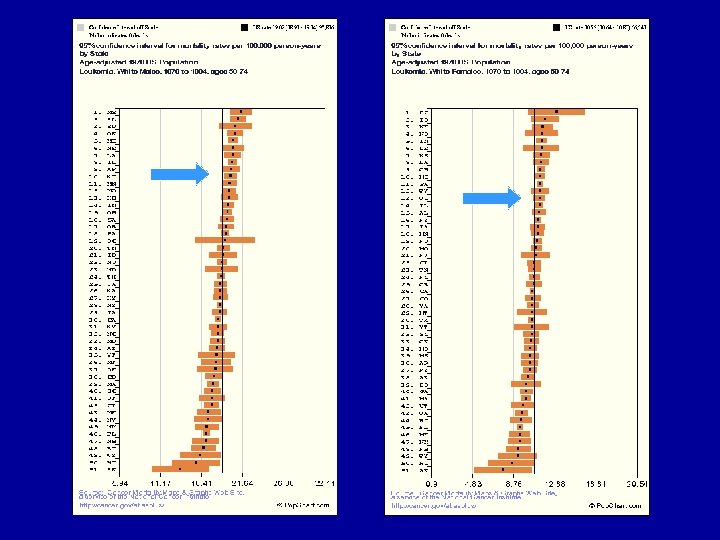
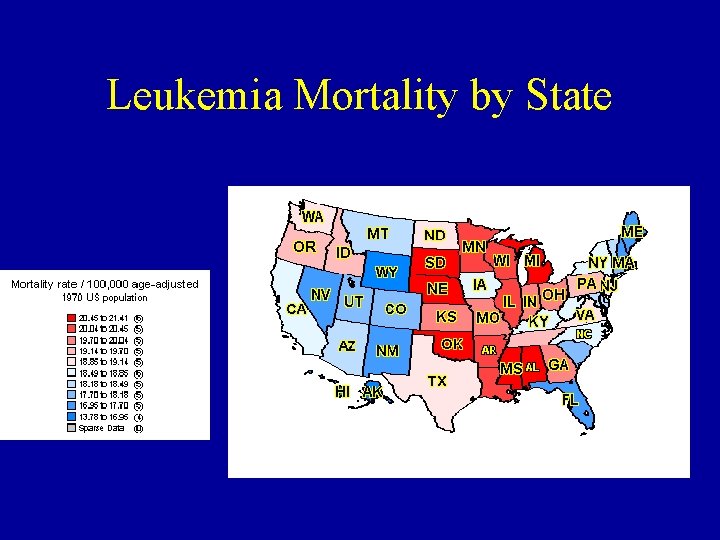
Leukemia Mortality by State

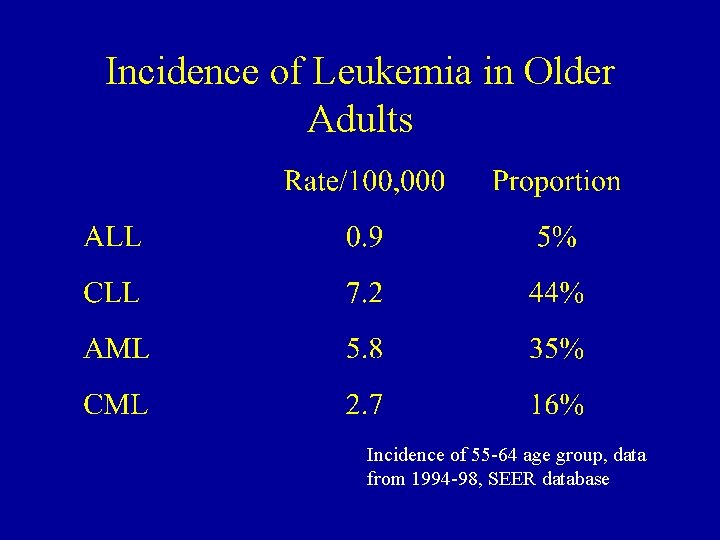
Incidence of Leukemia in Older Adults Incidence of 55 -64 age group, data from 1994 -98, SEER database

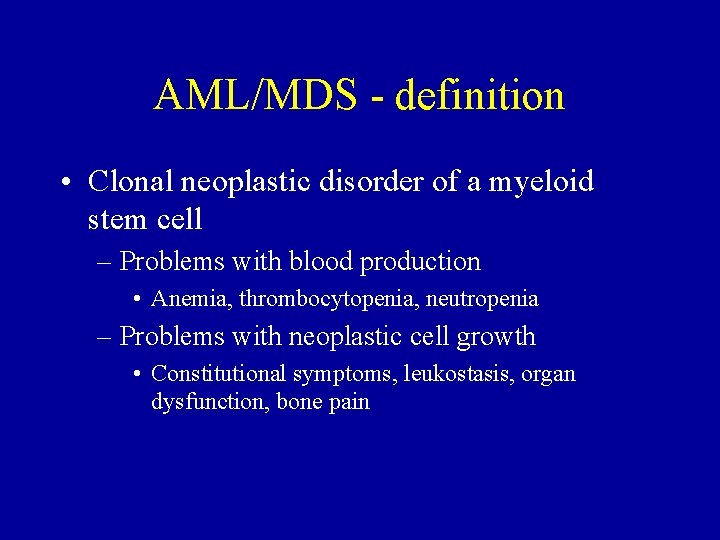
AML/MDS - definition • Clonal neoplastic disorder of a myeloid stem cell – Problems with blood production • Anemia, thrombocytopenia, neutropenia – Problems with neoplastic cell growth • Constitutional symptoms, leukostasis, organ dysfunction, bone pain

