MYELODYSPLASTIC SYNDROMES AND MYELOPROLIFERATIVE DISORDERS Jaya V Juturi

















- Slides: 91

MYELODYSPLASTIC SYNDROMES AND MYELOPROLIFERATIVE DISORDERS Jaya V. Juturi MD 4/24/08 2005 i 3 dln

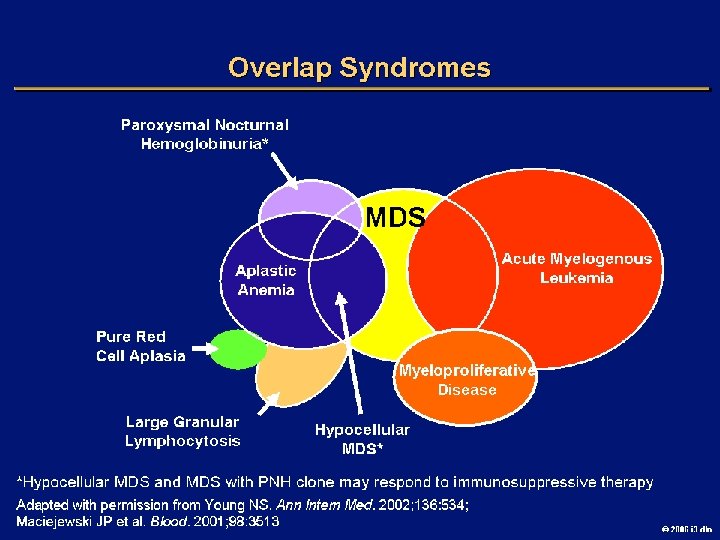
OBJECTIVES l CASE l DEFINITION l EPIDEMIOLOGY l OVERLAP SYNDROMES l CLASSIFICATION l IPSS l GOALS OF THERAPY l TREATMENT OVERVIEW 2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

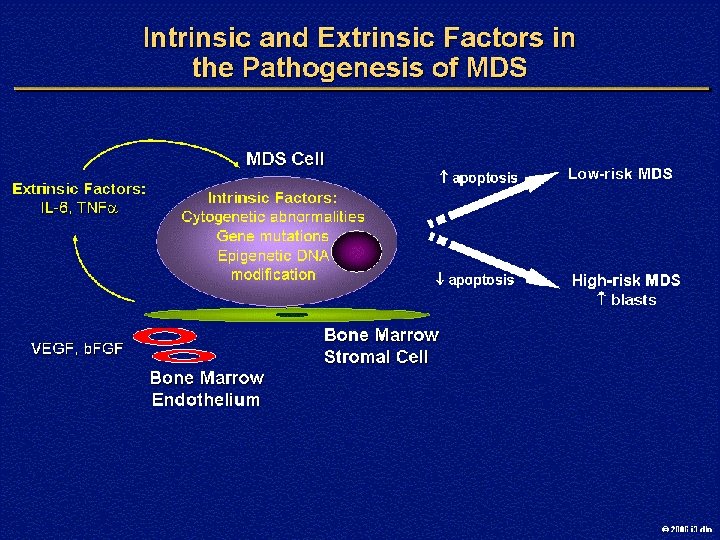
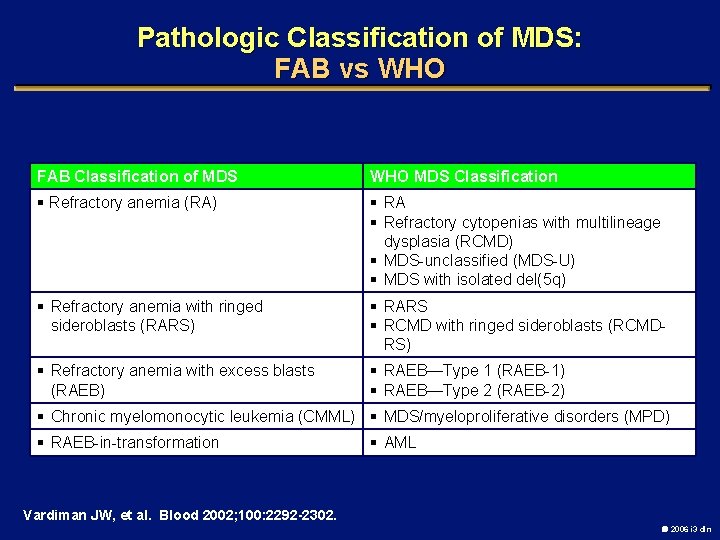
Pathologic Classification of MDS: FAB vs WHO FAB Classification of MDS WHO MDS Classification § Refractory anemia (RA) § RA § Refractory cytopenias with multilineage dysplasia (RCMD) § MDS-unclassified (MDS-U) § MDS with isolated del(5 q) § Refractory anemia with ringed sideroblasts (RARS) § RARS § RCMD with ringed sideroblasts (RCMDRS) § Refractory anemia with excess blasts (RAEB) § RAEB—Type 1 (RAEB-1) § RAEB—Type 2 (RAEB-2) § Chronic myelomonocytic leukemia (CMML) § MDS/myeloproliferative disorders (MPD) § RAEB-in-transformation § AML Vardiman JW, et al. Blood 2002; 100: 2292 -2302. 2006 i 3 dln

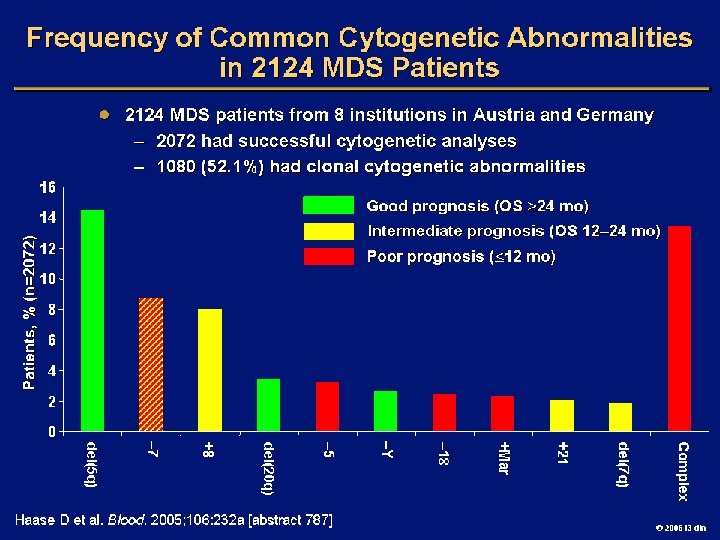
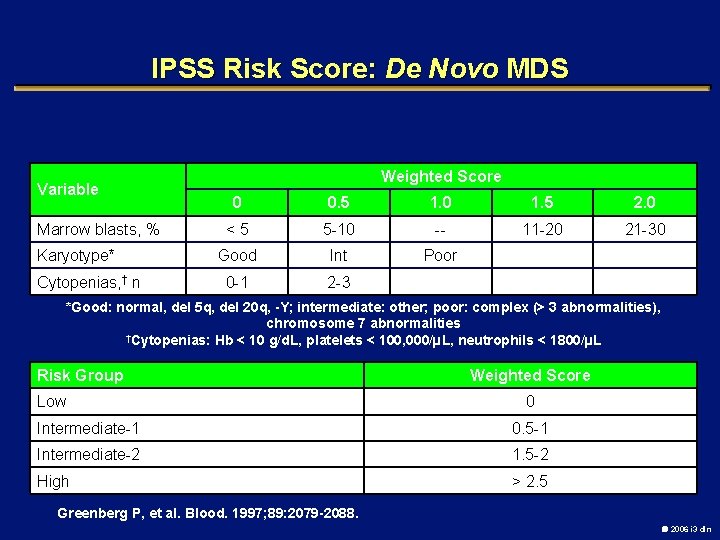
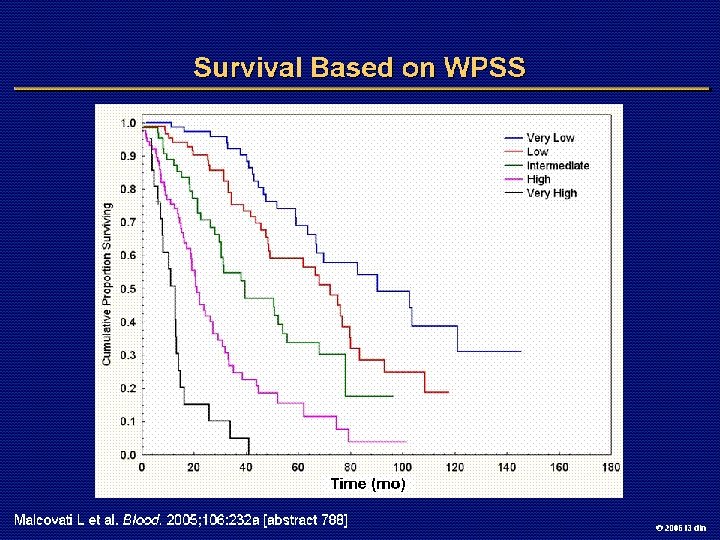
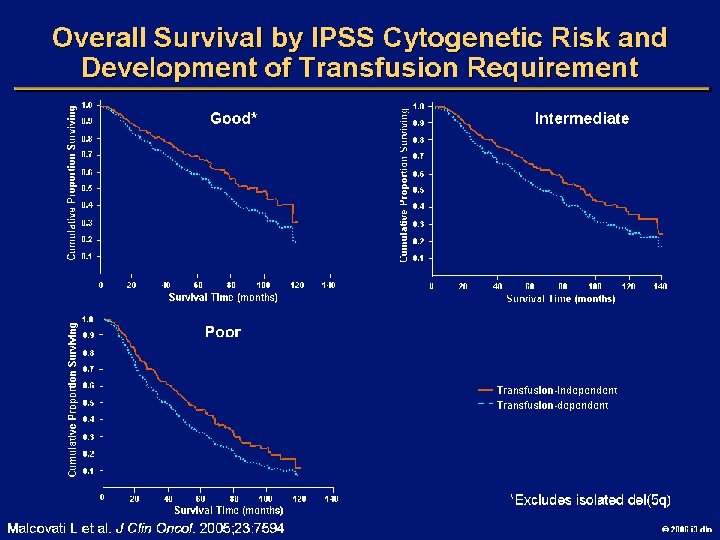
IPSS Risk Score: De Novo MDS Variable Marrow blasts, % Karyotype* Cytopenias, † n Weighted Score 0 0. 5 1. 0 1. 5 2. 0 <5 5 -10 -- 11 -20 21 -30 Good Int Poor 0 -1 2 -3 *Good: normal, del 5 q, del 20 q, -Y; intermediate: other; poor: complex (> 3 abnormalities), chromosome 7 abnormalities †Cytopenias: Hb < 10 g/d. L, platelets < 100, 000/µL, neutrophils < 1800/µL Risk Group Low Weighted Score 0 Intermediate-1 0. 5 -1 Intermediate-2 1. 5 -2 High > 2. 5 Greenberg P, et al. Blood. 1997; 89: 2079 -2088. 2006 i 3 dln

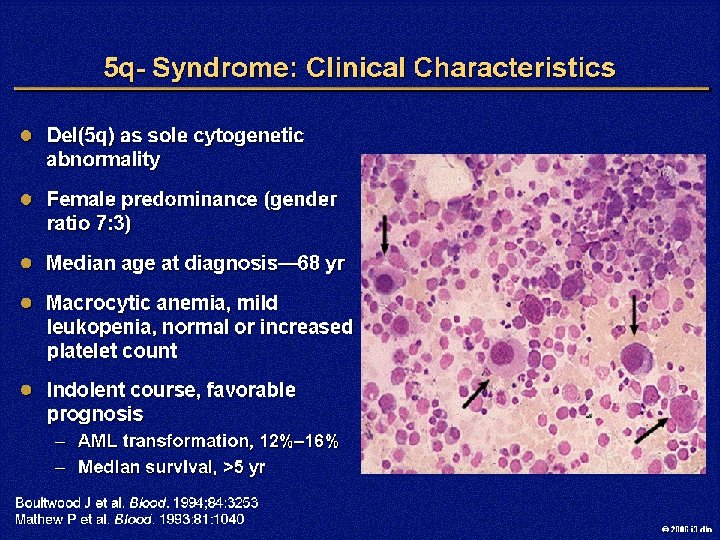
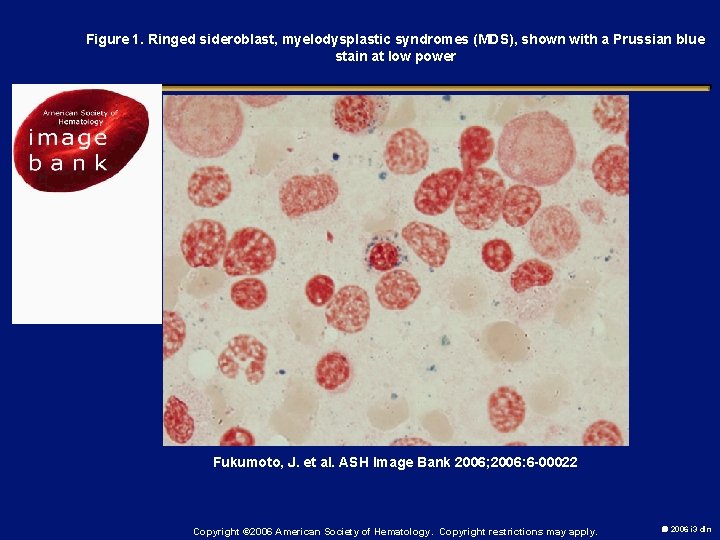
Figure 1. Ringed sideroblast, myelodysplastic syndromes (MDS), shown with a Prussian blue stain at low power Fukumoto, J. et al. ASH Image Bank 2006; 2006: 6 -00022 Copyright © 2006 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

