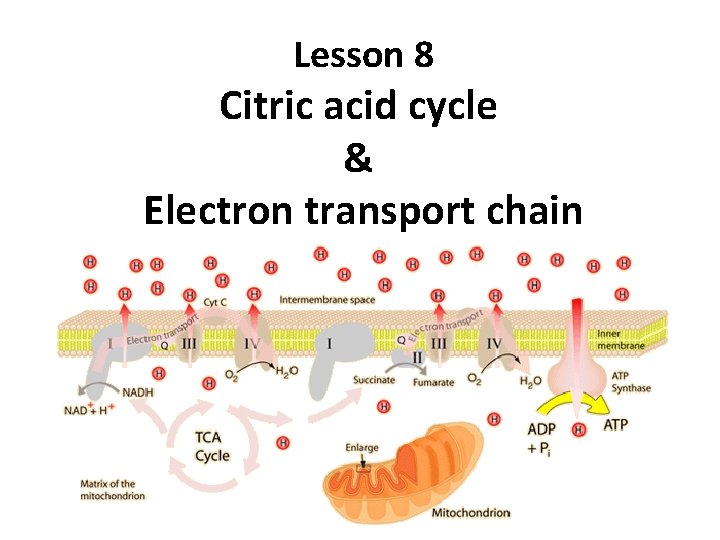
Lesson 8 Citric acid cycle Electron transport chain

- Slides: 46

Lesson 8 Citric acid cycle & Electron transport chain

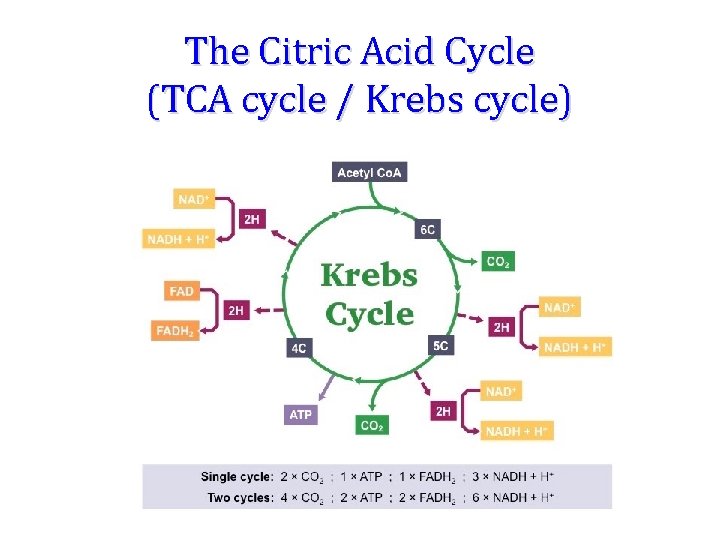
The Citric Acid Cycle (TCA cycle / Krebs cycle)

Introduction ü Also called citric acid cycle or the Krebs cycle (after its discoverer, Hans Krebs). ü TCA cycle is a series of reactions catalyzed by different enzymes in which acetyl Co. A is oxidized into CO 2, H 2 O and energy. ü It occurs in the mitochondrial matrix aerobically. ü The enzymes involved in the TCA cycle are present in the mitochondrial matrix either free or attached to the inner surface of the mitochondrial membrane.

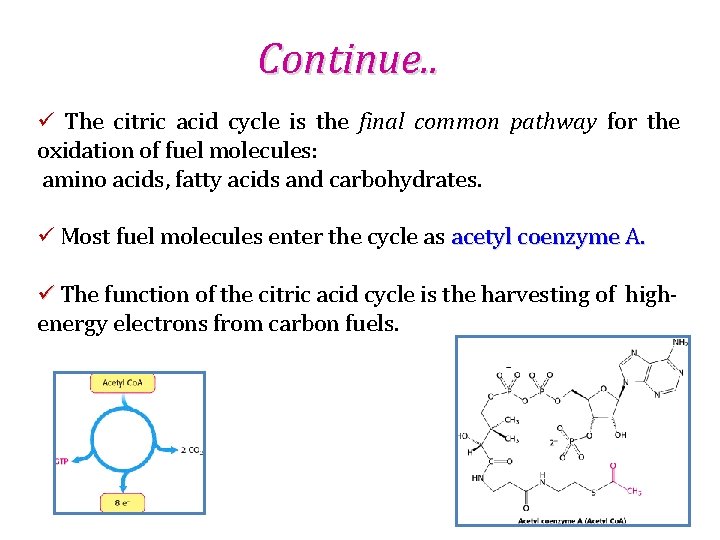
Continue. . ü The citric acid cycle is the final common pathway for the oxidation of fuel molecules: amino acids, fatty acids and carbohydrates. ü Most fuel molecules enter the cycle as acetyl coenzyme A. ü The function of the citric acid cycle is the harvesting of high- energy electrons from carbon fuels.

Continue. . ü The citric acid cycle itself neither generates a large amount of ATP nor includes oxygen as a reactant. ü Instead, the citric acid cycle removes electrons from acetyl Co. A and uses these electrons to form NADH and FADH 2. ü The citric acid cycle includes a series of oxidation-reduction reactions that result in the oxidation of an acetyl group to two molecules of carbon dioxide. The citric acid cycle oxidizes two-carbon units, producing two molecules of CO 2, one molecule of GTP, and high-energy electrons in the form of NADH and FADH 2.

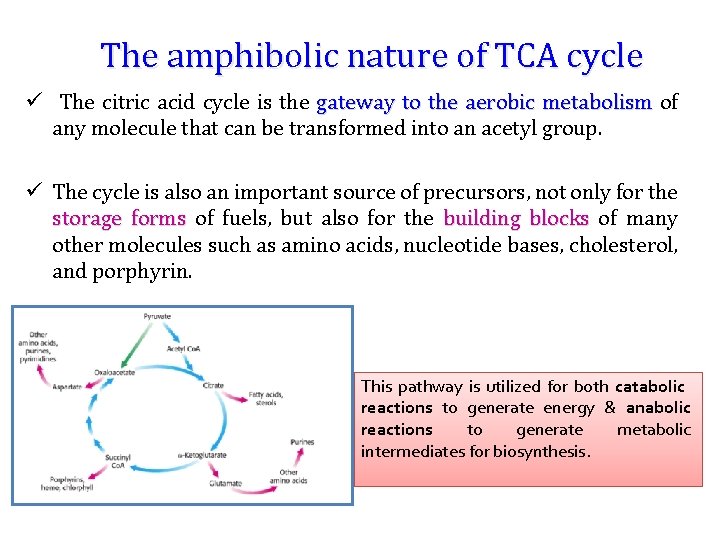
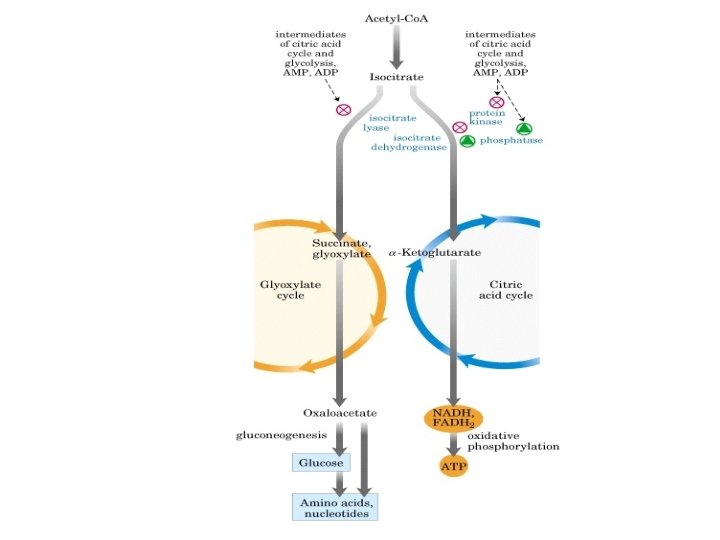
The amphibolic nature of TCA cycle ü The citric acid cycle is the gateway to the aerobic metabolism of any molecule that can be transformed into an acetyl group. ü The cycle is also an important source of precursors, not only for the storage forms of fuels, but also for the building blocks of many other molecules such as amino acids, nucleotide bases, cholesterol, and porphyrin. This pathway is utilized for both catabolic reactions to generate energy & anabolic reactions to generate metabolic intermediates for biosynthesis.