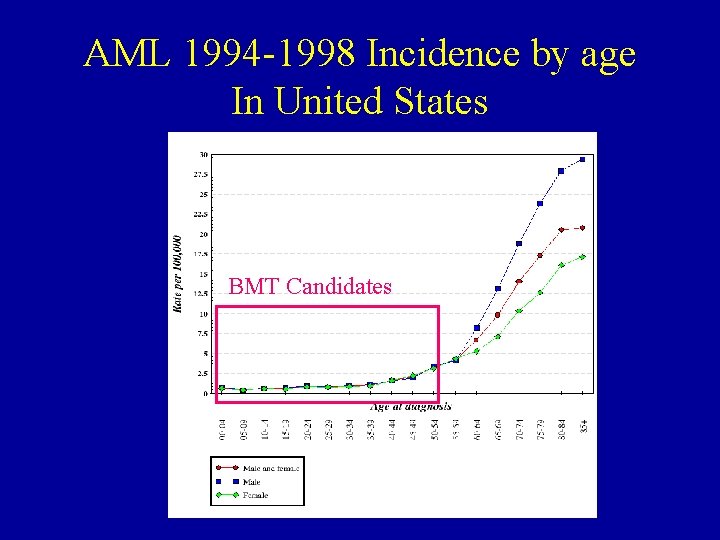
AML 1994 -1998 Incidence by age In United States BMT Candidates

Pathogenesis - “the usual” • Complex interaction between agents, environment, genetics. • Accumulation of genetic events (mutations, deletions, etc), multistep process • Faulty genetic program impedes differentiation, favors growth • Process continues until organ dysfunction, cell growth causes symptoms

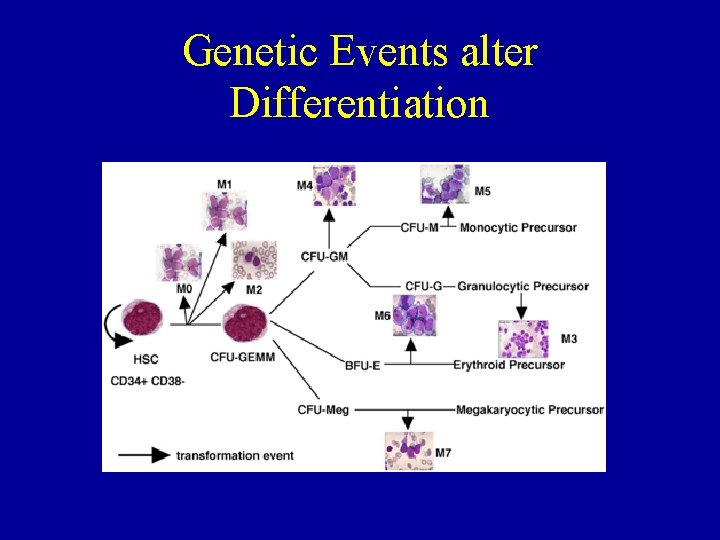
Genetic Events alter Differentiation

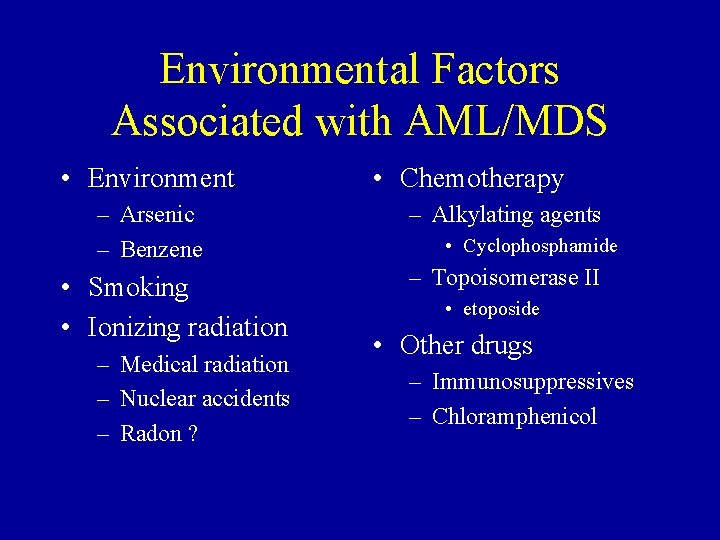
Environmental Factors Associated with AML/MDS • Environment – Arsenic – Benzene • Smoking • Ionizing radiation – Medical radiation – Nuclear accidents – Radon ? • Chemotherapy – Alkylating agents • Cyclophosphamide – Topoisomerase II • etoposide • Other drugs – Immunosuppressives – Chloramphenicol

Genetic Factors • • • Down Syndrome Fanconi Syndrome Bloom Syndrome Klinefelter Syndrome Ataxia-telangiectasia Dyskeratosis Congentia • Congenital aneuploidy • Identical Sib with AML • Combined Immunodeficiency • Li-Fraumeni syndrome

