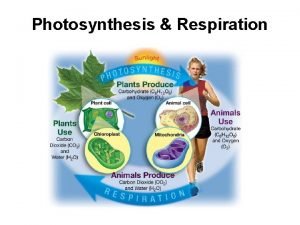
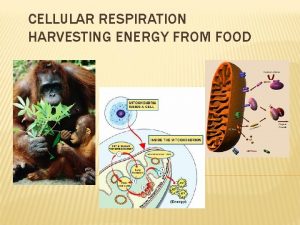
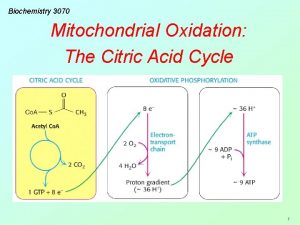
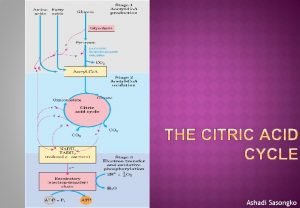
Citric acid cycle Definition The citric acid cycle


























- Slides: 69

Citric acid cycle

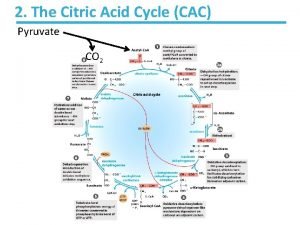
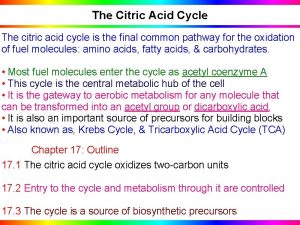
Definition The citric acid cycle is the final common pathway for the oxidation of fuel molecules such as amino acids, fatty acids, and carbohydrates. Most fuel molecules enter the cycle as acetyl coenzyme A. The reactions of the citric acid cycle take place inside mitochondria, in contrast with those of glycolysis, that take place in the cytosol. Under aerobic conditions, the pyruvate generated from glucose is oxidatively decarboxylated to form acetyl Co. A.

Overview of the citric acid cycle Citric acid cycle also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle. The cycle is also an important source of precursors, not only for the storage forms of fuels, but also for the building blocks of many other molecules such as amino acids, nucleotide bases, cholesterol, and porphyrin (the organic component of heme) What is the function of TCA cycle in transforming fuel molecules into ATP?

Remember that fuel molecules are carbon compounds that are capable of being oxidized of losing electrons. The citric acid cycle includes a series of oxidation reduction reactions that result in the oxidation of an acetyl group to 2 molecules of carbon dioxide. A four carbon compound (oxaloacetate) condenses with a two carbon acetyl unit to yield a six carbon tricarboxylic acid (citrate). An isomer of citrate is then oxidatively decarboxylated. The resulting five carbon compound (α ketoglutarate) also is oxidatively decaroxylated to yield a four carbon compound (succinate). Oxaloacetate is then regenerated from succinate

Two carbon atoms enter the cycle as an acetyl unit and two carbon atoms lave the cycle in the form of two molecules of carbon dioxide. Three hydride ions (so, six electrons) re transferred to three molecules of nicotinamide adenine dinucleotide (NAD+), whereas one pair of hydrogen atoms (therefore, 2 electrons) is transferred to one molecule of flavin adenine dinucleotide (FAD). The function of the citric acid cycle I the harvesting of high energy electrons from carbon fuels. NOTE: that TCA cycle itself neither generates a large amount of ATP nor includes oxygen as a reactant.

1 The citric acid cycle begins with the condensation of a four carbon unit, oxaloacetate, and a two carbon unit, the acetyl group of acetyl Co. A. Oxaloacetate reacts with acetyl Co. A and H 2 O to yield citrate (C 6), which isomerized to isocitrate (C 6). 2 Oxidative decarboxylation of this intermediate gives α ketoglutarate (C 5). 3 The second molecule of carbon dioxide comes off in the next reaction, in which α ketoglutarate is oxidatively decarboxylated to succinyl Co. A(C 4).

4 The thioester bond of succinyl Co. A is cleaved by inorthophosphate to yield succinate, and a high phosphoryl transfer potential compound in the form of GTP is along with generated. 5 Succinate is oxidized to fumarate (C 4), which is then hydrated to form malate (C 4). 6 Finally, malate is oxidized to regenerate oxaloacetate (C 4).

Therefore, 2 carbon atoms from acetyl Co. A enter the cycle, and 2 carbon atoms leave the cycle as CO 2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and α ketoglutara Dehydrogenase. In the four oxidation reduction reactions in the cycle, 3 pairs of electrons are transferred to NAD+ and one pair to FAD. These reduced electrons carriers are subsequently oxidized by the electron transport chain to generate approximately 9 molecules of ATP.

In addition, 1 molecule of a compound having a high phosphoryl transfer potential is directly formed in the citric acid cycle. Hence, a total of 10 molecules of compounds having high phosphoryl transfer potential are generated for each two carbon fragment that is completely oxidized to H 2 O and CO 2.

The disruption of pyruvate metabolism is the cause Beriberi and poisoning by Mercury and Arsenic Beriberi, a neurologic and cardiovascular disorder, is caused by a dietary deficiency of thiamine (vitamin B 1). The disease is characterized by neurologic and cardiac symptoms. Damage to the peripheral nervous system is expressed as pain in the limbs, kness, and unclear skin sensation. Heart may enlarged and the cardiac output inadequate. In Beriberi the levels of pyruvate and α ketoglutarate in the blood are higher than normal.

The increase in the level of pyruvate in the blood is especially obvious After the ingestion of glucose. A related finding is that the activity of the pyruvate and α ketoglutarate dehydrogenase complexes in vivo are abnormally low. The low transketolase activity of red cells in Beriberi is an easily measured and reliable diagnostic indicator of the disease. The binding of mercury or arsenite to the dihydropoyl groups inhibits the complex and leads to central nervous system pathologies. Treatment for these poisons is the administration of 2, 3 dimercaptopropanol was developed after World War 1 as an antidote to lewisite, an arsenic based chemical weapon. This compound was initially called BAL, for British anti lewisite.