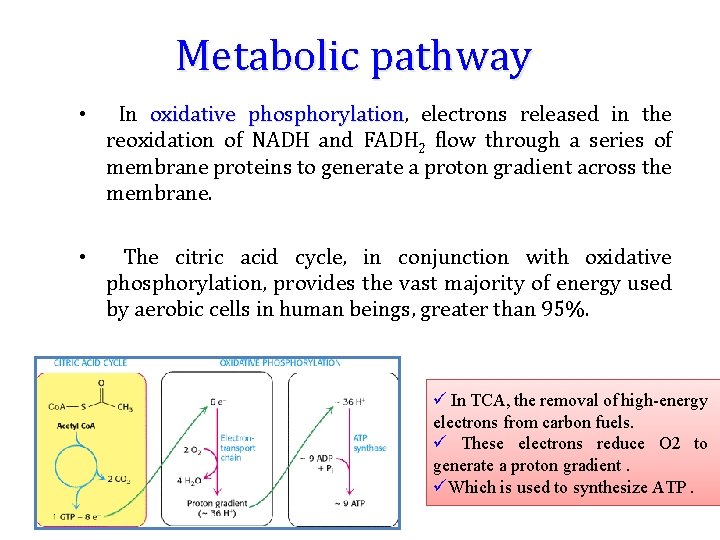
Metabolic pathway • In oxidative phosphorylation, phosphorylation electrons released in the reoxidation of NADH and FADH 2 flow through a series of membrane proteins to generate a proton gradient across the membrane. • The citric acid cycle, in conjunction with oxidative phosphorylation, provides the vast majority of energy used by aerobic cells in human beings, greater than 95%. ü In TCA, the removal of high-energy electrons from carbon fuels. ü These electrons reduce O 2 to generate a proton gradient. üWhich is used to synthesize ATP.

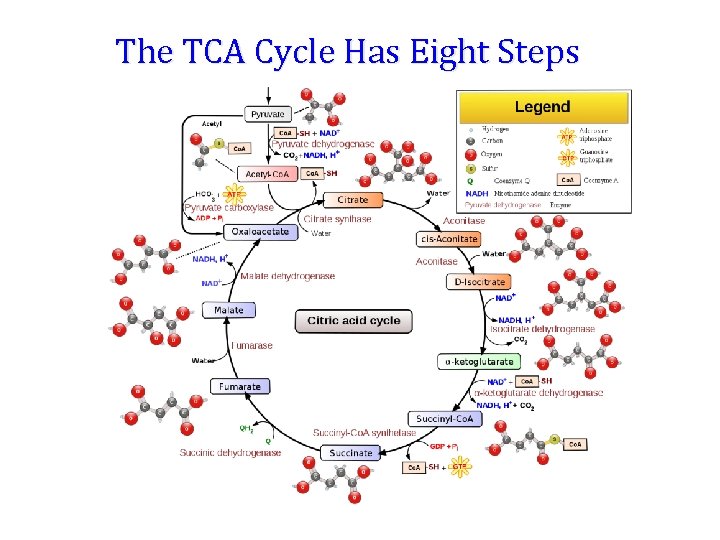
The TCA Cycle Has Eight Steps

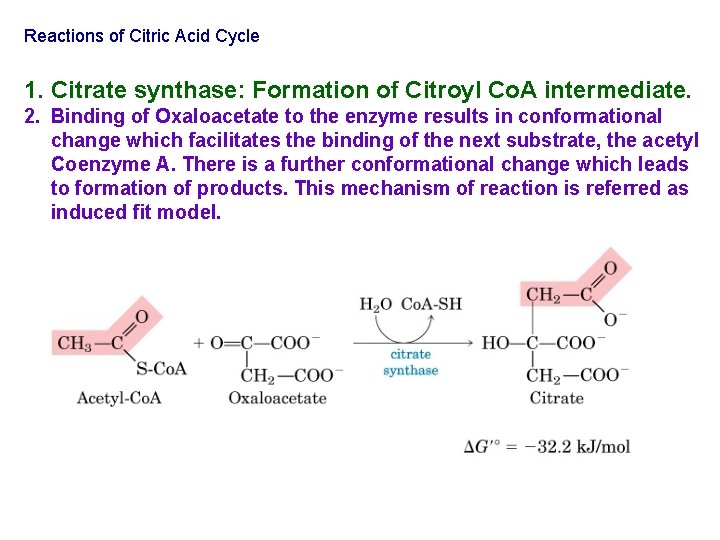
Reactions of Citric Acid Cycle 1. Citrate synthase: Formation of Citroyl Co. A intermediate. 2. Binding of Oxaloacetate to the enzyme results in conformational change which facilitates the binding of the next substrate, the acetyl Coenzyme A. There is a further conformational change which leads to formation of products. This mechanism of reaction is referred as induced fit model.

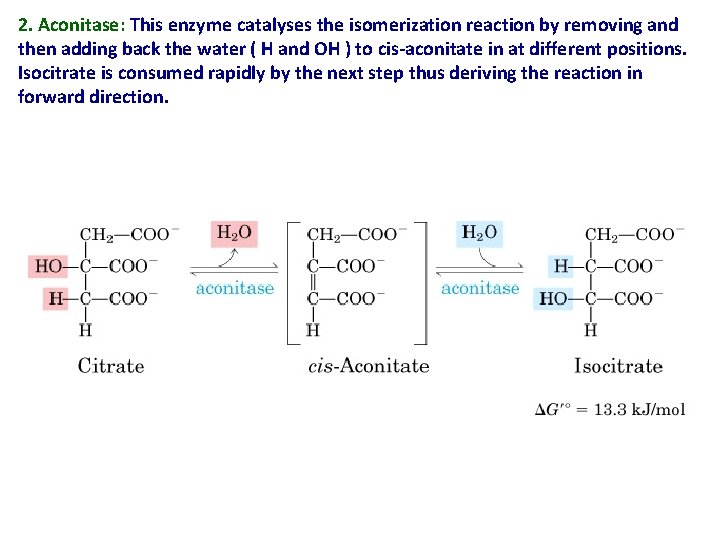
2. Aconitase: This enzyme catalyses the isomerization reaction by removing and then adding back the water ( H and OH ) to cis-aconitate in at different positions. Isocitrate is consumed rapidly by the next step thus deriving the reaction in forward direction.

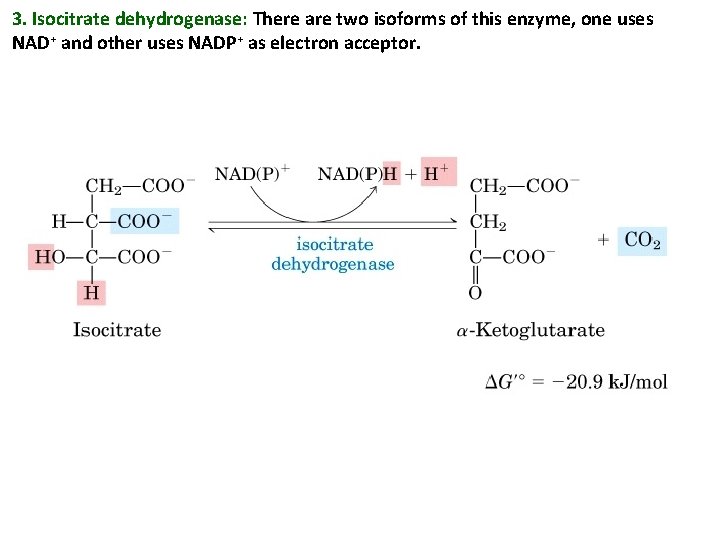
3. Isocitrate dehydrogenase: There are two isoforms of this enzyme, one uses NAD+ and other uses NADP+ as electron acceptor.