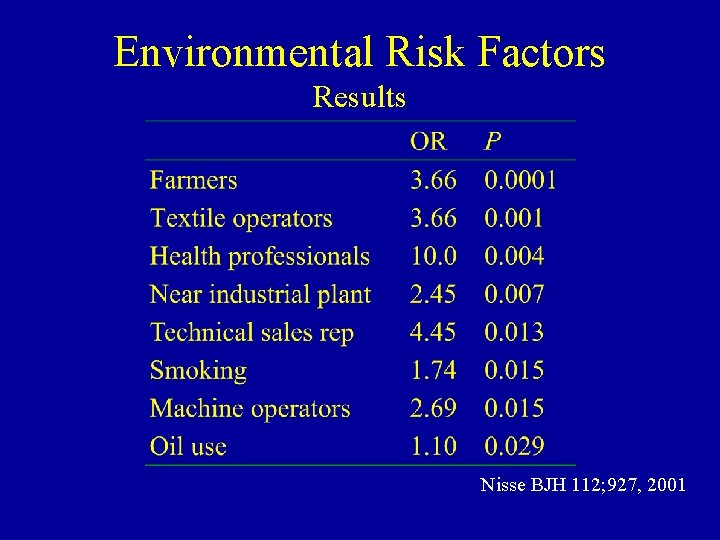
Environmental Risk Factors for MDS Nisse BJH 112; 927, 2001 • 204 MDS patients and controls • Interviewed at home – demographic data, lifetime residence, medical history, proximity to nuclear, chemical, industrial plants, carcinogen exposure. • Occupational history with exposure to list of compounds. • Reviewed validity with occupational experts (reviewers blinded)

Environmental Risk Factors Results Nisse BJH 112; 927, 2001

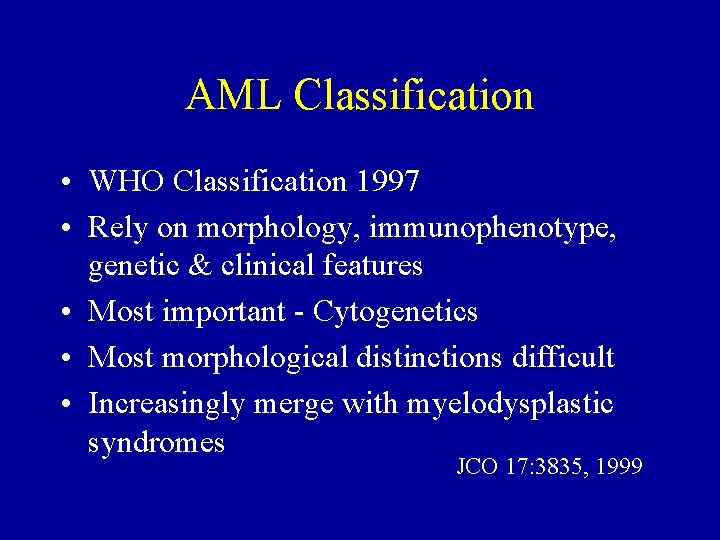
AML Classification • WHO Classification 1997 • Rely on morphology, immunophenotype, genetic & clinical features • Most important - Cytogenetics • Most morphological distinctions difficult • Increasingly merge with myelodysplastic syndromes JCO 17: 3835, 1999

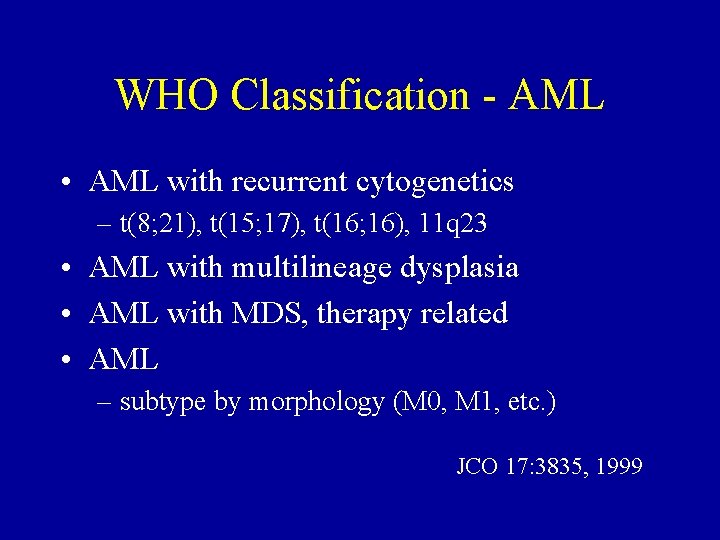
WHO Classification - AML • AML with recurrent cytogenetics – t(8; 21), t(15; 17), t(16; 16), 11 q 23 • AML with multilineage dysplasia • AML with MDS, therapy related • AML – subtype by morphology (M 0, M 1, etc. ) JCO 17: 3835, 1999

WHO Classification - MDS • Refractory anemia – with ringed sideroblasts – without ringed sideroblasts • Refractory cytopenia with multilineage dysplasia • Refractory anemia with excess blasts • 5 q- syndrome • Myelodysplastic syndrome, NOS JCO 17: 3835, 1999

