Module 10 Myelodysplastic Syndromes Myeloproliferative Neoplasms and Hematologic



















![Studies with Peg. IFN Study MDACC[3, 4] § Peg. IFN α-2 a Population § Studies with Peg. IFN Study MDACC[3, 4] § Peg. IFN α-2 a Population §](https://slidetodoc.com/presentation_image_h2/b6171b1491861307c06ff74b8720e6ee/image-46.jpg)

- Slides: 50

Module 10: Myelodysplastic Syndromes, Myeloproliferative Neoplasms and Hematologic Disorder-Related Anemia Sarah K Swanson, ANP-BC Rami Komrokji, MD

Disclosure for Ms Swanson No relevant conflicts of interest to disclose.

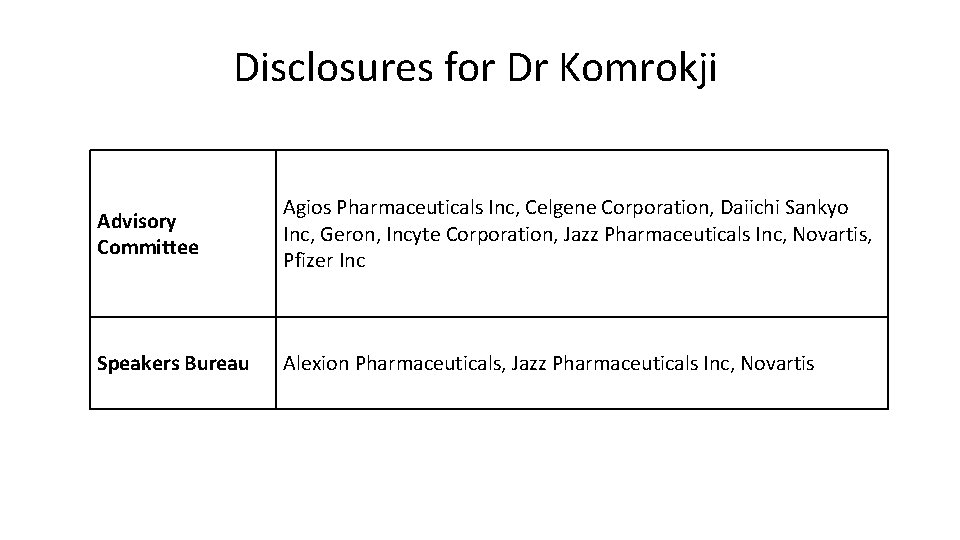
Disclosures for Dr Komrokji Advisory Committee Agios Pharmaceuticals Inc, Celgene Corporation, Daiichi Sankyo Inc, Geron, Incyte Corporation, Jazz Pharmaceuticals Inc, Novartis, Pfizer Inc Speakers Bureau Alexion Pharmaceuticals, Jazz Pharmaceuticals Inc, Novartis

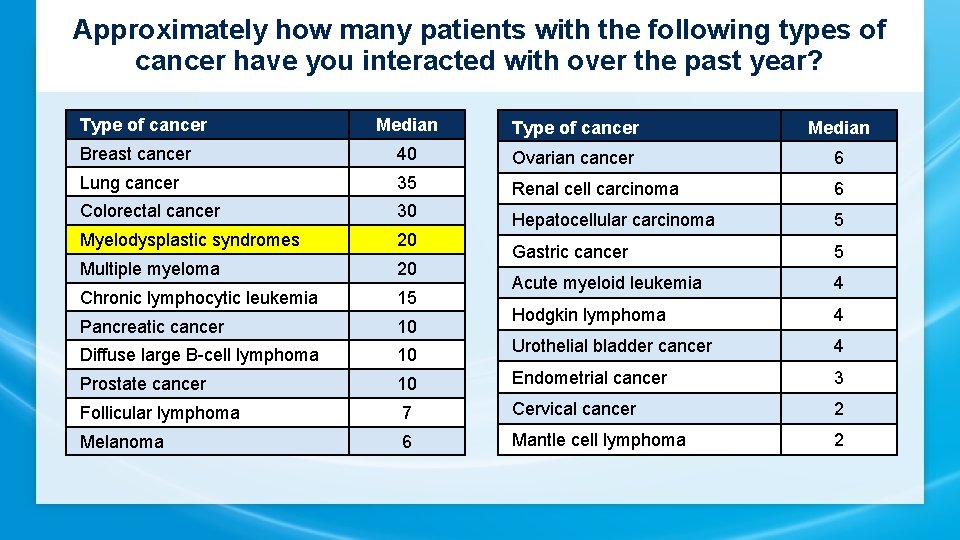
Approximately how many patients with the following types of cancer have you interacted with over the past year? Type of cancer Median Breast cancer 40 Ovarian cancer 6 Lung cancer 35 Renal cell carcinoma 6 Colorectal cancer 30 Myelodysplastic syndromes 20 Hepatocellular carcinoma 5 Multiple myeloma 20 Gastric cancer 5 Chronic lymphocytic leukemia 15 Acute myeloid leukemia 4 Pancreatic cancer 10 Hodgkin lymphoma 4 Diffuse large B-cell lymphoma 10 Urothelial bladder cancer 4 Prostate cancer 10 Endometrial cancer 3 Follicular lymphoma 7 Cervical cancer 2 Melanoma 6 Mantle cell lymphoma 2

Day in the Life: Myeloproliferative Neoplasms/MDS • 91 M, Observation, ETOH use • 97 M, azacitidine, has a pet duck • 80 F, azacitidine, continues to cook Sunday dinner for her large family • 74 F, MDS/CMML, azacitidine, eccentric • 91 M, azacitidine, anemia • 76 M, MDS, decitabine, unable to tolerate full dose, has done well for months on 25% dose reduction • 87 M, MDS, azacitidine, he is in charge! Walks back to treatment room with his own chart, finds his own chair, making this place feel like home and grandpa is visiting! Always sharing recipes and talking about what’s for dinner.

Ms Swanson Case • 66 y/o gentleman who presented with fatigue, dyspnea and exercise intolerance to his primary care provider • Looking back, he believes it had all started about a year prior when he had been hiking in the Rocky Mountains at the time, and was having a difficult time with what would have normally been very manageable for him • He was found to have anemia on a blood draw by his PCP

• He went on to have a bone marrow biopsy which showed low grade MDS with ringed sideroblasts • His Hgb continued to decline dropping into the 6 -7 g/dl range and he required weekly blood transfusions • He also required chelating agents because of his rising ferritin due to his increasing transfusion needs • It was at that time that he was started on darbepoetin alfa with increasing doses but with little response

• His exercise intolerance began to impact his Qo. L as did the need to go to the hospital for frequent blood transfusions • This would have been an ideal patient for luspatercept, an injection that he would receive every 21 days • Because of his progressive symptomatic anemia, he eventually started therapy with a hypomethylating agent

Myelodysplastic Syndromes and Myeloproliferative Neoplasms Rami Komrokji, MD Senior Member & Professor of Oncologic Sciences Section Head – Leukemia & MDS Vice Chair - Malignant Hematology Department H Lee Moffitt Cancer Center & Research Institute Tampa, Florida

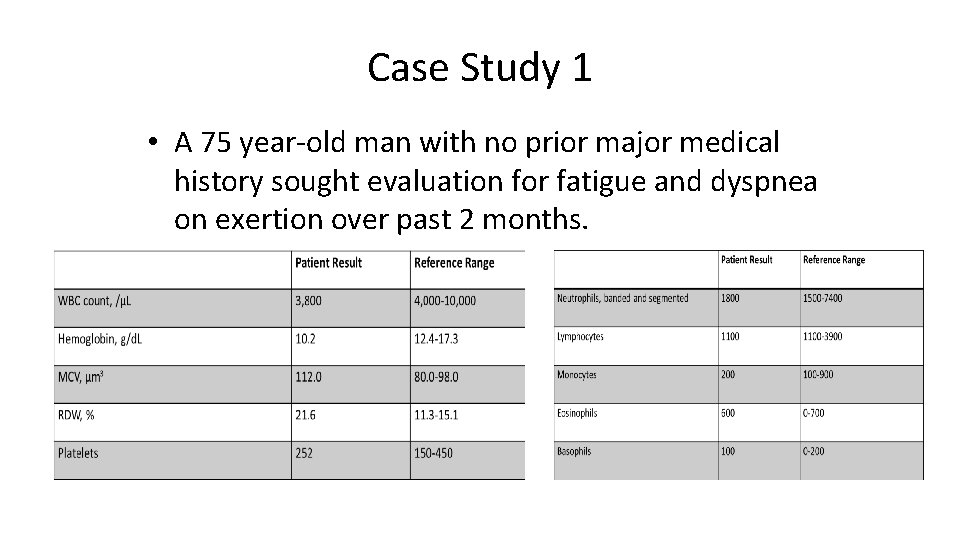
Case Study 1 • A 75 year-old man with no prior major medical history sought evaluation for fatigue and dyspnea on exertion over past 2 months.

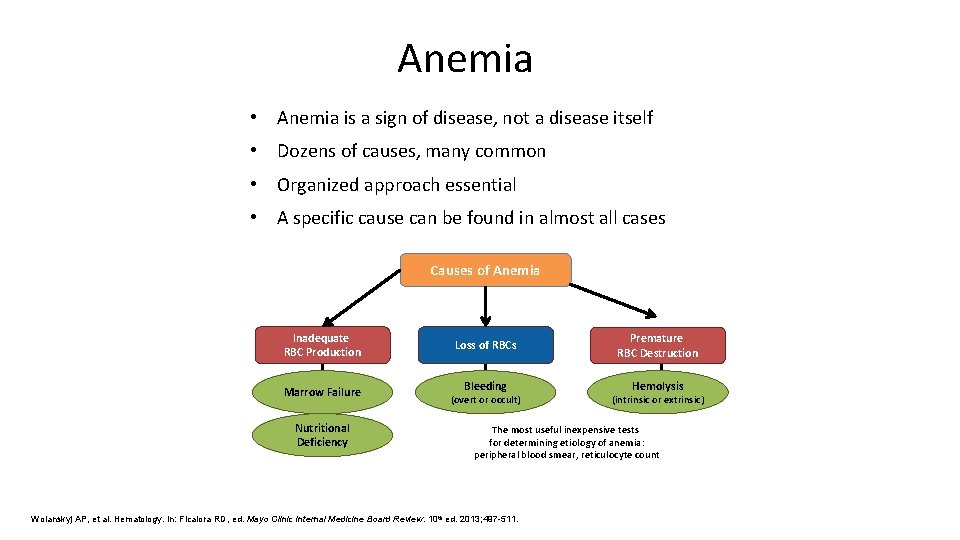
Anemia • Anemia is a sign of disease, not a disease itself • Dozens of causes, many common • Organized approach essential • A specific cause can be found in almost all cases Causes of Anemia Inadequate RBC Production Marrow Failure Nutritional Deficiency Loss of RBCs Bleeding (overt or occult) Premature RBC Destruction Hemolysis (intrinsic or extrinsic) The most useful inexpensive tests for determining etiology of anemia: peripheral blood smear, reticulocyte count Wolanskyj AP, et al. Hematology. In: Ficalora RD, ed. Mayo Clinic Internal Medicine Board Review. 10 th ed. 2013; 497 -511.