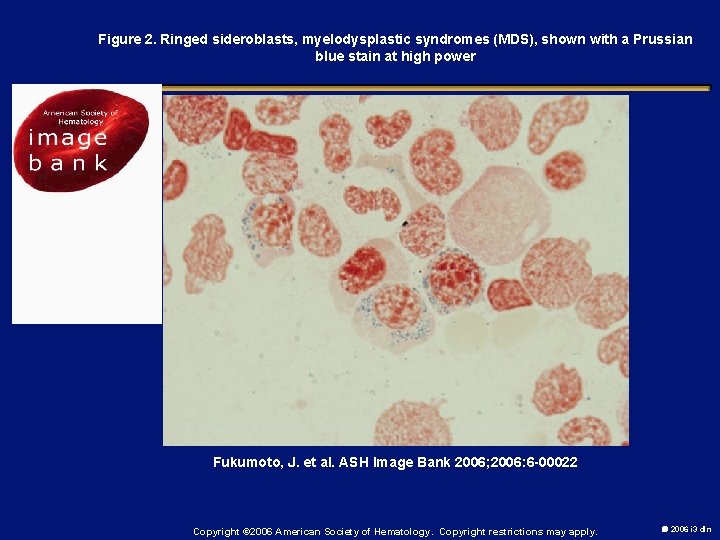
Figure 2. Ringed sideroblasts, myelodysplastic syndromes (MDS), shown with a Prussian blue stain at high power Fukumoto, J. et al. ASH Image Bank 2006; 2006: 6 -00022 Copyright © 2006 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

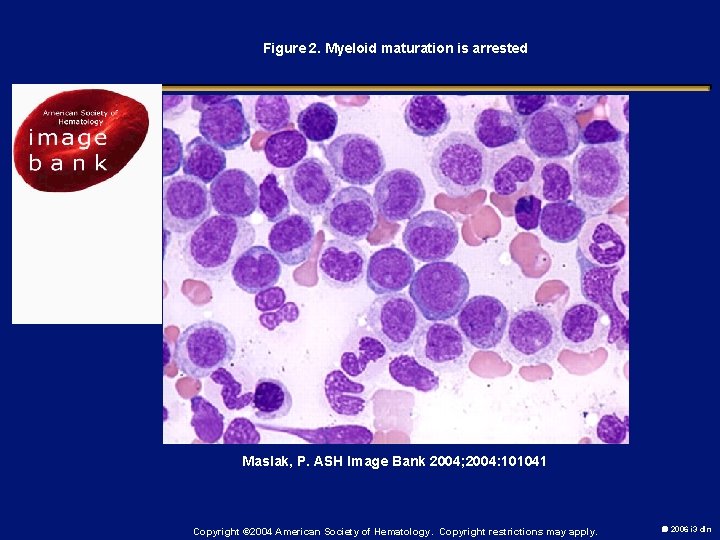
Figure 2. Myeloid maturation is arrested Maslak, P. ASH Image Bank 2004; 2004: 101041 Copyright © 2004 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

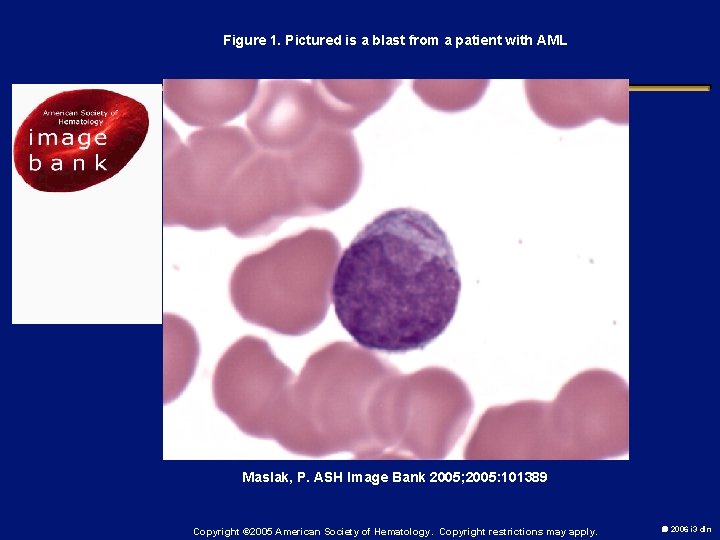
Figure 1. Pictured is a blast from a patient with AML Maslak, P. ASH Image Bank 2005; 2005: 101389 Copyright © 2005 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

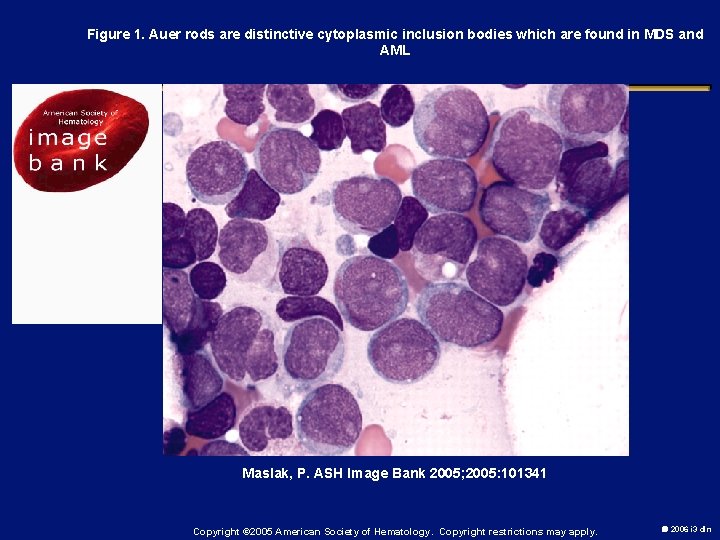
Figure 1. Auer rods are distinctive cytoplasmic inclusion bodies which are found in MDS and AML Maslak, P. ASH Image Bank 2005; 2005: 101341 Copyright © 2005 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

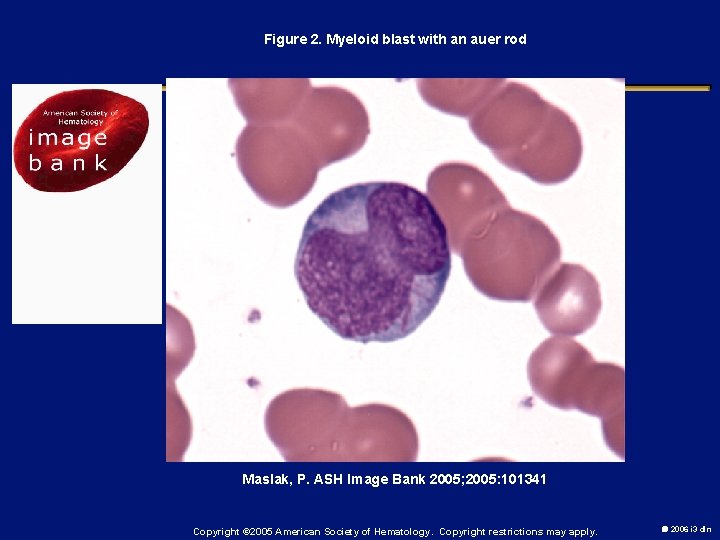
Figure 2. Myeloid blast with an auer rod Maslak, P. ASH Image Bank 2005; 2005: 101341 Copyright © 2005 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

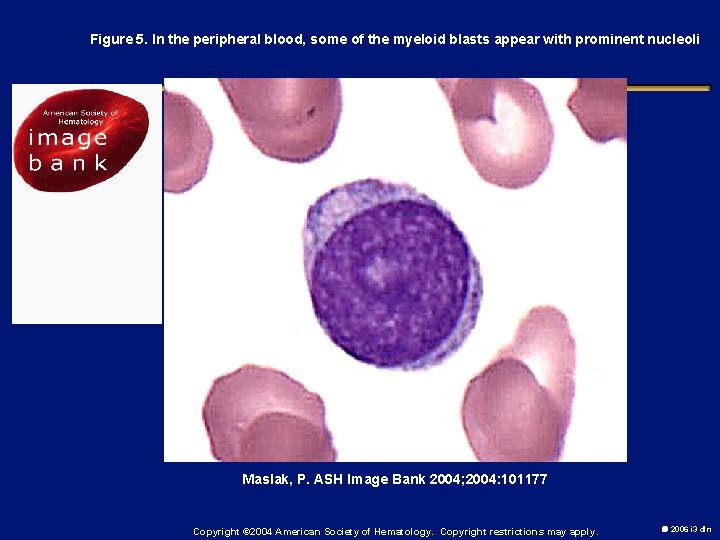
Figure 5. In the peripheral blood, some of the myeloid blasts appear with prominent nucleoli Maslak, P. ASH Image Bank 2004; 2004: 101177 Copyright © 2004 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