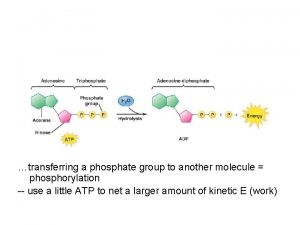
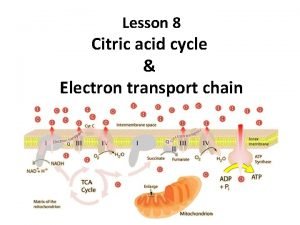
Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH 2 to O 2 by a series of electron carrier. This process take place in mitochondria. The flow of electrons from NADH or FADH 2 to O 2 through protein complexes located in the mitochondrial inner membrane leads to the pumping of protons out of the mitochondrial matrix. The resulting uneven distribution of protons generates a PH gradient and a transmembrane electrical potential that creates a proton motive force.

ATP is synthesized when protons flow back to the mitochondrial matrix through an enzyme complex. Therefore, the oxidation of fuels and the phosphorylation of ADP are coupled by a proton gradient across the inner mitochondrial membrane. - Oxidative phosphorylation is the culmination of a series of energy transformations that are called cellular respiration. 1 carbon fuels are oxidized in the citric acid cycle to yield electrons with high transfer potential. 2 This electron motive force is converted into a proton motive force.

3 finally, the proton motive force is converted into phosphoryl transfer potential. The conversion of electron motive force into proton motive force is carried out by 3 electron driven proton pumps NADH. Q oxidoreductase, Q cytochrome c oxidoreductase, and cytochrome c oxidase. These large transmembrane complexes contain multiple oxidation reduction centers, including quinones, flavins, iron sulfur clusters, hemes, and copper ions. The final phase of oxidative phosphorylation is carried out by ATP synthase, an ATP synthesizing assembly that is driven by the flow of protons back into the mitochondrial matrix.

Oxidative phosphorylation brightly shows that proton gradients are an interconvertible currency of free energy in biological systems. Toxic derivatives of molecular oxygen such as superoxide radical are scavenge by protective enzymes Although cytochrome c oxidase and other proteins that reduce O 2 are remarkably successful in not releasing intermediates, small amounts of superoxide anion and hydrogen peroxide are unavoidably formed. Superoxide, hydrogen peroxide, and species that can be generated from them such as OH· are collectively referred to as reactive oxygen species or ROS.

What are the cellular defense strategies against oxidative damage by ROS? The enzyme superoxide dismutase. This enzyme scavenges superoxide radicals by catalyzing the conversion of two of these radicals into hydrogen peroxide and molecular oxygen. 2 O 2· + 2 H+ ↔ O 2 + H 2 O 2 Eukaryotes contain 2 forms of this enzyme, manganese containing version located in mitochondria and copper zinc dependent cytosolic form. a) The oxidized form of the enzyme is reduced by superoxide to form O 2.

b) The reduced form of the enzyme, formed in this reaction then reacts with a second superoxide ion to form peroxide, which takes 2 protons along the reaction path to yield hydrogen peroxide. The hydrogen peroxide formed by superoxide dismutase and by other processes is scavenged by catalase, a ubiquitous heme protein that catalyzes the dismutation of hydrogen peroxide into water and O 2. 2 H 2 O 2 ↔ O 2 + 2 H 2 O Superoxide dismutase and catalase are remarkable efficient, performing their reactions at or near the diffusion limited rate. Other cellular defenses against oxidative damage include the antioxidant vitamins, vitamins E and C.

Because it is lipophilic, vitamin E is especially useful in protecting membranes from lipid peroxidation. The importance of the cell’s defense against ROS is demonstrated by the presence of superoxide dismutase In all aerobic organisms. Oxidative damage is believed to cause, at least in part, a growing of diseases such as, Atherogenesis, Diabetes, Parkinson disease, acute renal failure and cervical cancer

Coordinated control of ATP production Glycolysis, the citric acid cycle, and oxidative phosphorylation compose the major pathways for cellular ATP production. Control of oxidative phosphorylation by the ATP mass action ratio [ATP] / [ADP][Pi] depends, of course on an adequate supply of electrons to fuel the electron transport chain. This aspect of the system’s control is, in turn, dependent on the [NADH] / [NAD+] ratio, which is maintained high by the combined action of glycolysis and the citric acid cycle in converting 10 molecules of NAD+ to NADH per molecule of glucose oxidized. Therefore, that coordinated control is necessary for the three processes.

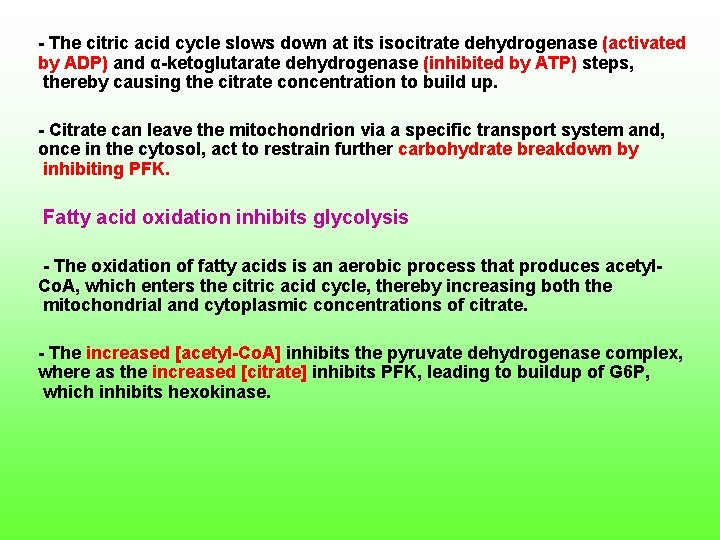
This is provided by the regulation of each of the control points of glycolysis [hexokinase, phosphofructokinase (PFK), and pyruvate kinase] and the citric acid cycle [ pyruvate dehydrogenase, citrate synthase, isocitrate dehydrogenase, and α ketoglutarate dehydrogenase] by adenine nuclotides or NADH or both as well as by certain metabolites. Citrate inhibits glycolysis The main control points of glycolysis and citric acid cycle are regulated by several effectors besides adenine nuclotides or NADH. One particularly interesting regulatory effect is the inhibition of PFK by citrate. When demand for ATP decreases, [ATP] increases and [ADP] decreases.

