Podemos seleccionar los pacientes para tratamiento adyuvante en









- Slides: 72

Podemos seleccionar los pacientes para tratamiento adyuvante en cáncer de colon? Dr. Ramon Salazar Medical Oncology Dpt Catalan Institute of Oncology L’Hospitalet de Llobregat, Barcelona ICO L’Hospitalet Institut Català d’Oncologia

Conflicts of Interest • None ICO L’Hospitalet

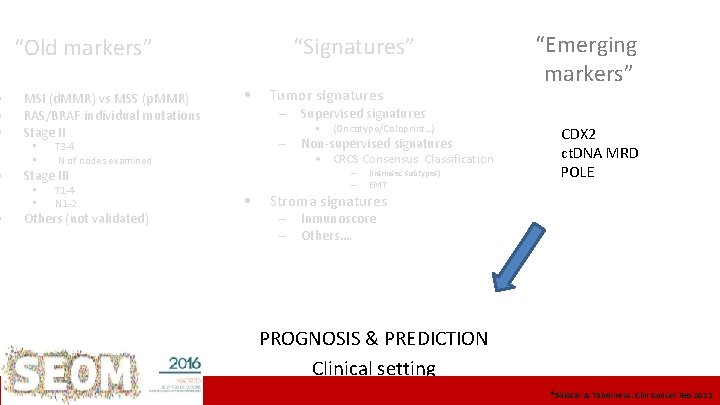
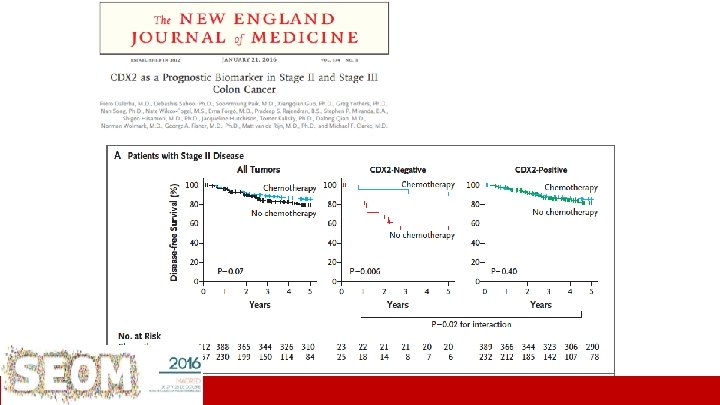
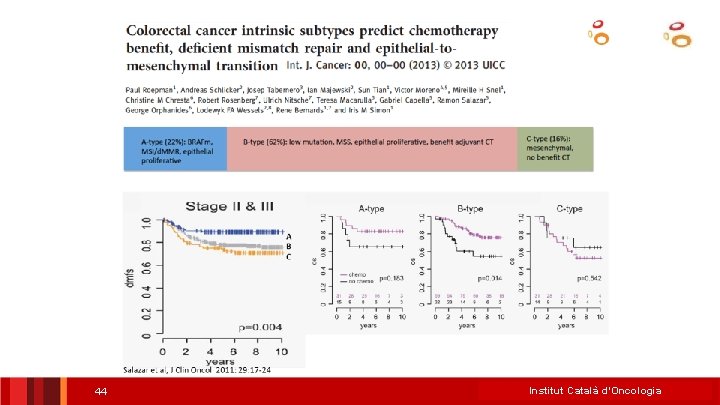
• • • “Signatures” “Old markers” • Stage II • • – Supervised signatures T 3 -4 N of nodes examined • MSI (d. MMR) vs MSS (p. MMR) • St II > St III T 1 -4 N 1 -2 Others (not validated) (Oncotype/Coloprint…) – Non-supervised signatures • CRCS Consensus Classification RAS/BRAF individual mutations Stage III • • Tumor signatures “Emerging markers” • – (Intrinsec subtypes) – EMT CDX 2 ct. DNA MRD POLE Stroma signatures – Inmunoscore – Others…. PROGNOSIS & PREDICTION Clinical setting ICO L’Hospitalet Institut&Català d’Oncologia *Salazar Tabernero. Clin Cancer Res 2015

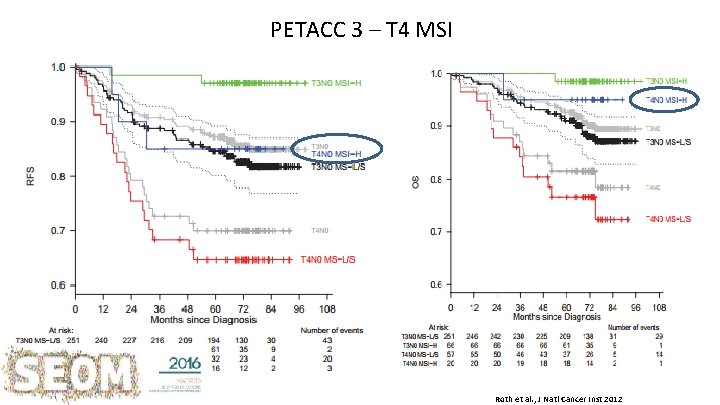
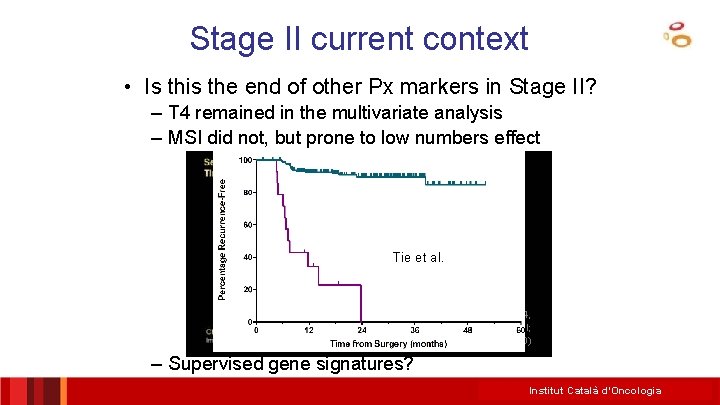
PETACC 3 – T 4 MSI Roth et al. , J Natl Cancer Inst 2012

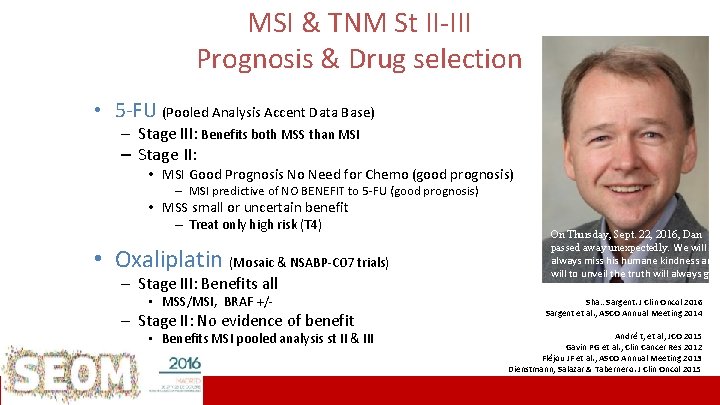
MSI & TNM St II-III Prognosis & Drug selection • 5 -FU (Pooled Analysis Accent Data Base) – Stage III: Benefits both MSS than MSI – Stage II: • MSI Good Prognosis No Need for Chemo (good prognosis) – MSI predictive of NO BENEFIT to 5 -FU (good prognosis) • MSS small or uncertain benefit – Treat only high risk (T 4) • Oxaliplatin (Mosaic & NSABP-C 07 trials) – Stage III: Benefits all • MSS/MSI, BRAF +/- – Stage II: No evidence of benefit • Benefits MSI pooled analysis st II & III ICO L’Hospitalet On Thursday, Sept. 22, 2016, Dan passed away unexpectedly. We will always miss his humane kindness and will to unveil the truth will always guid us Sha…Sargent. J Clin Oncol 2016 Sargent et al. , ASCO Annual Meeting 2014 André t, et al, JCO 2015 Gavin PG et al. , Clin Cancer Res 2012 Fléjou JF et al. , ASCO Annual Meeting 2013 Dienstmann, Salazar & Tabernero. J Clin Oncol 2015

• • • “Signatures” “Old markers” MSI (d. MMR) vs MSS (p. MMR) RAS/BRAF individual mutations Stage II • • • T 3 -4 N of nodes examined Tumor signatures – Supervised signatures – Non-supervised signatures • CRCS Consensus Classification Stage III • • • (Oncotype/Coloprint…) – T 1 -4 N 1 -2 Others (not validated) • – (Intrinsec subtypes) EMT “Emerging markers” CDX 2 ct. DNA MRD POLE Stroma signatures – – Inmunoscore Others…. PROGNOSIS & PREDICTION Clinical setting ICO L’Hospitalet *Salazar & Tabernero. Clin Cancer Res 2015

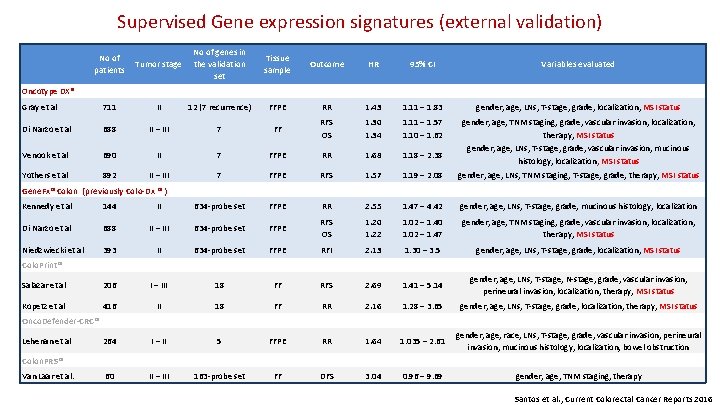
Supervised Gene expression signatures (external validation) No of patients Tumor stage No of genes in the validation set Tissue sample Outcome HR 95% CI Variables evaluated Gray et al 711 II 12 (7 recurrence) FFPE RR 1. 43 1. 11 – 1. 83 gender, age, LNs, T-stage, grade, localization, MSI status Di Narzo et al 688 II – III 7 FF RFS OS 1. 30 1. 34 1. 11 – 1. 57 1. 10 – 1. 62 gender, age, TNM staging, grade, vascular invasion, localization, therapy, MSI status Venook et al 690 II 7 FFPE RR 1. 68 1. 18 – 2. 38 gender, age, LNs, T-stage, grade, vascular invasion, mucinous histology, localization, MSI status Yothers et al 892 II – III 7 FFPE RFS 1. 57 1. 19 – 2. 08 gender, age, LNs, TNM staging, T-stage, grade, therapy, MSI status Oncotype DX® Gene. Fx® Colon (previously Colo-Dx ® ) Kennedy et al 144 II 634 -probe set FFPE RR 2. 55 1. 47 – 4. 42 gender, age, LNs, T-stage, grade, mucinous histology, localization Di Narzo et al 688 II – III 634 -probe set FFPE RFS OS 1. 20 1. 22 1. 02 – 1. 40 1. 02 – 1. 47 gender, age, TNM staging, grade, vascular invasion, localization, therapy, MSI status Niedzwiecki et al 393 II 634 -probe set FFPE RFI 2. 13 1. 30 – 3. 5 gender, age, LNs, T-stage, grade, localization, MSI status Colo. Print® Salazar et al 206 I – III 18 FF RFS 2. 69 1. 41 – 5. 14 gender, age, LNs, T-stage, N-stage, grade, vascular invasion, perineural invasion, localization, therapy, MSI status Kopetz et al 416 II 18 FF RR 2. 16 1. 28 – 3. 65 gender, age, LNs, T-stage, grade, localization, therapy, MSI status 1. 035 – 2. 61 gender, age, race, LNs, T-stage, grade, vascular invasion, perineural invasion, mucinous histology, localization, bowel obstruction Onco. Defender-CRC® Lehenan et al 264 I – II 5 FFPE RR 1. 64 60 II – III 163 -probe set FF DFS 3. 04 Colon. PRS® Van Laar et al. 0. 96 – 9. 69 gender, age, TNM staging, therapy Santos et al. , Current Colorectal Cancer Reports 2016