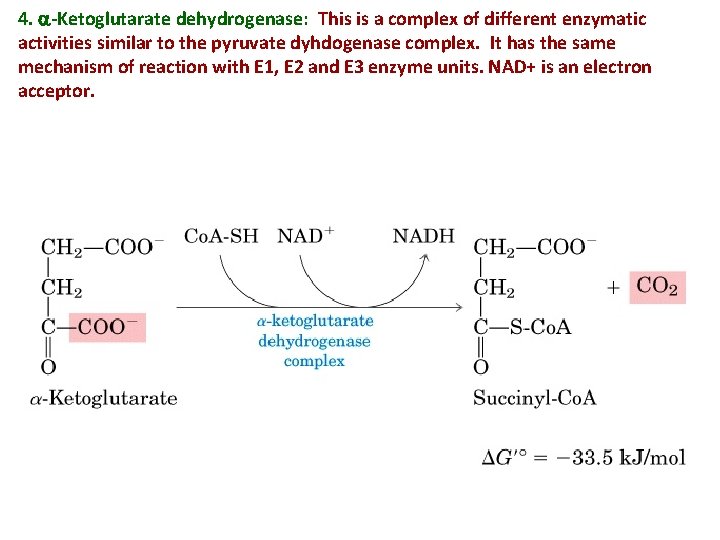
4. a-Ketoglutarate dehydrogenase: This is a complex of different enzymatic activities similar to the pyruvate dyhdogenase complex. It has the same mechanism of reaction with E 1, E 2 and E 3 enzyme units. NAD+ is an electron acceptor.

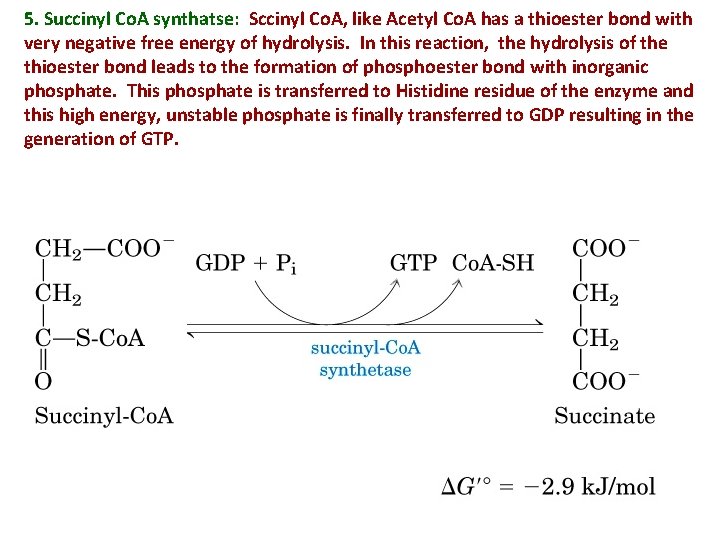
5. Succinyl Co. A synthatse: Sccinyl Co. A, like Acetyl Co. A has a thioester bond with very negative free energy of hydrolysis. In this reaction, the hydrolysis of the thioester bond leads to the formation of phosphoester bond with inorganic phosphate. This phosphate is transferred to Histidine residue of the enzyme and this high energy, unstable phosphate is finally transferred to GDP resulting in the generation of GTP.

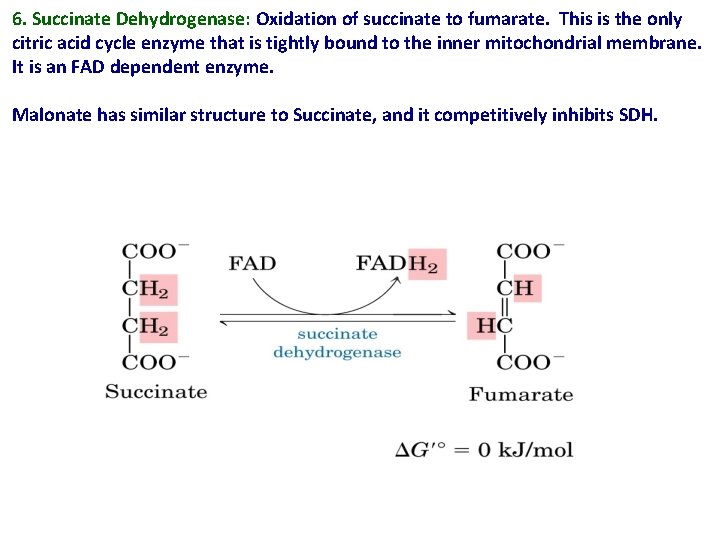
6. Succinate Dehydrogenase: Oxidation of succinate to fumarate. This is the only citric acid cycle enzyme that is tightly bound to the inner mitochondrial membrane. It is an FAD dependent enzyme. Malonate has similar structure to Succinate, and it competitively inhibits SDH.

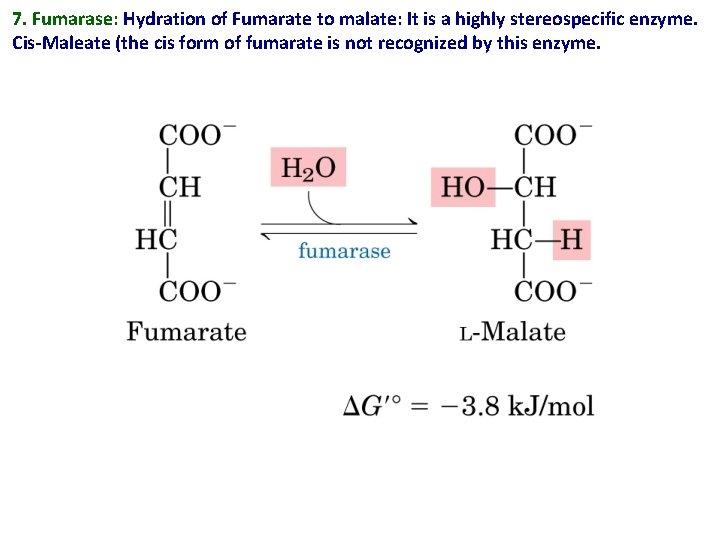
7. Fumarase: Hydration of Fumarate to malate: It is a highly stereospecific enzyme. Cis-Maleate (the cis form of fumarate is not recognized by this enzyme.