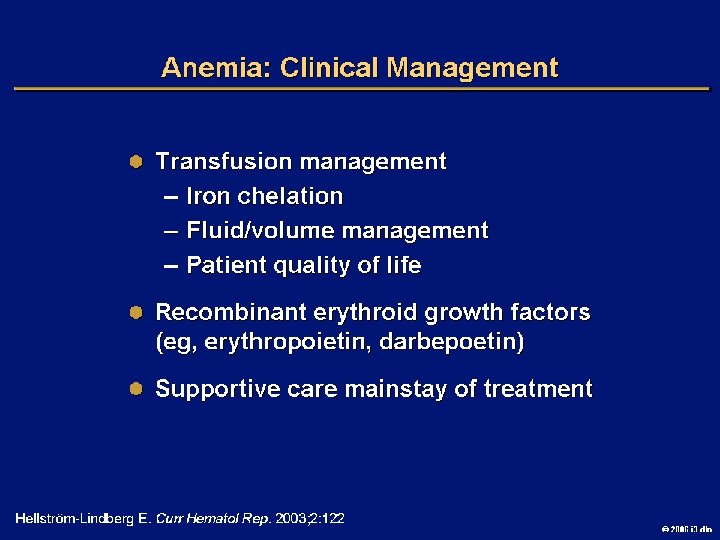
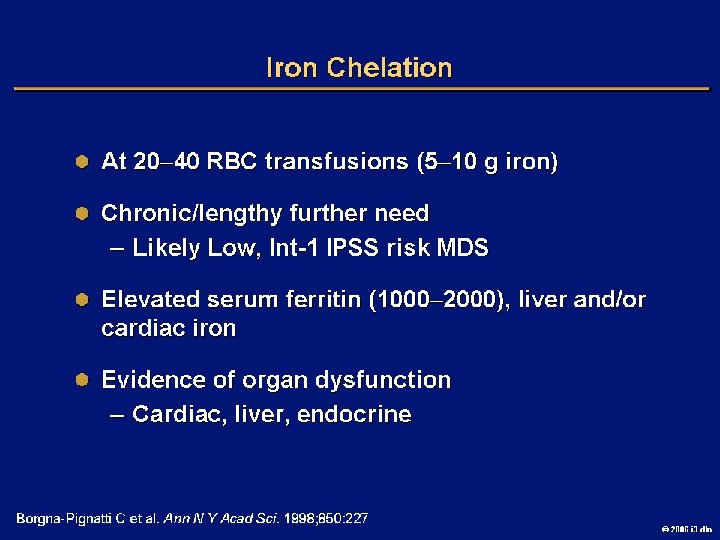
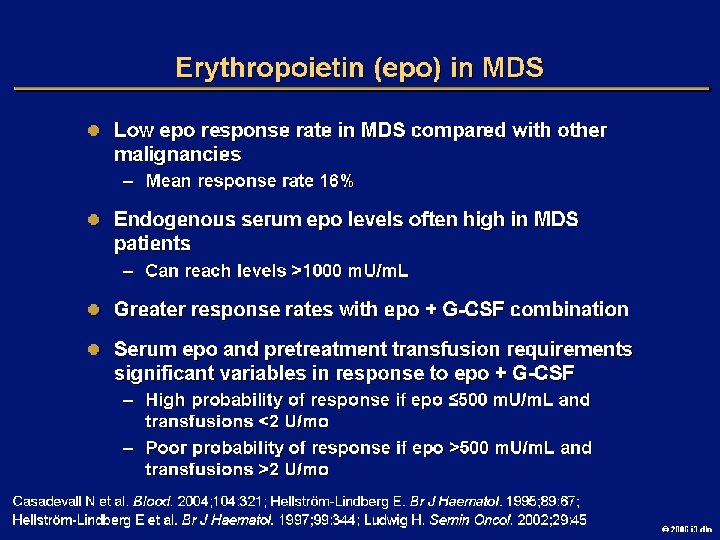
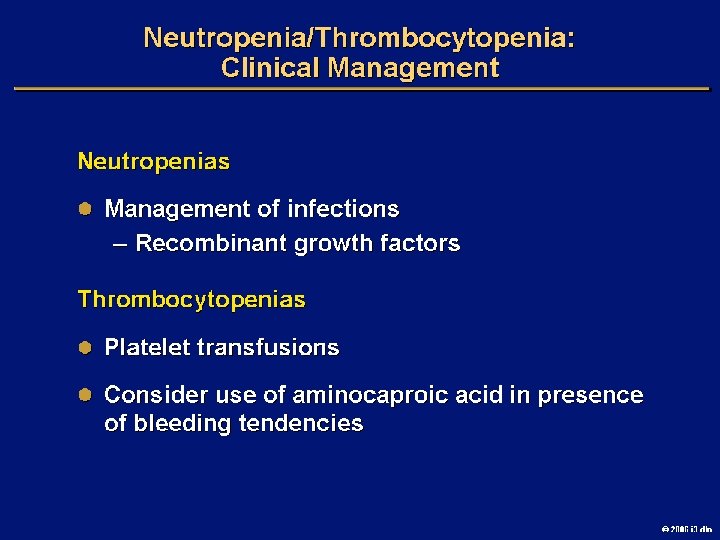
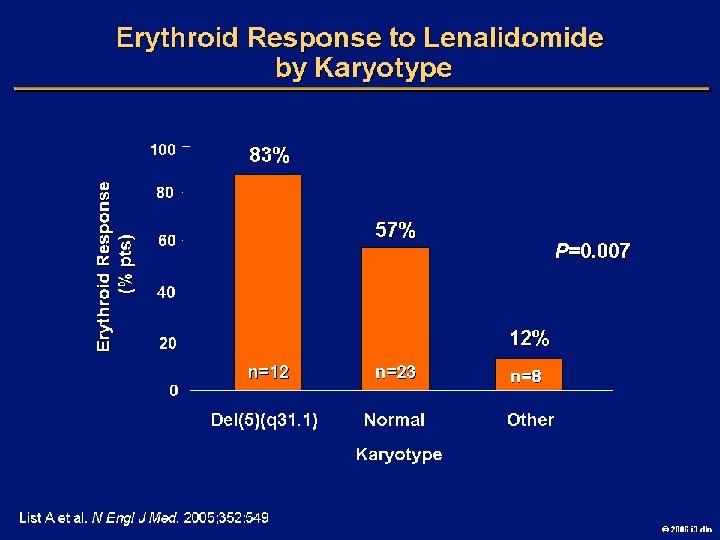
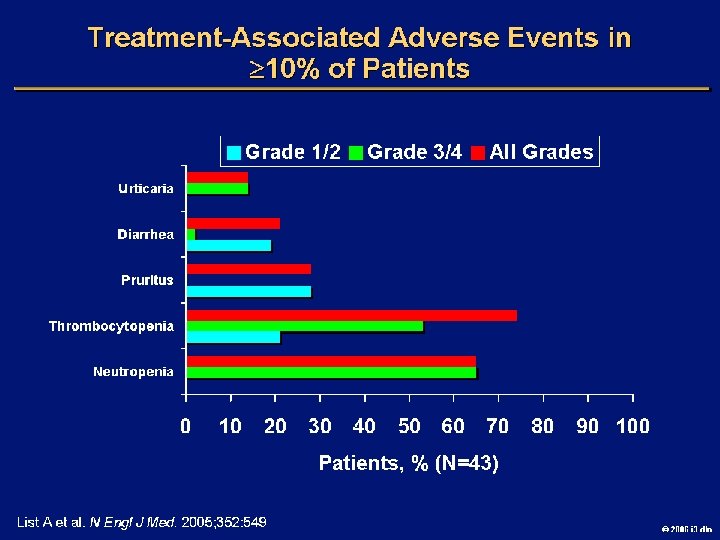
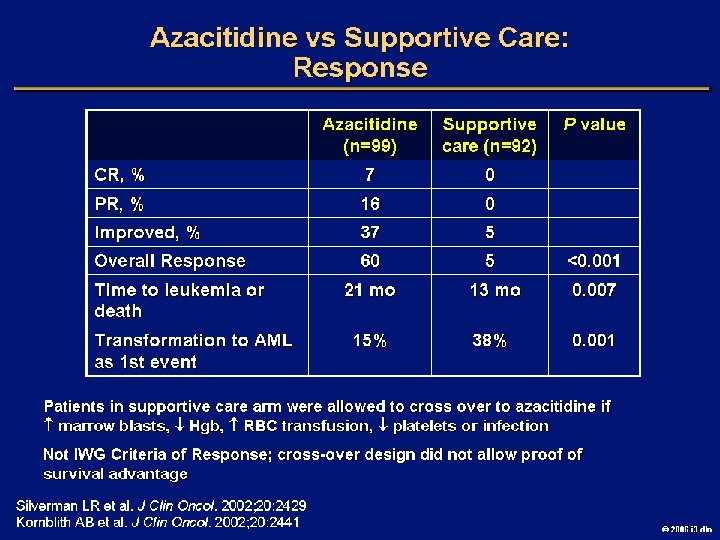
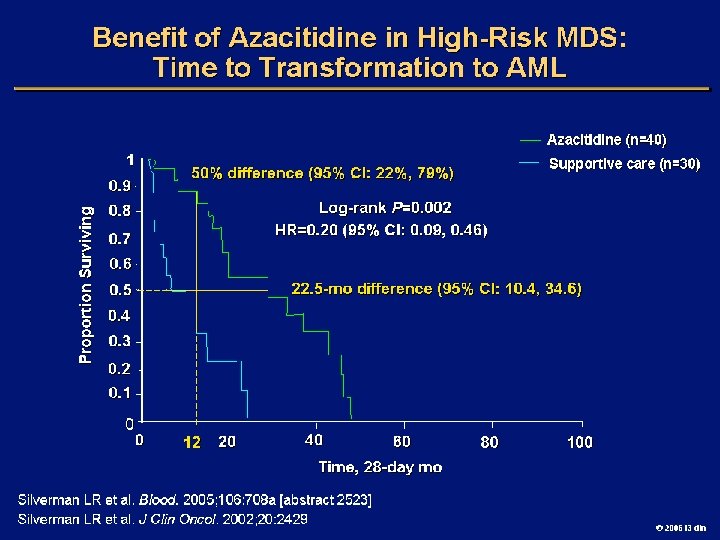
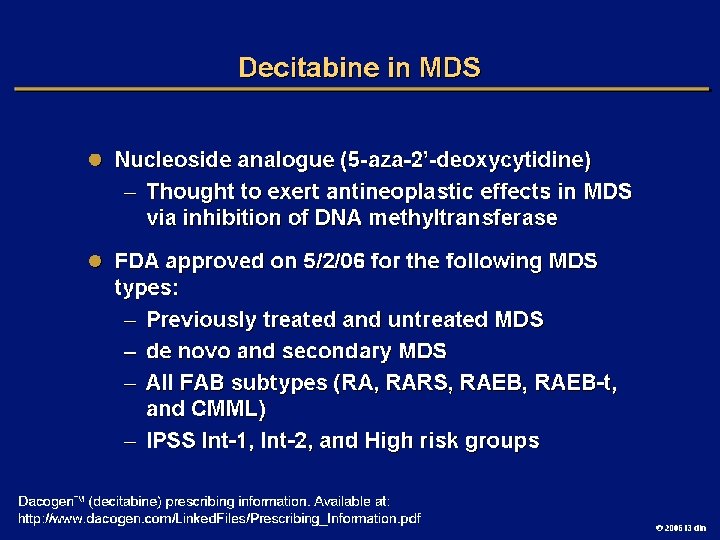
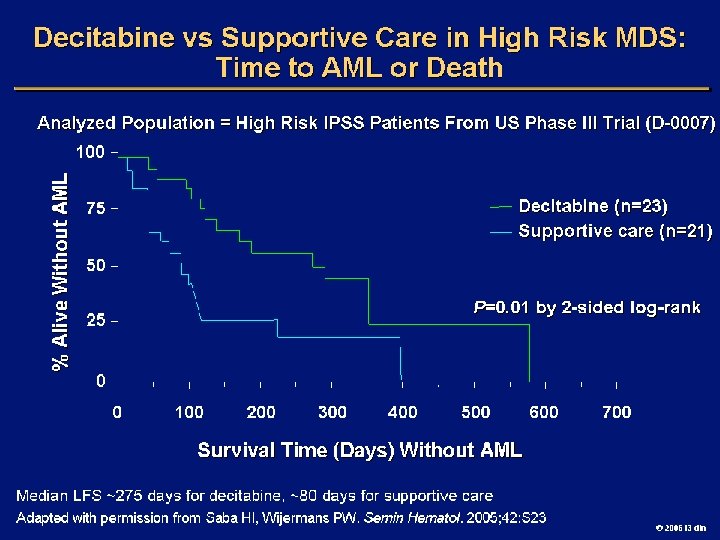
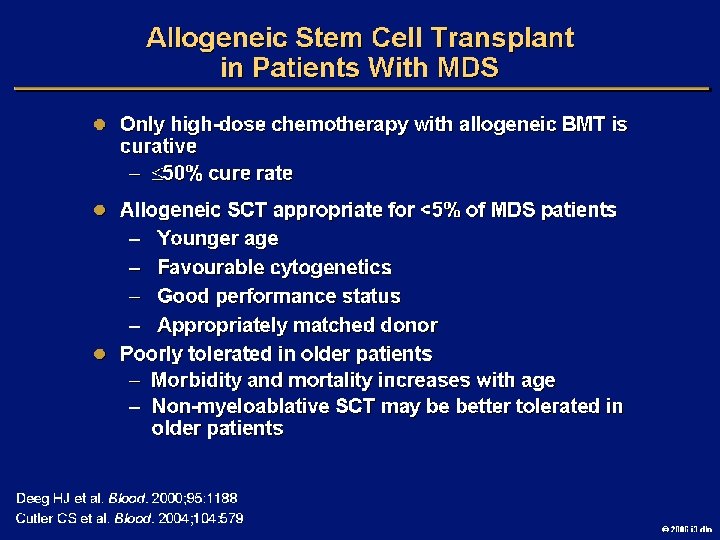
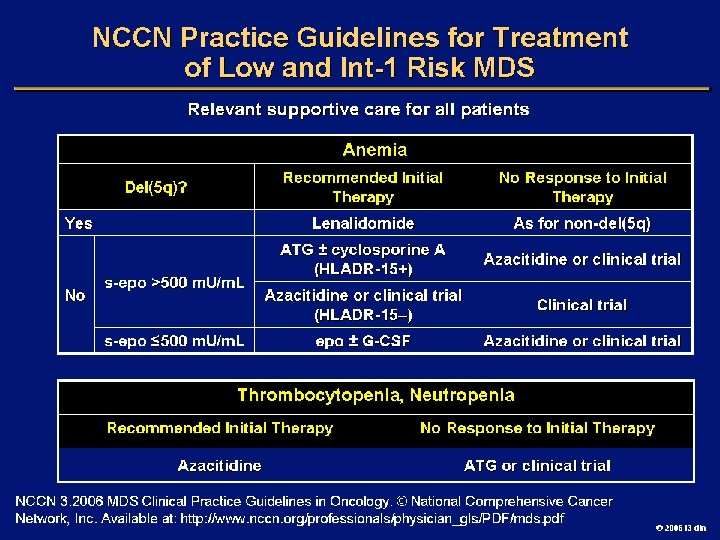
Goals of MDS Therapy l Select best treatment – Response according to predictive variables – Consider type and severity of cytopenia(s), age, and possible comorbidities l IPSS intermediate-1/low risk – Improve blood counts, quality of life; decrease infections – Decrease transfusion requirement, potentially improve survival l Intermediate-2/high-risk IPSS – Prolong survival, delay progression to AML Cheson BD, et al. Blood. 2000; 96: 3671 -3674. MDS Guidelines. Available at: http: // www. NCCN. org. 2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