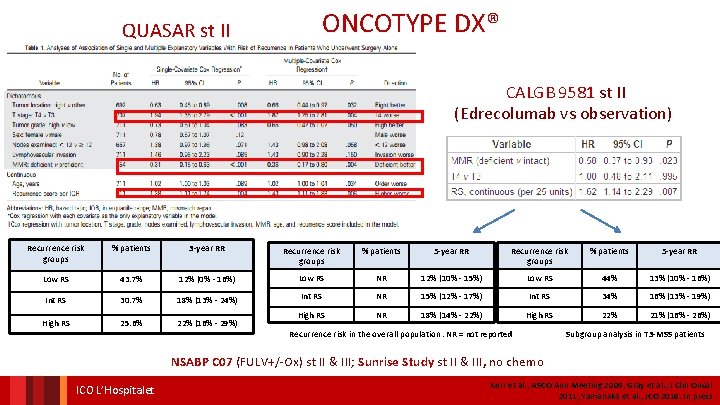
ONCOTYPE DX® QUASAR st II CALGB 9581 st II (Edrecolumab vs observation) Recurrence risk groups % patients 3 -year RR Recurrence risk groups % patients 5 -year RR Low RS 43. 7% 12% (0% - 16%) Low RS NR 12% (10% - 15%) Low RS 44% 13% (10% - 16%) Int RS 30. 7% 18% (13% - 24%) Int RS NR 15% (12% - 17%) Int RS 34% 16% (13% - 19%) High RS 25. 6% 22% (16% - 29%) High RS NR 18% (14% - 22%) High RS 22% 21% (16% - 26%) Recurrence risk in the overall population. NR = not reported Subgroup analysis in T 3 -MSS patients NSABP C 07 (FULV+/-Ox) st II & III; Sunrise Study st II & III, no chemo ICO L’Hospitalet Kerr et al. , ASCO Ann Meeting 2009; Gray et al. , J Clin Oncol 2011; Yamanaka et al. , JCO 2016. In press

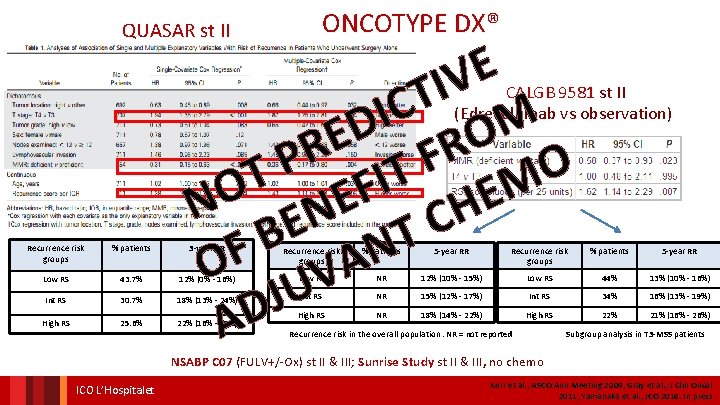
ONCOTYPE DX® QUASAR st II E V I T C I M D O E R R F P FIT O T M O E E N EN H C T B N F A O UV J D A CALGB 9581 st II (Edrecolumab vs observation) Recurrence risk groups % patients 3 -year RR Recurrence risk groups % patients 5 -year RR Low RS 43. 7% 12% (0% - 16%) Low RS NR 12% (10% - 15%) Low RS 44% 13% (10% - 16%) Int RS 30. 7% 18% (13% - 24%) Int RS NR 15% (12% - 17%) Int RS 34% 16% (13% - 19%) High RS 25. 6% 22% (16% - 29%) High RS NR 18% (14% - 22%) High RS 22% 21% (16% - 26%) Recurrence risk in the overall population. NR = not reported Subgroup analysis in T 3 -MSS patients NSABP C 07 (FULV+/-Ox) st II & III; Sunrise Study st II & III, no chemo ICO L’Hospitalet Kerr et al. , ASCO Ann Meeting 2009; Gray et al. , J Clin Oncol 2011; Yamanaka et al. , JCO 2016. In press

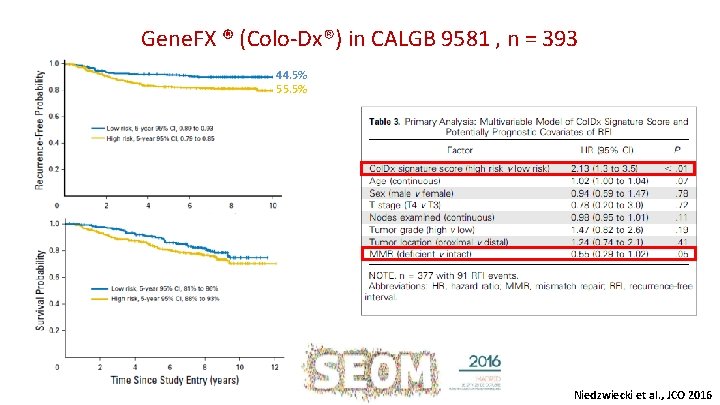
Gene. FX ® (Colo-Dx®) in CALGB 9581 , n = 393 44. 5% 55. 5% Niedzwiecki et al. , JCO 2016

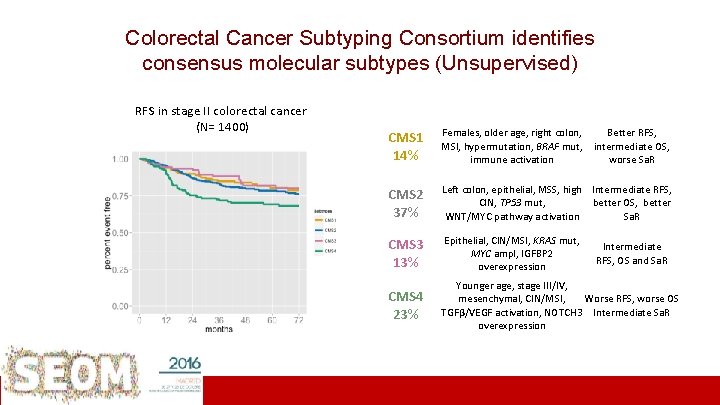
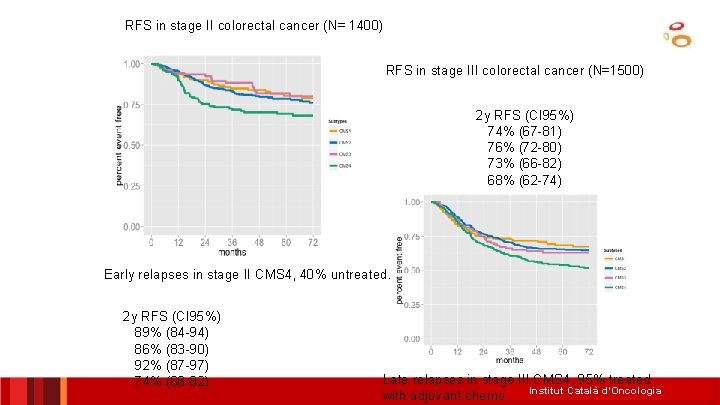
RFS in stage III colorectal cancer (N=1500)identifies Consortium Colorectal Cancer Subtyping consensus molecular subtypes (Unsupervised) RFS in stage II colorectal cancer (N= 1400) CMS 1 Females, older age, right colon, MSI, hypermutation, BRAF mut, 14% immune activation CMS 2 37% Better RFS, intermediate OS, worse Sa. R Left colon, epithelial, MSS, high Intermediate RFS, CIN, TP 53 mut, better OS, better WNT/MYC pathway activation Sa. R CMS 3 Epithelial, CIN/MSI, KRAS mut, Intermediate MYC ampl, IGFBP 2 RFS, OS and Sa. R Late relapses in stage III CMS 4 (95% treated 13% overexpression with adjuvant chemo) CMS 4 23% Younger age, stage III/IV, 2 y RFS (CI 95%) mesenchymal, CIN/MSI, Worse RFS, worse OS TGFβ/VEGF activation, NOTCH 3 Intermediate Sa. R 74% (67 -81) overexpression 76% (72 -80) 73% (66 -82) 68% (62 -74) ICO L’Hospitalet Rodrigo Dienstmann ASCO 2015 Guinney et al. Nature Medicine 2015

ICO L’Hospitalet

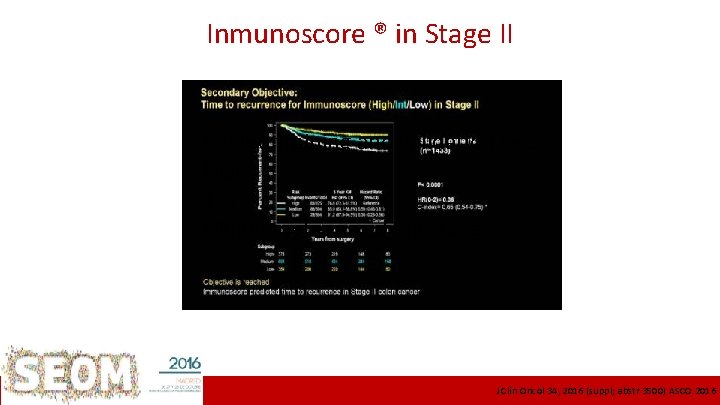
Inmunoscore ® in Stage II Pooled training and validation sets MSI? T 4? Multivariate mixed I-III stages ICO L’Hospitalet J Clin Oncol 34, 2016 (suppl; abstr 3500) ASCO 2016

• • • “Signatures” “Old markers” MSI (d. MMR) vs MSS (p. MMR) RAS/BRAF individual mutations Stage II • • • T 3 -4 N of nodes examined Tumor signatures – Supervised signatures – Non-supervised signatures • CRCS Consensus Classification Stage III • • • (Oncotype/Coloprint…) – T 1 -4 N 1 -2 Others (not validated) • – (Intrinsec subtypes) EMT “Emerging markers” CDX 2 ct. DNA MRD POLE Stroma signatures – – Inmunoscore Others…. PROGNOSIS & PREDICTION Clinical setting ICO L’Hospitalet *Salazar & Tabernero. Clin Cancer Res 2015

ICO L’Hospitalet Dalerba et al. N Engl J Med 374; 3 January 21, 2016

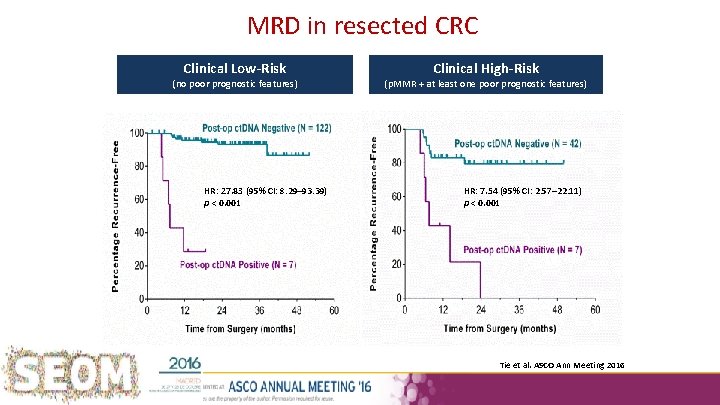
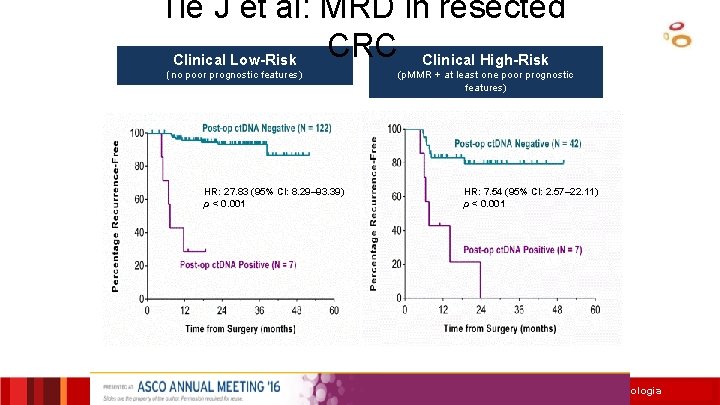
MRD in resected CRC Clinical Low-Risk (no poor prognostic features) HR: 27. 83 (95% CI: 8. 29– 93. 39) p < 0. 001 Clinical High-Risk (p. MMR + at least one poor prognostic features) HR: 7. 54 (95% CI: 2. 57– 22. 11) p < 0. 001 Tie et al. ASCO Ann Meeting 2016