The citric acid cycle slows down at its isocitrate dehydrogenase (activated by ADP) and α ketoglutarate dehydrogenase (inhibited by ATP) steps, thereby causing the citrate concentration to build up. Citrate can leave the mitochondrion via a specific transport system and, once in the cytosol, act to restrain further carbohydrate breakdown by inhibiting PFK. Fatty acid oxidation inhibits glycolysis The oxidation of fatty acids is an aerobic process that produces acetyl Co. A, which enters the citric acid cycle, thereby increasing both the mitochondrial and cytoplasmic concentrations of citrate. The increased [acetyl Co. A] inhibits the pyruvate dehydrogenase complex, where as the increased [citrate] inhibits PFK, leading to buildup of G 6 P, which inhibits hexokinase.

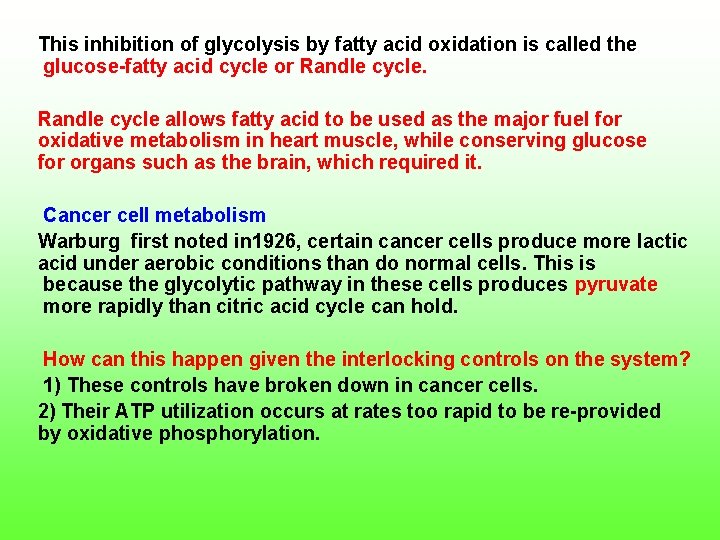
This inhibition of glycolysis by fatty acid oxidation is called the glucose fatty acid cycle or Randle cycle allows fatty acid to be used as the major fuel for oxidative metabolism in heart muscle, while conserving glucose for organs such as the brain, which required it. Cancer cell metabolism Warburg first noted in 1926, certain cancer cells produce more lactic acid under aerobic conditions than do normal cells. This is because the glycolytic pathway in these cells produces pyruvate more rapidly than citric acid cycle can hold. How can this happen given the interlocking controls on the system? 1) These controls have broken down in cancer cells. 2) Their ATP utilization occurs at rates too rapid to be re provided by oxidative phosphorylation.

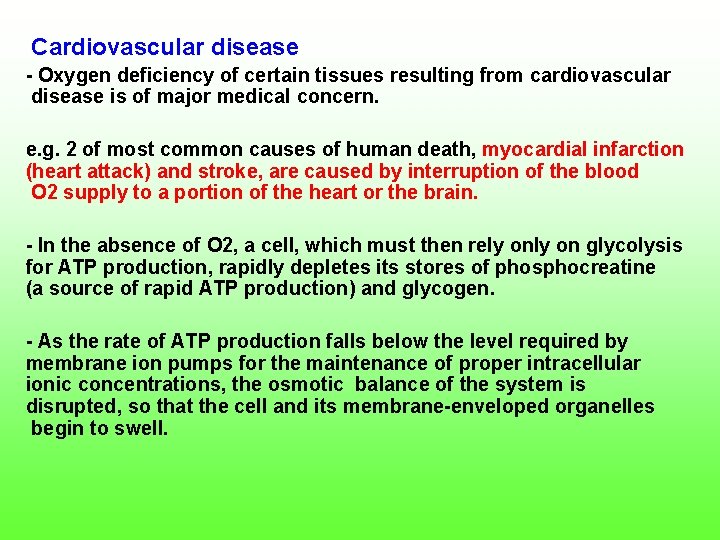
Cardiovascular disease Oxygen deficiency of certain tissues resulting from cardiovascular disease is of major medical concern. e. g. 2 of most common causes of human death, myocardial infarction (heart attack) and stroke, are caused by interruption of the blood O 2 supply to a portion of the heart or the brain. In the absence of O 2, a cell, which must then rely on glycolysis for ATP production, rapidly depletes its stores of phosphocreatine (a source of rapid ATP production) and glycogen. As the rate of ATP production falls below the level required by membrane ion pumps for the maintenance of proper intracellular ionic concentrations, the osmotic balance of the system is disrupted, so that the cell and its membrane enveloped organelles begin to swell.

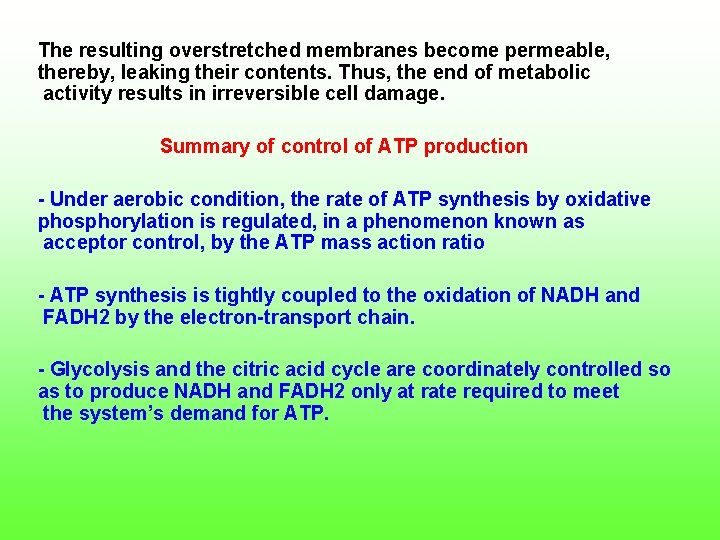
The resulting overstretched membranes become permeable, thereby, leaking their contents. Thus, the end of metabolic activity results in irreversible cell damage. Summary of control of ATP production Under aerobic condition, the rate of ATP synthesis by oxidative phosphorylation is regulated, in a phenomenon known as acceptor control, by the ATP mass action ratio ATP synthesis is tightly coupled to the oxidation of NADH and FADH 2 by the electron transport chain. Glycolysis and the citric acid cycle are coordinately controlled so as to produce NADH and FADH 2 only at rate required to meet the system’s demand for ATP.

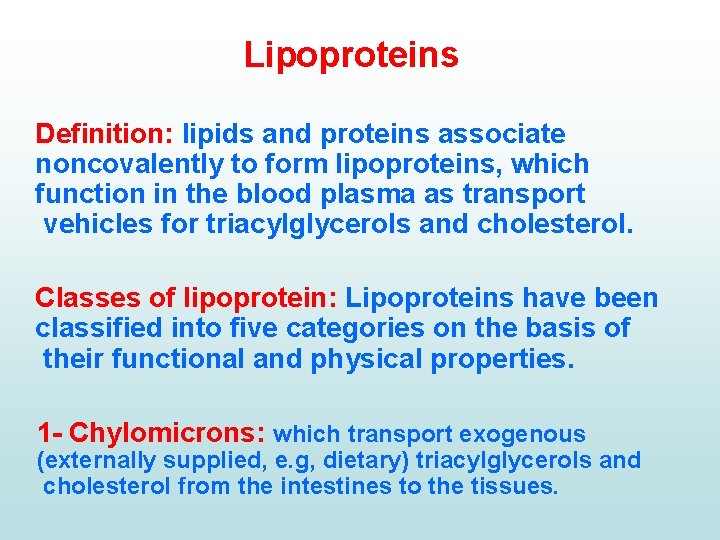
Lipoproteins Definition: lipids and proteins associate noncovalently to form lipoproteins, which function in the blood plasma as transport vehicles for triacylglycerols and cholesterol. Classes of lipoprotein: Lipoproteins have been classified into five categories on the basis of their functional and physical properties. 1 Chylomicrons: which transport exogenous (externally supplied, e. g, dietary) triacylglycerols and cholesterol from the intestines to the tissues.

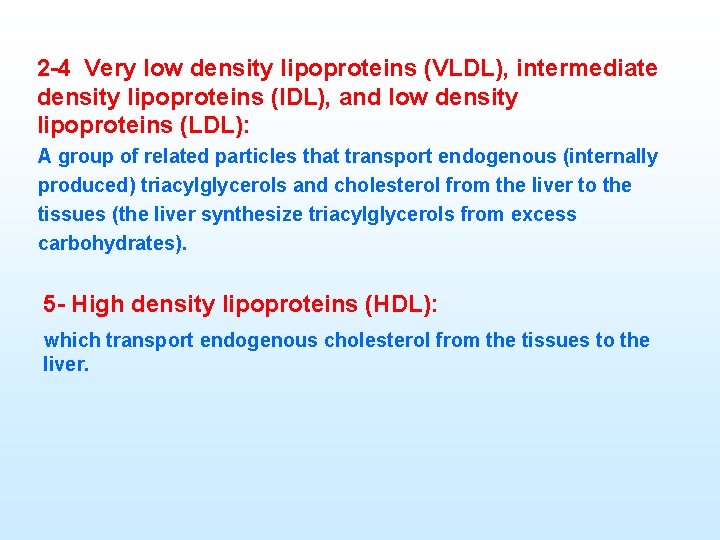
2 4 Very low density lipoproteins (VLDL), intermediate density lipoproteins (IDL), and low density lipoproteins (LDL): A group of related particles that transport endogenous (internally produced) triacylglycerols and cholesterol from the liver to the tissues (the liver synthesize triacylglycerols from excess carbohydrates). 5 High density lipoproteins (HDL): which transport endogenous cholesterol from the tissues to the liver.

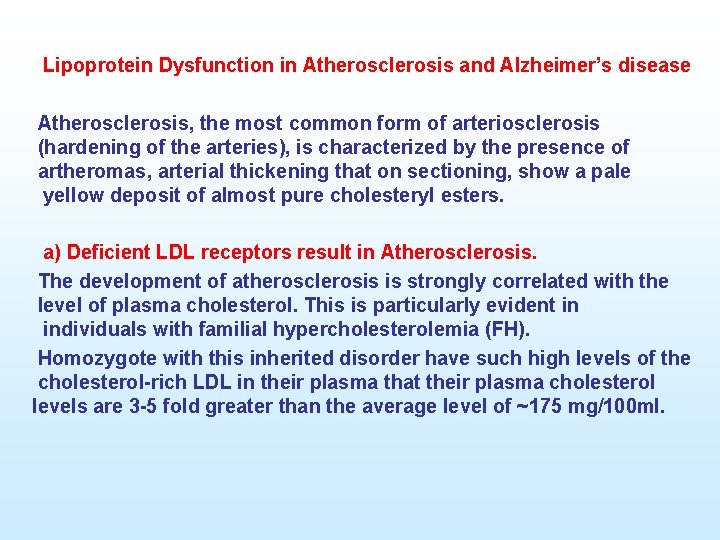
Lipoprotein Dysfunction in Atherosclerosis and Alzheimer’s disease Atherosclerosis, the most common form of arteriosclerosis (hardening of the arteries), is characterized by the presence of artheromas, arterial thickening that on sectioning, show a pale yellow deposit of almost pure cholesteryl esters. a) Deficient LDL receptors result in Atherosclerosis. The development of atherosclerosis is strongly correlated with the level of plasma cholesterol. This is particularly evident in individuals with familial hypercholesterolemia (FH). Homozygote with this inherited disorder have such high levels of the cholesterol rich LDL in their plasma that their plasma cholesterol levels are 3 5 fold greater than the average level of ~175 mg/100 ml.