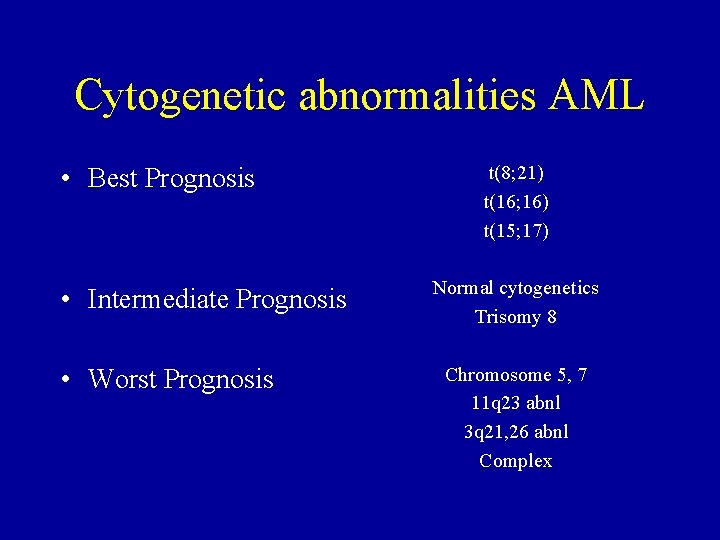
Cytogenetic abnormalities AML • Best Prognosis • Intermediate Prognosis • Worst Prognosis t(8; 21) t(16; 16) t(15; 17) Normal cytogenetics Trisomy 8 Chromosome 5, 7 11 q 23 abnl 3 q 21, 26 abnl Complex

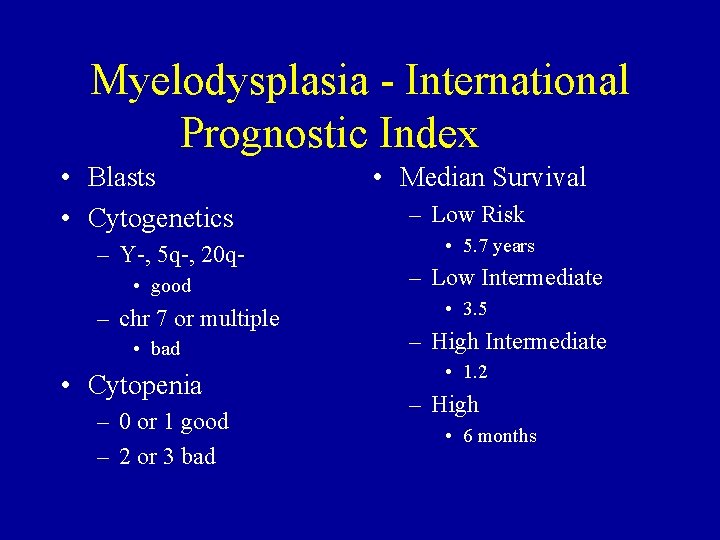
Myelodysplasia - International Prognostic Index • Blasts • Cytogenetics – Y-, 5 q-, 20 q • good – chr 7 or multiple • bad • Cytopenia – 0 or 1 good – 2 or 3 bad • Median Survival – Low Risk • 5. 7 years – Low Intermediate • 3. 5 – High Intermediate • 1. 2 – High • 6 months

Initial Treatment Strategy for AML • Rapidly control metabolic imbalances • Transfuse (Hgb > 8, Plt > 10 - 20 k) • Control WBC if needed – Hydroxyurea – Leukapheresis • Evaluate cardiorespiratory function • Given chemotherapy when “tuned”

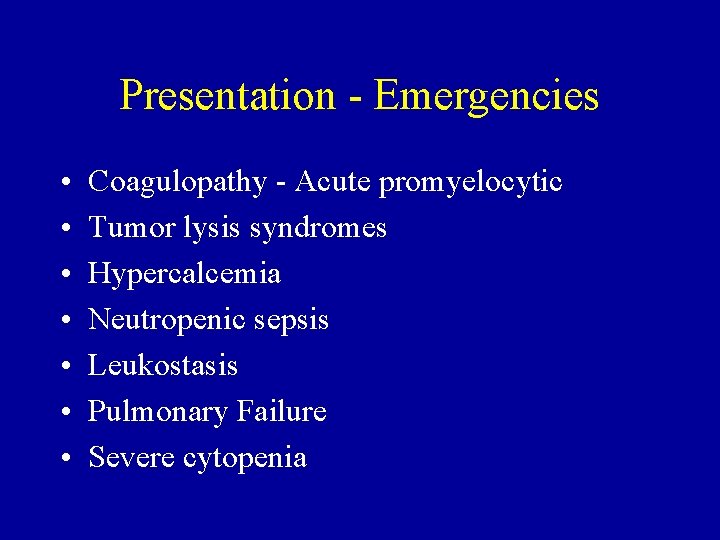
Presentation - Emergencies • • Coagulopathy - Acute promyelocytic Tumor lysis syndromes Hypercalcemia Neutropenic sepsis Leukostasis Pulmonary Failure Severe cytopenia