Myeloproliferative disorders 2005 i 3 dln

OBJECTIVES l DEFINITION l CLASSIFICATION l OVERLAP SYNDROMES l BIOLOGY l CML l P. VERA l ET l MF 2006 i 3 dln

Myeloproliferative disorders l Clonal haematopoeitic disorders l Proliferation of one of myeloid lineages – – – Granulocytic Erythroid Megakaryocytic l Relatively normal maturation 2006 i 3 dln

Myeloproliferative disorders l Ch Myeloid leukemia (BCR-ABL positive) l Polycythemia Vera l Essential Thrombocythemia l Myelofibrosis – Specific clincopathologic criteria for diagnosis and – – – distinct diseases, have common features Increased number of one or more myeloid cell lines Hepatosplenomegaly Hypercatabolism Clonal marrow hyperplasia without dysplasia Predisposition to evolve 2006 i 3 dln

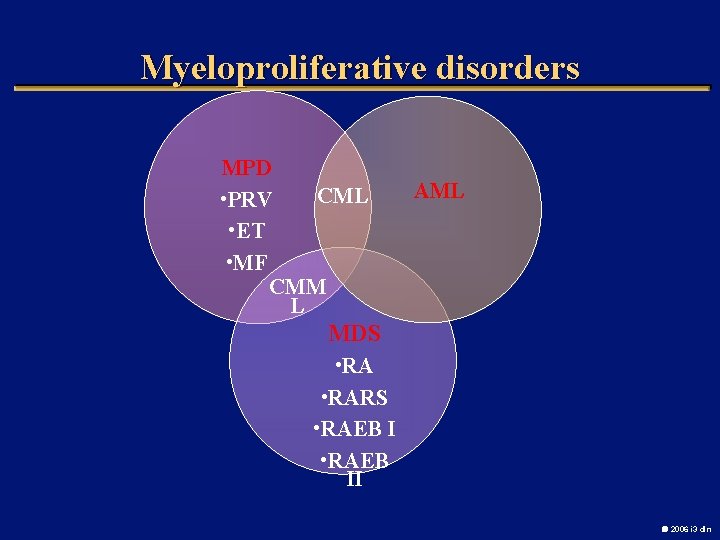
Myeloproliferative disorders MPD CML • PRV • ET • MF CMM L AML MDS • RARS • RAEB II 2006 i 3 dln

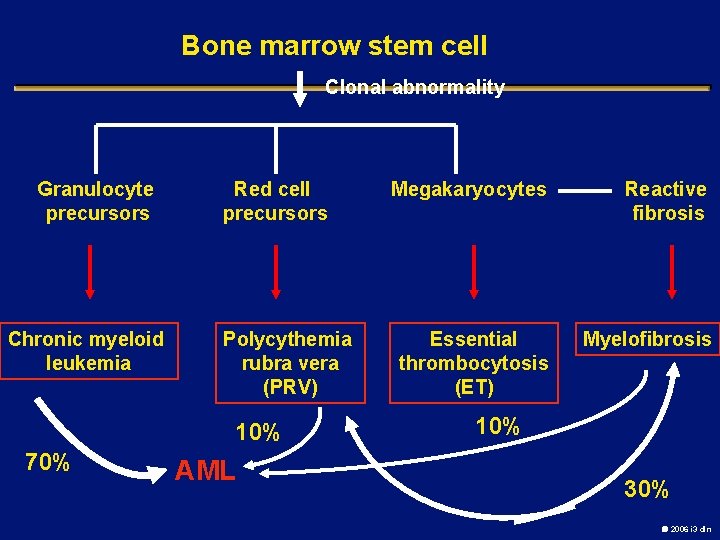
Bone marrow stem cell Clonal abnormality Granulocyte precursors Chronic myeloid leukemia Red cell precursors Polycythemia rubra vera (PRV) 10% 70% AML Megakaryocytes Reactive fibrosis Essential thrombocytosis (ET) Myelofibrosis 10% 30% 2006 i 3 dln

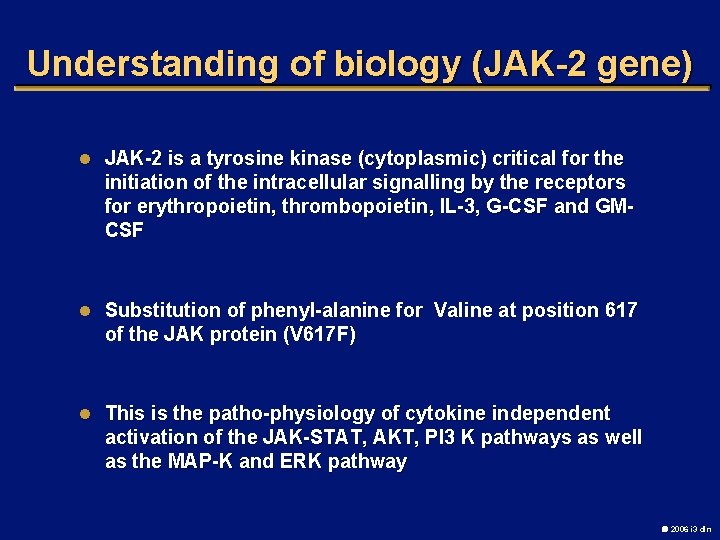
Understanding of biology (JAK-2 gene) l JAK-2 is a tyrosine kinase (cytoplasmic) critical for the initiation of the intracellular signalling by the receptors for erythropoietin, thrombopoietin, IL-3, G-CSF and GMCSF l Substitution of phenyl-alanine for Valine at position 617 of the JAK protein (V 617 F) l This is the patho-physiology of cytokine independent activation of the JAK-STAT, AKT, PI 3 K pathways as well as the MAP-K and ERK pathway 2006 i 3 dln

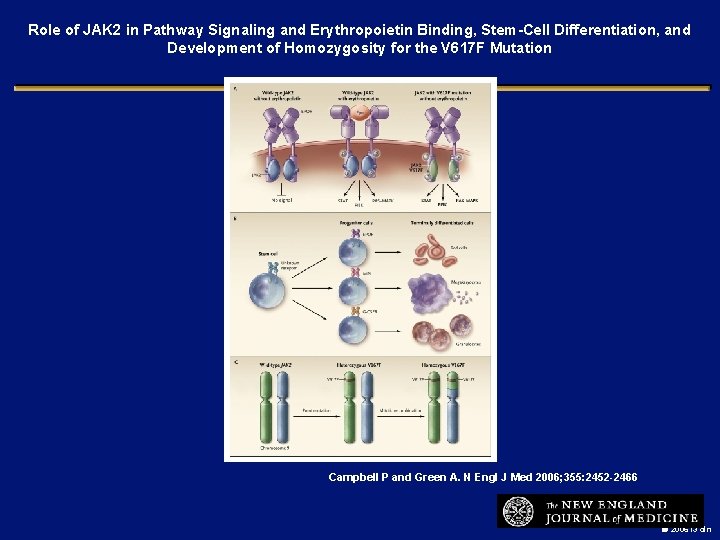
Role of JAK 2 in Pathway Signaling and Erythropoietin Binding, Stem-Cell Differentiation, and Development of Homozygosity for the V 617 F Mutation Campbell P and Green A. N Engl J Med 2006; 355: 2452 -2466 2006 i 3 dln

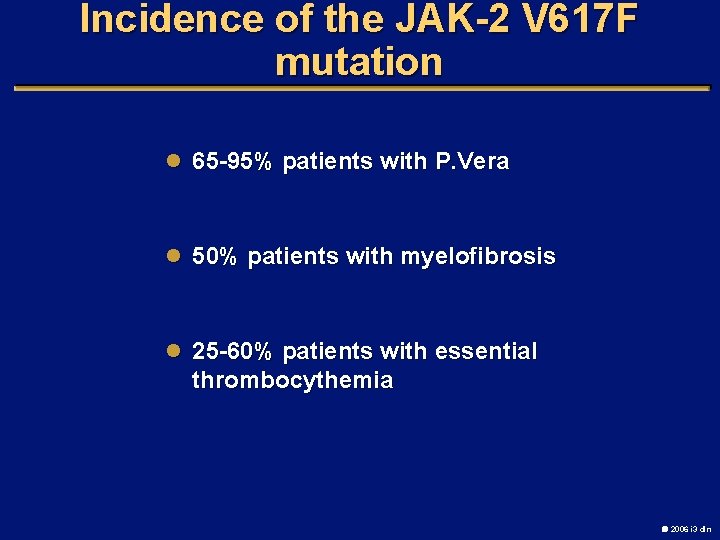
Incidence of the JAK-2 V 617 F mutation l 65 -95% patients with P. Vera l 50% patients with myelofibrosis l 25 -60% patients with essential thrombocythemia 2006 i 3 dln

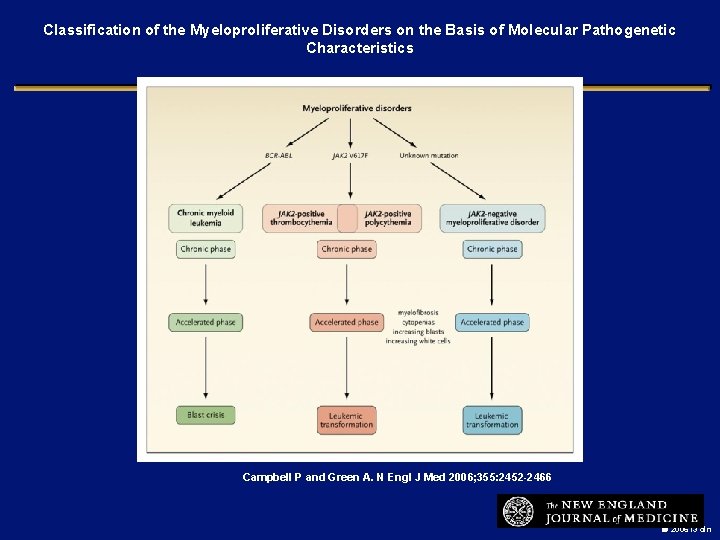
Classification of the Myeloproliferative Disorders on the Basis of Molecular Pathogenetic Characteristics Campbell P and Green A. N Engl J Med 2006; 355: 2452 -2466 2006 i 3 dln

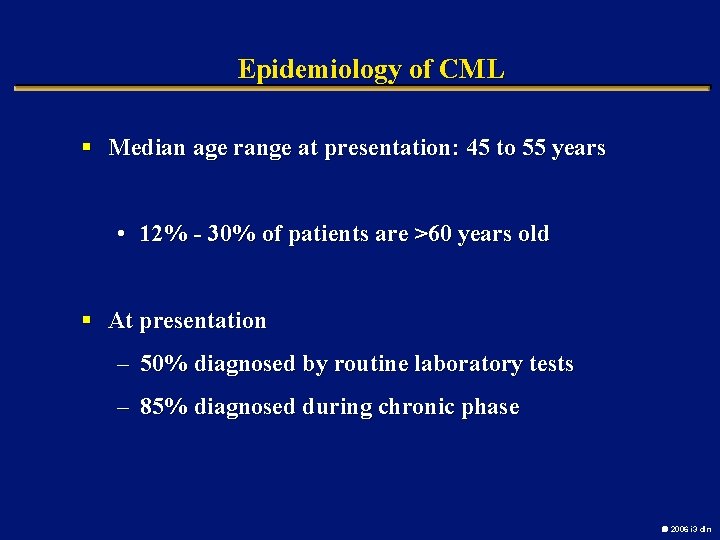
Epidemiology of CML § Median age range at presentation: 45 to 55 years • 12% - 30% of patients are >60 years old § At presentation – 50% diagnosed by routine laboratory tests – 85% diagnosed during chronic phase 2006 i 3 dln