However, fat greater damage is caused by the rapid formation of atheromas that, in homozygotes, cause death from myocardial infraction as early as the age of 5. Heterozygote, which comprise ~1 person in 500, are less severely cause problems; they develop symptoms of coronary artery disease after the age 30. Cells taken from FH homozygote completely lack functional LDLR, whereas those taken from heterozygote have about half of the normal balance. Homozygotes and to a less extent, heterozygotes are therefore unable to utilize the cholesterol in LDL. Rather, their cells must synthesize most of the cholesterol for their needs. The high level of plasma LDL in these individuals results from 2 causes:

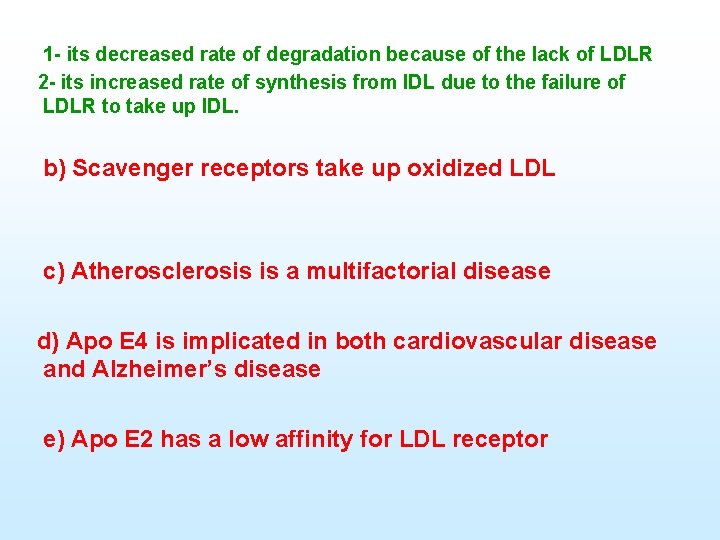
1 its decreased rate of degradation because of the lack of LDLR 2 its increased rate of synthesis from IDL due to the failure of LDLR to take up IDL. b) Scavenger receptors take up oxidized LDL c) Atherosclerosis is a multifactorial disease d) Apo E 4 is implicated in both cardiovascular disease and Alzheimer’s disease e) Apo E 2 has a low affinity for LDL receptor

Summary Lipoproteins: lipids are transported in the circulation by plasma lipoproteins. These are essentially droplets of triacylglycerols cholesteryl esters coated with a monolayer of phospholipids, cholesterol, and apolipoproteins. The amphiphilic apolipoprotein helices float on the lipoprotein surface in hydrophobic contact with its lipid interior. Chylomicrons and VLDL function to respectively transport triacylglycerols and cholesterol from the intestines and the liver to the tissues. HDL transports mainly cholesterol from the tissues to the liver, the only organ capable of disposing of significant quantities of cholesterol.

The triacylglycerols of chylomicrons and VLDL are degraded by lipoprotein lipase that lines the capillaries. LDL, the cholesterol containing degradation product of VLDL, binds to cell surface LDL receptors (LDLR) and is taken into cells by receptor mediated endocytosis. The presence of excess intracellular cholesterol inhibits the synthesis of both LDLR and cholesterol. A major cause of atherosclerosis is an excess of plasma LDL, a phenomenon that is particularly evident in individuals with familial hypercholesterolemia, who lack functional LDLRs. The excess LDL becomes oxidized and is taken up by the macrophages that inhibit atherosclerotic plaques via their scavenger receptors.

Atherosclerosis a multifactorial disease, is also correlated with a low concentration of HDL, which functions as a cholesterol scavenger. The apo. E 2 and apo. E 4 variants of apo. E are implicated in cardiovascular disease, whereas apo. E 4 is also implicated in Alzheimer’s disease.

Bile acids The quantitatively most important pathway for the excretion of cholesterol in mammals is the formation of bile salts (the conjugate bases of bile acids). The major bile salts, cholate, and chenodeoxycholate, are synthesized in the liver and secreted as glycine or taurine conjugates into the gallbladder. And then, they are secreted into the small intestine, where they act as emulsifying agents in the digestion and absorption of fats and fat soluble vitamins. An efficient recycling system allows the bile salts to reenter the bloodstream and return to the liver for reuse several times each day.

The < 1 g / day of bile salts that normally escape this recycling system are further metabolized by microorganisms in the large intestine and excreted. This is the body’s only route for cholesterol excretion.

Porphyrin and Porphyries Porphyrin Myoglobin and each of the four subunits of hemoglobin noncovalently bind a single heme group (haem in British English) Heme is responsible for the red color of blood and is the site at which each globin monomer binds 1 molecule of O 2 (globins are the heme free proteins of Hb and Mb). The hetero cyclic ring system of heme is a porphyrin derivative; it consists of four pyrrole rings linked by methene bridge.

The porphyrin in heme. With its particular arrangement of 4 methyl, 2 propionate, and 2 vinyl substituents, is known as protoporphyrin IX. Heme, then, is protoporphyrin IX with a centrally bound iron atom In Hb and Mb, the iron atom normally remains in the Fe (II) (ferrous) n state whether or not the heme is oxygenated (binds O 2). Cells which synthesize heme are predominantly erythroblasts/reticulocytes in the bone marrow (80%) and hepatocytes (20%)

Porphyries The porphyries are a group of metabolic disorders characterized by deficiencies in the activity of enzymes involved in the biosynthesis of heme. In erythropoietic protoporphyria (EPP), in the majority of cases an autosomal dominant disease, there is a mutation of the gene that encodes ferrochelatase (FECH). FECH deficiency is associated with increased concentrations of protoporphyrin in erythrocytes, plasma, skin and liver. Retention of protoporphyrin in skin predisposes to acute photosensitivity.

As a result of absorption of ultraviolet light (400 nm) by protoporphyrin in plasma and erythrocytes when blood circulates through the dermal vessels, free radicals are formed, erythrocytes become unstable and injury to the skin is induced. A significant increase in the hepatobiliary excretion on protoporphyrin can damage the liver through both cholestatic phenomena and oxidative stress predisposing to hepatobiliary disease of varying degrees of severity.

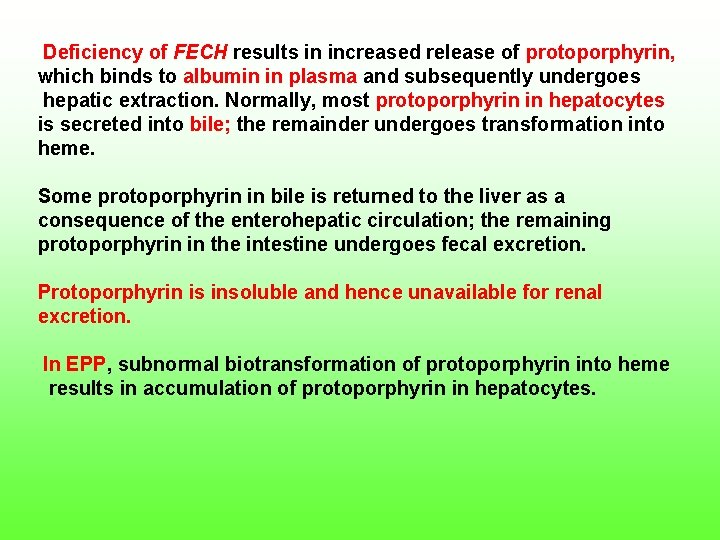
Deficiency of FECH results in increased release of protoporphyrin, which binds to albumin in plasma and subsequently undergoes hepatic extraction. Normally, most protoporphyrin in hepatocytes is secreted into bile; the remainder undergoes transformation into heme. Some protoporphyrin in bile is returned to the liver as a consequence of the enterohepatic circulation; the remaining protoporphyrin in the intestine undergoes fecal excretion. Protoporphyrin is insoluble and hence unavailable for renal excretion. In EPP, subnormal biotransformation of protoporphyrin into heme results in accumulation of protoporphyrin in hepatocytes.

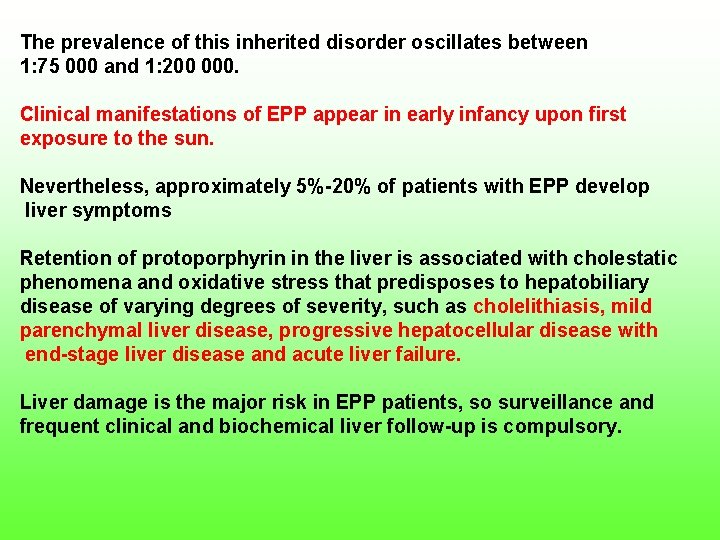
The prevalence of this inherited disorder oscillates between 1: 75 000 and 1: 200 000. Clinical manifestations of EPP appear in early infancy upon first exposure to the sun. Nevertheless, approximately 5% 20% of patients with EPP develop liver symptoms Retention of protoporphyrin in the liver is associated with cholestatic phenomena and oxidative stress that predisposes to hepatobiliary disease of varying degrees of severity, such as cholelithiasis, mild parenchymal liver disease, progressive hepatocellular disease with end stage liver disease and acute liver failure. Liver damage is the major risk in EPP patients, so surveillance and frequent clinical and biochemical liver follow up is compulsory.

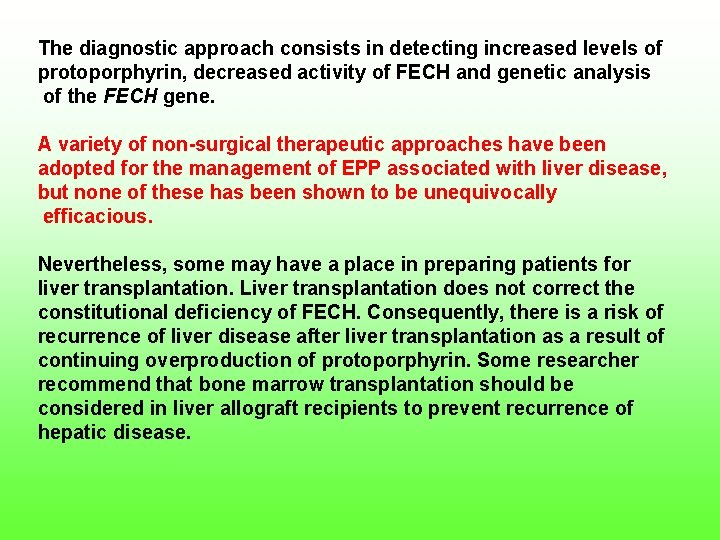
The diagnostic approach consists in detecting increased levels of protoporphyrin, decreased activity of FECH and genetic analysis of the FECH gene. A variety of non surgical therapeutic approaches have been adopted for the management of EPP associated with liver disease, but none of these has been shown to be unequivocally efficacious. Nevertheless, some may have a place in preparing patients for liver transplantation. Liver transplantation does not correct the constitutional deficiency of FECH. Consequently, there is a risk of recurrence of liver disease after liver transplantation as a result of continuing overproduction of protoporphyrin. Some researcher recommend that bone marrow transplantation should be considered in liver allograft recipients to prevent recurrence of hepatic disease.

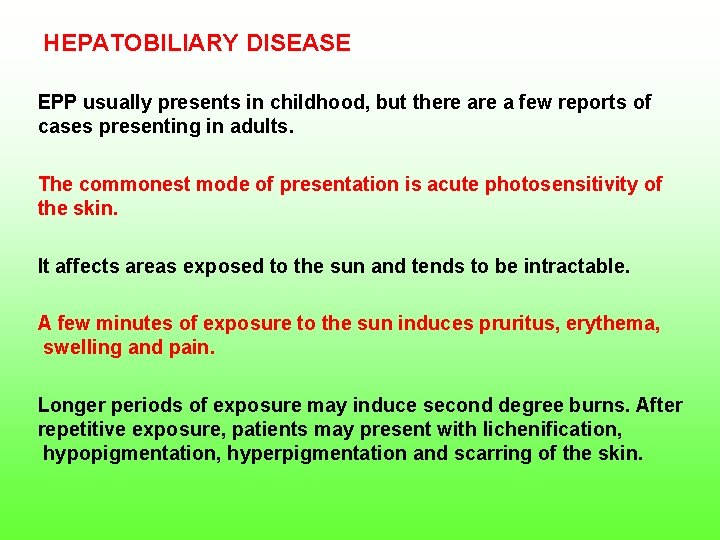
HEPATOBILIARY DISEASE EPP usually presents in childhood, but there a few reports of cases presenting in adults. The commonest mode of presentation is acute photosensitivity of the skin. It affects areas exposed to the sun and tends to be intractable. A few minutes of exposure to the sun induces pruritus, erythema, swelling and pain. Longer periods of exposure may induce second degree burns. After repetitive exposure, patients may present with lichenification, hypopigmentation, hyperpigmentation and scarring of the skin.

Clinical findings suggestive of liver disease appear in approximately 5% 20% of patients. The susceptibility of individual patients with EPP to protoporphyrin induced liver damage is highly variable. The host factors responsible for this variable susceptibility are unknown. The spectrum of hepatobiliary disease associated with EPP is wide. It includes cholelithiasis, mild parenchymal liver disease, progressive hepatocellular disease and end stage liver disease

Pathophysiology Liver damage in EPP has been attributed to precipitation of insoluble protoporphyrin in bile canaliculi and to protoporphyrin-induced oxidative stress. The latter arises as a consequence of excess unmetabolized protoporphyrin interacting with the hepatocellular membrane and inducing impaired function of the Na+/K+ -ATPase pump within the membrane. The accumulation of excess protoporphyrin, that does not undergo biliary excretion, exacerbates cholestasis and further reduces the excretion of protoporphyrin. These pathophysiological phenomena may result in hepatic inflammation, progressive hepatocellular disease, hepatic fibrosis, and, eventually, cirrhosis.

Clinical manifestations Cholelithiasis: Insoluble protoporphyrin in bile may act as nuclei for stone formation. Cholelithiasis is frequent in EPP (10%-20%), due to the accumulation of free protoporphyrin and increased biliary protoporphyrin concentration. Clinical manifestations of cholelithiasis and choledocolithiasis are similar to lithiasis by cholesterol or bilirubin. Many patients with EPP and gallstones undergo cholecystectomy. Subsequent analysis of the stones reveals that they are birefringent and contain high concentrations of protoporphyrin. Some authors believe that EPP should be suspected when cholelithiasis presents in childhood.

Mild parenchymal liver disease: Most patients (20%) present with a mild liver disease, characterized by increased levels of aminotransferases and/or cholestatic enzymes. Typically, in patients with mild disease, there are no symptoms. Patients can also present with splenomegaly and hepatomegaly. Liver biopsy in such patients may reveal features of appreciable hepatocellular injury.

Progressive hepatocellular disease: Symptoms include upper abdominal pain and jaundice. There may be associated rapid deterioration of photosensitivity because of decreased secretion of protoporphyrin into bile, secondary to cholestasis and hemolysis. It is rare for the initial presentation of EPP to be manifestations of progressive hepatocellular disease. When jaundice is clinically evident, hepatocellular disease is advanced and hepatic clearance function is appreciably reduced. Blood protoporphyrin levels increase further, but fecal protoporphyrin excretion decreases.

End stage liver disease: Only 5% of liver damage presents as an acute liver insufficiency. Progressive hepatocellular disease ultimately leads to cholestatic hepatocellular failure, which often has an acute onset, and a rapidly progressive, irreversible course. Progressive hepatocellular disease in EPP is usually fatal within months if liver transplantation is not undertaken.

There is no consensus regarding optimal surveillance for patients with EPP. Liver biopsy is the gold standard to assess the degree of hepatic damage. Results of noninvasive methods, such as serum biochemical liver tests, do not correlate closely with the degree of hepatic injury. In a review, Anstey and Hift[11] proposed the following indications for liver biopsy in patients with EPP: (1) Presence of null mutations or autosomal recessive disease; (2) Family history of EPP-related liver disease; (3) Presence of risk factors for the development of liver disease, such as markers of viral hepatitis, factors suggestive of non-alcoholic fatty liver disease, and alcohol abuse; (4) Abnormal results of serum biochemical liver tests; (5) Evidence of hepatocellular decompensation; and (6) To relieve a patient’s anxiety or to comply with a patient’s preference.

The optimal frequency of blood tests to monitor patients with EPP has not been established. Some authors advocate serum biochemical liver tests every 6 mo; others prefer to have these tests done annually up to the age of 20 years and then biennially

Treatment approaches A variety of non-surgical therapeutic approaches have been adopted for the management of EPP associated with progressive hepatocellular disease. However, none of these has been shown to be unequivocally efficacious. Nevertheless, some may have a place in preparing patients for liver transplantation. The pathophysiology of this disease suggests several potential therapeutic targets. Such targets include attempts to induce bile flow, to render bile less toxic, to reduce protoporphyrin production in the bone marrow, to reduce the circulating pool of protoporphyrin, to promote hepatocellular metabolism and transport of protoporphyrin, to protect hepatocytes from toxic damage, and to interrupt the enterohepatic circulation.

Ursodeoxycholic acid: This bile acid is administered to promote biliary secretion of protoporphyrin. Results of its use in EPP are controversial. However, it is known to alter the composition of bile, to protect hepatocytes from the cytotoxic effect of hydrophobic bile acids, and to stimulate biliary secretion by several distinct mechanisms.

Parenteral iron and transfusion of erythrocytes: The objective of administering iron and/or erythrocytes is to suppress erythropoiesis and, hence, reduce the protoporphyrin level. In theory, iron therapy should not work since it stimulates heme synthesis via 5 aminolevulinate synthase. However, results of this approach in patients with EPP are contradictory. It has been reported that iron therapy may exacerbate hepatic dysfunction, whereas in some case reports the correction of iron deficiency has improved EPP. Nevertheless, the mechanism of this favorable response to iron therapy remains unknown, so more studies with a significant number of patients are necessary to clarify the role of iron therapy in the medical treatment of EPP. Because of the lack of definitive clinical data, the contradictory reports and theoretical possibility of exacerbating hepatic dysfunction, the decision to use this therapy should be individualized.

Infusions of hematin: Hematin appears to reduce excess protoporphyrin production in the bone marrow. It has been administered to patients with EPP (3 -4 mg/kg iv) who develop a crisis after liver transplantation. Plasmapheresis: Circulating levels of protoporphyrin can be decreased by plasma exchange. Extracorporeal albumin dialysis: This type of dialysis is used to decrease circulating levels of albumin-bound toxins. Accordingly, in patients with EPP, damage to the hepatobiliary system may be reduced by using this approach to reduce plasma concentrations of protoporphyrin. It may also reduce levels of protoporphyrin in erythrocytes, when protoporphyrin subsequently diffuses out of these cells into the plasma. A greater reduction in erythrocyte protoporphyrin levels following treatment with a molecular adsorbent recirculating system (9. 1%) than following plasmapheresis (0. 8%) or treatment with the Prometheus system (5. 9%) has been documented in a case study of a patient with EPP and liver disease.