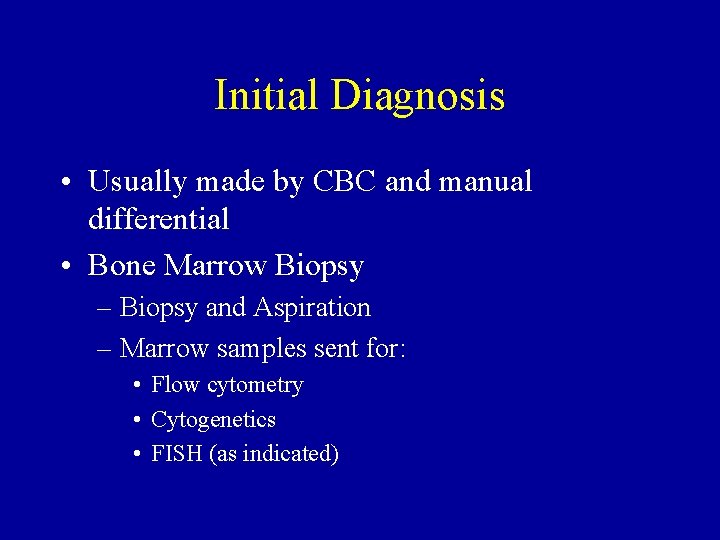
Initial Diagnosis • Usually made by CBC and manual differential • Bone Marrow Biopsy – Biopsy and Aspiration – Marrow samples sent for: • Flow cytometry • Cytogenetics • FISH (as indicated)

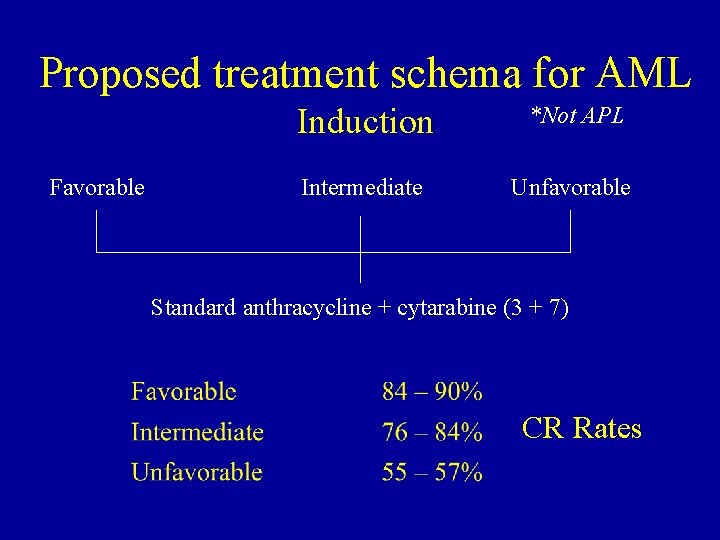
Proposed treatment schema for AML Favorable Induction *Not APL Intermediate Unfavorable Standard anthracycline + cytarabine (3 + 7) CR Rates

Next step - “Consolidation” • No furtherapy – 100% relapse, median 4. 1 mo (Cassileth, JCO 6: 583) • Chemotherapy alone – Standard high dose cytarabine 2 or 3 times • Autologous Bone Marrow Transplantation • Allogeneic Bone Marrow Transplantation

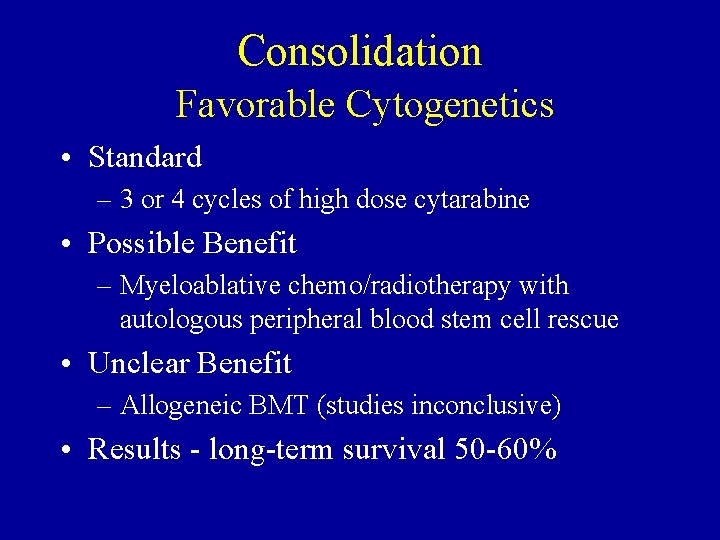
Consolidation Favorable Cytogenetics • Standard – 3 or 4 cycles of high dose cytarabine • Possible Benefit – Myeloablative chemo/radiotherapy with autologous peripheral blood stem cell rescue • Unclear Benefit – Allogeneic BMT (studies inconclusive) • Results - long-term survival 50 -60%

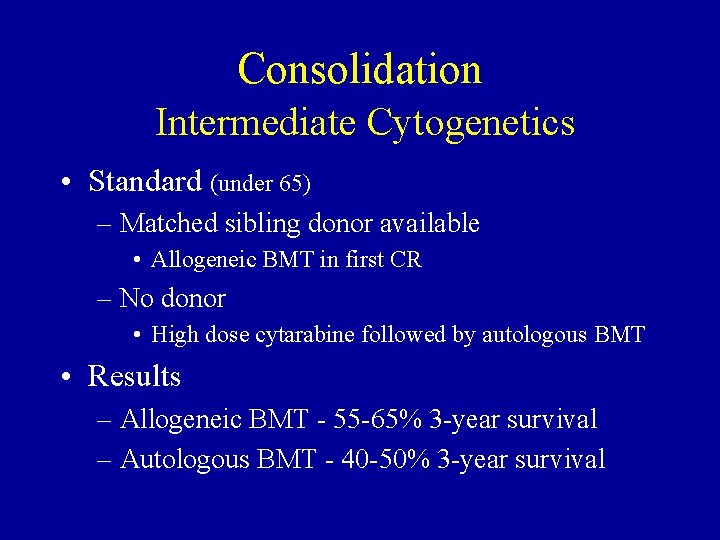
Consolidation Intermediate Cytogenetics • Standard (under 65) – Matched sibling donor available • Allogeneic BMT in first CR – No donor • High dose cytarabine followed by autologous BMT • Results – Allogeneic BMT - 55 -65% 3 -year survival – Autologous BMT - 40 -50% 3 -year survival