Presentation Insidious onset Anorexia and weight loss Symptoms of anemia Splenomegaly –maybe massive Pt. maybe asymptomatic 2006 i 3 dln

The Philadelphia Chromosome: t(9; 22) Translocation 9 9+ Philadelphia Ph chromosome 22 bcr-abl Fusion protein with tyrosine kinase activity 2006 i 3 dln

2006 i 3 dln

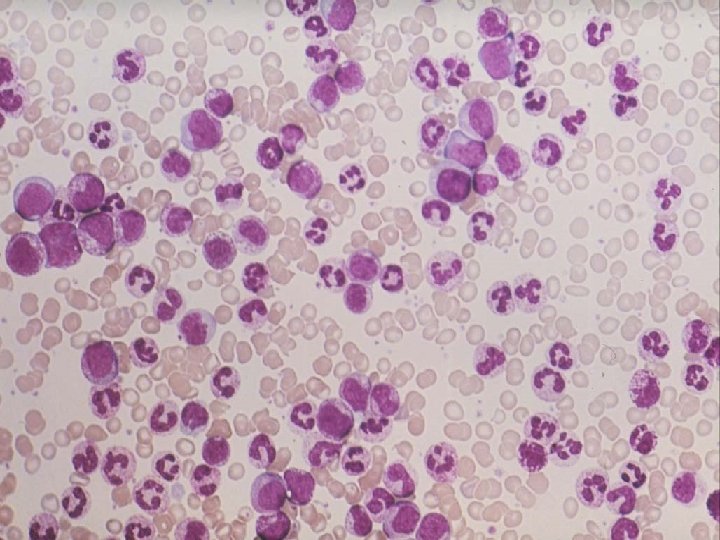
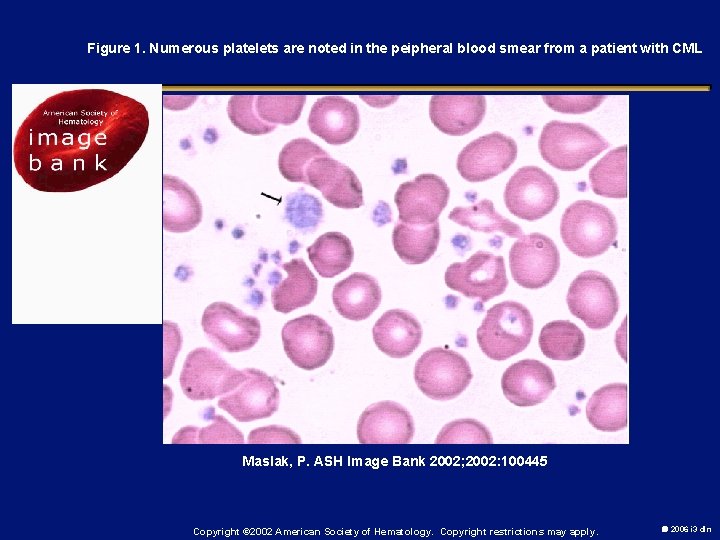
Figure 1. Numerous platelets are noted in the peipheral blood smear from a patient with CML Maslak, P. ASH Image Bank 2002; 2002: 100445 Copyright © 2002 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

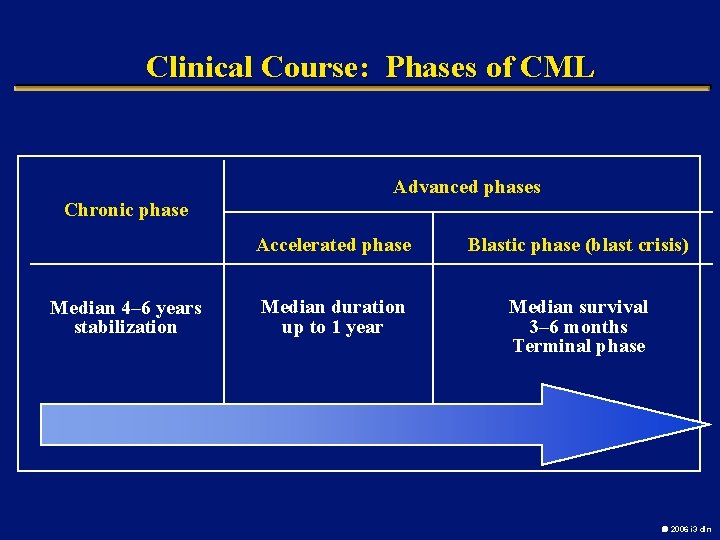
Clinical Course: Phases of CML Advanced phases Chronic phase Median 4– 6 years stabilization Accelerated phase Blastic phase (blast crisis) Median duration up to 1 year Median survival 3– 6 months Terminal phase 2006 i 3 dln

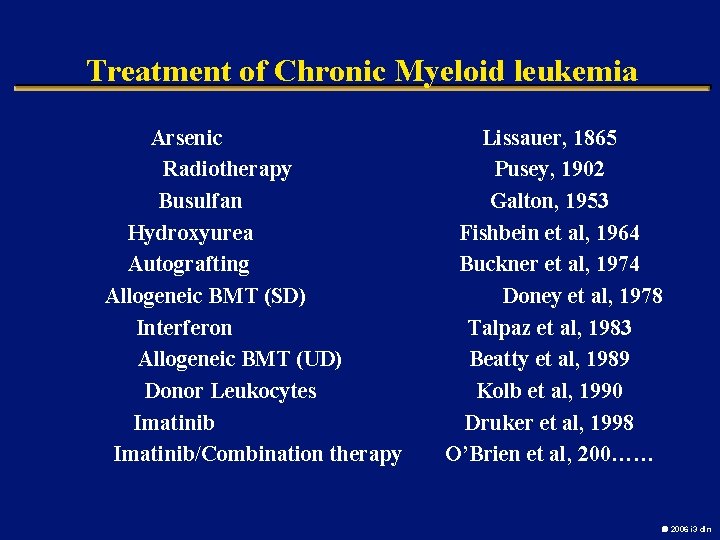
Treatment of Chronic Myeloid leukemia Arsenic Radiotherapy Busulfan Hydroxyurea Autografting Allogeneic BMT (SD) Interferon Allogeneic BMT (UD) Donor Leukocytes Imatinib/Combination therapy Lissauer, 1865 Pusey, 1902 Galton, 1953 Fishbein et al, 1964 Buckner et al, 1974 Doney et al, 1978 Talpaz et al, 1983 Beatty et al, 1989 Kolb et al, 1990 Druker et al, 1998 O’Brien et al, 200…… 2006 i 3 dln

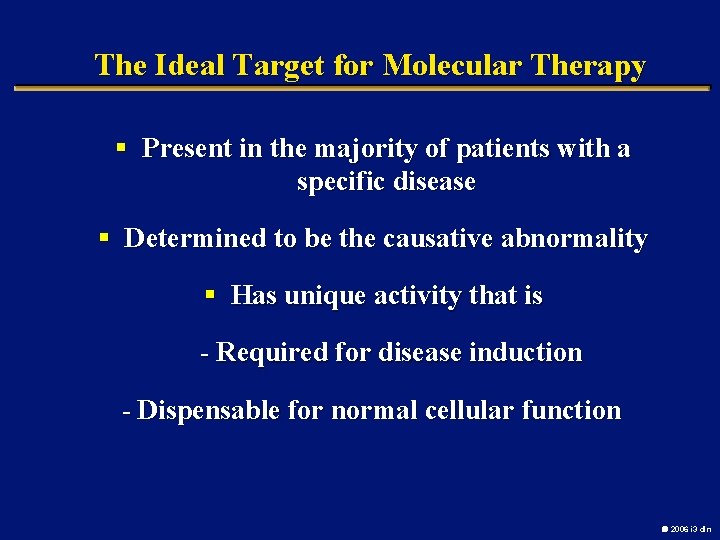
The Ideal Target for Molecular Therapy § Present in the majority of patients with a specific disease § Determined to be the causative abnormality § Has unique activity that is - Required for disease induction - Dispensable for normal cellular function 2006 i 3 dln

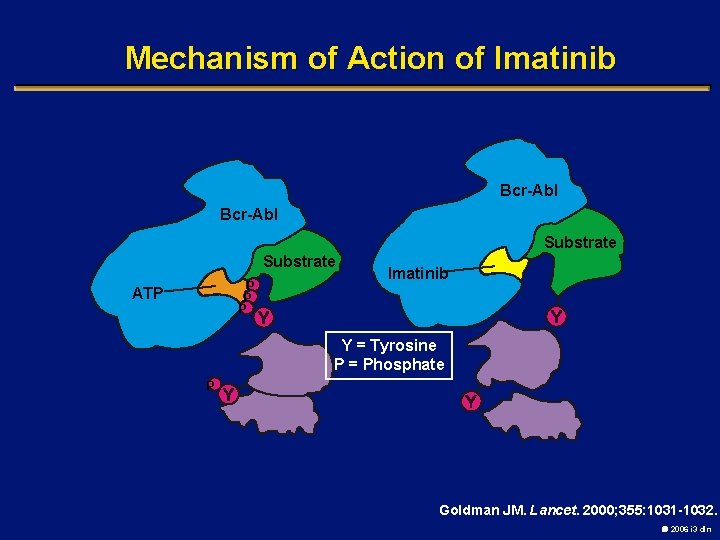
Mechanism of Action of Imatinib Bcr-Abl Substrate P P P ATP Imatinib Y = Tyrosine P = Phosphate P Goldman JM. Lancet. 2000; 355: 1031 -1032. 2006 i 3 dln

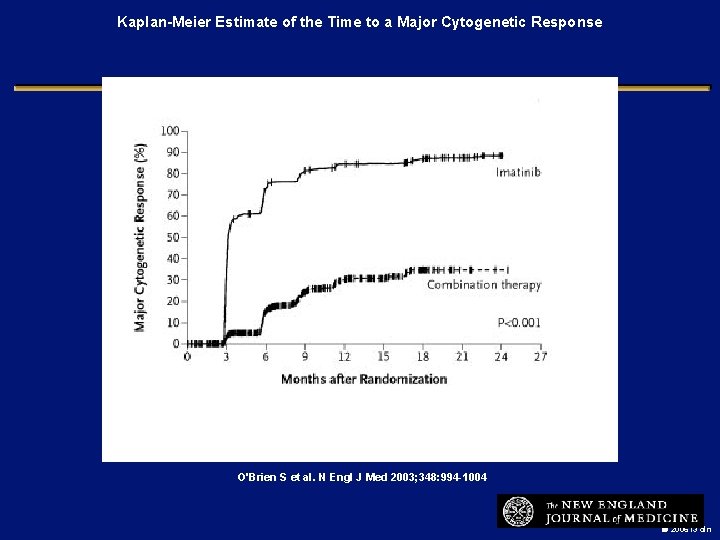
Kaplan-Meier Estimate of the Time to a Major Cytogenetic Response O'Brien S et al. N Engl J Med 2003; 348: 994 -1004 2006 i 3 dln

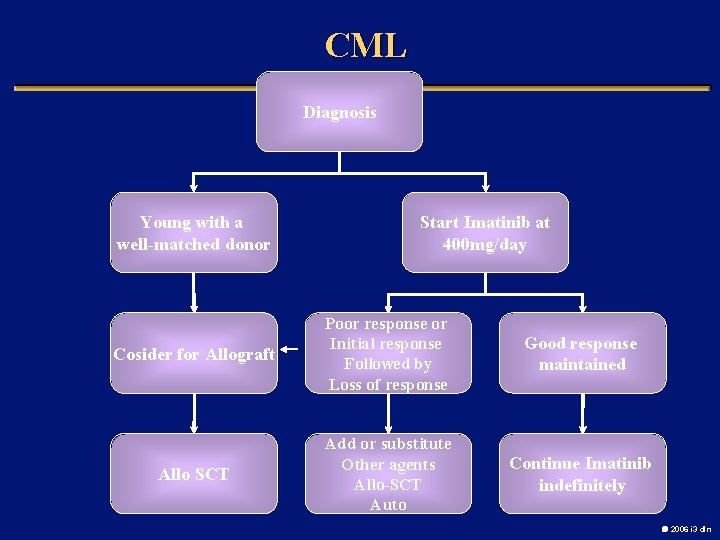
CML Diagnosis Young with a well-matched donor Start Imatinib at 400 mg/day Cosider for Allograft Poor response or Initial response Followed by Loss of response Good response maintained Allo SCT Add or substitute Other agents Allo-SCT Auto Continue Imatinib indefinitely 2006 i 3 dln

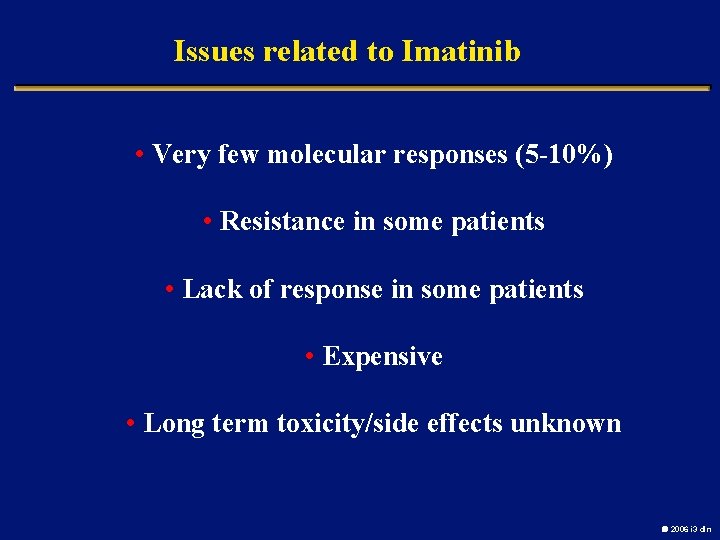
Issues related to Imatinib • Very few molecular responses (5 -10%) • Resistance in some patients • Lack of response in some patients • Expensive • Long term toxicity/side effects unknown 2006 i 3 dln

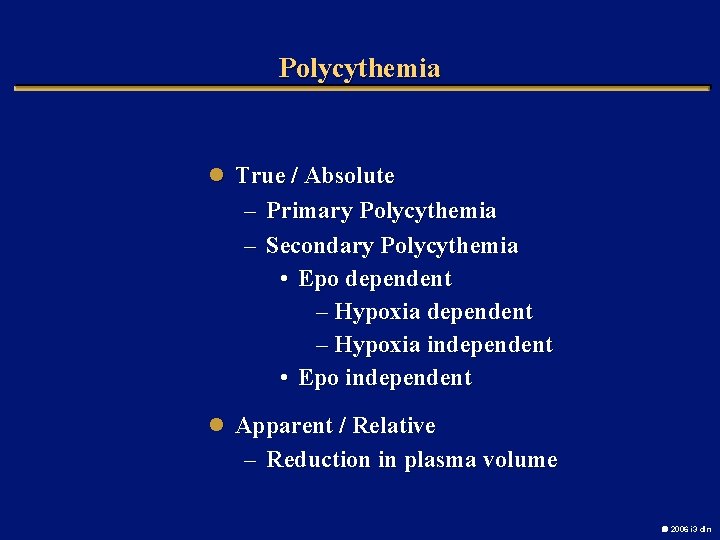
Polycythemia l True / Absolute – Primary Polycythemia – Secondary Polycythemia • Epo dependent – Hypoxia independent • Epo independent l Apparent / Relative – Reduction in plasma volume 2006 i 3 dln

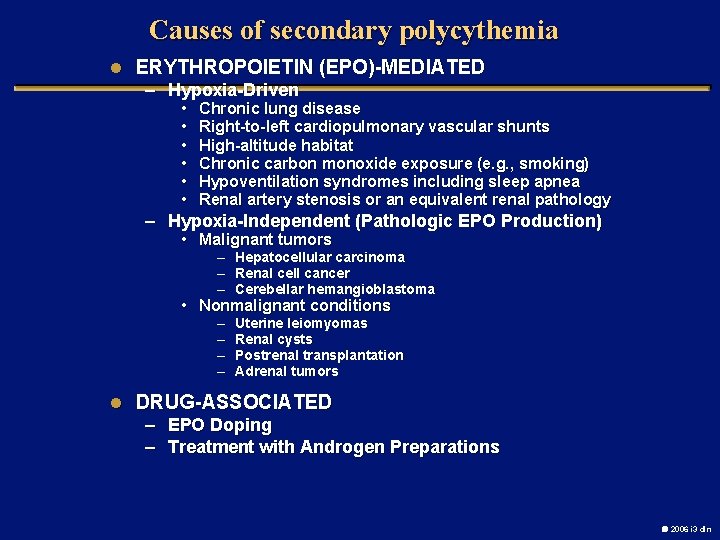
Causes of secondary polycythemia l ERYTHROPOIETIN (EPO)-MEDIATED – Hypoxia-Driven • • • Chronic lung disease Right-to-left cardiopulmonary vascular shunts High-altitude habitat Chronic carbon monoxide exposure (e. g. , smoking) Hypoventilation syndromes including sleep apnea Renal artery stenosis or an equivalent renal pathology – Hypoxia-Independent (Pathologic EPO Production) • Malignant tumors – Hepatocellular carcinoma – Renal cell cancer – Cerebellar hemangioblastoma • Nonmalignant conditions – – Uterine leiomyomas Renal cysts Postrenal transplantation Adrenal tumors l DRUG-ASSOCIATED – EPO Doping – Treatment with Androgen Preparations 2006 i 3 dln

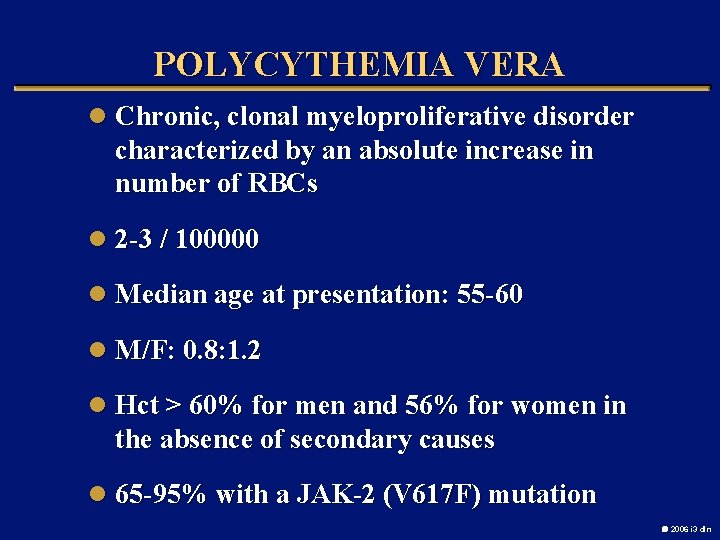
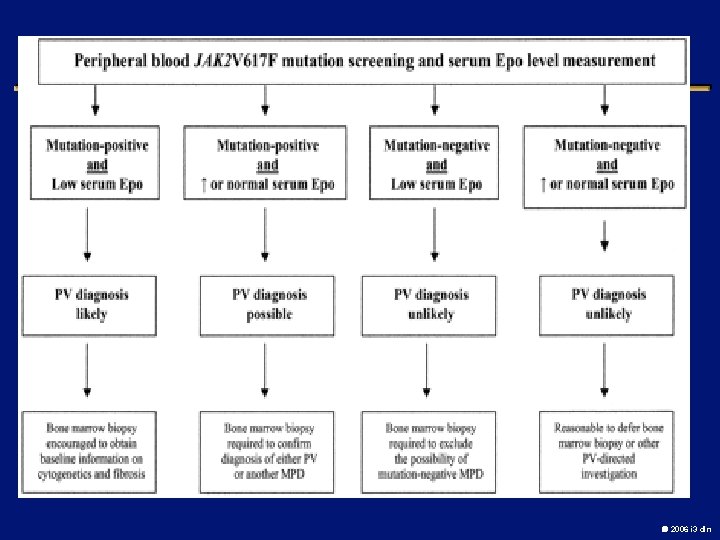
POLYCYTHEMIA VERA l Chronic, clonal myeloproliferative disorder characterized by an absolute increase in number of RBCs l 2 -3 / 100000 l Median age at presentation: 55 -60 l M/F: 0. 8: 1. 2 l Hct > 60% for men and 56% for women in the absence of secondary causes l 65 -95% with a JAK-2 (V 617 F) mutation 2006 i 3 dln

Clinical features l Headache, pruritus, dyspnea, blurred vision, facial plethora l Persistent leukocytosis and or thrombocytosis l Microcytosis secondary to iron deficiency l Splenomegaly l Unusual thrombosis (e. g. , Budd-Chiari syndrome) l Erythromelalgia (acral dysesthesia and erythema) 2006 i 3 dln

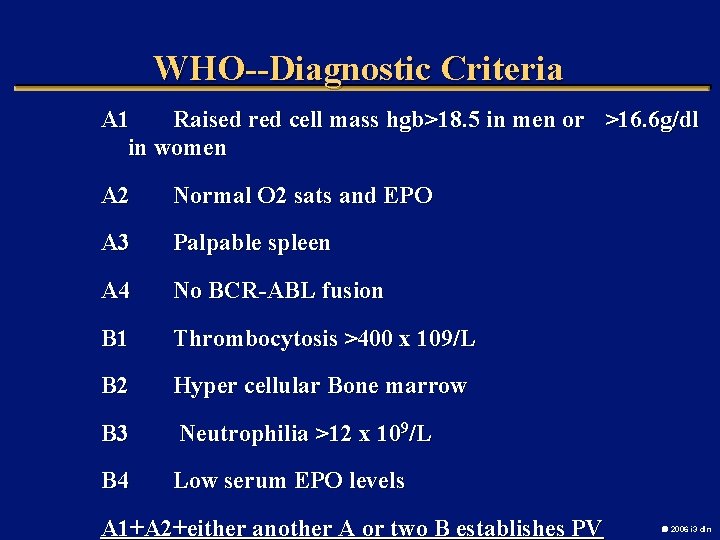
WHO--Diagnostic Criteria A 1 Raised red cell mass hgb>18. 5 in men or >16. 6 g/dl in women A 2 Normal O 2 sats and EPO A 3 Palpable spleen A 4 No BCR-ABL fusion B 1 Thrombocytosis >400 x 109/L B 2 Hyper cellular Bone marrow B 3 Neutrophilia >12 x 109/L B 4 Low serum EPO levels A 1+A 2+either another A or two B establishes PV 2006 i 3 dln

2006 i 3 dln

Treatment l The mainstay of therapy in PV remains phlebotomy to keep the hematocrit below 45 percent in men and 42 percent in women l Additional hydroxyurea in high-risk pts for thrombosis (age over 70, prior thrombosis, platelet count >400, 000/micro. L, presence of cardiovascular risk factors) l Aspirin (75 -100 mg/d) if no contraindication, reduces thromboses l IFNa (3 mu three times per week) in patients with refractory pruritus, pregnancy l Normalize hematocrit values for several days prior to any elective surgery 2006 i 3 dln

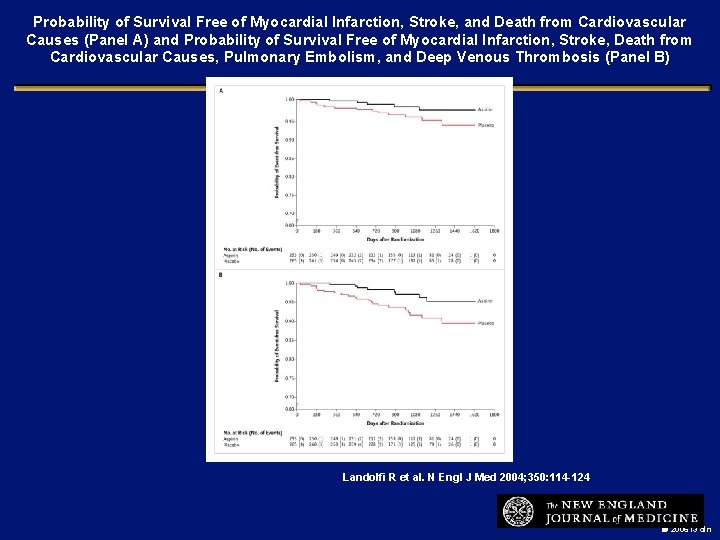
Probability of Survival Free of Myocardial Infarction, Stroke, and Death from Cardiovascular Causes (Panel A) and Probability of Survival Free of Myocardial Infarction, Stroke, Death from Cardiovascular Causes, Pulmonary Embolism, and Deep Venous Thrombosis (Panel B) Landolfi R et al. N Engl J Med 2004; 350: 114 -124 2006 i 3 dln

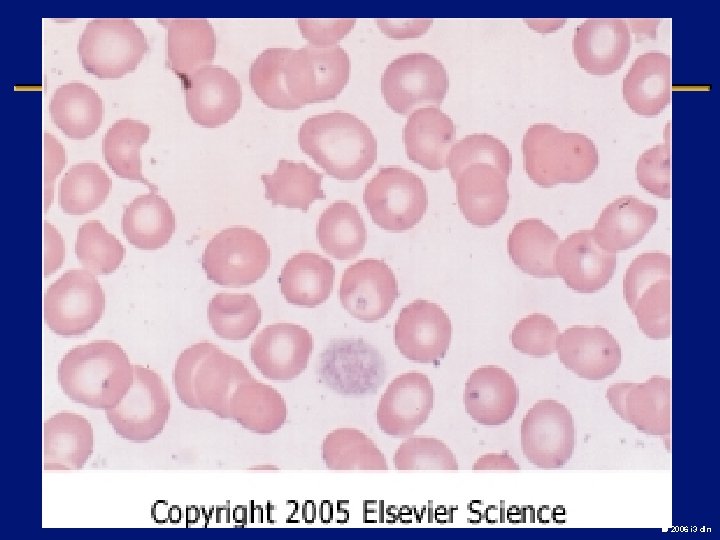
Essential Thrombocythemia (ET) l Most common clonal MPD l Persistent elevation of Plt>600 /micro. L l Lack of positive diagnostic criteria l Median age at diagnosis: 60, however 20% cases <40 yrs l JAK-2 mtation found upto 50% of the pts 2006 i 3 dln

Clinical Features l Vasomotor – – Headache Lightheadedness Syncope Erythromelalgia (burning pain of the hands or feet associated with erythema and warmth) – Transient visual disturbances (eg, amaurosis fujax, scintillating scotomata, ocular migraine) – Livedo reticularis l Thrombosis and Hemorrhage l Transformation rare 2006 i 3 dln

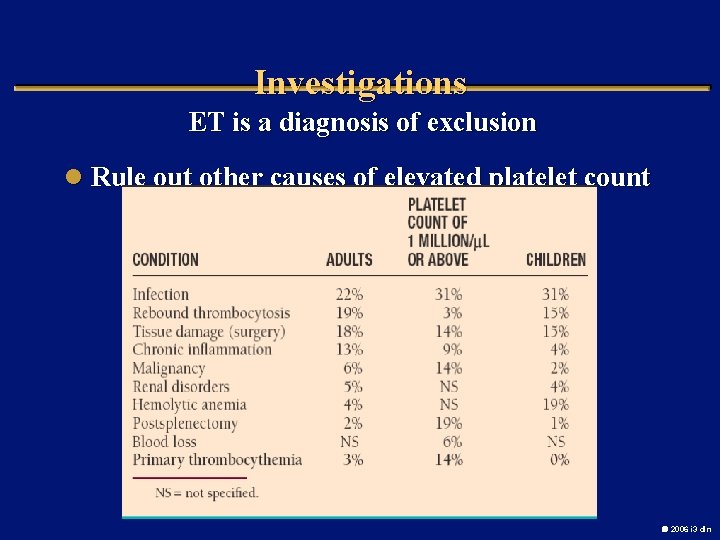
Investigations ET is a diagnosis of exclusion l Rule out other causes of elevated platelet count 2006 i 3 dln

Diagnostic criteria for ET l Platelet count >600 x 109/L for at least 2 months l Megakaryocytic hyperplasia on bone marrow aspiration and biopsy l No cause for reactive thrombocytosis l Absence of the Philadelphia chromosome l Normal red blood cell (RBC) mass or a HCT <0. 48 l Presence of stainable iron in a bone marrow aspiration l No evidence of myelofibrosis l No evidence of MDS 2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

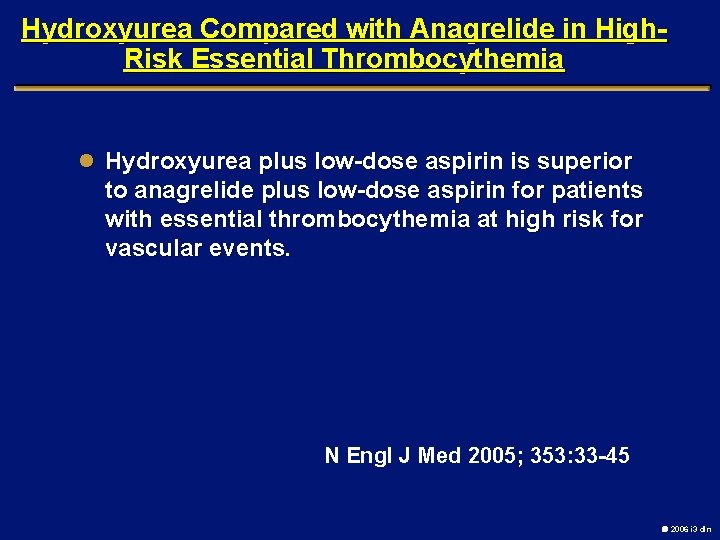
Hydroxyurea Compared with Anagrelide in High. Risk Essential Thrombocythemia l Hydroxyurea plus low-dose aspirin is superior to anagrelide plus low-dose aspirin for patients with essential thrombocythemia at high risk for vascular events. N Engl J Med 2005; 353: 33 -45 2006 i 3 dln

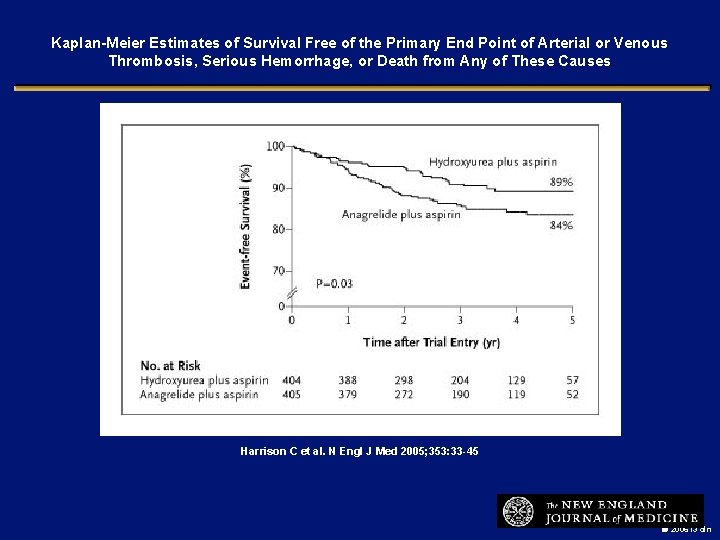
Kaplan-Meier Estimates of Survival Free of the Primary End Point of Arterial or Venous Thrombosis, Serious Hemorrhage, or Death from Any of These Causes Harrison C et al. N Engl J Med 2005; 353: 33 -45 2006 i 3 dln

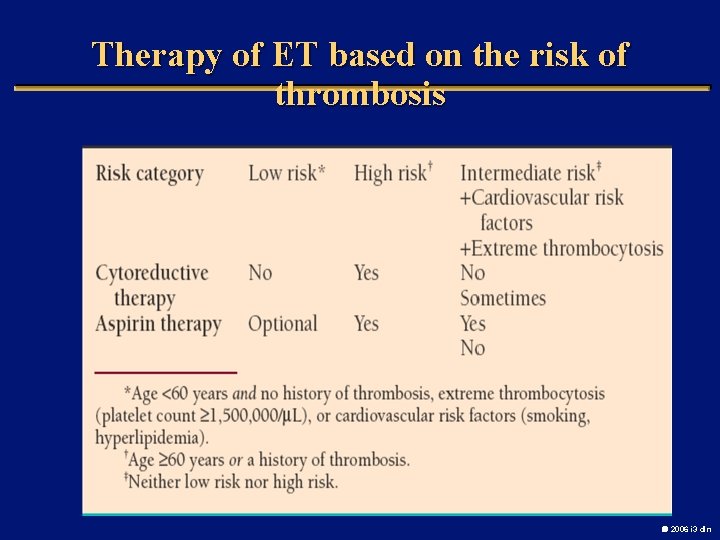
Therapy of ET based on the risk of thrombosis 2006 i 3 dln

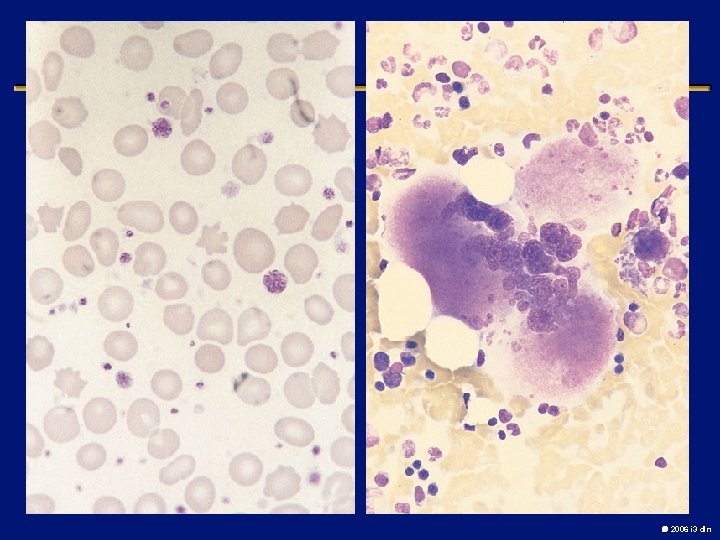
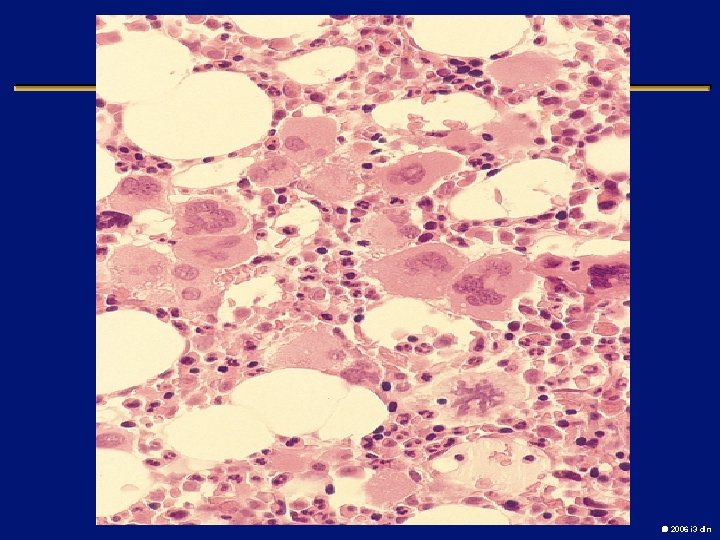
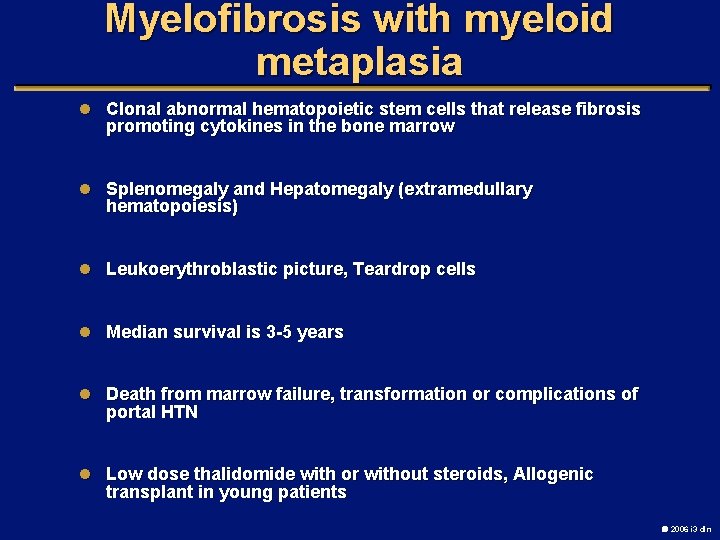
Myelofibrosis with myeloid metaplasia l Clonal abnormal hematopoietic stem cells that release fibrosis promoting cytokines in the bone marrow l Splenomegaly and Hepatomegaly (extramedullary hematopoiesis) l Leukoerythroblastic picture, Teardrop cells l Median survival is 3 -5 years l Death from marrow failure, transformation or complications of portal HTN l Low dose thalidomide with or without steroids, Allogenic transplant in young patients 2006 i 3 dln