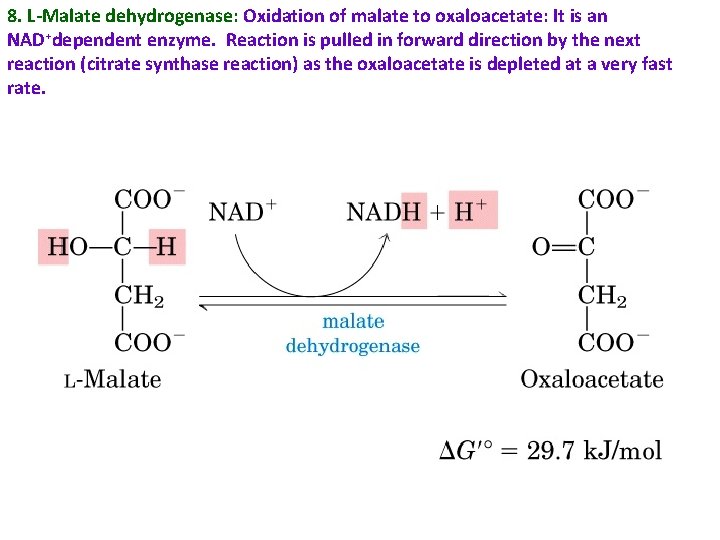
8. L-Malate dehydrogenase: Oxidation of malate to oxaloacetate: It is an NAD+dependent enzyme. Reaction is pulled in forward direction by the next reaction (citrate synthase reaction) as the oxaloacetate is depleted at a very fast rate.

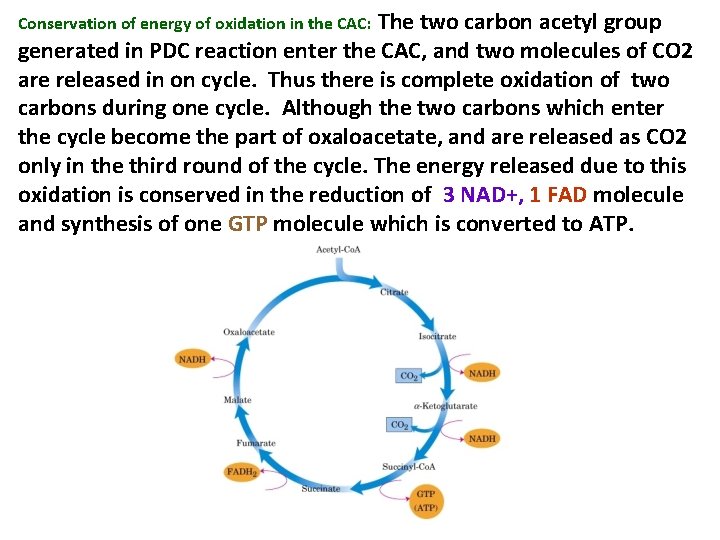
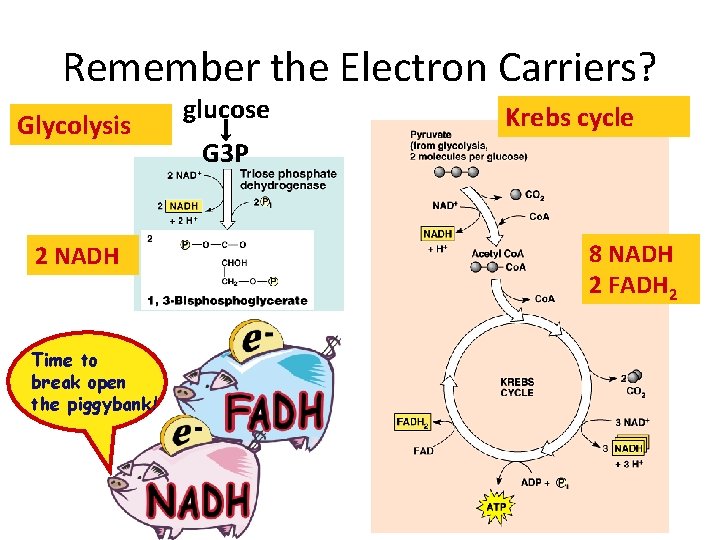
The two carbon acetyl group generated in PDC reaction enter the CAC, and two molecules of CO 2 are released in on cycle. Thus there is complete oxidation of two carbons during one cycle. Although the two carbons which enter the cycle become the part of oxaloacetate, and are released as CO 2 only in the third round of the cycle. The energy released due to this oxidation is conserved in the reduction of 3 NAD+, 1 FAD molecule and synthesis of one GTP molecule which is converted to ATP. Conservation of energy of oxidation in the CAC:


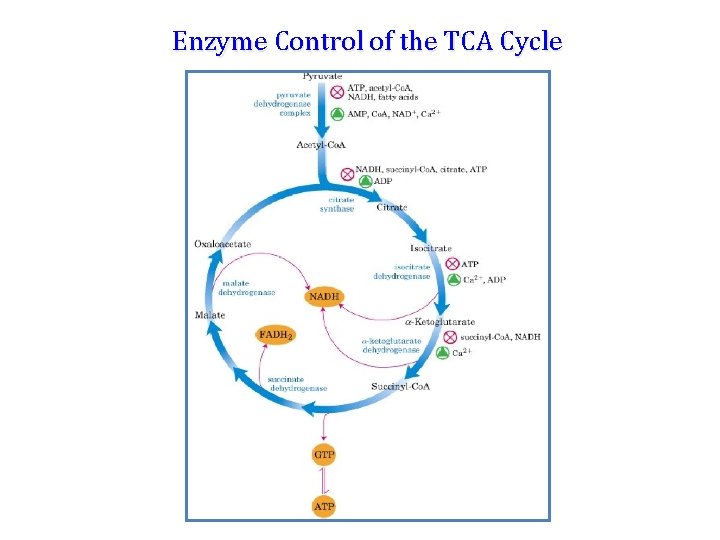
Enzyme Control of the TCA Cycle

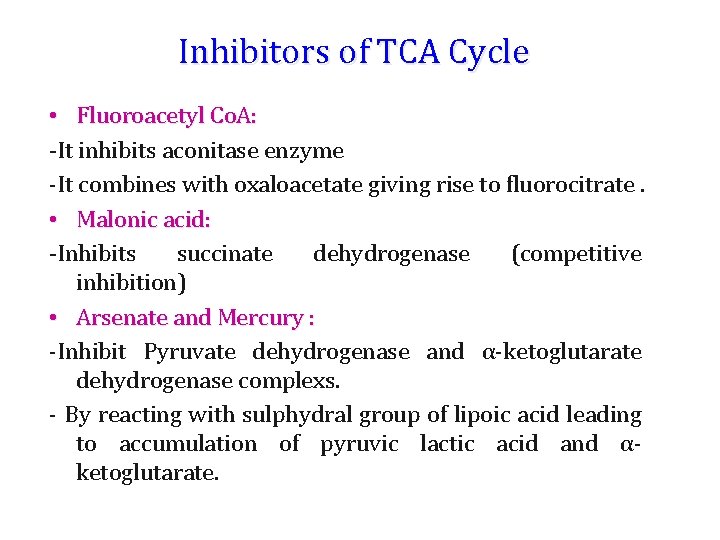
Inhibitors of TCA Cycle • Fluoroacetyl Co. A: -It inhibits aconitase enzyme -It combines with oxaloacetate giving rise to fluorocitrate. • Malonic acid: -Inhibits succinate dehydrogenase (competitive inhibition) • Arsenate and Mercury : -Inhibit Pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexs. - By reacting with sulphydral group of lipoic acid leading to accumulation of pyruvic lactic acid and αketoglutarate.

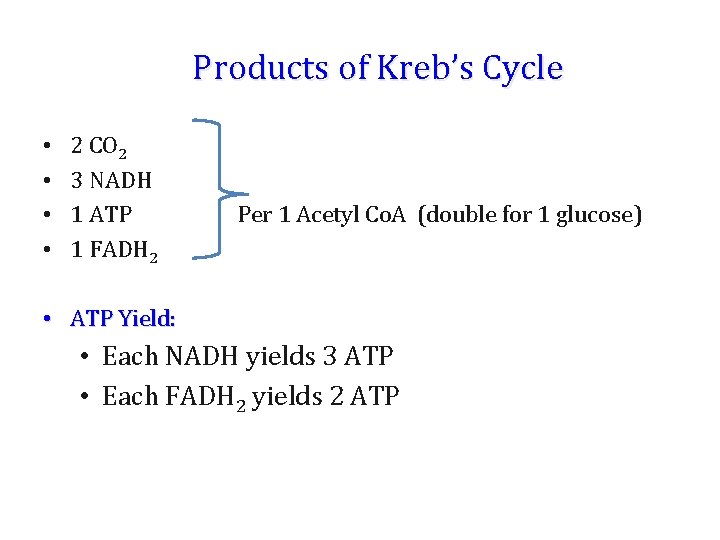
Products of Kreb’s Cycle • • 2 CO 2 3 NADH 1 ATP 1 FADH 2 Per 1 Acetyl Co. A (double for 1 glucose) • ATP Yield: • Each NADH yields 3 ATP • Each FADH 2 yields 2 ATP

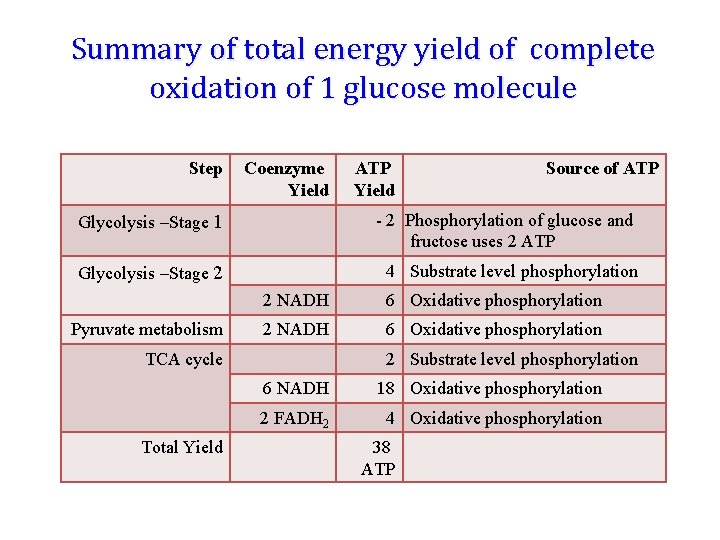
Summary of total energy yield of complete oxidation of 1 glucose molecule Step Coenzyme Yield ATP Yield Source of ATP Glycolysis –Stage 1 - 2 Phosphorylation of glucose and fructose uses 2 ATP Glycolysis –Stage 2 4 Substrate level phosphorylation Pyruvate metabolism 2 NADH 6 Oxidative phosphorylation TCA cycle Total Yield 2 Substrate level phosphorylation 6 NADH 18 Oxidative phosphorylation 2 FADH 2 4 Oxidative phosphorylation 38 ATP

Cellular Respiration Stage 4: Electron Transport Chain

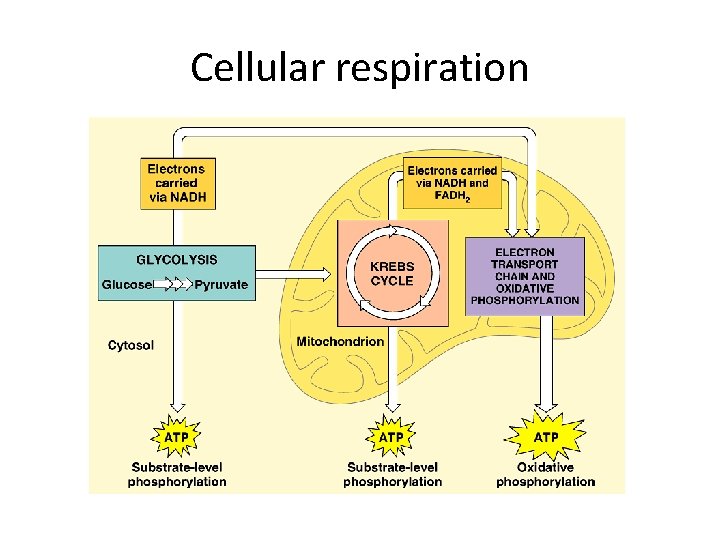
Cellular respiration

What’s the point? The point is to make ATP! ATP 2006 -2007

ATP accounting so far… • Glycolysis 2 ATP • Kreb’s cycle 2 ATP • Life takes a lot of energy to run, need to extract more energy than 4 ATP! There’s got to be a better way! I need a lot more ATP! A working muscle recycles over 10 million ATPs per second

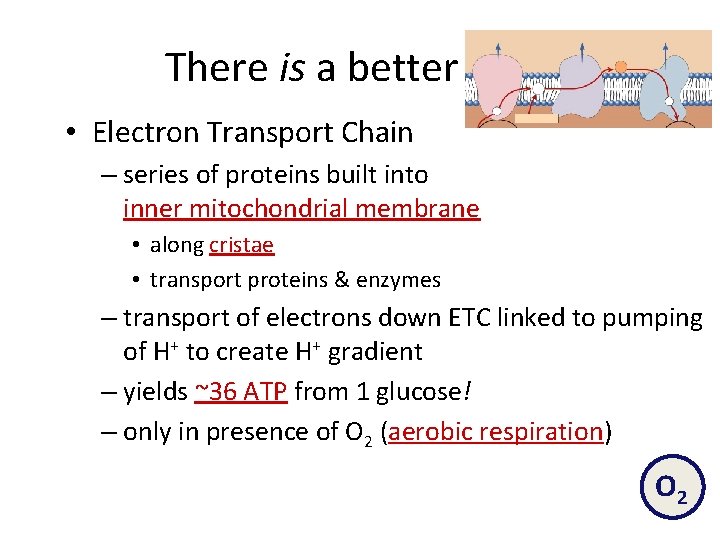
There is a better way! • Electron Transport Chain – series of proteins built into inner mitochondrial membrane • along cristae • transport proteins & enzymes – transport of electrons down ETC linked to pumping of H+ to create H+ gradient – yields ~36 ATP from 1 glucose! – only in presence of O 2 (aerobic respiration) O 2

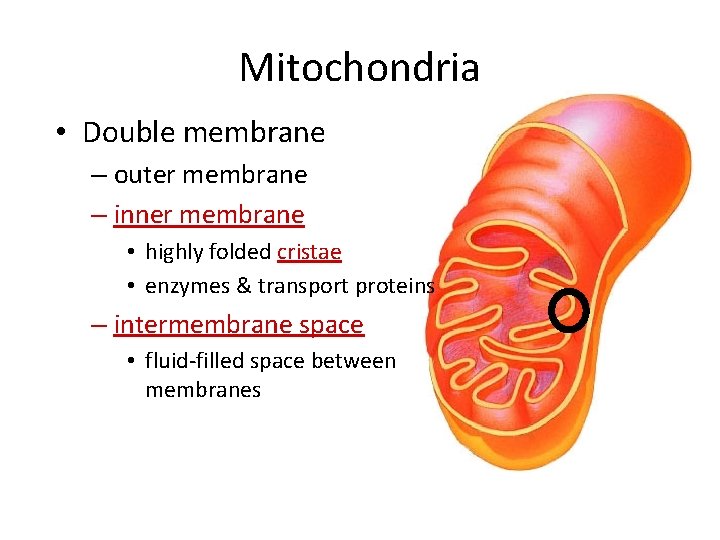
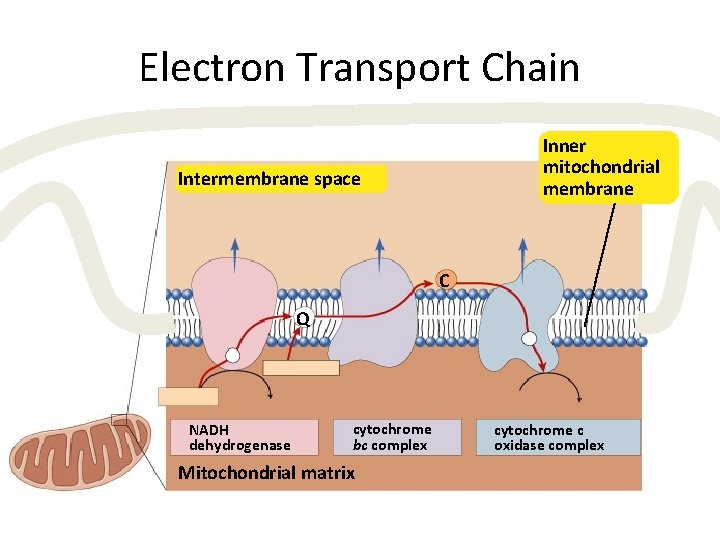
Mitochondria • Double membrane – outer membrane – inner membrane • highly folded cristae • enzymes & transport proteins – intermembrane space • fluid-filled space between membranes

Electron Transport Chain Inner mitochondrial membrane Intermembrane space C Q NADH dehydrogenase cytochrome bc complex Mitochondrial matrix cytochrome c oxidase complex

Remember the Electron Carriers? Glycolysis 2 NADH Time to break open the piggybank! glucose Krebs cycle G 3 P 8 NADH 2 FADH 2

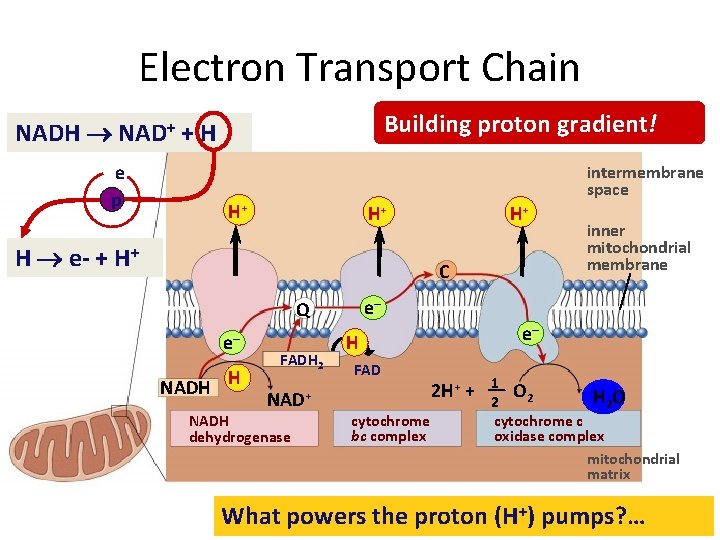
Electron Transport Chain Building proton gradient! NADH NAD+ + H e p intermembrane space H+ H+ H e- + H+ H+ C e– Q e– NADH H FADH 2 NAD+ NADH dehydrogenase inner mitochondrial membrane e– H FAD cytochrome bc complex 2 H+ + 1 O 2 H 2 O 2 cytochrome c oxidase complex mitochondrial matrix What powers the proton (H+) pumps? …

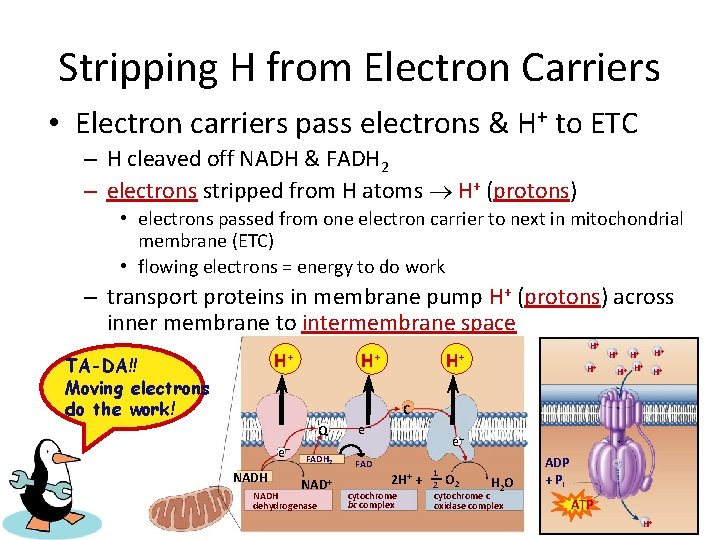
Stripping H from Electron Carriers • Electron carriers pass electrons & H+ to ETC – H cleaved off NADH & FADH 2 – electrons stripped from H atoms H+ (protons) • electrons passed from one electron carrier to next in mitochondrial membrane (ETC) • flowing electrons = energy to do work – transport proteins in membrane pump H+ (protons) across inner membrane to intermembrane space + H H+ TA-DA!! Moving electrons do the work! + H H+ H+ H H+ H+ C Q e– NADH FADH 2 NAD+ NADH dehydrogenase e– e– FAD 2 H+ cytochrome bc complex + 1 H 2 O 2 cytochrome c oxidase complex ADP + Pi ATP H+

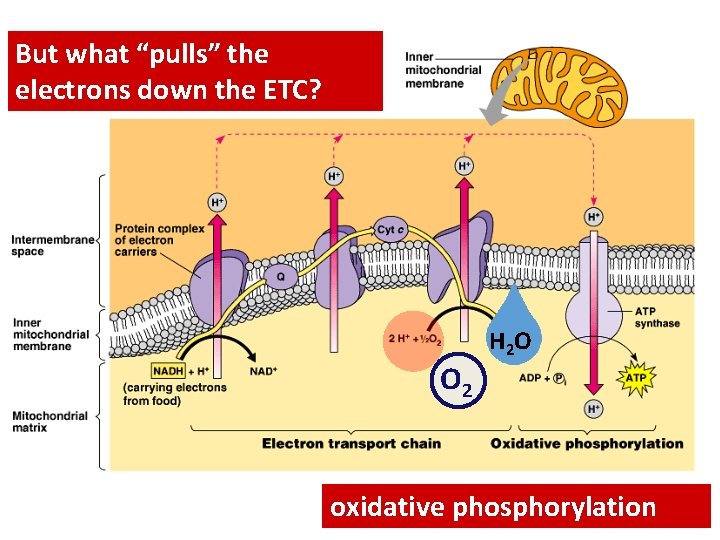
But what “pulls” the electrons down the ETC? O 2 H 2 O oxidative phosphorylation

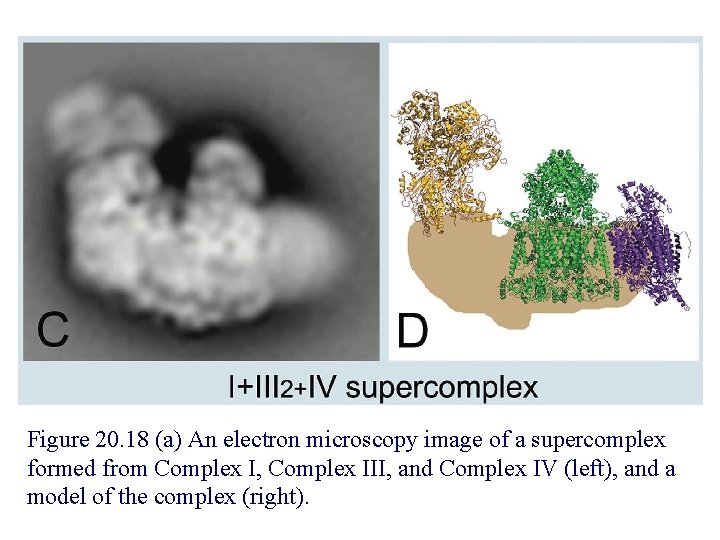
Figure 20. 18 (a) An electron microscopy image of a supercomplex formed from Complex I, Complex III, and Complex IV (left), and a model of the complex (right).

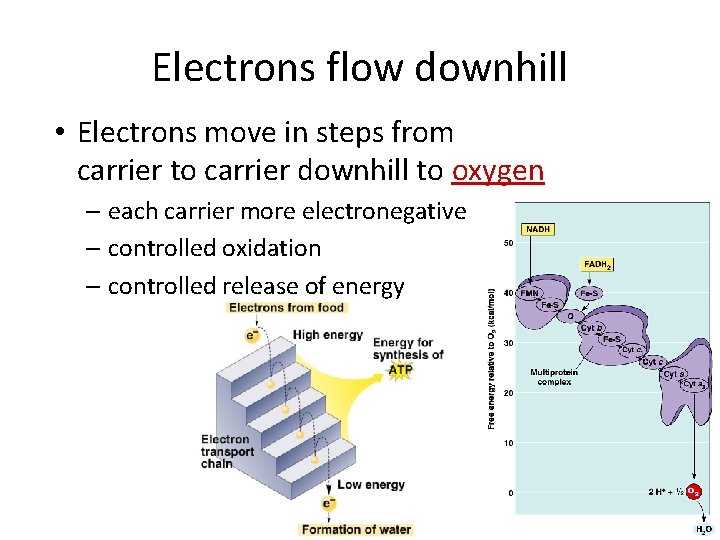
Electrons flow downhill • Electrons move in steps from carrier to carrier downhill to oxygen – each carrier more electronegative – controlled oxidation – controlled release of energy

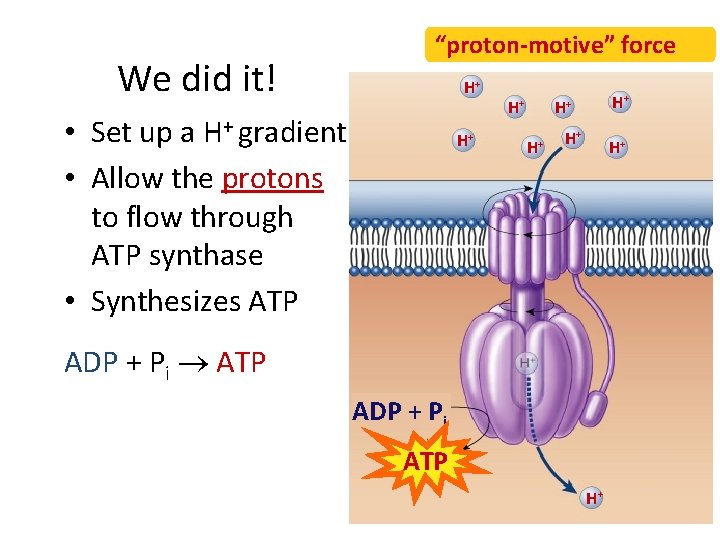
We did it! “proton-motive” force H+ • Set up a H+ gradient • Allow the protons to flow through ATP synthase • Synthesizes ATP H+ H+ ADP + Pi ATP ADP + Pi ATP H+

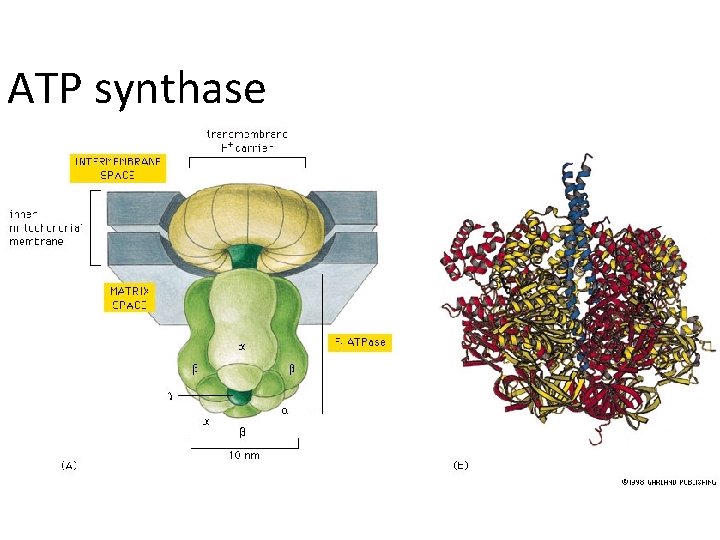
ATP synthase

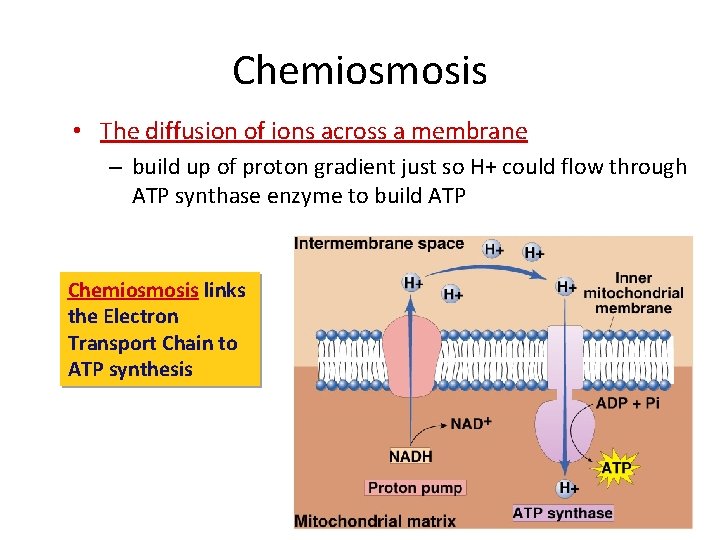
Chemiosmosis • The diffusion of ions across a membrane – build up of proton gradient just so H+ could flow through ATP synthase enzyme to build ATP Chemiosmosis links the Electron Transport Chain to ATP synthesis

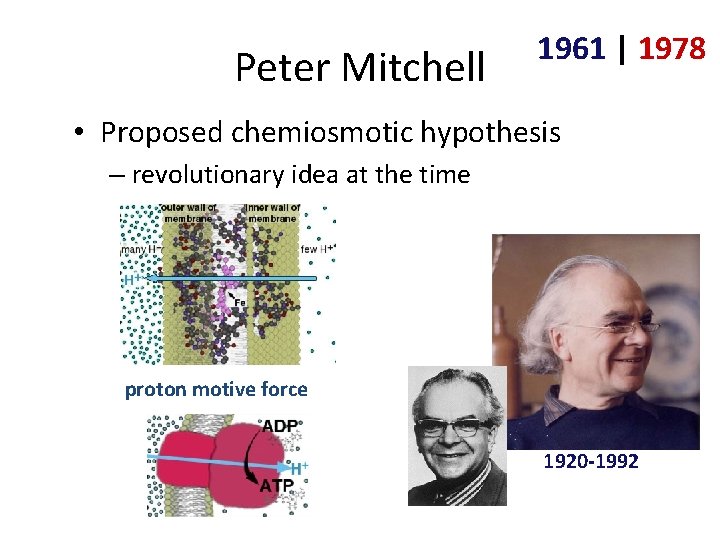
Peter Mitchell 1961 | 1978 • Proposed chemiosmotic hypothesis – revolutionary idea at the time proton motive force 1920 -1992

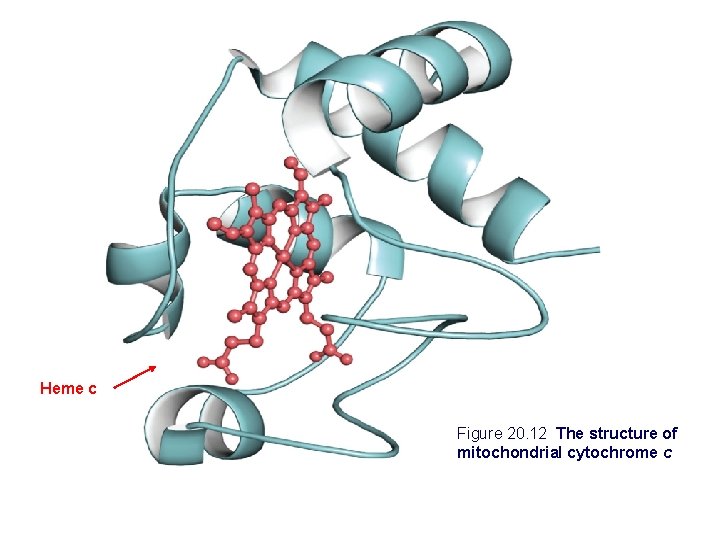
Heme c Figure 20. 12 The structure of mitochondrial cytochrome c

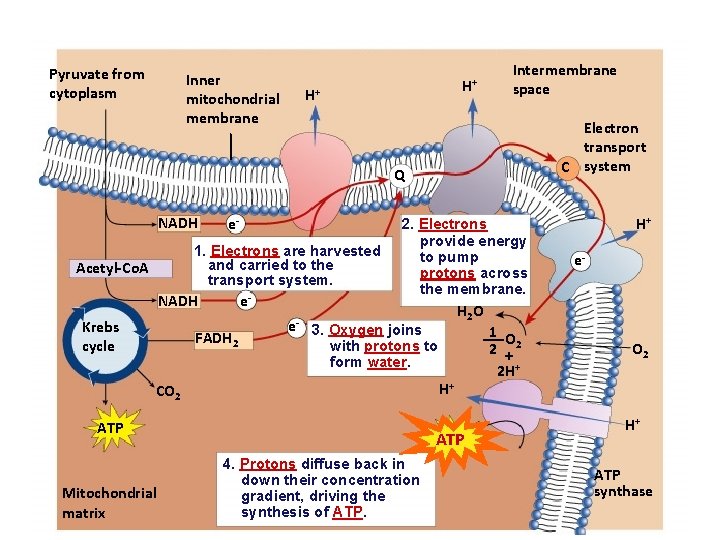
Pyruvate from cytoplasm Inner mitochondrial membrane H+ H+ Intermembrane space Electron transport C system Q NADH e- 1. Electrons are harvested and carried to the transport system. Acetyl-Co. A NADH Krebs cycle e- FADH 2 e- 2. Electrons provide energy to pump protons across the membrane. 3. Oxygen joins with protons to form water. H+ CO 2 ATP Mitochondrial matrix H 2 O ATP 4. Protons diffuse back in down their concentration gradient, driving the synthesis of ATP. 1 O 2 +2 2 H+ H+ e- O 2 H+ ATP synthase

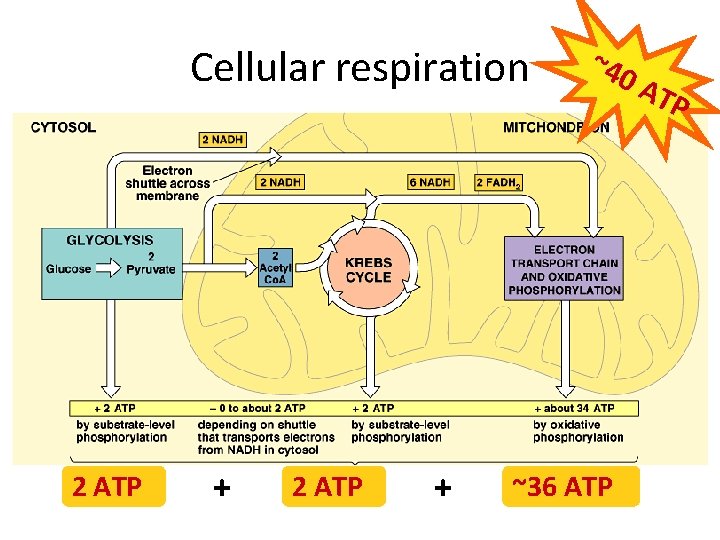
Cellular respiration 2 ATP + ~40 ~36 ATP AT P

Summary of cellular respiration C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + ~40 ATP Where did the glucose come from? Where did the O 2 come from? Where did the CO 2 go? Where did the H 2 O come from? Where did the ATP come from? What else is produced that is not listed in this equation? • Why do we breathe? • •

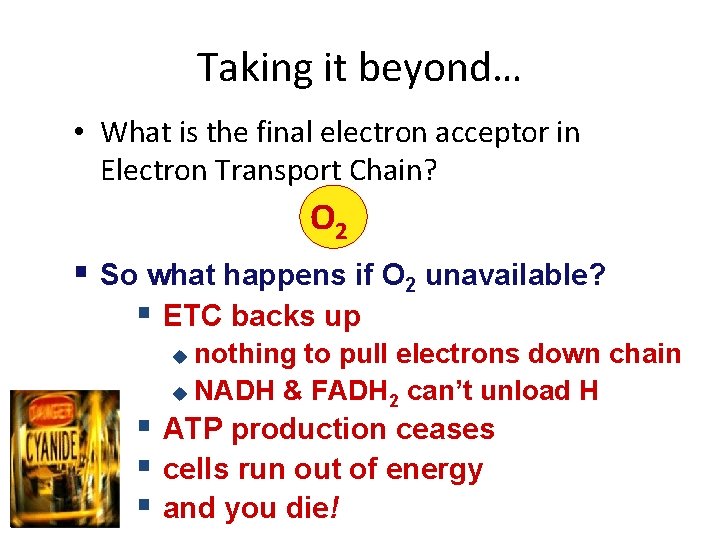
Taking it beyond… • What is the final electron acceptor in Electron Transport Chain? O 2 § So what happens if O 2 unavailable? § ETC backs up nothing to pull electrons down chain u NADH & FADH 2 can’t unload H u § ATP production ceases § cells run out of energy § and you die!

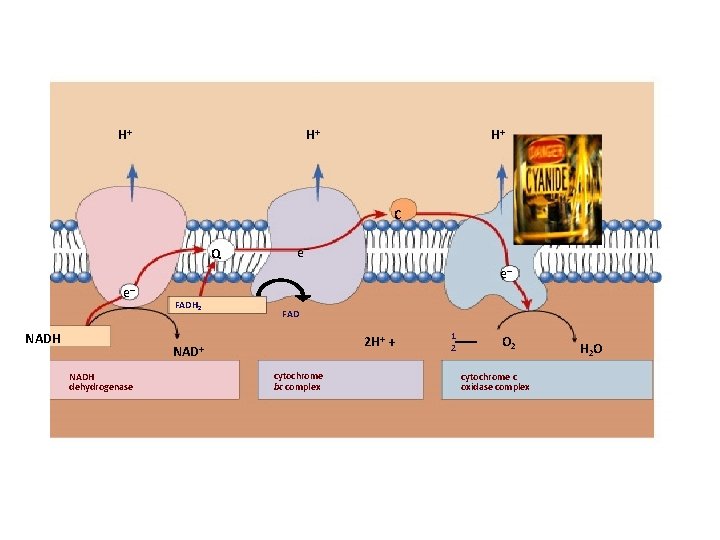
H+ H+ H+ C Q e– e– e– NADH FADH 2 FAD 2 H+ + NADH dehydrogenase cytochrome bc complex 1 2 O 2 cytochrome c oxidase complex H 2 O

What’s the point? The point is to make ATP! ATP 2006 -2007