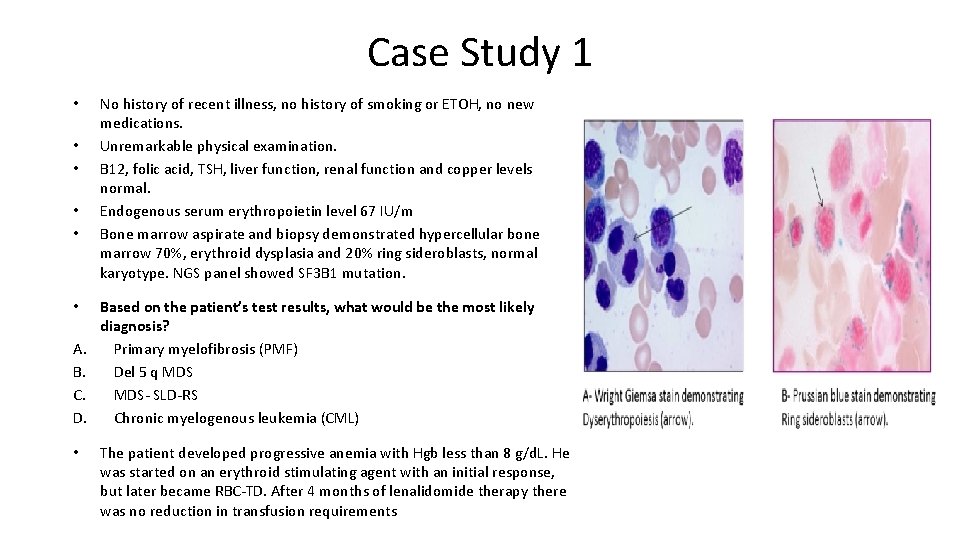
Case Study 1 • • • A. B. C. D. • No history of recent illness, no history of smoking or ETOH, no new medications. Unremarkable physical examination. B 12, folic acid, TSH, liver function, renal function and copper levels normal. Endogenous serum erythropoietin level 67 IU/m Bone marrow aspirate and biopsy demonstrated hypercellular bone marrow 70%, erythroid dysplasia and 20% ring sideroblasts, normal karyotype. NGS panel showed SF 3 B 1 mutation. Based on the patient’s test results, what would be the most likely diagnosis? Primary myelofibrosis (PMF) Del 5 q MDS- SLD-RS Chronic myelogenous leukemia (CML) The patient developed progressive anemia with Hgb less than 8 g/d. L. He was started on an erythroid stimulating agent with an initial response, but later became RBC-TD. After 4 months of lenalidomide therapy there was no reduction in transfusion requirements

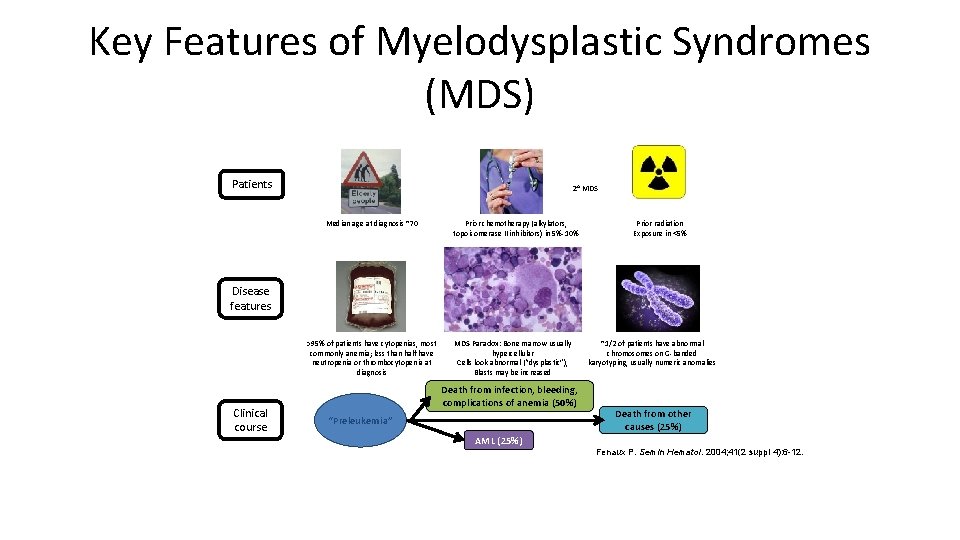
Key Features of Myelodysplastic Syndromes (MDS) Patients 2 o MDS Median age at diagnosis ~70 Prior chemotherapy (alkylators, topoisomerase II inhibitors) in 5%-10% Prior radiation Exposure in <5% Disease features >95% of patients have cytopenias, most commonly anemia; less than half have neutropenia or thrombocytopenia at diagnosis Clinical course MDS Paradox: Bone marrow usually hypercellular Cells look abnormal (“dysplastic”), Blasts may be increased Death from infection, bleeding, complications of anemia (50%) “Preleukemia” ~1/2 of patients have abnormal chromosomes on G-banded karyotyping, usually numeric anomalies Death from other causes (25%) AML (25%) Fenaux P. Semin Hematol. 2004; 41(2 suppl 4): 6 -12.

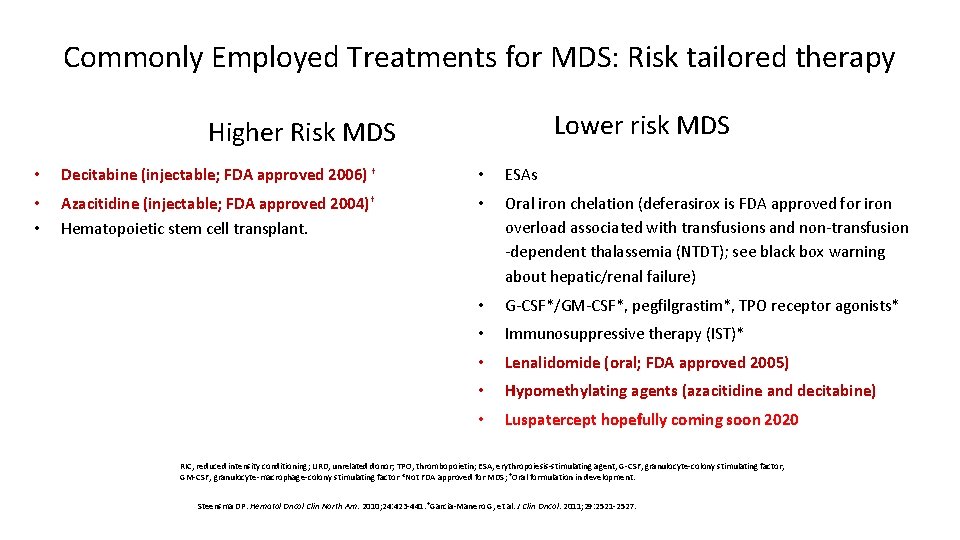
Commonly Employed Treatments for MDS: Risk tailored therapy Lower risk MDS Higher Risk MDS • Decitabine (injectable; FDA approved 2006) † • ESAs • • Azacitidine (injectable; FDA approved 2004)† Hematopoietic stem cell transplant. • Oral iron chelation (deferasirox is FDA approved for iron overload associated with transfusions and non-transfusion -dependent thalassemia (NTDT); see black box warning about hepatic/renal failure) • G-CSF*/GM-CSF*, pegfilgrastim*, TPO receptor agonists* • Immunosuppressive therapy (IST)* • Lenalidomide (oral; FDA approved 2005) • Hypomethylating agents (azacitidine and decitabine) • Luspatercept hopefully coming soon 2020 RIC, reduced intensity conditioning; URD, unrelated donor; TPO, thrombopoietin; ESA, erythropoiesis-stimulating agent, G-CSF, granulocyte-colony stimulating factor, GM-CSF, granulocyte-macrophage-colony stimulating factor *Not FDA approved for MDS; †Oral formulation in development. Steensma DP. Hematol Oncol Clin North Am. 2010; 24: 423 -441. †Garcia-Manero G, et al. J Clin Oncol. 2011; 29: 2521 -2527.

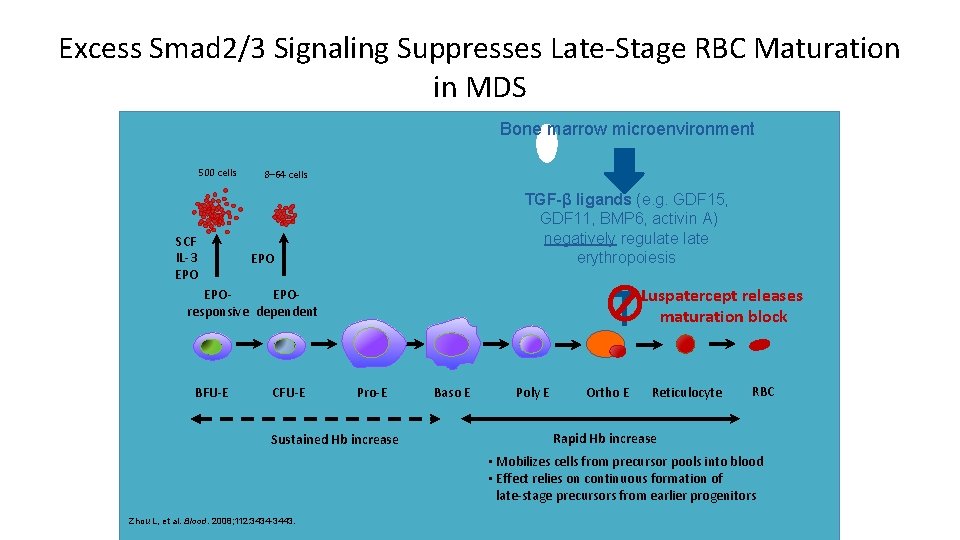
Excess Smad 2/3 Signaling Suppresses Late-Stage RBC Maturation in MDS Bone marrow microenvironment 500 cells SCF IL-3 EPO 8– 64 cells TGF-β ligands (e. g. GDF 15, GDF 11, BMP 6, activin A) negatively regulate erythropoiesis EPO Luspatercept releases maturation block EPOEPOresponsive dependent BFU-E CFU-E Pro-E Sustained Hb increase Baso E Poly E Ortho E Reticulocyte RBC Rapid Hb increase • Mobilizes cells from precursor pools into blood • Effect relies on continuous formation of late-stage precursors from earlier progenitors Zhou L, et al. Blood. 2008; 112: 3434 -3443.

The MEDALIST Trial: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Luspatercept to Treat Patients With Very Low-, or Intermediate-Risk Myelodysplastic Syndromes (MDS) Associated Anemia With Ring Sideroblasts (RS) Who Require Red Blood Cell (RBC) Transfusions Pierre Fenaux, Uwe Platzbecker, Ghulam J. Mufti, Guillermo Garcia-Manero, Rena Buckstein, Valeria Santini, María Díez-Campelo, Carlo Finelli, Mario Cazzola, Osman Ilhan, Mikkael A. Sekeres, José F. Falantes, Beatriz Arrizabalaga, Flavia Salvi, Valentina Giai, Paresh Vyas, David Bowen, Dominik Selleslag, Amy E. De. Zern, Joseph G. Jurcic, Ulrich Germing, Katharina S. Götze, Bruno Quesnel, Odile Beyne-Rauzy, Thomas Cluzeau, Maria Teresa Voso, Dominiek Mazure, Edo Vellenga, Peter L. Greenberg, Eva Hellström. Lindberg, Amer M. Zeidan, Abderrahmane Laadem, Aziz Benzohra, Jennie Zhang, Anita Rampersad, Peter G. Linde, Matthew L. Sherman, Rami S. Komrokji, Alan F. List

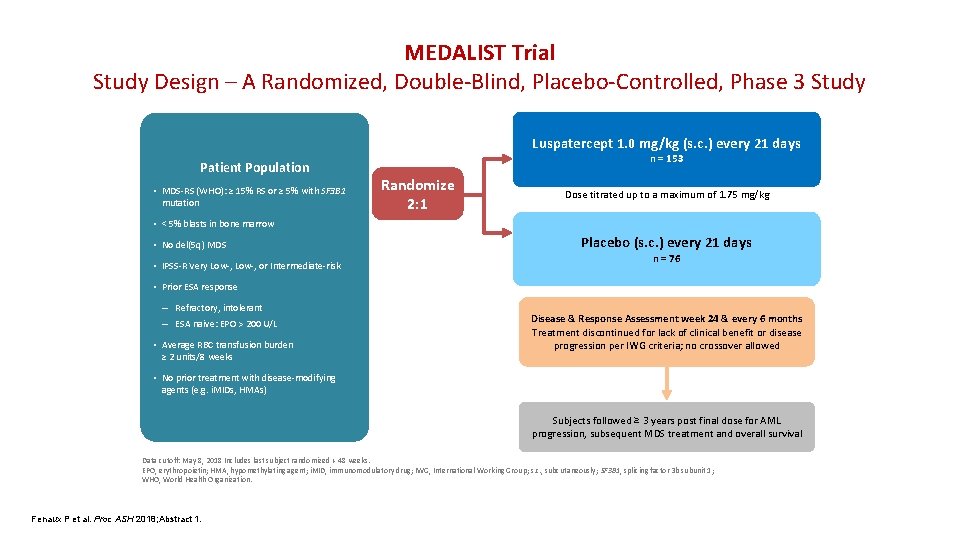
MEDALIST Trial Study Design – A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study Luspatercept 1. 0 mg/kg (s. c. ) every 21 days Patient Population • MDS-RS (WHO): ≥ 15% RS or ≥ 5% with SF 3 B 1 mutation n = 153 Randomize 2: 1 Dose titrated up to a maximum of 1. 75 mg/kg • < 5% blasts in bone marrow • No del(5 q) MDS • IPSS-R Very Low-, or Intermediate-risk Placebo (s. c. ) every 21 days n = 76 • Prior ESA response – Refractory, intolerant – ESA naive: EPO > 200 U/L • Average RBC transfusion burden ≥ 2 units/8 weeks Disease & Response Assessment week 24 & every 6 months Treatment discontinued for lack of clinical benefit or disease progression per IWG criteria; no crossover allowed • No prior treatment with disease-modifying agents (e. g. i. MIDs, HMAs) Subjects followed ≥ 3 years post final dose for AML progression, subsequent MDS treatment and overall survival Data cutoff: May 8, 2018 Includes last subject randomized + 48 weeks. EPO, erythropoietin; HMA, hypomethylating agent; i. MID, immunomodulatory drug; IWG, International Working Group; s. c. , subcutaneously; SF 3 B 1, splicing factor 3 b subunit 1; WHO, World Health Organization. Fenaux P et al. Proc ASH 2018; Abstract 1.

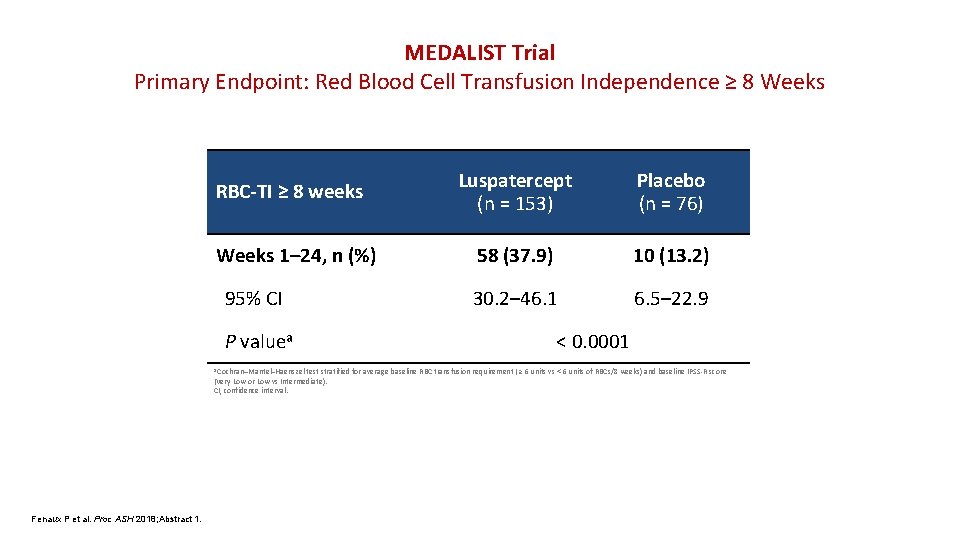
MEDALIST Trial Primary Endpoint: Red Blood Cell Transfusion Independence ≥ 8 Weeks RBC-TI ≥ 8 weeks Weeks 1– 24, n (%) 95% CI P valuea a Cochran–Mantel–Haenszel Luspatercept (n = 153) Placebo (n = 76) 58 (37. 9) 10 (13. 2) 30. 2– 46. 1 6. 5– 22. 9 < 0. 0001 test stratified for average baseline RBC transfusion requirement ( ≥ 6 units vs < 6 units of RBCs/8 weeks) and baseline IPSS-R score (Very Low or Low vs Intermediate). CI, confidence interval. Fenaux P et al. Proc ASH 2018; Abstract 1.

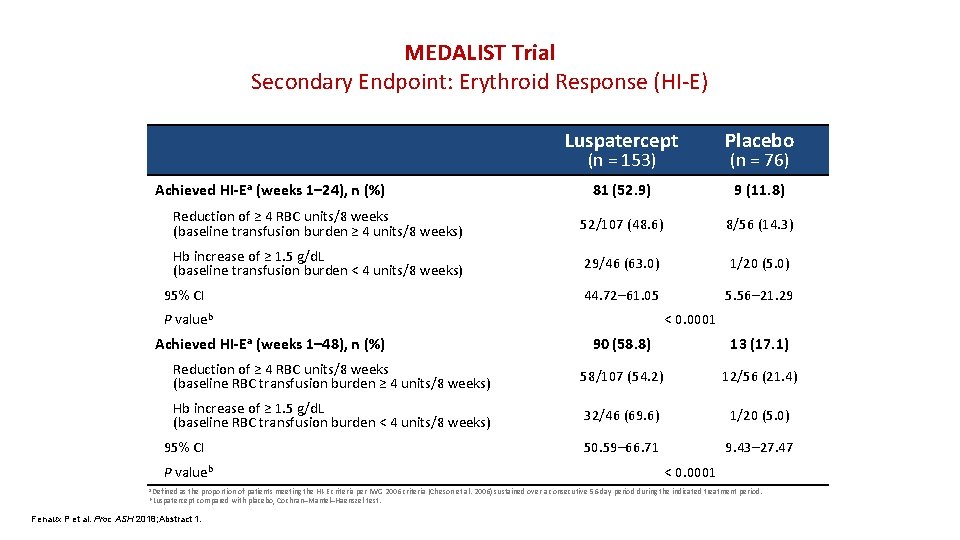
MEDALIST Trial Secondary Endpoint: Erythroid Response (HI-E) Luspatercept Placebo 81 (52. 9) 9 (11. 8) Reduction of ≥ 4 RBC units/8 weeks (baseline transfusion burden ≥ 4 units/8 weeks) 52/107 (48. 6) 8/56 (14. 3) Hb increase of ≥ 1. 5 g/d. L (baseline transfusion burden < 4 units/8 weeks) 29/46 (63. 0) 1/20 (5. 0) 44. 72– 61. 05 5. 56– 21. 29 (n = 153) Achieved HI-Ea (weeks 1– 24), n (%) 95% CI P valueb Achieved HI-Ea (weeks 1– 48), n (%) (n = 76) < 0. 0001 90 (58. 8) 13 (17. 1) Reduction of ≥ 4 RBC units/8 weeks (baseline RBC transfusion burden ≥ 4 units/8 weeks) 58/107 (54. 2) 12/56 (21. 4) Hb increase of ≥ 1. 5 g/d. L (baseline RBC transfusion burden < 4 units/8 weeks) 32/46 (69. 6) 1/20 (5. 0) 50. 59– 66. 71 9. 43– 27. 47 95% CI P valueb a Defined < 0. 0001 as the proportion of patients meeting the HI-E criteria per IWG 2006 criteria (Cheson et al. 2006) sustained over a consecutive 56 -day period during the indicated treatment period. compared with placebo, Cochran–Mantel–Haenszel test. b Luspatercept Fenaux P et al. Proc ASH 2018; Abstract 1.

Ms Swanson Case • 60 y/o gentleman with a history of HLD and HTN • Patient had a long history of essential thrombocytosis initially diagnosed in 1989 • He was treated with anagrelide on clinical trial, which had been continued after the trial closed

• He presented to our institution in 2010 to establish care • A bone marrow biopsy was recommended at that time which showed he had progressed to myelofibrosis • He never tested positive for the JAK 2 mutation • Continued on anagrelide with progressively decreasing dose until 2017 when it was discontinued due to worsening anemia

• In 2017 he retired from a long career as an engineer • He and his wife had plans to travel within the US and abroad • He remained off any therapy from June 2017 until May 2019 aside from supportive care • During this time he was noted to have progressive anemia as well as worsening splenomegaly. Spleen was palpable at 14 cm below the LCM Hemoglobin: 6/20/2017 11. 7 (L) 7/24/2017 11. 4 (L) 2/1/2018 10. 9 (L) 8/2/2018 10. 7 (L) 2/7/2019 10. 8 (L) 5/8/2019 10. 1 (L) Abdominal US FINDINGS: The spleen is enlarged, measuring 21. 6 x 8. 6 x 21. 7 cm (previously 14. 4 x 6. 6 x 14. 6 cm on 11/27/2013). The spleen parenchyma has normal homogeneous echotexture, with no discrete lesion detected. The rest of the imaged left upper quadrant soft tissues are unremarkable.

• In May of 2019 we recommended he have Nex. Gen sequencing to assess for any pathogenic mutation • It was at this time that he tested positive for the CALR mutation • He also became increasingly symptomatic with bloating, early satiety, night sweats and feeling of “catching” of his spleen with activity • Also noted to have an MPN-SAF score of 9

Pre-Therapy Counseling Points • Discuss the dangers of abruptly stopping the medication, including the abrupt return of symptoms • Recommend eligible patients get the shingles vaccine prior to starting because of risk of zoster infection • Recommend patients see a dermatologist for an annual skin exam because of increase risk of skin cancers • Plan to see your PCP 8 -12 weeks after starting therapy to assess your cholesterol level, ruxolitinib has been associated with increase in cholesterol levels • Discuss other common side effects including cytopenias, diarrhea, fatigue, headache

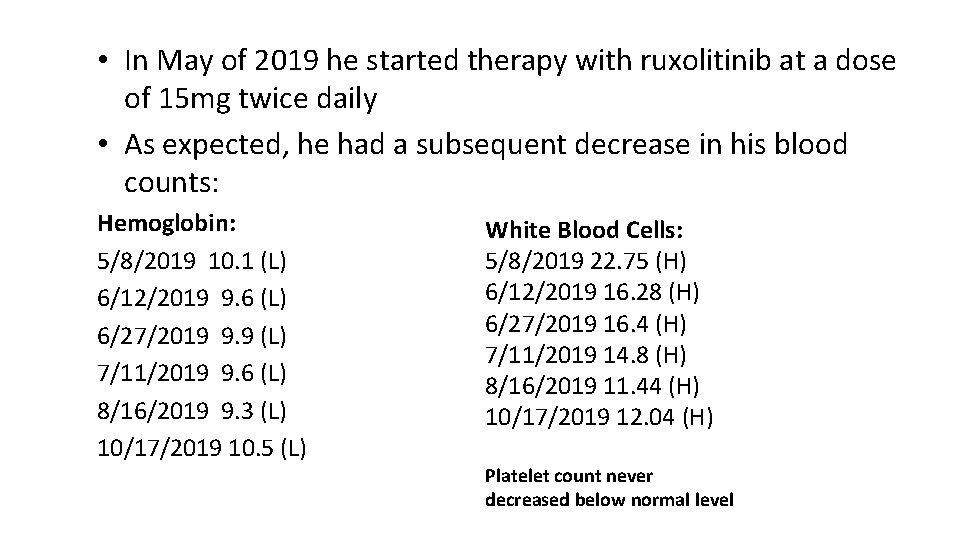
• In May of 2019 he started therapy with ruxolitinib at a dose of 15 mg twice daily • As expected, he had a subsequent decrease in his blood counts: Hemoglobin: 5/8/2019 10. 1 (L) 6/12/2019 9. 6 (L) 6/27/2019 9. 9 (L) 7/11/2019 9. 6 (L) 8/16/2019 9. 3 (L) 10/17/2019 10. 5 (L) White Blood Cells: 5/8/2019 22. 75 (H) 6/12/2019 16. 28 (H) 6/27/2019 16. 4 (H) 7/11/2019 14. 8 (H) 8/16/2019 11. 44 (H) 10/17/2019 12. 04 (H) Platelet count never decreased below normal level

• Spleen size decreased on palpation from 14 cm to 8 cm below the LCM. • Has not required a dose modification even though he did develop some anemia, his platelet count remained normal. • Patient notes an overall improvement in symptoms including bloating, early satiety and spleen size. Also with complete resolution of night sweats. • MPN-SAF score decreased to 3. • He and his wife travelled to Hawaii last month and he states he was able to fully enjoy his trip.

Myelodysplastic Syndromes and Myeloproliferative Neoplasms Rami Komrokji, MD Senior Member & Professor of Oncologic Sciences Section Head – Leukemia & MDS Vice Chair - Malignant Hematology Department H Lee Moffitt Cancer Center & Research Institute Tampa, Florida

Case Study 2 • Patient characteristics – Age, 58 years – Generally fit, no major health problems – Healthy lifestyle - low-fat diet, moderate activity level • Presentation – Fatigue, night sweats, generalized bone/joint pain – Abdominal discomfort that seems to be worsening • Physical exam – Splenomegaly by palpation (extends 10 cm below the left costal margin)



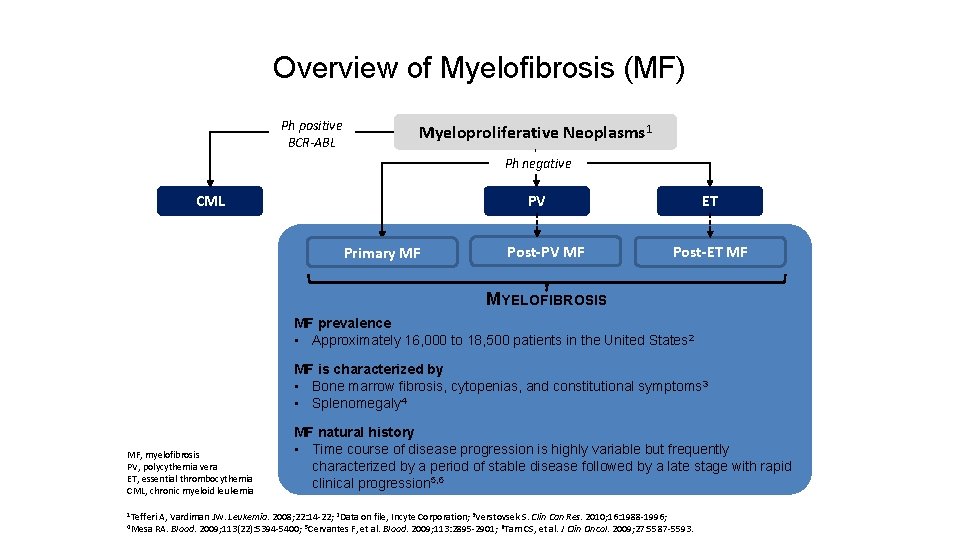
Overview of Myelofibrosis (MF) Ph positive BCR-ABL Myeloproliferative Neoplasms 1 Ph negative CML PV Primary MF Post-PV MF ET Post-ET MF MYELOFIBROSIS MF prevalence • Approximately 16, 000 to 18, 500 patients in the United States 2 MF is characterized by • Bone marrow fibrosis, cytopenias, and constitutional symptoms 3 • Splenomegaly 4 MF, myelofibrosis PV, polycythemia vera ET, essential thrombocythemia CML, chronic myeloid leukemia 1 Tefferi 4 Mesa MF natural history • Time course of disease progression is highly variable but frequently characterized by a period of stable disease followed by a late stage with rapid clinical progression 5, 6 A, Vardiman JW. Leukemia. 2008; 22: 14 -22; 2 Data on file, Incyte Corporation; 3 Verstovsek S. Clin Can Res. 2010; 16: 1988 -1996; RA. Blood. 2009; 113(22): 5394 -5400; 5 Cervantes F, et al. Blood. 2009; 113: 2895 -2901; 6 Tam CS, et al. J Clin Oncol. 2009; 27: 5587 -5593.

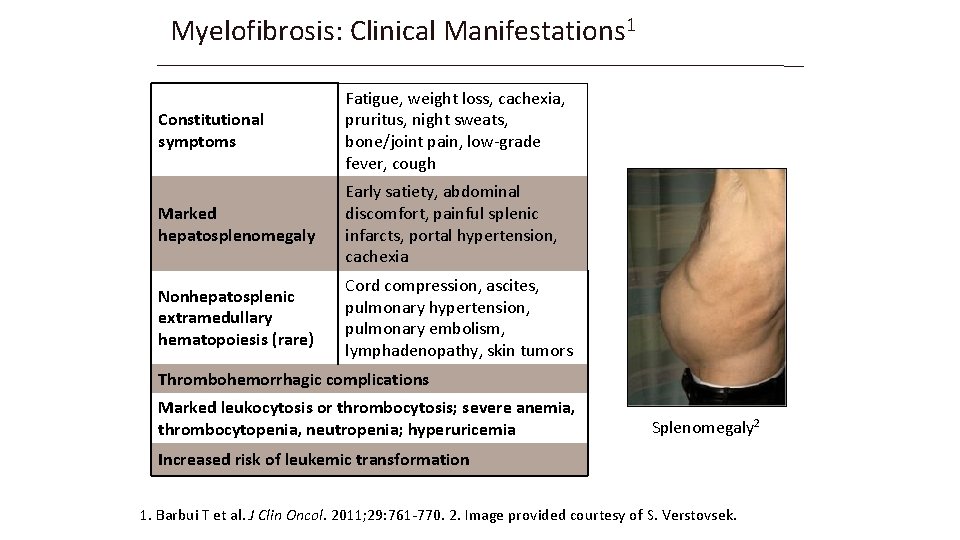
Myelofibrosis: Clinical Manifestations 1 Constitutional symptoms Fatigue, weight loss, cachexia, pruritus, night sweats, bone/joint pain, low-grade fever, cough Marked hepatosplenomegaly Early satiety, abdominal discomfort, painful splenic infarcts, portal hypertension, cachexia Nonhepatosplenic extramedullary hematopoiesis (rare) Cord compression, ascites, pulmonary hypertension, pulmonary embolism, lymphadenopathy, skin tumors Thrombohemorrhagic complications Marked leukocytosis or thrombocytosis; severe anemia, thrombocytopenia, neutropenia; hyperuricemia Splenomegaly 2 Increased risk of leukemic transformation 1. Barbui T et al. J Clin Oncol. 2011; 29: 761 -770. 2. Image provided courtesy of S. Verstovsek.

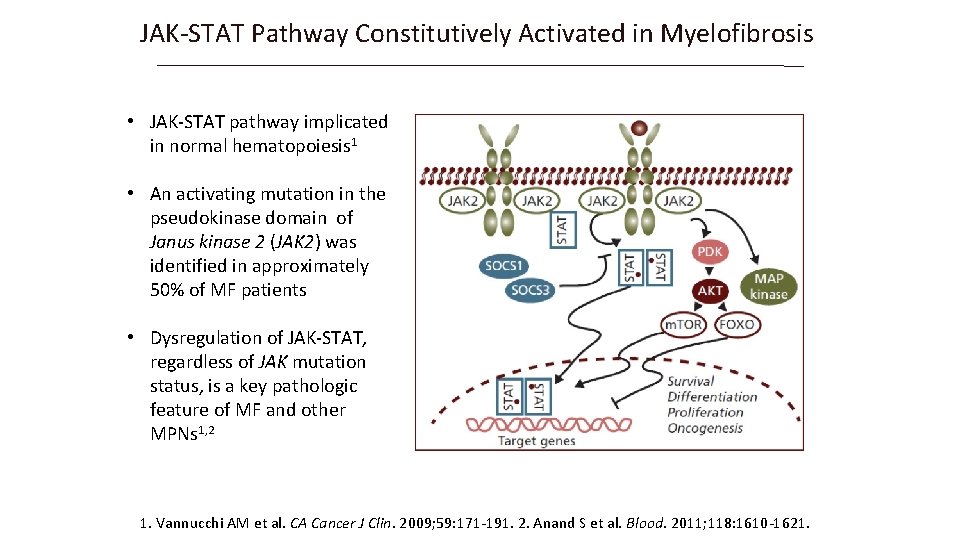
JAK-STAT Pathway Constitutively Activated in Myelofibrosis • JAK-STAT pathway implicated in normal hematopoiesis 1 • An activating mutation in the pseudokinase domain of Janus kinase 2 (JAK 2) was identified in approximately 50% of MF patients • Dysregulation of JAK-STAT, regardless of JAK mutation status, is a key pathologic feature of MF and other MPNs 1, 2 1. Vannucchi AM et al. CA Cancer J Clin. 2009; 59: 171 -191. 2. Anand S et al. Blood. 2011; 118: 1610 -1621.

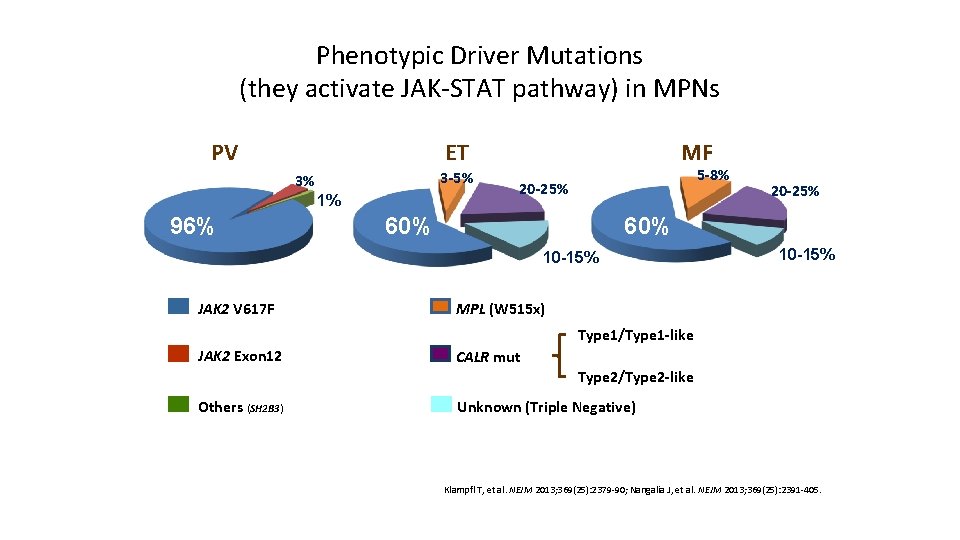
Phenotypic Driver Mutations (they activate JAK-STAT pathway) in MPNs PV 3 -5% 3% 1% 96% MF ET 5 -8% 20 -25% 60% 10 -15% JAK 2 V 617 F 20 -25% 10 -15% MPL (W 515 x) Type 1/Type 1 -like JAK 2 Exon 12 CALR mut Type 2/Type 2 -like Others (SH 2 B 3) Unknown (Triple Negative) Klampfl T, et al. NEJM 2013; 369(25): 2379 -90; Nangalia J, et al. NEJM 2013; 369(25): 2391 -405.

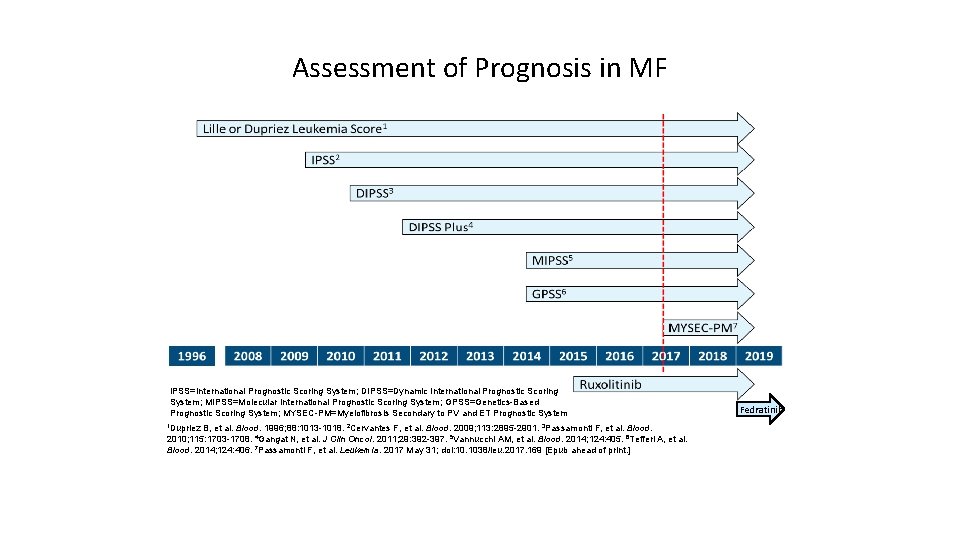
Assessment of Prognosis in MF IPSS=International Prognostic Scoring System; DIPSS=Dynamic International Prognostic Scoring System; MIPSS=Molecular International Prognostic Scoring System; GPSS=Genetics-Based Prognostic Scoring System; MYSEC-PM=Myelofibrosis Secondary to PV and ET Prognostic System 1 Dupriez B, et al. Blood. 1996; 88: 1013 -1018. 2 Cervantes F, et al. Blood. 2009; 113: 2895 -2901. 3 Passamonti F, et al. Blood. 2010; 115: 1703 -1708. 4 Gangat N, et al. J Clin Oncol. 2011; 29: 392 -397. 5 Vannucchi AM, et al. Blood. 2014; 124: 405. 6 Tefferi A, et al. Blood. 2014; 124: 406. 7 Passamonti F, et al. Leukemia. 2017 May 31; doi: 10. 1038/leu. 2017. 169 [Epub ahead of print. ] Fedratinib


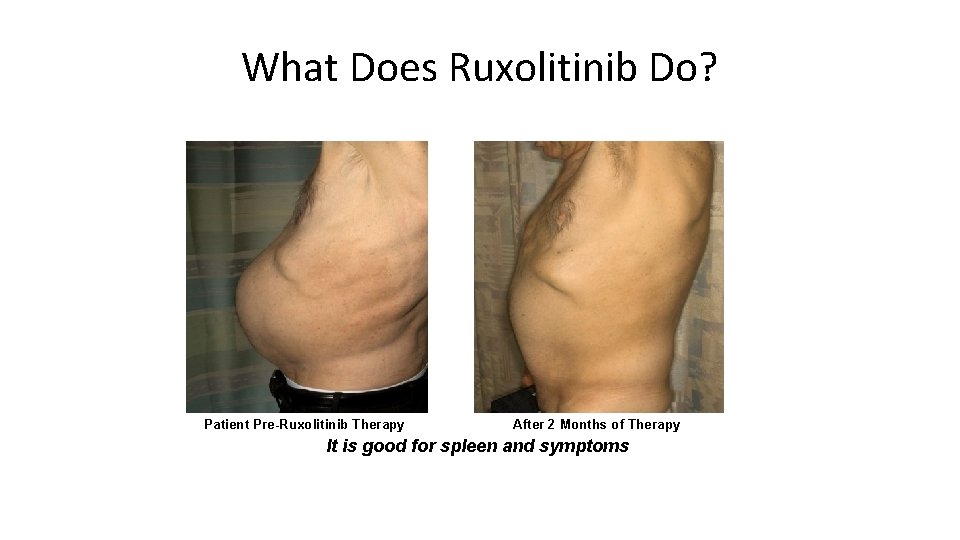
What Does Ruxolitinib Do? Patient Pre-Ruxolitinib Therapy After 2 Months of Therapy It is good for spleen and symptoms

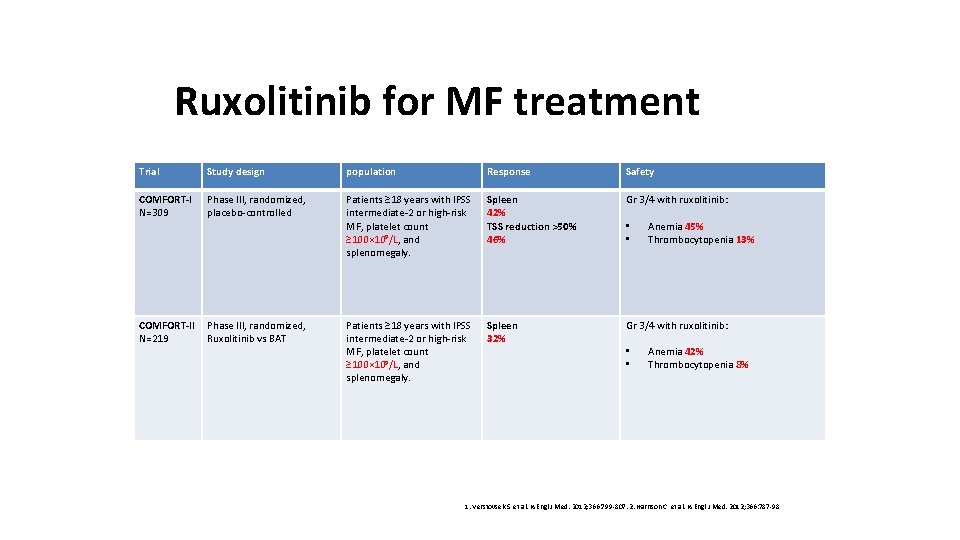
Ruxolitinib for MF treatment Trial Study design population Response Safety COMFORT-I N=309 Phase III, randomized, placebo-controlled Patients ≥ 18 years with IPSS intermediate-2 or high-risk MF, platelet count ≥ 100× 10 9/L, and splenomegaly. Spleen 42% TSS reduction >50% 46% Gr 3/4 with ruxolitinib: COMFORT-II N=219 Phase III, randomized, Ruxolitinib vs BAT Patients ≥ 18 years with IPSS intermediate-2 or high-risk MF, platelet count ≥ 100× 10 9/L, and splenomegaly. Spleen 32% Gr 3/4 with ruxolitinib: • • Anemia 45% Thrombocytopenia 13% Anemia 42% Thrombocytopenia 8% 1. Verstovsek S, et al. N Engl J Med. 2012; 366: 799 -807. 2. Harrison C, et al. N Engl J Med. 2012; 366: 787 -98

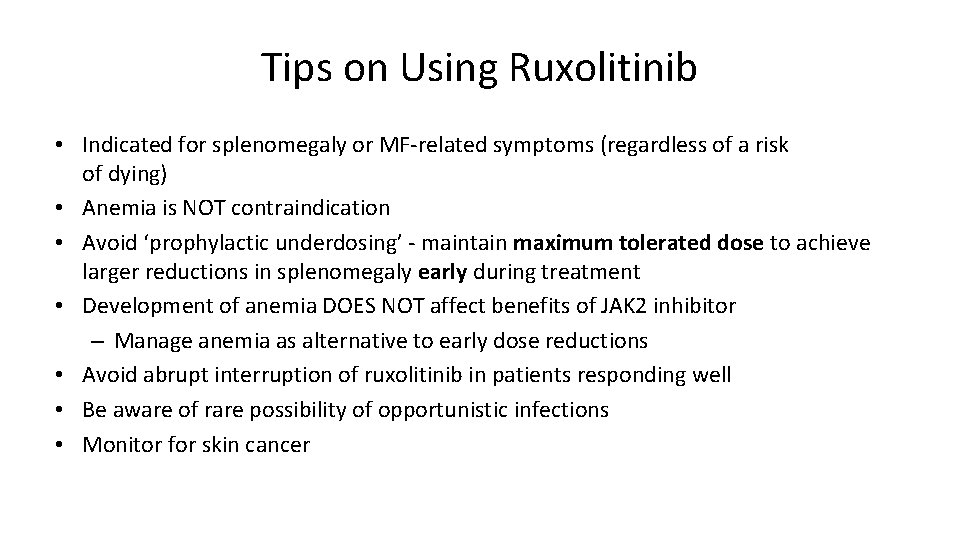
Tips on Using Ruxolitinib • Indicated for splenomegaly or MF-related symptoms (regardless of a risk of dying) • Anemia is NOT contraindication • Avoid ‘prophylactic underdosing’ - maintain maximum tolerated dose to achieve larger reductions in splenomegaly early during treatment • Development of anemia DOES NOT affect benefits of JAK 2 inhibitor – Manage anemia as alternative to early dose reductions • Avoid abrupt interruption of ruxolitinib in patients responding well • Be aware of rare possibility of opportunistic infections • Monitor for skin cancer

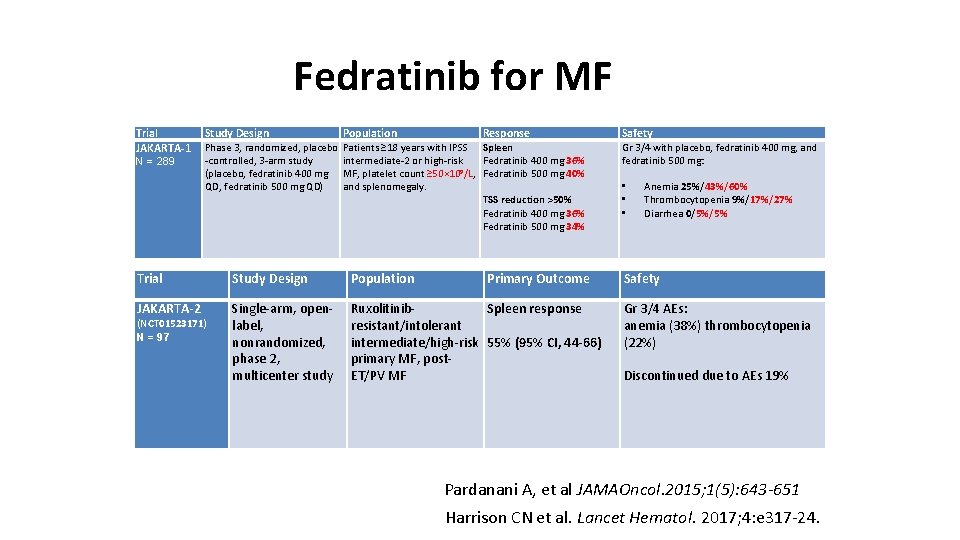
Fedratinib for MF Trial JAKARTA-1 N = 289 Study Design Population Phase 3, randomized, placebo -controlled, 3 -arm study (placebo, fedratinib 400 mg QD, fedratinib 500 mg QD) Patients ≥ 18 years with IPSS Spleen intermediate-2 or high-risk Fedratinib 400 mg 36% 9 MF, platelet count ≥ 50× 10 /L, Fedratinib 500 mg 40% and splenomegaly. TSS reduction >50% Fedratinib 400 mg 36% Fedratinib 500 mg 34% Response Trial Study Design Population JAKARTA-2 Single-arm, openlabel, nonrandomized, phase 2, multicenter study Ruxolitinib. Spleen response resistant/intolerant intermediate/high-risk 55% (95% CI, 44 -66) primary MF, post. ET/PV MF (NCT 01523171) N = 97 Primary Outcome Safety Gr 3/4 with placebo, fedratinib 400 mg, and fedratinib 500 mg: • • • Anemia 25%/43%/60% Thrombocytopenia 9%/17%/27% Diarrhea 0/5%/5% Safety Gr 3/4 AEs: anemia (38%) thrombocytopenia (22%) Discontinued due to AEs 19% Pardanani A, et al JAMAOncol. 2015; 1(5): 643 -651 Harrison CN et al. Lancet Hematol. 2017; 4: e 317 -24.

Case Study 3 • • 57 year old gentleman noted to have polycythemia on routine blood testing. He has no smoking history, no COPD or sleep apnea, no testosterone use. He had drenching night sweats, 30 LB weight loss over 2 months, and mild splenomegaly on imaging. He had one prior episode of documented TIA. Bone marrow biopsy showed mildly hypercellular bone marrow 50 -60% with atypical megakaryocytes, normal karyotype and JAK-2 V 617 F mutation detected (VAF 46%) and IDH-1 mutation (VAF 26%). Based on the patient’s test results, what would be the most likely diagnosis? A. Primary myelofibrosis (PMF) B. Polycythemia vera (PV) C. Essential thrombocythemia (ET) D. Chronic myelogenous leukemia (CML)

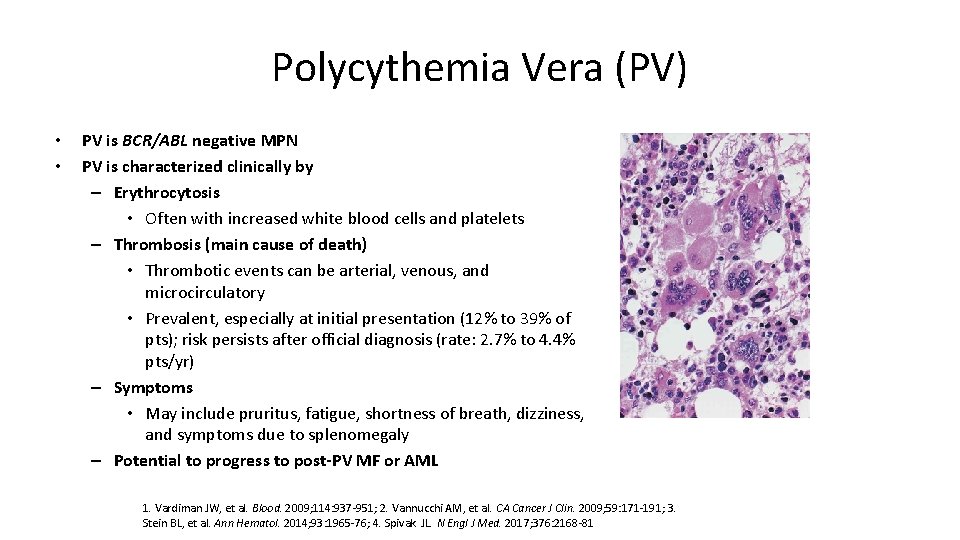
Polycythemia Vera (PV) • • PV is BCR/ABL negative MPN PV is characterized clinically by – Erythrocytosis • Often with increased white blood cells and platelets – Thrombosis (main cause of death) • Thrombotic events can be arterial, venous, and microcirculatory • Prevalent, especially at initial presentation (12% to 39% of pts); risk persists after official diagnosis (rate: 2. 7% to 4. 4% pts/yr) – Symptoms • May include pruritus, fatigue, shortness of breath, dizziness, and symptoms due to splenomegaly – Potential to progress to post-PV MF or AML 1. Vardiman JW, et al. Blood. 2009; 114: 937 -951; 2. Vannucchi AM, et al. CA Cancer J Clin. 2009; 59: 171 -191; 3. Stein BL, et al. Ann Hematol. 2014; 93: 1965 -76; 4. Spivak JL. N Engl J Med. 2017; 376: 2168 -81

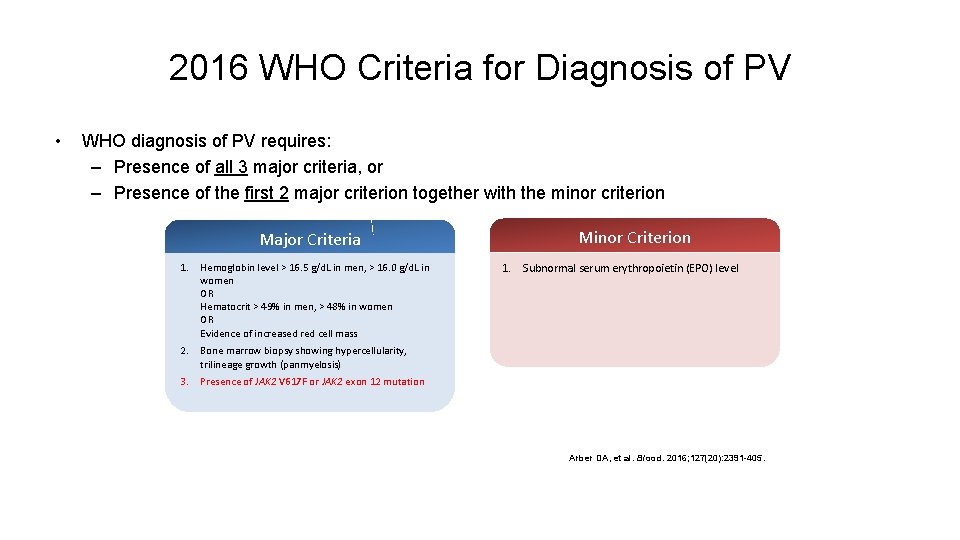
2016 WHO Criteria for Diagnosis of PV • WHO diagnosis of PV requires: – Presence of all 3 major criteria, or – Presence of the first 2 major criterion together with the minor criterion Major Criteria 1. Hemoglobin level > 16. 5 g/d. L in men, > 16. 0 g/d. L in women OR Hematocrit > 49% in men, > 48% in women OR Evidence of increased red cell mass 2. Bone marrow biopsy showing hypercellularity, trilineage growth (panmyelosis) 3. Presence of JAK 2 V 617 F or JAK 2 exon 12 mutation Minor Criterion 1. Subnormal serum erythropoietin (EPO) level Arber DA, et al. Blood. 2016; 127(20): 2391 -405.

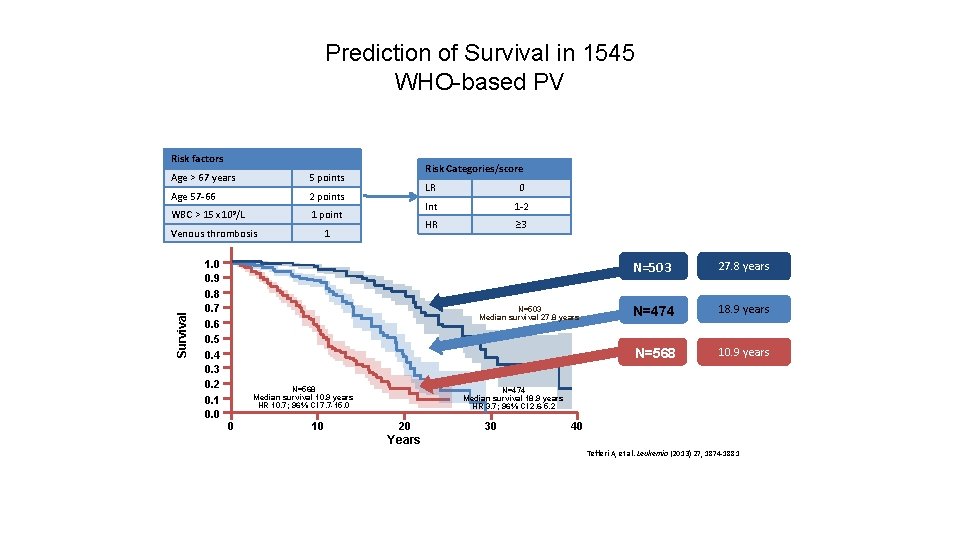
Prediction of Survival in 1545 WHO-based PV Risk factors Age > 67 years 5 points Age 57 -66 2 points WBC > 15 x 109/L 1 point Survival Venous thrombosis 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 Risk Categories/score 1 LR 0 Int 1 -2 HR ≥ 3 N=503 Median survival 27. 8 years N=568 Median survival 10. 9 years HR 10. 7; 96% CI 7. 7 -15. 0 0 10 N=503 27. 8 years N=474 18. 9 years N=568 10. 9 years N=474 Median survival 18. 9 years HR 3. 7; 96% CI 2. 6 -5. 2 20 30 40 Years Tefferi A, et al. Leukemia (2013) 27, 1874 -1881

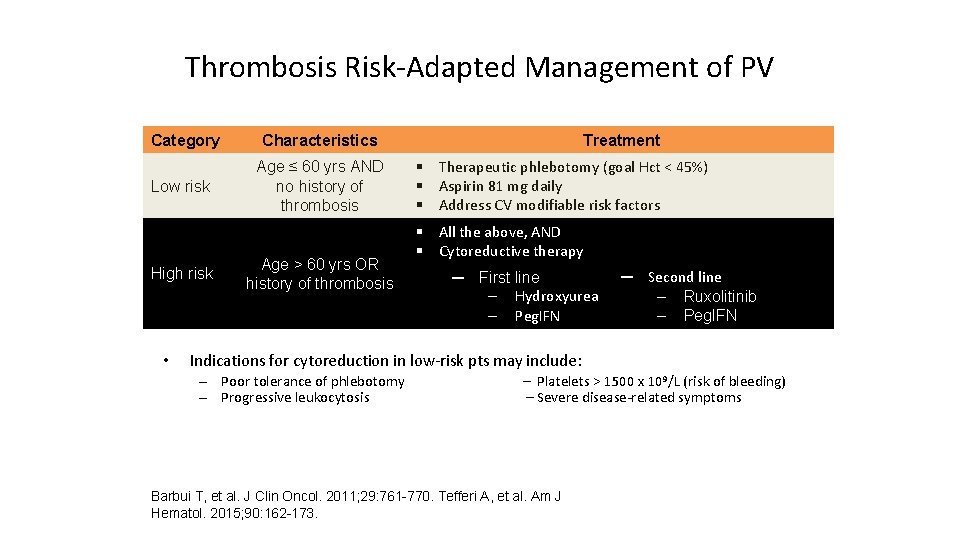
Thrombosis Risk-Adapted Management of PV Category Low risk High risk • Characteristics Age ≤ 60 yrs AND no history of thrombosis Age > 60 yrs OR history of thrombosis Treatment § Therapeutic phlebotomy (goal Hct < 45%) § Aspirin 81 mg daily § Address CV modifiable risk factors § All the above, AND § Cytoreductive therapy ─ First line ‒ Hydroxyurea ‒ Peg. IFN ─ Second line ‒ Ruxolitinib ‒ Peg. IFN Indications for cytoreduction in low-risk pts may include: – Poor tolerance of phlebotomy – Progressive leukocytosis – Platelets > 1500 x 109/L (risk of bleeding) – Severe disease-related symptoms Barbui T, et al. J Clin Oncol. 2011; 29: 761 -770. Tefferi A, et al. Am J Hematol. 2015; 90: 162 -173.
![Studies with Peg IFN Study MDACC3 4 Peg IFN α2 a Population Studies with Peg. IFN Study MDACC[3, 4] § Peg. IFN α-2 a Population §](https://slidetodoc.com/presentation_image_h2/b6171b1491861307c06ff74b8720e6ee/image-46.jpg)
Studies with Peg. IFN Study MDACC[3, 4] § Peg. IFN α-2 a Population § N = 43 § ~ 50% previous cytoreductives § Median FU: 83 mos Findings § Median response duration: hematologic: 65 mos; molecular: 58 mos ‒ Failure to achieve CMR: more likely to have/acquire nondriver mutations § Thrombosis and progression can occur § Toxicity continued over time (new grade 3/4 events in 10% to 17% of PY); d/c for AEs: 22% § § PROUD-PV Phase III Trial of Ropeginterferon Alfa 2 b Vs. Hydroxyurea § PV patients treatment naïve or HU pretreated but not resistant 83 (Ropeg) and 70 (HU/BAT) patients completed the 36 month efficacy analysis time point § § § Gisslinger et al, ASH 2018, Abstract # 579 Mascarenhas JO, et al, ASH 2018. Abstract 577. After 36 months of treatment, maintenance of higher responder rates was shown in the Ropeg arm compared to HU/BAT for CHR (70. 5% vs. 51. 4%; p=0. 0122; RR [95% CI]: 1. 38 [1. 07 -1. 79]) and for CHR plus symptom improvement (52. 6% vs. 37. 8%; p=0. 0437; RR [95% CI]: 1. 42 [1. 01 -2. 00]). JAK 2 V 617 F: after 36 months 66. 0% of patients in the Ropeg arm and only 27. 0% in the HU/BAT arm had achieved MR (p<0. 0001; RR [95% CI]: 2. 31 [1. 56 -3. 42]). MR strongly correlated with CHR. Ropeg was able to reduce also non-JAK allele burden including TET 2.

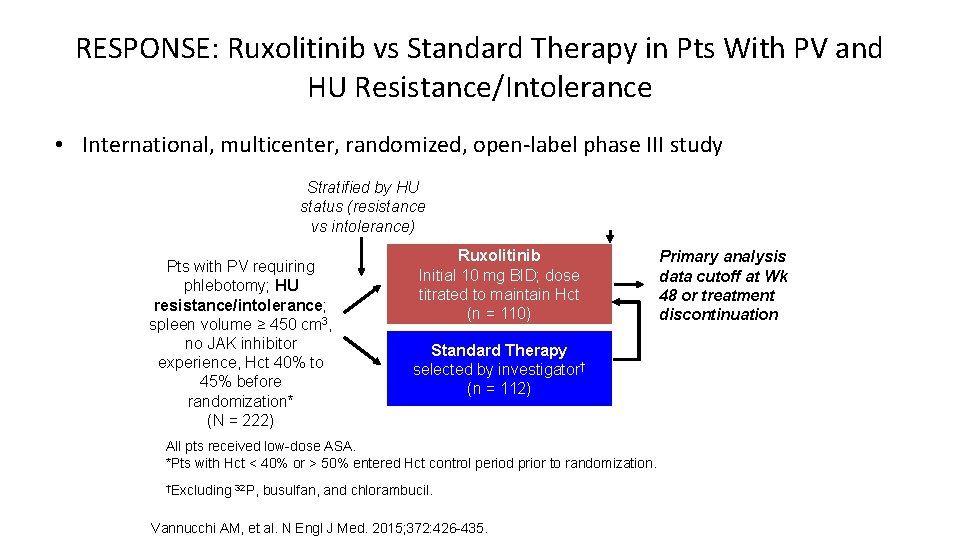
RESPONSE: Ruxolitinib vs Standard Therapy in Pts With PV and HU Resistance/Intolerance • International, multicenter, randomized, open-label phase III study Stratified by HU status (resistance vs intolerance) Pts with PV requiring phlebotomy; HU resistance/intolerance; spleen volume ≥ 450 cm 3, no JAK inhibitor experience, Hct 40% to 45% before randomization* (N = 222) Wk 32 Ruxolitinib Initial 10 mg BID; dose titrated to maintain Hct (n = 110) Standard Therapy selected by investigator† (n = 112) All pts received low-dose ASA. *Pts with Hct < 40% or > 50% entered Hct control period prior to randomization. †Excluding 32 P, busulfan, and chlorambucil. Vannucchi AM, et al. N Engl J Med. 2015; 372: 426 -435. Primary analysis data cutoff at Wk 48 or treatment discontinuation Crossover at PD or Wk 32 if primary endpoint not met

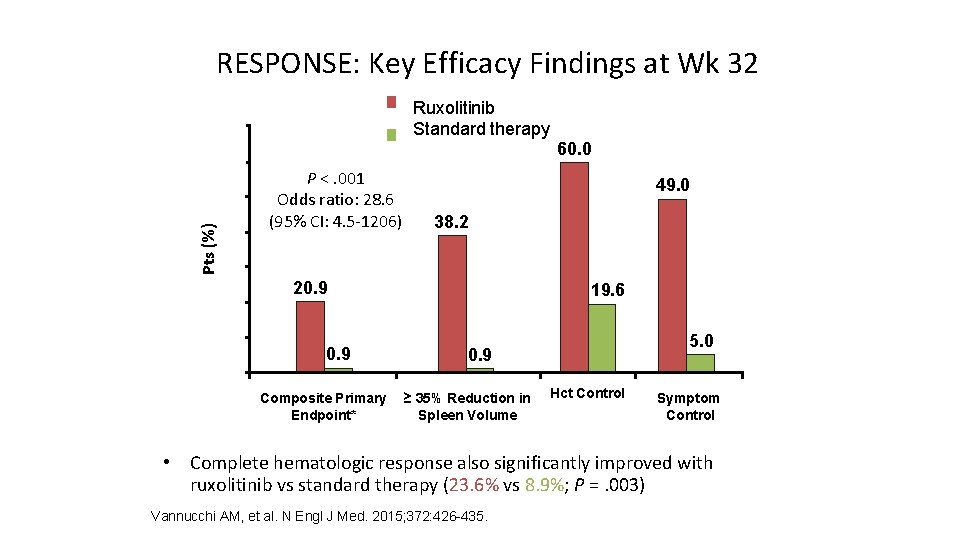
RESPONSE: Key Efficacy Findings at Wk 32 Ruxolitinib Standard therapy 70 60 Pts (%) 50 40 P <. 001 Odds ratio: 28. 6 (95% CI: 4. 5 -1206) 60. 0 49. 0 38. 2 30 20 10 0 20. 9 Composite Primary Endpoint* 19. 6 5. 0 0. 9 ≥ 35% Reduction in Spleen Volume Hct Control Symptom Control *Proportion with Hct control + spleen volume reduction ≥ 35%. • Complete hematologic response also significantly improved with ruxolitinib vs standard therapy (23. 6% vs 8. 9%; P =. 003) Vannucchi AM, et al. N Engl J Med. 2015; 372: 426 -435.

Summary • Anemia is major challenge in patients with MDS and remains an unmet need. • JAK-2 inhibitors are main choice for treatment of splenomegaly and constitutional symptoms in myelofibrosis. • Treatment of polycythemia vera is based on disease risk assessment.

Special Issues in Oncology Care Supportive care for patients with myelodysplastic syndromes and other hematologic disorders: Iron overload, prevention of thrombosis, growth factors
 Peripheral giant cell granuloma
Peripheral giant cell granuloma Endocrine and hematologic emergencies
Endocrine and hematologic emergencies Nursing assessment order
Nursing assessment order Hypochromic red cells
Hypochromic red cells Hematologic system assessment
Hematologic system assessment Finasteride side effects
Finasteride side effects What is geriatric syndromes
What is geriatric syndromes Neuroendocrine syndromes in gynecology
Neuroendocrine syndromes in gynecology Best language nih
Best language nih Cerebellaris
Cerebellaris Cerebellar syndromes
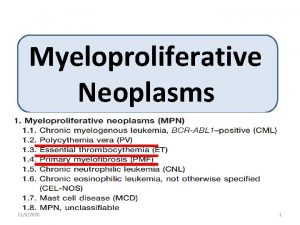
Cerebellar syndromes Myeloproliferative disorder
Myeloproliferative disorder Myeloproliferative neoplams
Myeloproliferative neoplams Cytosis neoplasm disorder
Cytosis neoplasm disorder Myelopoiesis
Myelopoiesis Myeloproliferative disorders
Myeloproliferative disorders Drugs causing leukocytosis
Drugs causing leukocytosis Myeloproliferative neoplasm
Myeloproliferative neoplasm C device module module 1
C device module module 1 Module 5 supply and demand introduction and demand
Module 5 supply and demand introduction and demand Module 5 intersections and roundabouts
Module 5 intersections and roundabouts Module 74 ap psychology
Module 74 ap psychology Module 7 weights and measurements
Module 7 weights and measurements Module 4 the challenges of middle and late adolescence
Module 4 the challenges of middle and late adolescence Business ethics and social responsibility module 5
Business ethics and social responsibility module 5 Module 77 prejudice and discrimination
Module 77 prejudice and discrimination Module 3: information and network security
Module 3: information and network security Module 12 sequences and series
Module 12 sequences and series Fraud and abuse module
Fraud and abuse module Module 10 expansion exploration and encounters
Module 10 expansion exploration and encounters Module 4 - open source software and licensing
Module 4 - open source software and licensing Learning: module 26: magnetic forces and fields
Learning: module 26: magnetic forces and fields Difference between psychoanalysis and psychodynamic
Difference between psychoanalysis and psychodynamic Ap psych schema
Ap psych schema Module 16 arc length and sector area answer key
Module 16 arc length and sector area answer key Module 3 topic 1 laws of nature
Module 3 topic 1 laws of nature Module 4 topic 1 assessing and managing risk
Module 4 topic 1 assessing and managing risk Module 4 topic 1 assessing and managing risk
Module 4 topic 1 assessing and managing risk Https://slidetodoc.com/php-and-my-sql-david-lash-module-3/
Https://slidetodoc.com/php-and-my-sql-david-lash-module-3/ Module 26 how we learn and classical conditioning
Module 26 how we learn and classical conditioning Expressive aphasia
Expressive aphasia Module 5 work and inventions
Module 5 work and inventions Module 29 biology cognition and learning
Module 29 biology cognition and learning Patterns and sequences module 4
Patterns and sequences module 4 Module 10 coordinate proof using slope and distance
Module 10 coordinate proof using slope and distance Module 43 stress and health
Module 43 stress and health Eighteen month old becca is in the telegraphic speech phase
Eighteen month old becca is in the telegraphic speech phase Module 8 raceways and fittings
Module 8 raceways and fittings Type nms cable contains
Type nms cable contains Module 5 intersections and roundabouts
Module 5 intersections and roundabouts Module 4 topic 5 turnabouts and parking
Module 4 topic 5 turnabouts and parking