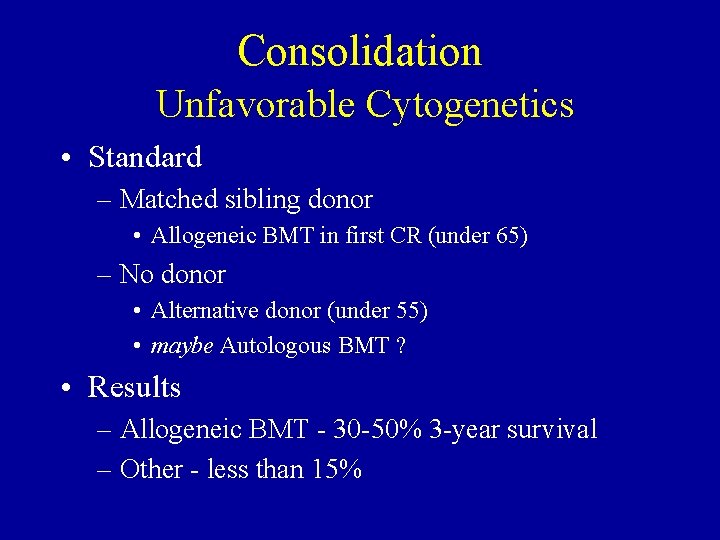
Consolidation Unfavorable Cytogenetics • Standard – Matched sibling donor • Allogeneic BMT in first CR (under 65) – No donor • Alternative donor (under 55) • maybe Autologous BMT ? • Results – Allogeneic BMT - 30 -50% 3 -year survival – Other - less than 15%

Bone Marrow Transplantation • Best - Matched Sibling (1/4 change/sib) • Matched Unrelated, Partial matched family an option - even more risky • Best case - Non-relapse Mortality 20 -30% • Autologous - Less risk, more relapse • Prolonged recovery - disability 1 year • Long term problems ?

Survival after AML (< 55 years) AML Pts on ECOG protocols since 1973

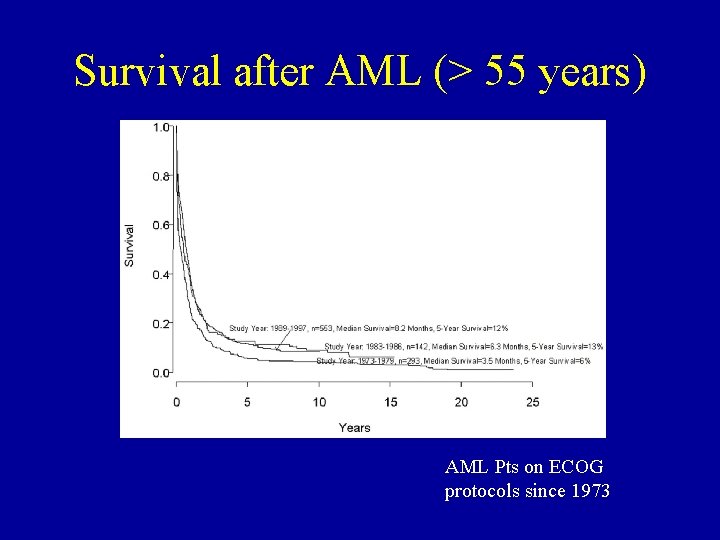
Survival after AML (> 55 years) AML Pts on ECOG protocols since 1973

Novel Approaches • • Immunoconjugates Signal Transduction modifiers Non-myeloablative transplantation Multidrug resistance modulation

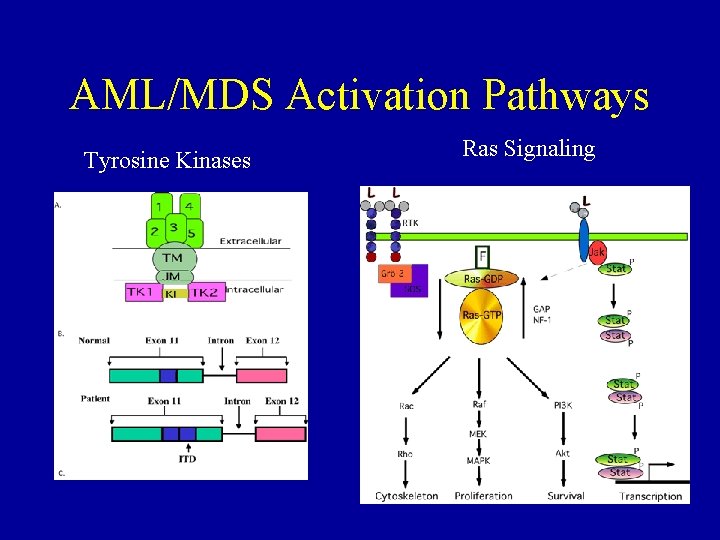
AML/MDS Activation Pathways Tyrosine Kinases Ras Signaling