POLE EDM TCGA ICO L’Hospitalet

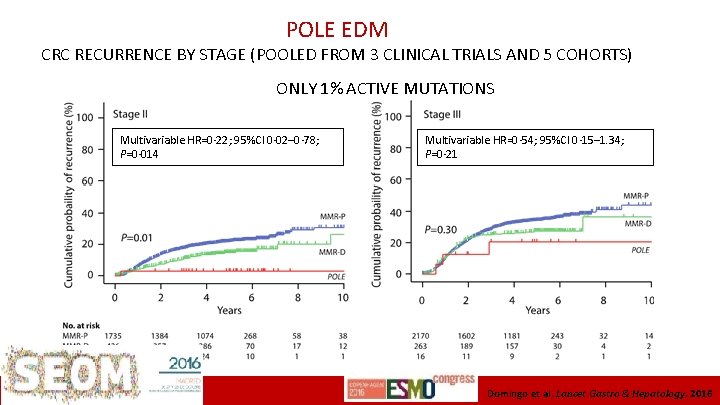
POLE EDM CRC RECURRENCE BY STAGE (POOLED FROM 3 CLINICAL TRIALS AND 5 COHORTS) ONLY 1% ACTIVE MUTATIONS Multivariable HR=0· 22; 95%CI 0· 02– 0· 78; P=0· 014 ICO L’Hospitalet Multivariable HR=0· 54; 95%CI 0· 15– 1. 34; P=0· 21 Domingo et al. Lancet Gastro & Hepatology, 2016

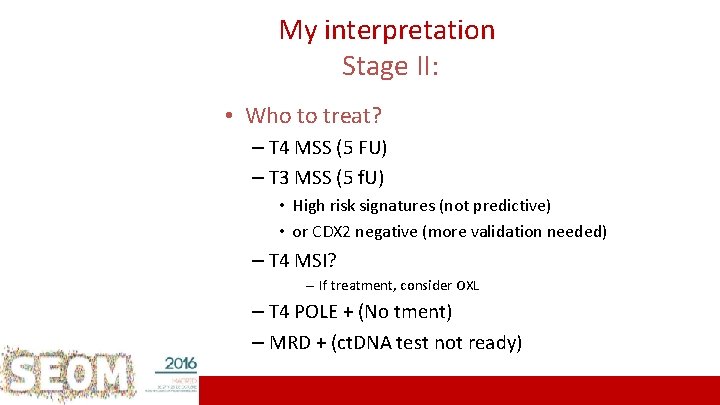
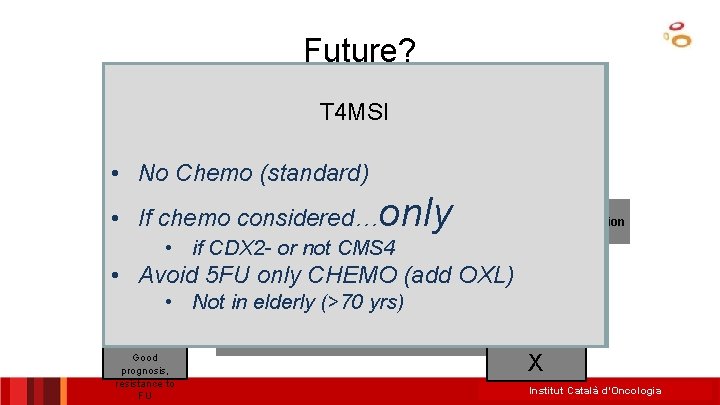
My interpretation Stage II: • Who to treat? – T 4 MSS (5 FU) – T 3 MSS (5 f. U) • High risk signatures (not predictive) • or CDX 2 negative (more validation needed) – T 4 MSI? – If treatment, consider OXL – T 4 POLE + (No tment) – MRD + (ct. DNA test not ready) 20 ICO L’Hospitalet

My interpretation Stage III: • Who to avoid treatment? – IIIa & • Low Risk Biomarkers? (Not Yet There) • Predictive Biomarkers? (Not Yet There) 21 ICO L’Hospitalet

22 ICO L’Hospitalet

On Thursday, Sept. 22, 2016, Dan passed away unexpectedly. We will always miss his humane kindness and his will to unveil the truth will always guide us 23 ICO L’Hospitalet

Thank-you…. 24

BACK UP SLIDES • • CIN, MSI (d. MMR) Individual mutations • – (RAS/BRAF…) Supervised signatures – • Gene signatures (tumor) (Oncotype/Coloprint…) Itrinsec signatures – CRCSC Consensus Classification – Composite PC 1 -EMT – Emerging biomarkers – – – Inmunoscore CDX 2 ct. DNA MRD PROGNOSIS & PREDICTION Clinical setting 25 ICO L’Hospitalet

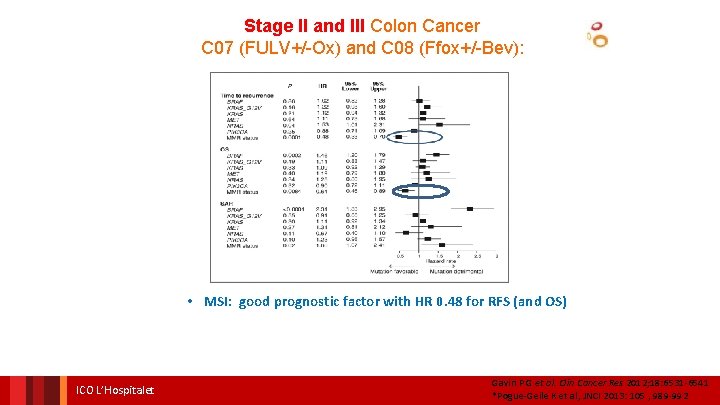
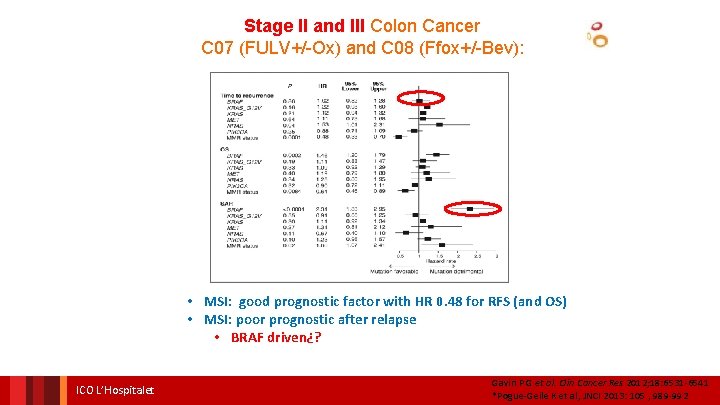
Stage II and III Colon Cancer C 07 (FULV+/-Ox) and C 08 (Ffox+/-Bev): • MSI: good prognostic factor with HR 0. 48 for RFS (and OS) ICO L’Hospitalet Gavin P G et al. Clin Cancer Res 2012; 18: 6531 -6541 *Pogue-Geile K et al, JNCI 2013: 105 , 989 -992

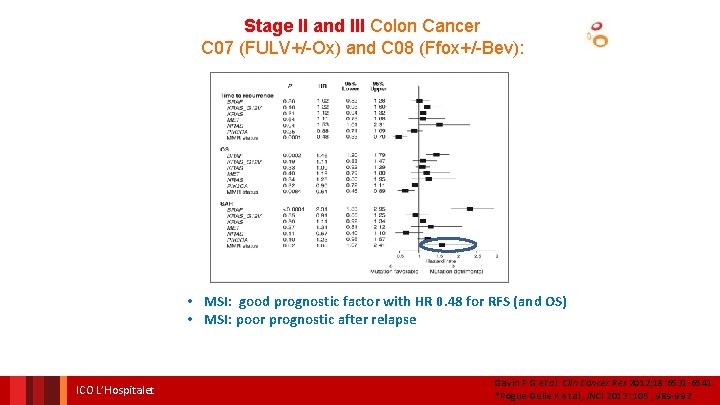
Stage II and III Colon Cancer C 07 (FULV+/-Ox) and C 08 (Ffox+/-Bev): • MSI: good prognostic factor with HR 0. 48 for RFS (and OS) • MSI: poor prognostic after relapse ICO L’Hospitalet Gavin P G et al. Clin Cancer Res 2012; 18: 6531 -6541 *Pogue-Geile K et al, JNCI 2013: 105 , 989 -992

Stage II and III Colon Cancer C 07 (FULV+/-Ox) and C 08 (Ffox+/-Bev): • MSI: good prognostic factor with HR 0. 48 for RFS (and OS) • MSI: poor prognostic after relapse • BRAF driven¿? ICO L’Hospitalet Gavin P G et al. Clin Cancer Res 2012; 18: 6531 -6541 *Pogue-Geile K et al, JNCI 2013: 105 , 989 -992

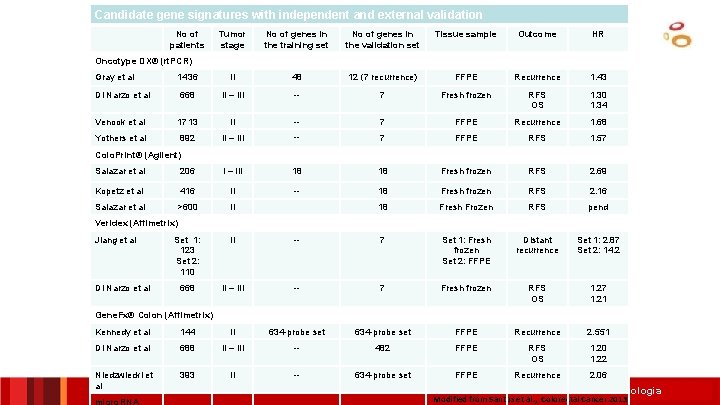
Candidate gene signatures with independent and external validation No of patients Tumor stage No of genes in the training set No of genes in the validation set Tissue sample Outcome HR Oncotype DX® (rt. PCR) Gray et al 1436 II 48 12 (7 recurrence) FFPE Recurrence 1. 43 Di Narzo et al 668 II – III -- 7 Fresh frozen RFS OS 1. 30 1. 34 Venook et al 1713 II -- 7 FFPE Recurrence 1. 68 Yothers et al 892 II – III -- 7 FFPE RFS 1. 57 Colo. Print® (Agilent) Salazar et al 206 I – III 18 18 Fresh frozen RFS 2. 69 Kopetz et al 416 II -- 18 Fresh frozen RFS 2. 16 Salazar et al >600 II 18 Fresh Frozen RFS pend Veridex (Affimetrix) Jiang et al Di Narzo et al Set 1: 123 Set 2: 110 II -- 7 Set 1: Fresh frozen Set 2: FFPE Distant recurrence Set 1: 2. 87 Set 2: 14. 2 668 II – III -- 7 Fresh frozen RFS OS 1. 27 1. 21 Gene. Fx® Colon (Affimetrix) Kennedy et al 144 II 634 -probe set FFPE Recurrence 2. 551 Di Narzo et al 688 II – III -- 482 FFPE RFS OS 1. 20 1. 22 Niedzwiecki et al 393 II -- 634 -probe set FFPE Recurrence 2. 06 micro. RNA Institut Català d’Oncologia Modified from Santos et al. , Colorectal Cancer 2013