2006 i 3 dln

2006 i 3 dln

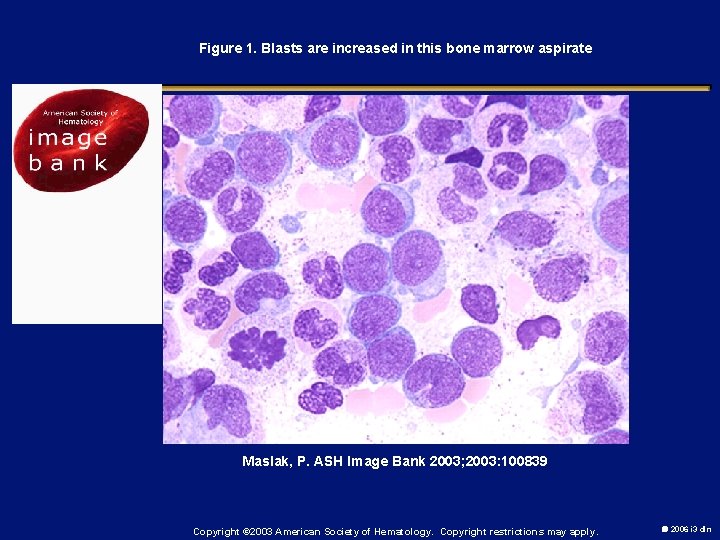
Figure 1. Blasts are increased in this bone marrow aspirate Maslak, P. ASH Image Bank 2003; 2003: 100839 Copyright © 2003 American Society of Hematology. Copyright restrictions may apply. 2006 i 3 dln

Case l 50 year old man with pruritus while showering l Otherwise excellent state of health l Non smoker and a palpable spleen tip on exam l FOB screen negative, normal O 2 sat l Hct 61%, wbc 11 k, MCV 79, Plt count 550, ferritin 12, GI screen Okay 2006 i 3 dln

Most appropriate therapy l Phlebotomy and anagrelide l Oral iron and Asa l Hydroxyurea and asa l Phlebotomy and low dose asa 2006 i 3 dln

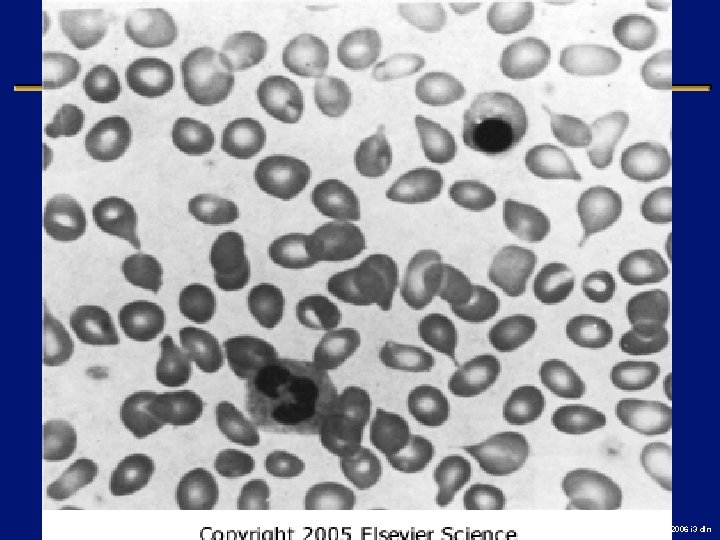
Case l 56 year old male with fatigue, fever and nose bleeds l RAEB-1 diagnosed 5 months ago l Supportive care with PRBC’s and EPO to date l Fever 103 F, ecchymoses and petechiae, no organomegaly or adenopathy l Hg. B 6 wbc 1. 2, plts 7 K 2006 i 3 dln

In addition to a transfusion and a bone marrow aspirate l Plasma exchange l Allogeneic stem cell transplant l Azacytidine l Combination induction chemotherapy 2006 i 3 dln

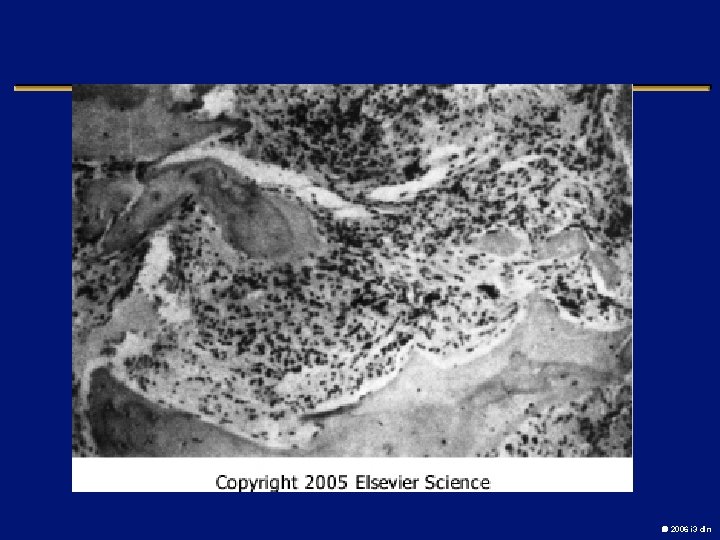
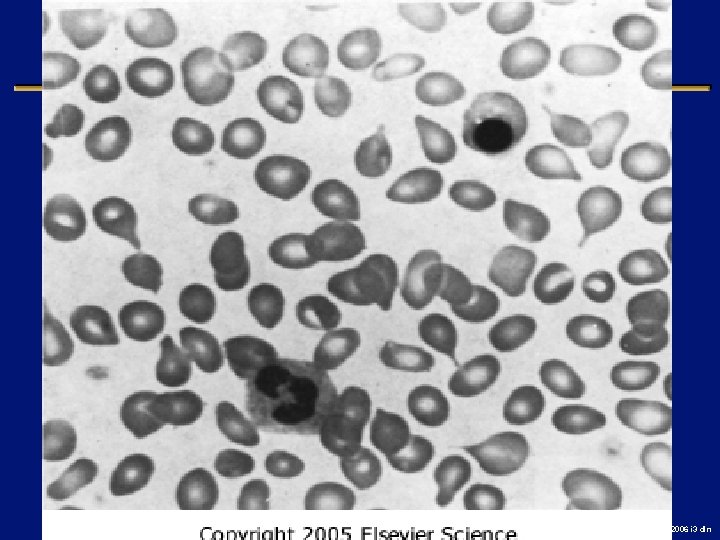
Case l 76 year old man with extreme fatigue, dyspnea, early satiety and night sweats l Lost 10% of baseline weight l large spleen, Hg. B 7. 6, WBC 4. 2, plts 1. 2 M l Bone marrow “dry tap”, PH chromosome neg l Improves with a transfusion 2006 i 3 dln

2006 i 3 dln

OPTIONS l Chronic transfusions l Splenectomy l Daily Imatinib Mesylate l Allogeneic stem cell transplant 2006 i 3 dln
 Myeloproliferative disease
Myeloproliferative disease Jaya jaya radha krishna yugala milana lyrics
Jaya jaya radha krishna yugala milana lyrics Finasteride side effects
Finasteride side effects What is geriatric syndromes
What is geriatric syndromes Neuroendocrine syndrome in gynecology
Neuroendocrine syndrome in gynecology Best language nih
Best language nih Anatonomina
Anatonomina Cerebellar syndromes
Cerebellar syndromes Myeloproliferative neoplams
Myeloproliferative neoplams Myeloproliferative disease
Myeloproliferative disease Myeloproliferative syndrome
Myeloproliferative syndrome Myelopoiesis
Myelopoiesis Myeloproliferative disorder
Myeloproliferative disorder Lumpy jaw meaning
Lumpy jaw meaning Struktur biro sdm polda
Struktur biro sdm polda Kariah maksud
Kariah maksud Smk cj
Smk cj Rancangan tempatan petaling jaya
Rancangan tempatan petaling jaya Struktur polda riau 2020
Struktur polda riau 2020 Rama raghava jaya seetha nayaka lyrics
Rama raghava jaya seetha nayaka lyrics Silsilah keluarga ciung wanara
Silsilah keluarga ciung wanara Pramuka praja muda karana
Pramuka praja muda karana Struktur organisasi polda
Struktur organisasi polda Perusahaan dagang kondang jaya
Perusahaan dagang kondang jaya Indonesia tanah air beta pusaka abadi nan jaya indonesia
Indonesia tanah air beta pusaka abadi nan jaya indonesia Pt langit jaya perkasa
Pt langit jaya perkasa Amarta jaya telekomindo
Amarta jaya telekomindo Pt anugrah alam jaya perkasa
Pt anugrah alam jaya perkasa Agivan
Agivan Jenis2 surat lamaran pekerjaan
Jenis2 surat lamaran pekerjaan Pt sihyong jaya persada
Pt sihyong jaya persada Majlis perbandaran seberang jaya
Majlis perbandaran seberang jaya Pt allindo jaya perkasa
Pt allindo jaya perkasa Jaya vaidyanathan
Jaya vaidyanathan Sri krsna caitanya prabhu nityananda
Sri krsna caitanya prabhu nityananda Bumd sarana jaya
Bumd sarana jaya Jaya srila prabhupada
Jaya srila prabhupada Unit 14 physiological disorders examples
Unit 14 physiological disorders examples Bipolar and other related disorders
Bipolar and other related disorders Bipolar and other related disorders
Bipolar and other related disorders Assistive technology for behavior
Assistive technology for behavior Puberty and autism spectrum disorders
Puberty and autism spectrum disorders Axis 1 and axis 2 disorders
Axis 1 and axis 2 disorders Neurotic stress-related and somatoform disorders
Neurotic stress-related and somatoform disorders Define a primary skin lesion and list three types
Define a primary skin lesion and list three types Physical disorders and health psychology
Physical disorders and health psychology Chapter 6 musculoskeletal system
Chapter 6 musculoskeletal system Chapter 46 digestive and endocrine disorders
Chapter 46 digestive and endocrine disorders Somatic symptom disorder
Somatic symptom disorder Chapter 29 endocrine and metabolic disorders
Chapter 29 endocrine and metabolic disorders Chapter 21 mental health diseases and disorders
Chapter 21 mental health diseases and disorders Personality disorder vs mental illness
Personality disorder vs mental illness Chapter 18 eating and feeding disorders
Chapter 18 eating and feeding disorders Chapter 17 reproductive system diseases and disorders
Chapter 17 reproductive system diseases and disorders Chapter 15 nervous system diseases and disorders
Chapter 15 nervous system diseases and disorders Chapter 15 anxiety and obsessive-compulsive disorders
Chapter 15 anxiety and obsessive-compulsive disorders Chapter 11 childhood and neurodevelopmental disorders
Chapter 11 childhood and neurodevelopmental disorders Milady chapter 10 nail disorders and diseases
Milady chapter 10 nail disorders and diseases Axis 1 and 2 disorders
Axis 1 and 2 disorders Dissociative
Dissociative Unit 14 physiological disorders and their care
Unit 14 physiological disorders and their care Neurotic personality disorder
Neurotic personality disorder Chapter 8 skin disorders and diseases
Chapter 8 skin disorders and diseases What conditions do fungal organisms favor for growth
What conditions do fungal organisms favor for growth Cardiovascular system diseases and disorders chapter 8
Cardiovascular system diseases and disorders chapter 8 Neurocognitive and neurodevelopmental disorders
Neurocognitive and neurodevelopmental disorders Mcq on phenylketonuria
Mcq on phenylketonuria Eating disorder nursing diagnosis
Eating disorder nursing diagnosis Milady chapter 8 skin disorders and diseases
Milady chapter 8 skin disorders and diseases Difference between mendelian and chromosomal disorders
Difference between mendelian and chromosomal disorders Chapter 47 urinary and reproductive disorders
Chapter 47 urinary and reproductive disorders Chapter 11 lesson 1 maintaining a healthy weight
Chapter 11 lesson 1 maintaining a healthy weight 10 diseases of lymphatic system
10 diseases of lymphatic system Unit 14 business level 3
Unit 14 business level 3 Neurocognitive disorders
Neurocognitive disorders Flinders model
Flinders model Somatoform disorders
Somatoform disorders Paraphilia disorders
Paraphilia disorders Types of sensory disorders
Types of sensory disorders Section 4-1 mental disorders answers
Section 4-1 mental disorders answers Section 4-1 mental disorders answers
Section 4-1 mental disorders answers Medical model psychology
Medical model psychology What are the 10 personality disorders
What are the 10 personality disorders Cluster b personality disorder
Cluster b personality disorder Avoidant personality traits
Avoidant personality traits Dsm v personality disorders
Dsm v personality disorders Psychological disorders characterized by inflexible
Psychological disorders characterized by inflexible Cluster c
Cluster c Cluster b personality traits
Cluster b personality traits Antisocial personality disorder cluster
Antisocial personality disorder cluster Eat disorder york
Eat disorder york Mild neurocognitive disorder
Mild neurocognitive disorder