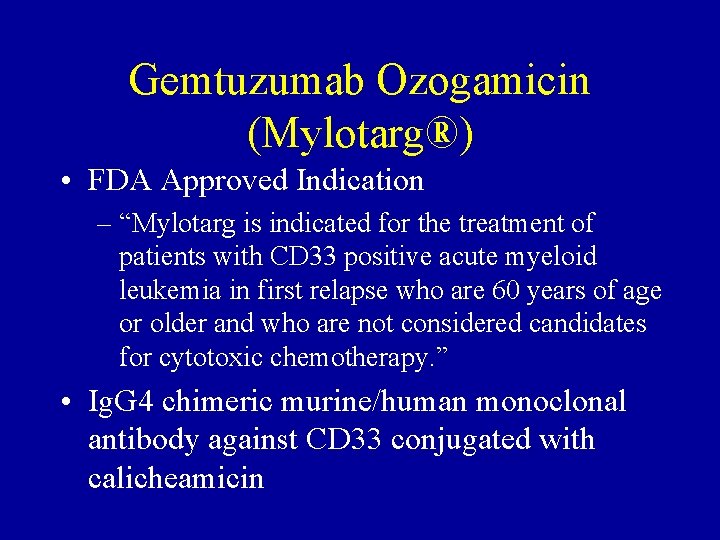
Gemtuzumab Ozogamicin (Mylotarg®) • FDA Approved Indication – “Mylotarg is indicated for the treatment of patients with CD 33 positive acute myeloid leukemia in first relapse who are 60 years of age or older and who are not considered candidates for cytotoxic chemotherapy. ” • Ig. G 4 chimeric murine/human monoclonal antibody against CD 33 conjugated with calicheamicin

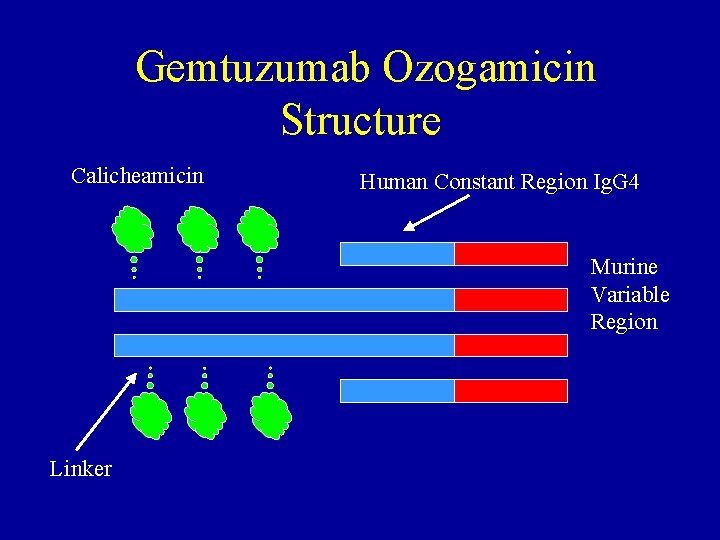
Gemtuzumab Ozogamicin Structure Calicheamicin Human Constant Region Ig. G 4 Murine Variable Region Linker

Mylotarg in relapsed AML CRp CR NR

Cooperative Group Trial - ECOG E 4999 • Patients with relapsed/refractory AML • AML CD 33 positive

Myeloablative SCT High dose radiation High dose chemo Stem cells Supportive Care Watch and Wait

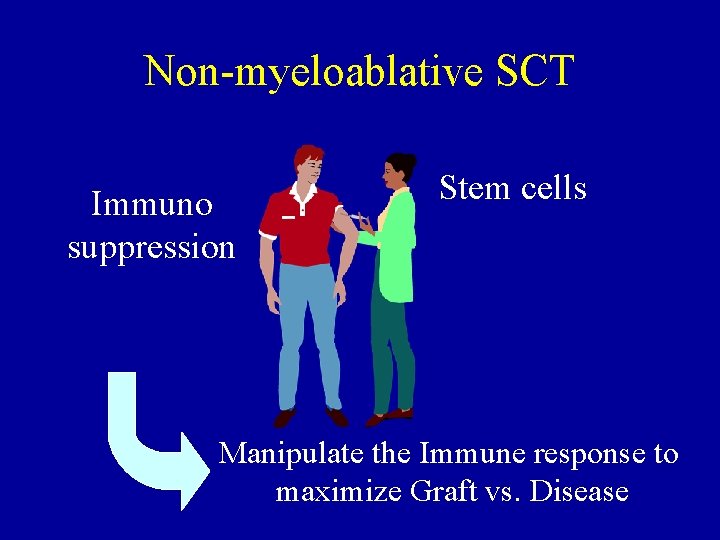
Non-myeloablative SCT Immuno suppression Stem cells Manipulate the Immune response to maximize Graft vs. Disease