Cholestyramine: This orally administered resin reduces circulating levels of protoporphyrin by binding to protoporphyrin in the intestine and, hence, interrupting the enterohepatic circulation. It is usually used in combination with other treatment approaches. Activated charcoal: Like cholestyramine, activated charcoal also binds to protoporphyrin in the intestine and prevents its absorption. It is cheap and readily available. It seems to be effective in reducing circulating protoporphyrin levels. N acetyl cysteine: In liver diseases free radicals are increased, thereby damaging the hepatic tissue[58]. In addition, nitric oxide has deleterious effects in the presence of reactive oxygen species, participating in the pathophysiology of different liver diseases. N-acetyl cysteine (NAC) modulates the expression of inducible oxide synthase in hepatocytes[61], and this action could be effective in the attenuation of oxidative and nitrosative stress in liver injury.

Based on these findings, it is suggested that antioxidant therapy might be beneficial in the treatment of liver damage of different etiologies. There is little experience with NAC in EPP, only clinical reports such a 32 -year-old man with EPP who developed progressive hepatocellular disease and was treated with NAC 300 mg/kg body weight per day Ⅳ infusion for 3 wk. This treatment was associated with an improvement in hepatocellular function, in particular, serum levels of hepatic enzymes normalized.

Bone marrow transplantation: The purpose of this approach is to remove the tissue primarily responsible for the overproduction of protoporphyrin. It is a frequently discussed option, but the incidence of associated adverse events has limited its use as a treatment for EPP. However, some authors advocate bone marrow transplantation as a complementary treatment in an attempt to avoid liver re transplantation in patients who have undergone liver transplantation for EPP associated liver disease.

Liver transplantation: More than 40 patients with EPP and hepatocellular disease, who have undergone liver transplantation, have been reported in the world literature[12]. Liver transplantation does not correct the constitutional deficiency of FECH. Consequently, there is a risk of recurrence of liver disease after liver transplantation as a result of the continuing overproduction of protoporphyrin. A review of 20 transplanted cases of EPP in the USA led the authors to recommend that bone marrow transplantation should be considered in liver allograft recipients to prevent recurrence of hepatic disease[45]. Patients with EPP are prone to certain perioperative complications of liver transplantation. Management involves adopting appropriate precautions or treatment options.

Phototoxic abdominal burns may be induced; the mechanism is analogous to that responsible for skin photosensitivity. Such burns can be avoided by fitting filters to lamps in the operating theater. Acute neuropathy is a major complication. It is associated with severe abdominal pain, an acute deterioration in hepatocellular function, and an increase in erythrocyte protoporphyrin levels. Treatment options include hematin infusions and/or plasmapheresis. Acute protoporphyrin mediated damage to the liver allograft may occur secondary to high circulating levels of protoporphyrin at the time of transplantation. This complication can be prevented by taking short term measures to reduce levels of protoporphyrin at the time of surgery[54, 65]: (1) Cholecystectomy; (2) Vaccines against viral hepatitis; and (3)Avoid hepatotoxic drugs. Avoid drugs that might develop a drug induced liver injury.

CONCLUSION In EPP there is a mutation of the gene that encodes FECH, the enzyme that catalyzes the insertion of ferrous iron into the protoporphyrin ring to form heme. EPP presents both recessive and dominant patterns of inheritance. Recently, a new pattern of EPP has been described related to gain of function mutations in the aminolevulinic acid synthase 2 gene. FECH deficiency is associated with increased concentrations of protoporphyrin in erythrocytes, plasma, skin and liver. A significant increase in the hepatobiliary excretion of protoporphyrin can damage the liver through both cholestatic phenomena and oxidative stress predisposing to a wide spectrum of hepatobiliary disease of varying degrees of severity that includes cholelithiasis, mild parenchymal liver disease, progressive hepatocellular disease and end stage liver disease.

The susceptibility of individual patients with EPP to protoporphyrin induced liver damage is highly variable. There is no consensus regarding optimal surveillance for patients with EPP. Liver biopsy is the gold standard to assess the degree of hepatic damage. A variety of non surgical therapeutic approaches have been adopted for the management of EPP associated with progressive liver disease, but none of these has been shown to be unequivocally efficacious. Liver transplantation does not correct the constitutional deficiency of FECH and, as a result of the continuing overproduction of protoporphyrin, there is a risk of recurrence of liver disease after liver transplantation.








 Tca cycle meaning
Tca cycle meaning Definition of citric acid cycle
Definition of citric acid cycle Types of organisms
Types of organisms How is citric acid cycle regulated
How is citric acid cycle regulated Citric acid cycle overall reaction
Citric acid cycle overall reaction Inputs of krebs cycle
Inputs of krebs cycle Oxaloacetate
Oxaloacetate Where does the citric acid cycle occur
Where does the citric acid cycle occur Oxidative deamination of glutamate
Oxidative deamination of glutamate Citrate synthase mechanism
Citrate synthase mechanism Citric acid cycle overall reaction
Citric acid cycle overall reaction Anaplerosis
Anaplerosis Citric acid cycle
Citric acid cycle Pathway of citric acid cycle
Pathway of citric acid cycle Biological significance of atp
Biological significance of atp Photosynthesis citric acid cycle
Photosynthesis citric acid cycle Citric acid cycle and electron transport chain
Citric acid cycle and electron transport chain Photosynthesis citric acid cycle
Photosynthesis citric acid cycle Oxidation of citric acid
Oxidation of citric acid Chemistry
Chemistry Citric acid discovery
Citric acid discovery Oxidation of citric acid
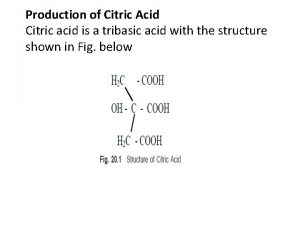
Oxidation of citric acid Citric acid formula
Citric acid formula Empirical formula of citric acid
Empirical formula of citric acid Citric acid
Citric acid Citric acid in water
Citric acid in water Chemical property of water
Chemical property of water Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Thang điểm glasgow
Thang điểm glasgow Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên tố là
Số nguyên tố là Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi 9-which acid is not considered a strong acid?
9-which acid is not considered a strong acid?