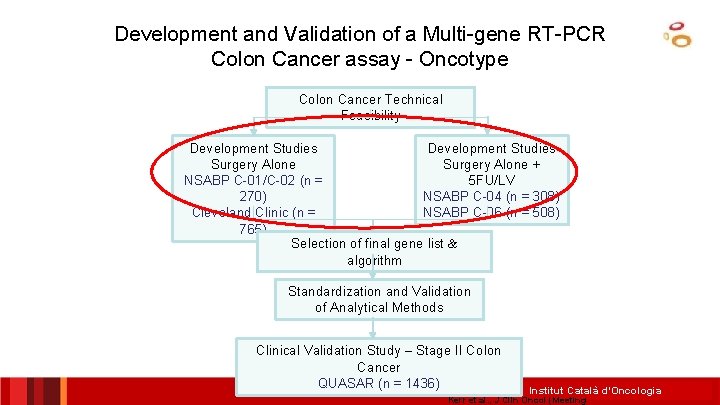
Development and Validation of a Multi-gene RT-PCR Colon Cancer assay - Oncotype Colon Cancer Technical Feasibility Development Studies Surgery Alone + NSABP C-01/C-02 (n = 5 FU/LV 270) NSABP C-04 (n = 308) Cleveland Clinic (n = NSABP C-06 (n = 508) 765) Selection of final gene list & algorithm Standardization and Validation of Analytical Methods Clinical Validation Study – Stage II Colon Cancer QUASAR (n = 1436) Institut Català d’Oncologia Kerr et al. , J Clin Oncol (Meeting

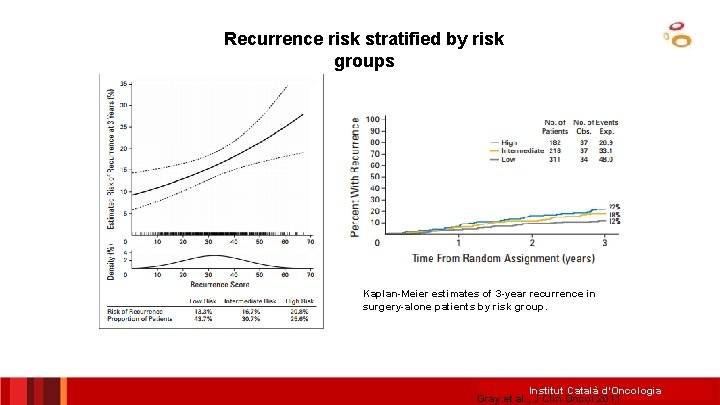
Recurrence risk stratified by risk groups Kaplan-Meier estimates of 3 -year recurrence in surgery-alone patients by risk group. Institut Català d’Oncologia Gray et al. , J Clin Oncol 2011

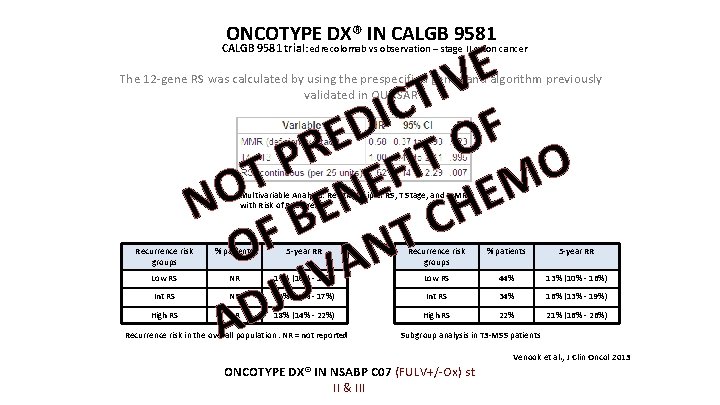
ONCOTYPE DX® IN CALGB 9581 E V I T C I D F E O R T P I O F T E M O E N N BE H C F T O N A V U J AD CALGB 9581 trial : edrecolomab vs observation – stage II colon cancer The 12 -gene RS was calculated by using the prespecified genes and algorithm previously validated in QUASAR Multivariable Analysis: Relationship of RS, T Stage, and MMR with Risk of Recurrence risk groups % patients 5 -year RR Low RS NR 12% (10% - 15%) Low RS 44% 13% (10% - 16%) NR 15% (12% - 17%) Int RS 34% 16% (13% - 19%) NR 18% (14% - 22%) High RS 22% 21% (16% - 26%) Int RS High RS Recurrence risk in the overall population. NR = not reported Subgroup analysis in T 3 -MSS patients Venook et al. , J Clin Oncol 2013 ONCOTYPE DX® IN NSABP C 07 (FULV+/-Ox) st II & III

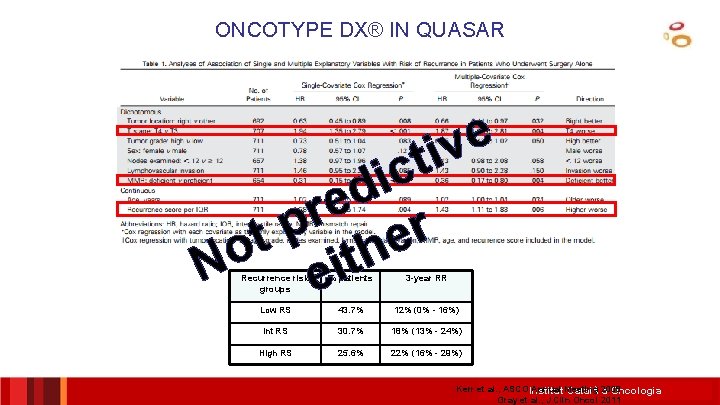
ONCOTYPE DX® IN QUASAR e v ti c i d e r p r t e o h t i N e Recurrence risk groups % patients 3 -year RR Low RS 43. 7% 12% (0% - 16%) Int RS 30. 7% 18% (13% - 24%) High RS 25. 6% 22% (16% - 29%) Kerr et al. , ASCO Institut Annual Meeting Català 2009 d’Oncologia Gray et al. , J Clin Oncol 2011

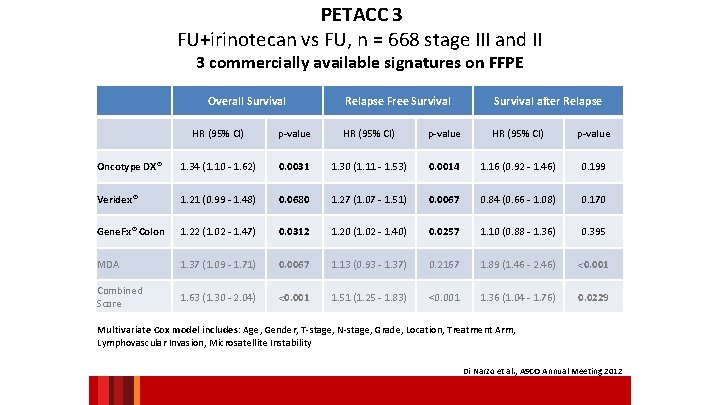
PETACC 3 FU+irinotecan vs FU, n = 668 stage III and II 3 commercially available signatures on FFPE Overall Survival Relapse Free Survival after Relapse HR (95% CI) p-value Oncotype DX® 1. 34 (1. 10 - 1. 62) 0. 0031 1. 30 (1. 11 - 1. 53) 0. 0014 1. 16 (0. 92 - 1. 46) 0. 199 Veridex® 1. 21 (0. 99 - 1. 48) 0. 0680 1. 27 (1. 07 - 1. 51) 0. 0067 0. 84 (0. 66 - 1. 08) 0. 170 Gene. Fx® Colon 1. 22 (1. 02 - 1. 47) 0. 0312 1. 20 (1. 02 - 1. 40) 0. 0257 1. 10 (0. 88 - 1. 36) 0. 395 MDA 1. 37 (1. 09 - 1. 71) 0. 0067 1. 13 (0. 93 - 1. 37) 0. 2167 1. 89 (1. 46 - 2. 46) <0. 001 Combined Score 1. 63 (1. 30 - 2. 04) <0. 001 1. 51 (1. 25 - 1. 83) <0. 001 1. 36 (1. 04 - 1. 76) 0. 0229 Multivariate Cox model includes: Age, Gender, T-stage, N-stage, Grade, Location, Treatment Arm, Lymphovascular Invasion, Microsatellite Instability Di Narzo et al. , ASCO Annual Meeting 2012

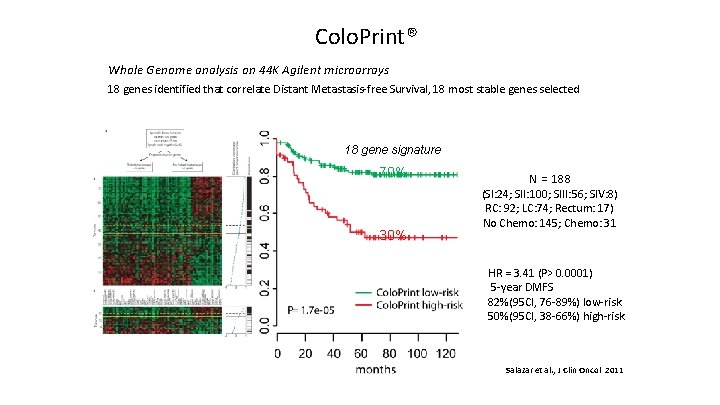
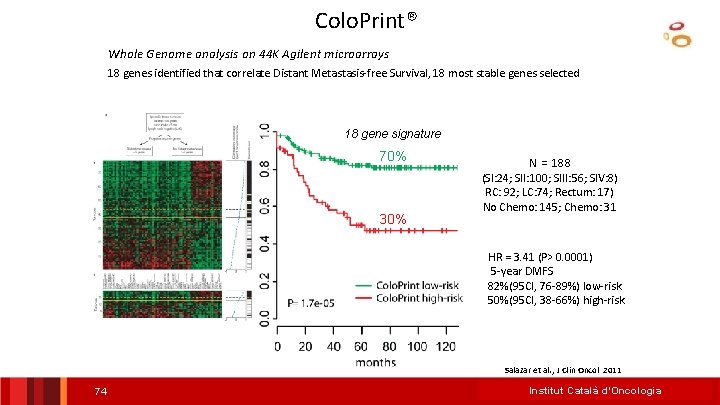
Colo. Print® Whole Genome analysis on 44 K Agilent microarrays 18 genes identified that correlate Distant Metastasis-free Survival, 18 most stable genes selected 18 gene signature DMFS 70% 30% N = 188 (SI: 24; SII: 100; SIII: 56; SIV: 8) RC: 92; LC: 74; Rectum: 17) No Chemo: 145; Chemo: 31 HR = 3. 41 (P> 0. 0001) 5 -year DMFS 82%(95 CI, 76 -89%) low-risk 50%(95 CI, 38 -66%) high-risk Salazar et al. , J Clin Oncol 2011 37

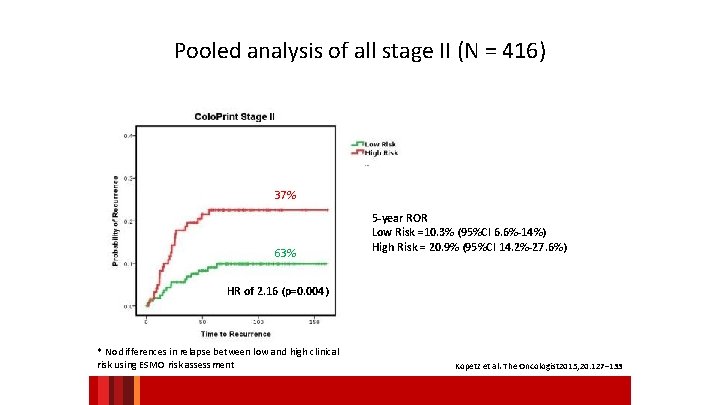
Pooled analysis of all stage II (N = 416) 37% 63% 5 -year ROR Low Risk =10. 3% (95%CI 6. 6%-14%) High Risk = 20. 9% (95%CI 14. 2%-27. 6%) HR of 2. 16 (p=0. 004) * No differences in relapse between low and high clinical risk using ESMO risk assessment Kopetz et al. The Oncologist 2015; 20: 127– 133

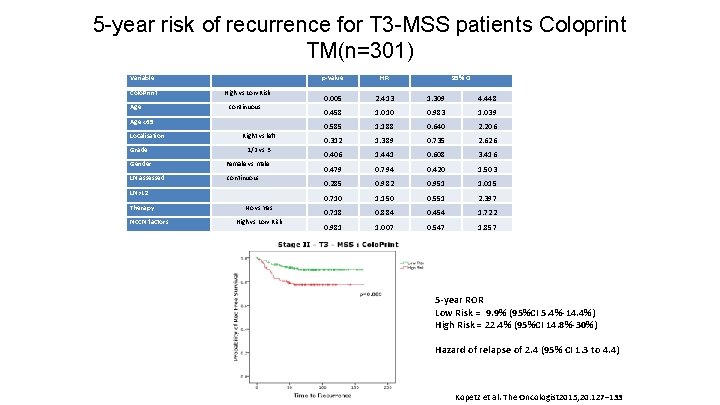
5 -year risk of recurrence for T 3 -MSS patients Coloprint TM(n=301) Variable Colo. Print High vs Low Risk Age continuous Age <65 Localisation Right vs left Grade 1/2 vs 3 Gender Female vs male LN assessed continuous LN>12 Therapy NCCN factors No vs Yes High vs Low Risk p-value HR 95% CI 0. 005 2. 413 1. 309 4. 448 0. 458 1. 010 0. 983 1. 039 0. 585 1. 188 0. 640 2. 206 0. 312 1. 389 0. 735 2. 626 0. 406 1. 441 0. 608 3. 416 0. 479 0. 794 0. 420 1. 503 0. 285 0. 982 0. 951 1. 015 0. 710 1. 150 0. 551 2. 397 0. 718 0. 884 0. 454 1. 722 0. 981 1. 007 0. 547 1. 857 5 -year ROR Low Risk = 9. 9% (95%CI 5. 4%-14. 4%) High Risk = 22. 4% (95%CI 14. 8%-30%) Hazard of relapse of 2. 4 (95% CI 1. 3 to 4. 4) Kopetz et al. The Oncologist 2015; 20: 127– 133

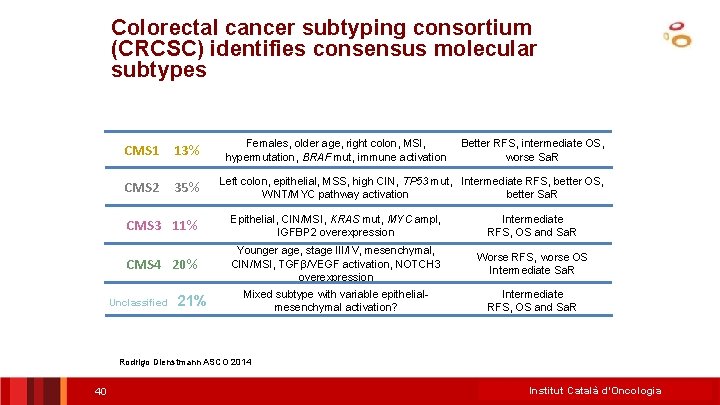
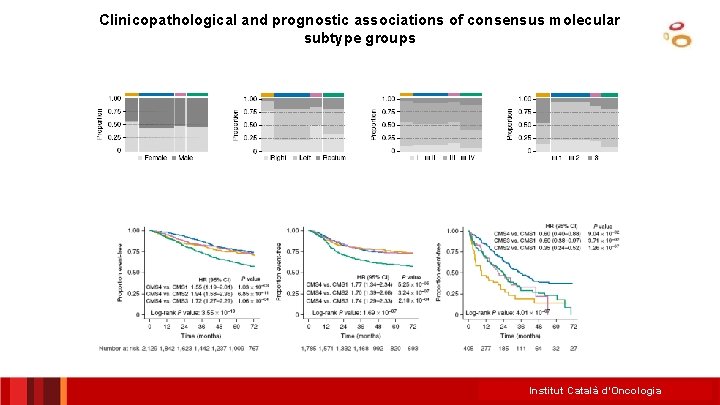
Colorectal cancer subtyping consortium (CRCSC) identifies consensus molecular subtypes CMS 1 13% CMS 2 35% Females, older age, right colon, MSI, hypermutation, BRAF mut, immune activation Better RFS, intermediate OS, worse Sa. R Left colon, epithelial, MSS, high CIN, TP 53 mut, Intermediate RFS, better OS, WNT/MYC pathway activation better Sa. R CMS 3 11% Epithelial, CIN/MSI, KRAS mut, MYC ampl, IGFBP 2 overexpression Intermediate RFS, OS and Sa. R CMS 4 20% Younger age, stage III/IV, mesenchymal, CIN/MSI, TGFβ/VEGF activation, NOTCH 3 overexpression Worse RFS, worse OS Intermediate Sa. R Mixed subtype with variable epithelialmesenchymal activation? Intermediate RFS, OS and Sa. R Unclassified 21% Rodrigo Dienstmann ASCO 2014 40 Institut Català d’Oncologia

41 Institut Català d’Oncologia

Clinicopathological and prognostic associations of consensus molecular subtype groups Institut Català d’Oncologia

RFS in stage II colorectal cancer (N= 1400) RFS in stage III colorectal cancer (N=1500) 2 y RFS (CI 95%) 74% (67 -81) 76% (72 -80) 73% (66 -82) 68% (62 -74) Early relapses in stage II CMS 4, 40% untreated. 2 y RFS (CI 95%) 89% (84 -94) 86% (83 -90) 92% (87 -97) 74% (68 -82) Late relapses in stage III CMS 4, 95% treated with adjuvant chemo. Institut Català d’Oncologia

44 Institut Català d’Oncologia

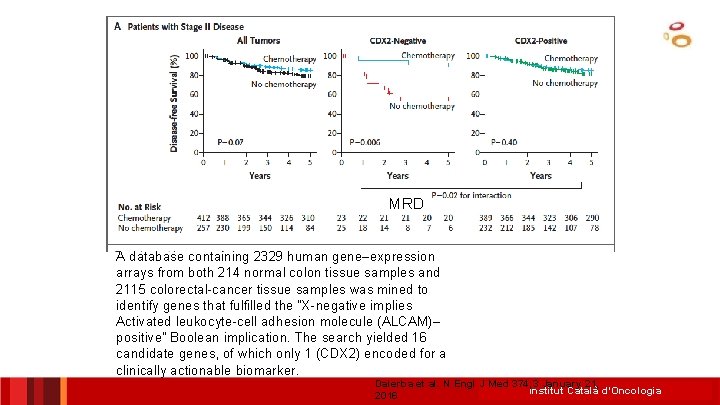
MRD A database containing 2329 human gene–expression arrays from both 214 normal colon tissue samples and 2115 colorectal-cancer tissue samples was mined to identify genes that fulfilled the “X-negative implies Activated leukocyte-cell adhesion molecule (ALCAM)– positive” Boolean implication. The search yielded 16 candidate genes, of which only 1 (CDX 2) encoded for a clinically actionable biomarker. Dalerba et al. N Engl J Med 374; 3 January 21, Institut Català d’Oncologia 2016

Tie J et al: MRD in resected CRC Clinical High-Risk Clinical Low-Risk (no poor prognostic features) HR: 27. 83 (95% CI: 8. 29– 93. 39) p < 0. 001 (p. MMR + at least one poor prognostic features) HR: 7. 54 (95% CI: 2. 57– 22. 11) p < 0. 001 Institut Català d’Oncologia

Stage II current context • Is this the end of other Px markers in Stage II? – T 4 remained in the multivariate analysis – MSI did not, but prone to low numbers effect Tie et al. J Clin Oncol 34, 2016 (suppl; abstr 3500) – Supervised gene signatures? Institut Català d’Oncologia

Future? Colon Cancer C Type/CMS 4/CCS 3/TGFb-like T 4 MSI Stage II (others run-ups: CDX 2) Stage III • No Chemo (standard) • MSI T 3 MSS chemo considered only if high. NO MSI MSS • Ifrisk chemo considered… signature and/or CDX 2 - and/or not. Determination needed • if CDX 2 or not CMS 4 mesenchimal-like (CMS 4) CT may be discussed • • Avoid 5 FU only CHEMO (add OXL) Other Markers: T 3/T 4, BRAF, KRAS? T 4 MSS Other follow-up w/o chemo considered if Factors: age, patient wish. . . Prognostic Not signature in elderly (>70 yrs) NO low • risk or CDX 2+ and/or CMS 4 signatures, intrinsec subtypes, FOLFO CT IHQ MK? * Good X prognosis, only resistance to FU Institut Català d’Oncologia

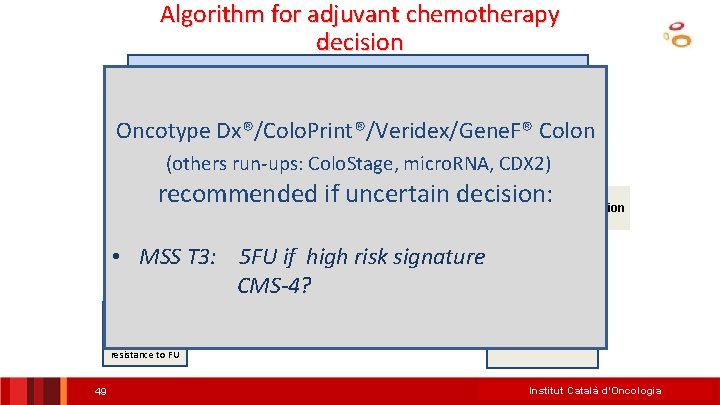
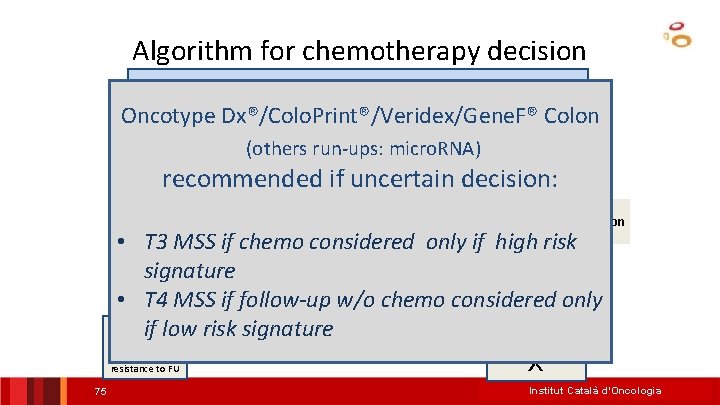
Algorithm for adjuvant chemotherapy decision Colon Cancer Stage III Oncotype Dx®/Colo. Print®/Veridex/Gene. F® Colon (others run-ups: Colo. Stage, micro. RNA, CDX 2) MSIrecommended if uncertain decision: MSS NO MSI Determination needed may be risk discussed • MSS T 3: CT 5 FU if high signature Other Markers: T 3/T 4, Other Factors: age, patient wish. . . Prognostic signatures? * CMS-4? NO CT Good prognosis, resistance to FU 49 FOLFOX Institut Català d’Oncologia

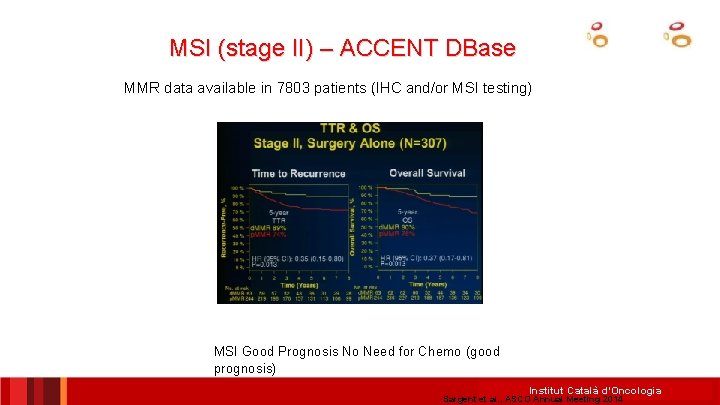
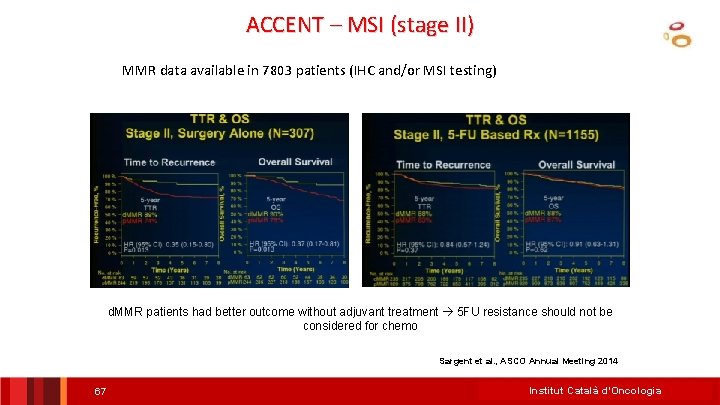
MSI (stage II) – ACCENT DBase MMR data available in 7803 patients (IHC and/or MSI testing) MSI Good Prognosis No Need for Chemo (good prognosis) Institut Català d’Oncologia Sargent et al. , ASCO Annual Meeting 2014

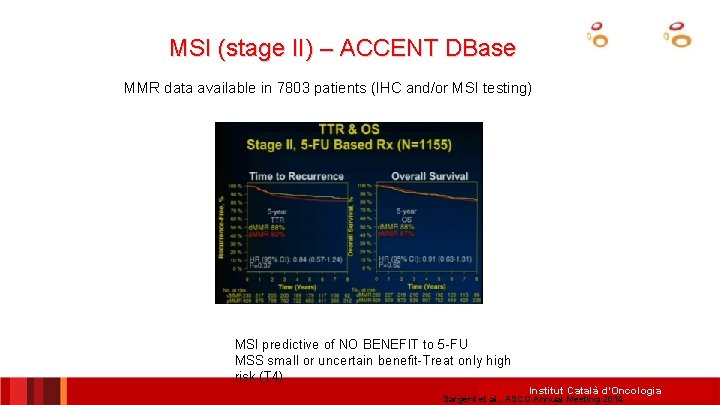
MSI (stage II) – ACCENT DBase MMR data available in 7803 patients (IHC and/or MSI testing) MSI predictive of NO BENEFIT to 5 -FU MSS small or uncertain benefit-Treat only high risk (T 4) Institut Català d’Oncologia Sargent et al. , ASCO Annual Meeting 2014

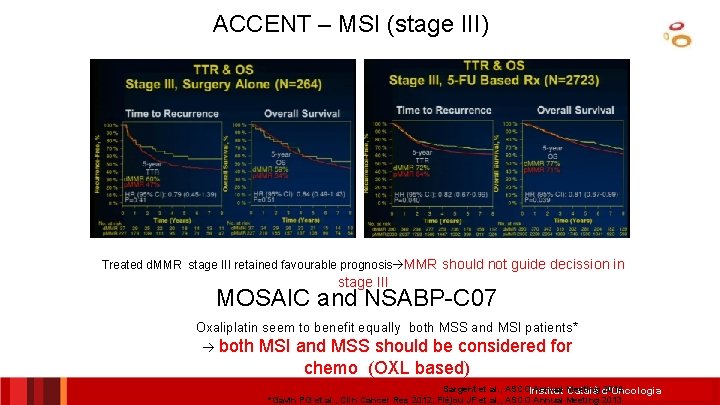
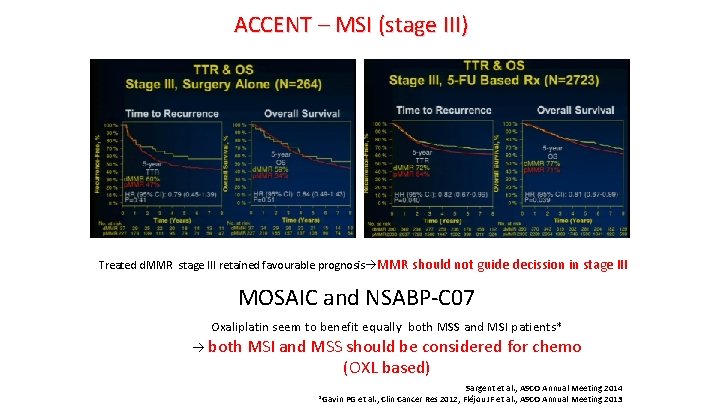
ACCENT – MSI (stage III) Treated d. MMR stage III retained favourable prognosis MMR should not guide decission in stage III MOSAIC and NSABP-C 07 Oxaliplatin seem to benefit equally both MSS and MSI patients* both MSI and MSS should be considered for chemo (OXL based) Sargent et al. , ASCOInstitut Annual Meeting 2014 Català d’Oncologia *Gavin PG et al. , Clin Cancer Res 2012; Fléjou JF et al. , ASCO Annual Meeting 2013

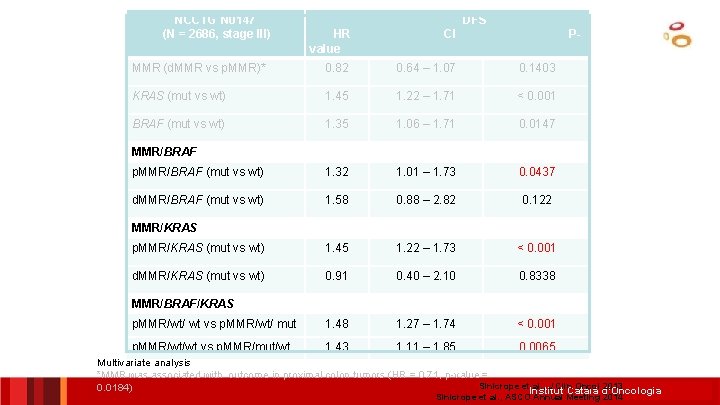
NCCTG N 0147 (N = 2686, stage III) DFS HR value CI P- MMR (d. MMR vs p. MMR)* 0. 82 0. 64 – 1. 07 0. 1403 KRAS (mut vs wt) 1. 45 1. 22 – 1. 71 < 0. 001 BRAF (mut vs wt) 1. 35 1. 06 – 1. 71 0. 0147 p. MMR/BRAF (mut vs wt) 1. 32 1. 01 – 1. 73 0. 0437 d. MMR/BRAF (mut vs wt) 1. 58 0. 88 – 2. 82 0. 122 p. MMR/KRAS (mut vs wt) 1. 45 1. 22 – 1. 73 < 0. 001 d. MMR/KRAS (mut vs wt) 0. 91 0. 40 – 2. 10 0. 8338 p. MMR/wt/ wt vs p. MMR/wt/ mut 1. 48 1. 27 – 1. 74 < 0. 001 p. MMR/wt/wt vs p. MMR/mut/wt 1. 43 1. 11 – 1. 85 0. 0065 MMR/BRAF MMR/KRAS MMR/BRAF/KRAS Multivariate analysis *MMR was associated with outcome in proximal colon tumors (HR = 0. 71, p-value = Sinicrope et al. , J Clin Oncol 2013 0. 0184) Institut Català d’Oncologia Sinicrope et al. , ASCO Annual Meeting 2014

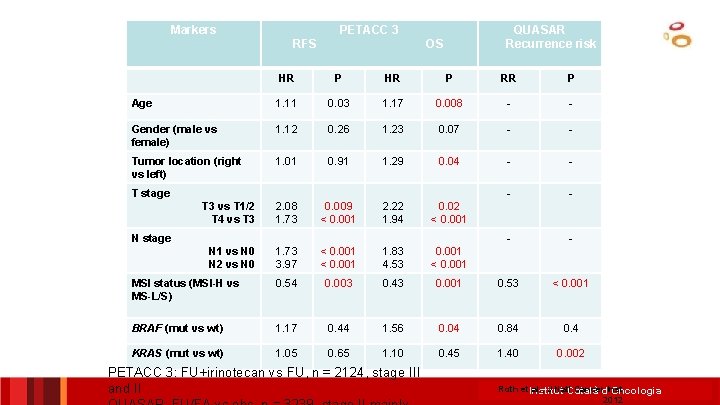
Markers PETACC 3 RFS QUASAR Recurrence risk OS HR P RR P Age 1. 11 0. 03 1. 17 0. 008 - - Gender (male vs female) 1. 12 0. 26 1. 23 0. 07 - - Tumor location (right vs left) 1. 01 0. 91 1. 29 0. 04 - - T 3 vs T 1/2 T 4 vs T 3 2. 08 1. 73 0. 009 < 0. 001 2. 22 1. 94 0. 02 < 0. 001 - - N 1 vs N 0 N 2 vs N 0 1. 73 3. 97 < 0. 001 1. 83 4. 53 0. 001 < 0. 001 MSI status (MSI-H vs MS-L/S) 0. 54 0. 003 0. 43 0. 001 0. 53 < 0. 001 BRAF (mut vs wt) 1. 17 0. 44 1. 56 0. 04 0. 84 0. 4 KRAS (mut vs wt) 1. 05 0. 65 1. 10 0. 45 1. 40 0. 002 T stage N stage PETACC 3: FU+irinotecan vs FU, n = 2124, stage III and II Roth et. Institut al. , J Natl. Català Cancerd’Oncologia Inst 2012

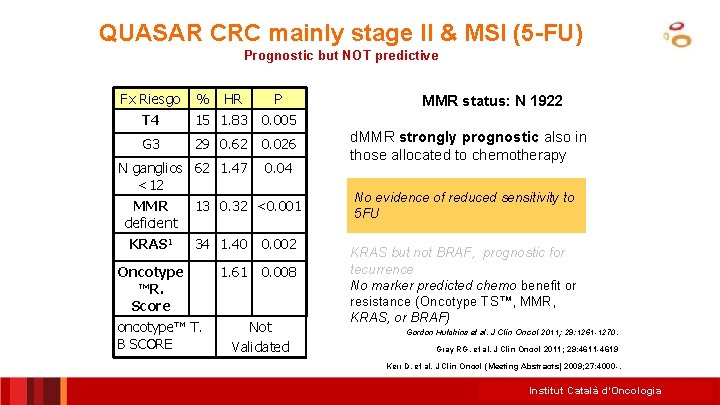
QUASAR CRC mainly stage II & MSI (5 -FU) Prognostic but NOT predictive Fx Riesgo % HR P T 4 15 1. 83 0. 005 G 3 29 0. 62 0. 026 N ganglios 62 1. 47 <12 0. 04 MMR deficient KRAS 1 13 0. 32 <0. 001 34 1. 40 0. 002 1. 61 0. 008 Oncotype ™R. Score oncotype™ T. B SCORE Not Validated MMR status: N 1922 d. MMR strongly prognostic also in those allocated to chemotherapy No evidence of reduced sensitivity to 5 FU KRAS but not BRAF, prognostic for tecurrence No marker predicted chemo benefit or resistance (Oncotype TS™, MMR, KRAS, or BRAF) Gordon Hutchins et al. J Clin Oncol 2011; 29: 1261 -1270. Gray RG, et al. J Clin Oncol 2011; 29: 4611 -4619 Kerr D, et al. J Clin Oncol (Meeting Abstracts) 2009; 27: 4000 -. Institut Català d’Oncologia

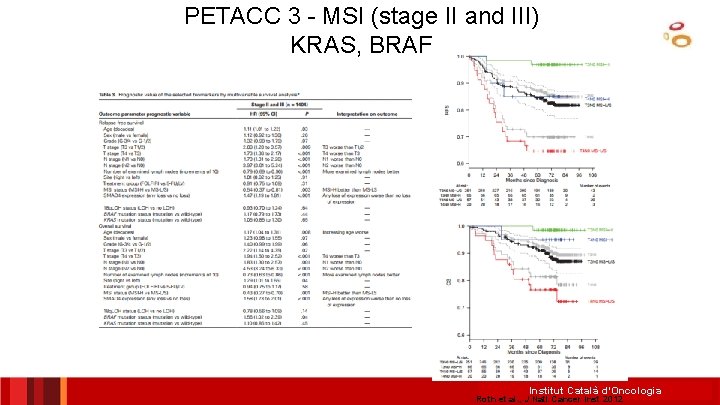
PETACC 3 - MSI (stage II and III) KRAS, BRAF Institut Català d’Oncologia Roth et al. , J Natl Cancer Inst 2012

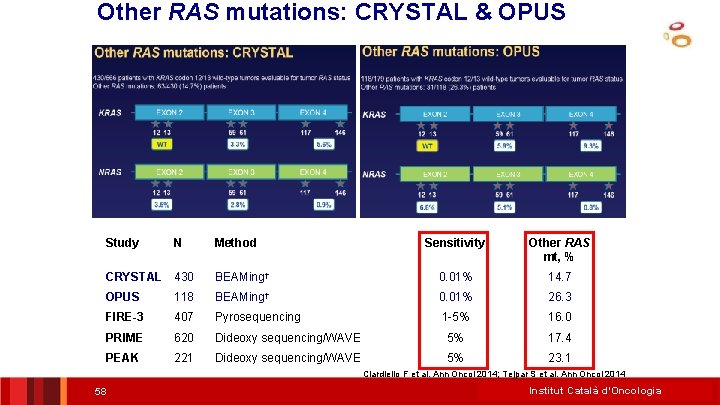
Other RAS mutations: CRYSTAL & OPUS Study N Method Sensitivity Other RAS mt, % CRYSTAL 430 BEAMing† 0. 01% 14. 7 OPUS 118 BEAMing† 0. 01% 26. 3 FIRE-3 407 Pyrosequencing 1 -5% 16. 0 PRIME 620 Dideoxy sequencing/WAVE 5% 17. 4 PEAK 221 Dideoxy sequencing/WAVE 5% 23. 1 Ciardiello F et al. Ann Oncol 2014; Tejpar S et al. Ann Oncol 2014 58 Institut Català d’Oncologia

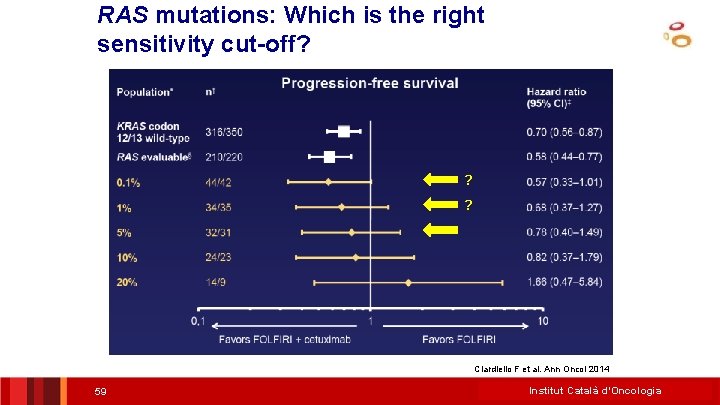
RAS mutations: Which is the right sensitivity cut-off? ? ? Ciardiello F et al. Ann Oncol 2014 59 Institut Català d’Oncologia

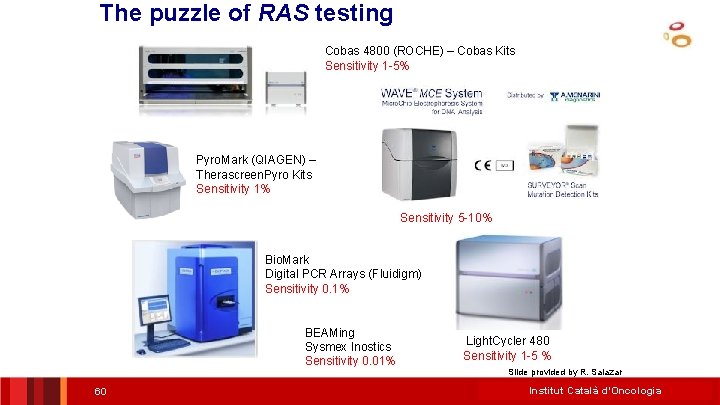
The puzzle of RAS testing Cobas 4800 (ROCHE) – Cobas Kits Sensitivity 1 -5% Pyro. Mark (QIAGEN) – Therascreen. Pyro Kits Sensitivity 1% Sensitivity 5 -10% Bio. Mark Digital PCR Arrays (Fluidigm) Sensitivity 0. 1% BEAMing Sysmex Inostics Sensitivity 0. 01% 60 Light. Cycler 480 Sensitivity 1 -5 % Slide provided by R. Salazar Institut Català d’Oncologia

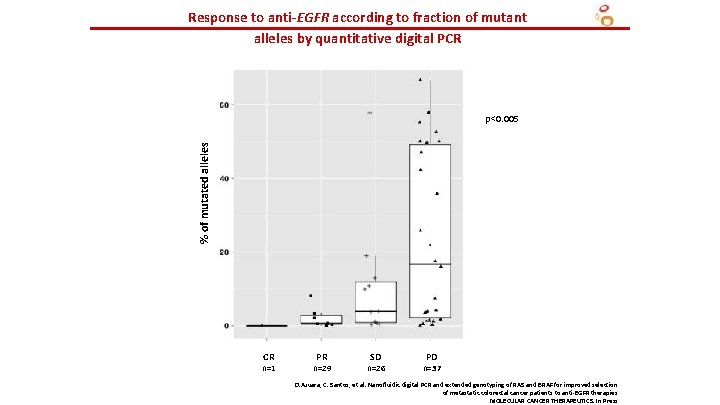
Response to anti-EGFR according to fraction of mutant alleles by quantitative digital PCR % of mutated alleles p<0. 005 CR n=1 PR n=29 SD n=26 PD n=37 D. Azuara, C. Santos, et al. Nanofluidic digital PCR and extended genotyping of RAS and BRAF for improved selection of metastatic colorectal cancer patients to anti-EGFR therapies MOLECULAR CANCER THERAPEUTICS. In Press

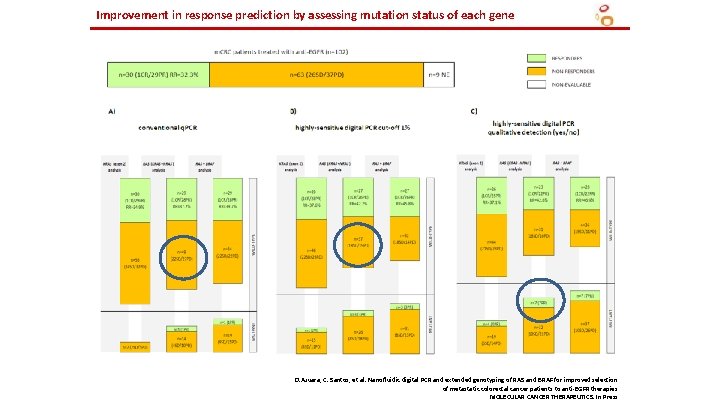
Improvement in response prediction by assessing mutation status of each gene D. Azuara, C. Santos, et al. Nanofluidic digital PCR and extended genotyping of RAS and BRAF for improved selection of metastatic colorectal cancer patients to anti-EGFR therapies MOLECULAR CANCER THERAPEUTICS. In Press

“Under pressure” clonal selection vs evolution Vilar E & Tabernero J, Nature 2012 63 Institut Català d’Oncologia

Treatment of Resistance (primary or secondary) in present or future clinical trials RAS mutants: Cetuximab + Irinotecan in KRAS G 13 D mutant CRC…. MEK inhibitors + pan HER inhibitors BRAF mutants: BRAF + EGFR + PIK 3 Ca or WNT inhibitors S 492 R mutants: Panitumumab C-MET amplifications C-MET inhibitors ? Tumor debulking in unresectable metastasic disease proof of principle cf. DNA load as surrogate endpoint of effective debulking of clones with resistance potential 64

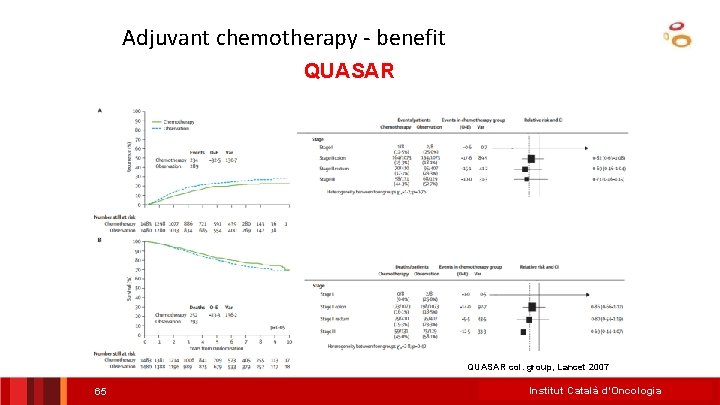
Adjuvant chemotherapy - benefit QUASAR col. group, Lancet 2007 65 Institut Català d’Oncologia

Risk stratification in stage II colon cancer • Clinicopathological features • Microsatellite instability, KRAS and BRAF • Supervised Prognostic signatures – Do gene signatures currently add information to the decision making progress? • Intrinsic molecular subtypes 66 Institut Català d’Oncologia

ACCENT – MSI (stage II) MMR data available in 7803 patients (IHC and/or MSI testing) d. MMR patients had better outcome without adjuvant treatment 5 FU resistance should not be considered for chemo Sargent et al. , ASCO Annual Meeting 2014 67 Institut Català d’Oncologia

ACCENT – MSI (stage III) Treated d. MMR stage III retained favourable prognosis MMR should not guide decission in stage III MOSAIC and NSABP-C 07 Oxaliplatin seem to benefit equally both MSS and MSI patients* both MSI and MSS should be considered for chemo (OXL based) Sargent et al. , ASCO Annual Meeting 2014 *Gavin PG et al. , Clin Cancer Res 2012; Fléjou JF et al. , ASCO Annual Meeting 2013

C 07 (FULV+/-Ox) and C 08 (Ffox+/-Bev): Stage II and III Colon Cancer • • MSI: prognostic factor with HR 0. 48 for RFS (and OS) MSI: oxaliplatin similar benefit in p. MMR and d. MMR BRAF (& MSI): poor prognostic after relapse MSI: predictive of BEV benefit OS HR 0. 52 p: 0. 02 favours BEV * Gavin P G et al. Clin Cancer Res 2012; 18: 6531 -6541 *Pogue-Geile K et al, JNCI 2013: 105 , 989 -992 69 Institut Català d’Oncologia

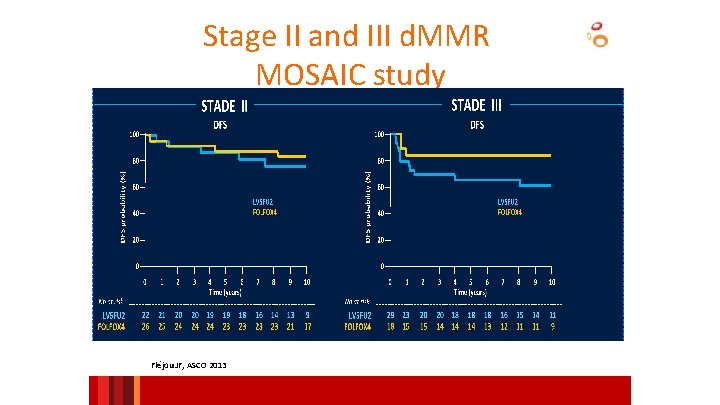
Stage II and III d. MMR MOSAIC study Fléjou JF, ASCO 2013

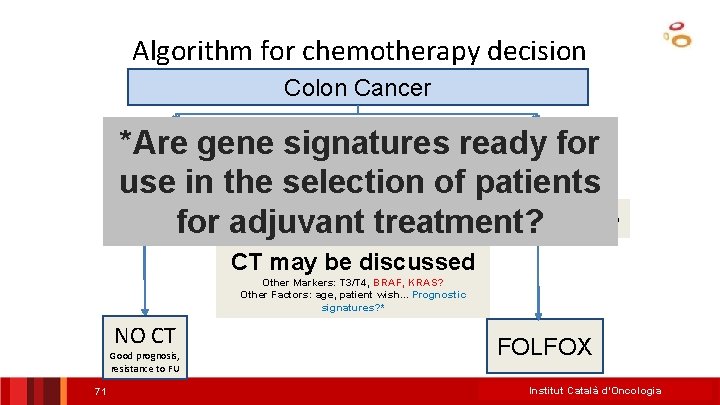
Algorithm for chemotherapy decision Colon Cancer Stage III *Are gene signatures ready for use in the selection of patients MSI MSS for adjuvant treatment? NO MSI Determination needed CT may be discussed Other Markers: T 3/T 4, BRAF, KRAS? Other Factors: age, patient wish. . . Prognostic signatures? * NO CT Good prognosis, resistance to FU 71 FOLFOX Institut Català d’Oncologia

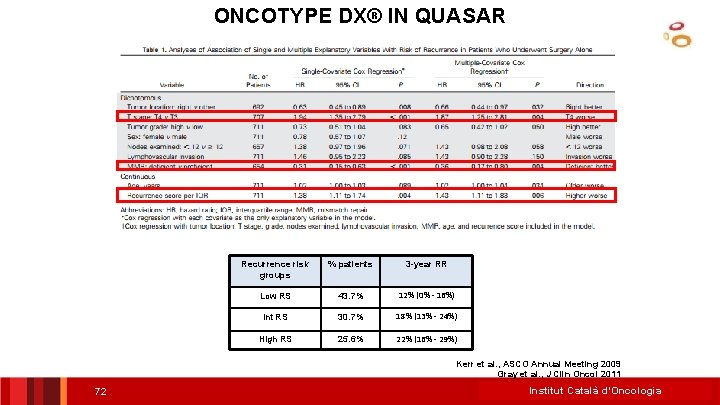
ONCOTYPE DX® IN QUASAR Recurrence risk groups % patients 3 -year RR Low RS 43. 7% 12% (0% - 16%) Int RS 30. 7% 18% (13% - 24%) High RS 25. 6% 22% (16% - 29%) Kerr et al. , ASCO Annual Meeting 2009 Gray et al. , J Clin Oncol 2011 72 Institut Català d’Oncologia

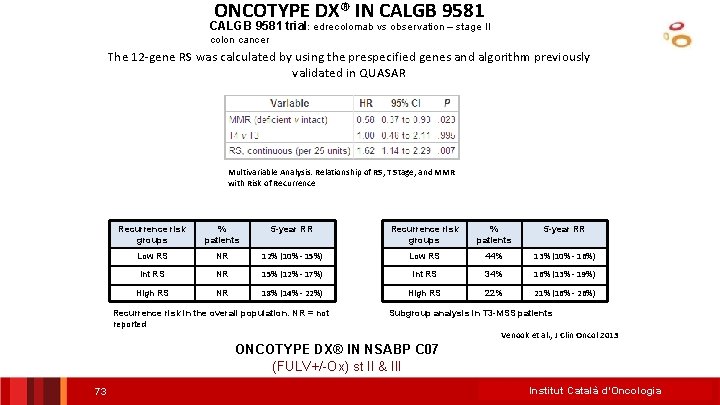
ONCOTYPE DX® IN CALGB 9581 trial: edrecolomab vs observation – stage II colon cancer The 12 -gene RS was calculated by using the prespecified genes and algorithm previously validated in QUASAR Multivariable Analysis: Relationship of RS, T Stage, and MMR with Risk of Recurrence risk groups % patients 5 -year RR Low RS NR 12% (10% - 15%) Low RS 44% 13% (10% - 16%) Int RS NR 15% (12% - 17%) Int RS 34% 16% (13% - 19%) High RS NR 18% (14% - 22%) High RS 22% 21% (16% - 26%) Recurrence risk in the overall population. NR = not reported Subgroup analysis in T 3 -MSS patients Venook et al. , J Clin Oncol 2013 ONCOTYPE DX® IN NSABP C 07 (FULV+/-Ox) st II & III 73 Institut Català d’Oncologia

Colo. Print® Whole Genome analysis on 44 K Agilent microarrays 18 genes identified that correlate Distant Metastasis-free Survival, 18 most stable genes selected 18 gene signature DMFS 70% 30% N = 188 (SI: 24; SII: 100; SIII: 56; SIV: 8) RC: 92; LC: 74; Rectum: 17) No Chemo: 145; Chemo: 31 HR = 3. 41 (P> 0. 0001) 5 -year DMFS 82%(95 CI, 76 -89%) low-risk 50%(95 CI, 38 -66%) high-risk Salazar et al. , J Clin Oncol 2011 74 Institut Català d’Oncologia

Algorithm for chemotherapy decision Colon Cancer Oncotype Dx®/Colo. Print®/Veridex/Gene. F® Colon (others run-ups: micro. RNA) Stage III recommended if uncertain decision: Stage II MSS NO MSI Determination needed • T 3 MSS if chemo considered only if high risk may be discussed signature. CTOther Markers: T 3/T 4, BRAF, KRAS? Other Factors: age, patient wish. . . Prognostic • T 4 MSS if follow-upsignatures? * w/o chemo considered only if low risk signature FOLFO NO CT Good prognosis, X resistance to FU 75 Institut Català d’Oncologia

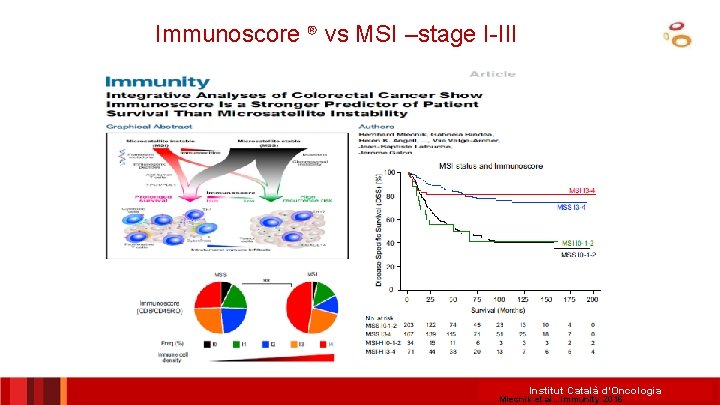
Immunoscore ® vs MSI –stage I-III Institut Català d’Oncologia Mlecnik et al. , Immunity 2016
 Obligaciones de los pacientes
Obligaciones de los pacientes Indica dónde haces estas diligencias.
Indica dónde haces estas diligencias. Seleccionar idioma
Seleccionar idioma Seleccionar idioma
Seleccionar idioma Audacity seleccionar fragmentos
Audacity seleccionar fragmentos Seleccionar catala
Seleccionar catala Uso de imagens de pacientes em redes sociais
Uso de imagens de pacientes em redes sociais Antecedentes de la teoria de colas
Antecedentes de la teoria de colas Tecnicas de inmovilizacion
Tecnicas de inmovilizacion Dimensiones ambulancia
Dimensiones ambulancia Escala de hipotonía de campbell
Escala de hipotonía de campbell Toxoplasma gondii
Toxoplasma gondii Comorbilidades
Comorbilidades Identificación inequívoca de pacientes
Identificación inequívoca de pacientes Bomba de heparina
Bomba de heparina Diagnostico diferencial tvp
Diagnostico diferencial tvp Crisis hipertensiva tipo emergencia
Crisis hipertensiva tipo emergencia Tratamiento para las drogas
Tratamiento para las drogas Adiccin
Adiccin Diagnosis
Diagnosis Mesa de demonios
Mesa de demonios Sífilis embarazo tratamiento
Sífilis embarazo tratamiento Tormenta tiroidea tratamiento
Tormenta tiroidea tratamiento Signos y sintomas de la tuberculosis pulmonar
Signos y sintomas de la tuberculosis pulmonar Bronquiectasias tratamiento
Bronquiectasias tratamiento Manejo hipopotasemia
Manejo hipopotasemia Hollín en la garganta
Hollín en la garganta Hitoplasma
Hitoplasma Tratamiento al final del tubo
Tratamiento al final del tubo Tratamiento primario acortado de la tuberculosis
Tratamiento primario acortado de la tuberculosis Rickettsiosis tratamiento
Rickettsiosis tratamiento Puñetazo precordial
Puñetazo precordial Nadrenalina
Nadrenalina Tratamiento de hemorragia interna
Tratamiento de hemorragia interna 5h y 5t
5h y 5t Hipocalcemia clasificacion
Hipocalcemia clasificacion Los conflictos intergrupales
Los conflictos intergrupales Tratamiento desnutrición
Tratamiento desnutrición Praxias rotacismo
Praxias rotacismo Corrimiento uretral
Corrimiento uretral Intrusión y extrusión dental
Intrusión y extrusión dental Avril pontigo edad
Avril pontigo edad Tratamiento antibiotico erisipela
Tratamiento antibiotico erisipela Tormenta tiroidea tratamiento
Tormenta tiroidea tratamiento Crisis hipertensiva tratamiento
Crisis hipertensiva tratamiento Hemorragia esteriorizada
Hemorragia esteriorizada Taquicardia sinusal tratamiento
Taquicardia sinusal tratamiento Neumomediastino tratamiento
Neumomediastino tratamiento Exacerbación de epoc
Exacerbación de epoc Disenteria en niños tratamiento
Disenteria en niños tratamiento Signo del campanario radiografia
Signo del campanario radiografia Complejo de von meyenburg
Complejo de von meyenburg Toxoplasmosis cerebral tratamiento
Toxoplasmosis cerebral tratamiento Anemia perniciosa tratamiento
Anemia perniciosa tratamiento Tratamiento de anemia megaloblastica
Tratamiento de anemia megaloblastica Amebas tratamiento
Amebas tratamiento Alcalosis respiratoria
Alcalosis respiratoria Foco tricuspideo
Foco tricuspideo Adrenalina nebulizada
Adrenalina nebulizada Upp grados
Upp grados Dosis de levotiroxina según tsh
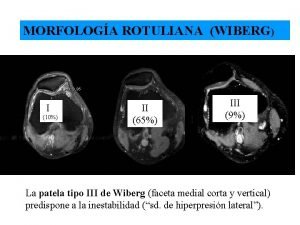
Dosis de levotiroxina según tsh Rotula tipo 3 di wiberg
Rotula tipo 3 di wiberg Meningitis viral antibiótico
Meningitis viral antibiótico Fenoximetilpenicilin
Fenoximetilpenicilin Criterios de centor
Criterios de centor Miriam salvo
Miriam salvo Tratamiento fiebre tifoidea adultos
Tratamiento fiebre tifoidea adultos Anemia microcítica hipocrómica tratamiento
Anemia microcítica hipocrómica tratamiento Diagnóstico diferencial de la hipertensión arterial
Diagnóstico diferencial de la hipertensión arterial Hemorragia alveolar difusa tratamiento
Hemorragia alveolar difusa tratamiento Tratamiento coartacion aortica
Tratamiento coartacion aortica Tratamiento de la información
Tratamiento de la información Tratamiento targa
Tratamiento targa