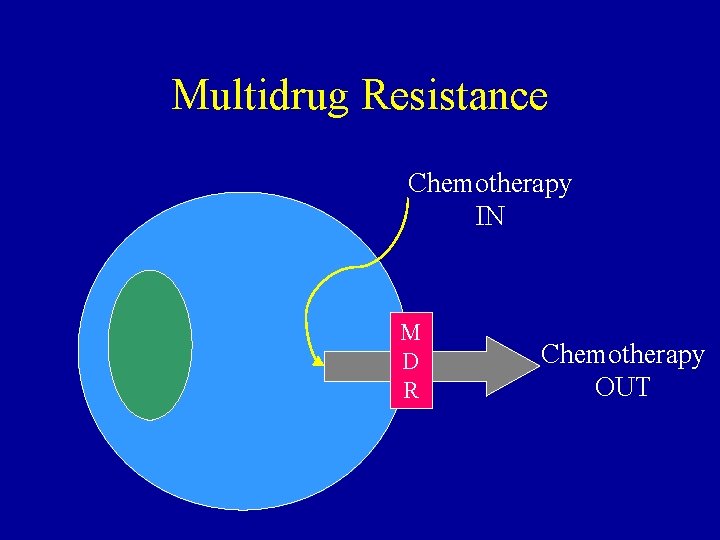
Multidrug Resistance Chemotherapy IN M D R Chemotherapy OUT

Multidrug Resistance Chemotherapy IN M D R MDR Block

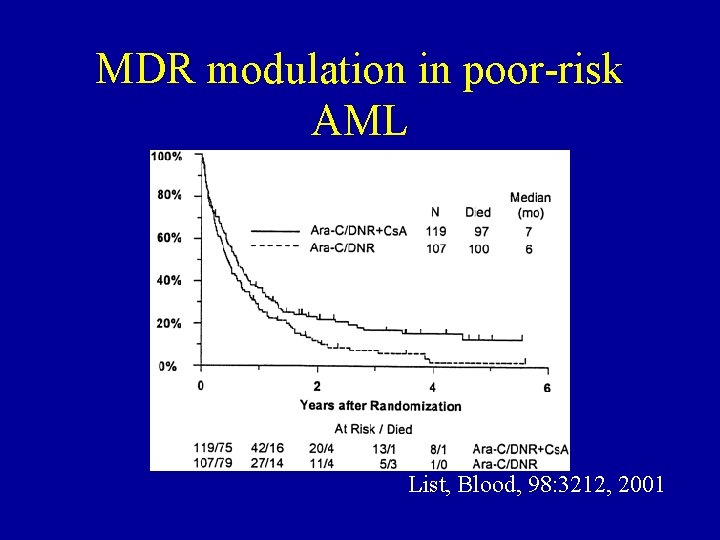
MDR modulation in poor-risk AML List, Blood, 98: 3212, 2001

Treatment of MDS • “Standard Care” - supportive • BMT - only curative option • Under investigation – Immunomodulation - ATG, thalidomide, Ontak – Differentiation - Doxercalciferol, arsenic, amifostine, decitabine – Signaling Pathway inhibitors – Novel BMT regimens

Conclusions • • • AML/MDS are diseases of older people Prognosis is best predicted by cytogenetics Outcome has improved for patients under 55 Outcome has not improved for older people HSC transplantation is indicated for most younger patients • New agents really do look promising
 Acute promyelocytic leukemia symptoms
Acute promyelocytic leukemia symptoms Platelet clumper
Platelet clumper Acute mylogenous leukemia
Acute mylogenous leukemia Treatments for acute renal failure
Treatments for acute renal failure Neuroendocrine syndromes in gynecology
Neuroendocrine syndromes in gynecology What is geriatric syndromes
What is geriatric syndromes Neuroendocrine syndrome in gynecology
Neuroendocrine syndrome in gynecology Best language nih
Best language nih Anatonomina
Anatonomina Cerebellar syndromes
Cerebellar syndromes Evolving design
Evolving design A framework for clustering evolving data streams
A framework for clustering evolving data streams Key evolving signature
Key evolving signature Evolving
Evolving What is the difference between lymphoma and leukemia
What is the difference between lymphoma and leukemia Prefix chondr
Prefix chondr Periodic acid schiff stain principle
Periodic acid schiff stain principle Chronic myeloid leukemia treatment
Chronic myeloid leukemia treatment Leukemia
Leukemia Leukemia statics
Leukemia statics Leukemia
Leukemia Zhang wang leukemia
Zhang wang leukemia How common is leukemia in adults
How common is leukemia in adults Leukemia aleukemik adalah
Leukemia aleukemik adalah Hemofília wikipedia
Hemofília wikipedia Conclusion of leukemia
Conclusion of leukemia Hairy cell leukemia
Hairy cell leukemia Lll leukemia
Lll leukemia Periodic acid schiff stain principle
Periodic acid schiff stain principle Nk leukemia
Nk leukemia Leukemia medical terminology
Leukemia medical terminology Chronic myeloid leukemia
Chronic myeloid leukemia Chronic myeloid leukemia
Chronic myeloid leukemia Leukemia statics
Leukemia statics Chronic myeloid leukemia
Chronic myeloid leukemia Dementia treatments and interventions near patterson
Dementia treatments and interventions near patterson Facial electrical treatments level 3
Facial electrical treatments level 3 Simultaneous treatment design
Simultaneous treatment design Hiv treatments
Hiv treatments Diabetes treatments
Diabetes treatments Advanced surface treatments
Advanced surface treatments Insomnia treatments
Insomnia treatments Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken