Citric acid cycle Synthesis of heme Hemoproteins Department






![Metabolic effects of glucagon [not on muscles] Glycolysis Gluconeogenesis Liver Synthesis of glycogen Glycogenolysis Metabolic effects of glucagon [not on muscles] Glycolysis Gluconeogenesis Liver Synthesis of glycogen Glycogenolysis](https://slidetodoc.com/presentation_image/3312bfae7af9d1911c66cf2c29acccb1/image-51.jpg)

- Slides: 78

Citric acid cycle Synthesis of heme. Hemoproteins © Department of Biochemistry (J. D. ) 2013 1

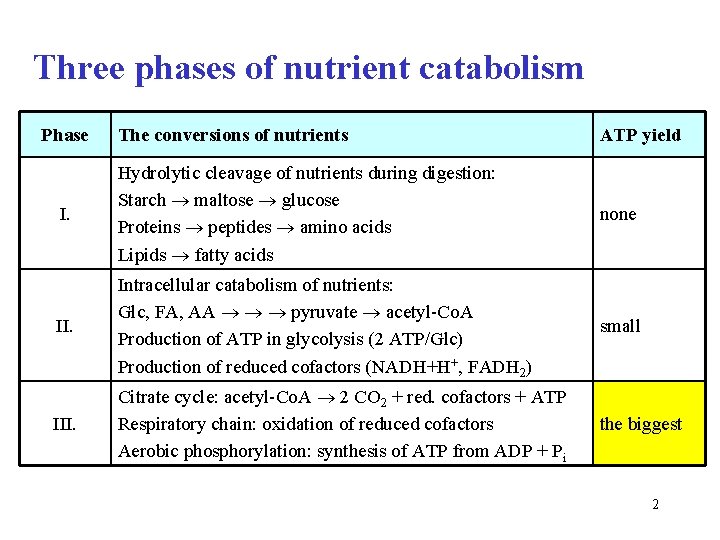
Three phases of nutrient catabolism Phase The conversions of nutrients ATP yield I. Hydrolytic cleavage of nutrients during digestion: Starch maltose glucose Proteins peptides amino acids Lipids fatty acids none II. Intracellular catabolism of nutrients: Glc, FA, AA pyruvate acetyl-Co. A Production of ATP in glycolysis (2 ATP/Glc) Production of reduced cofactors (NADH+H+, FADH 2) small III. Citrate cycle: acetyl-Co. A 2 CO 2 + red. cofactors + ATP Respiratory chain: oxidation of reduced cofactors Aerobic phosphorylation: synthesis of ATP from ADP + Pi the biggest 2

Sources of acetyl-Co. A • oxidative decarboxylation of pyruvate (from glucose and 6 AA) • β-oxidation of fatty acids • catabolism of some amino acids (Thr, Trp, Lys, Leu, Ile) • ketone bodies utilization in extrahepatal tissues: acetoacetate acetoacetyl-Co. A 2 acetyl-Co. A • catabolism of ethanol acetaldehyde acetate acetyl-Co. A 3

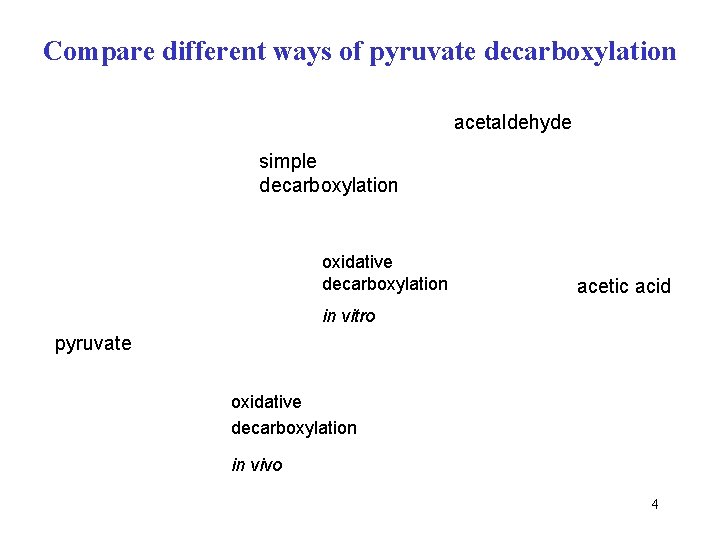
Compare different ways of pyruvate decarboxylation acetaldehyde simple decarboxylation oxidative decarboxylation acetic acid in vitro pyruvate oxidative decarboxylation in vivo 4

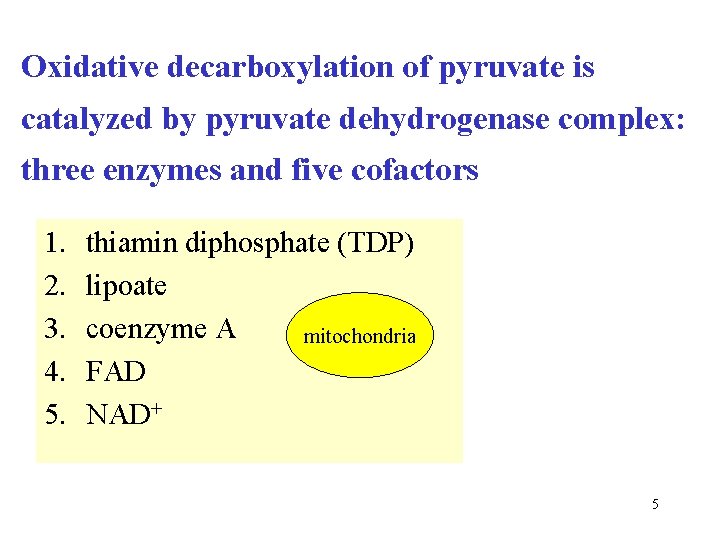
Oxidative decarboxylation of pyruvate is catalyzed by pyruvate dehydrogenase complex: three enzymes and five cofactors 1. 2. 3. 4. 5. thiamin diphosphate (TDP) lipoate coenzyme A mitochondria FAD NAD+ 5

(1) Decarboxylation of pyruvate thiazolium ring 6

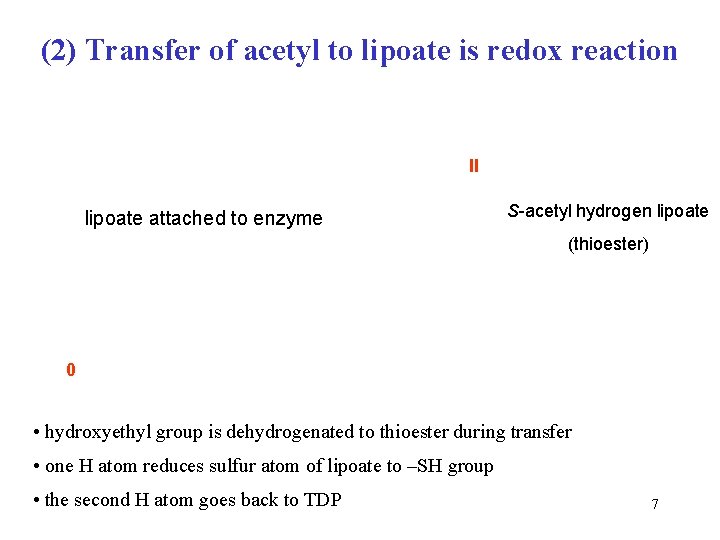
(2) Transfer of acetyl to lipoate is redox reaction II lipoate attached to enzyme S-acetyl hydrogen lipoate (thioester) 0 • hydroxyethyl group is dehydrogenated to thioester during transfer • one H atom reduces sulfur atom of lipoate to –SH group • the second H atom goes back to TDP 7

(3) Transfer of acetyl to coenzyme A dihydrogen lipoate 8

(4) Transfer of 2 H to NAD+ via FAD 9

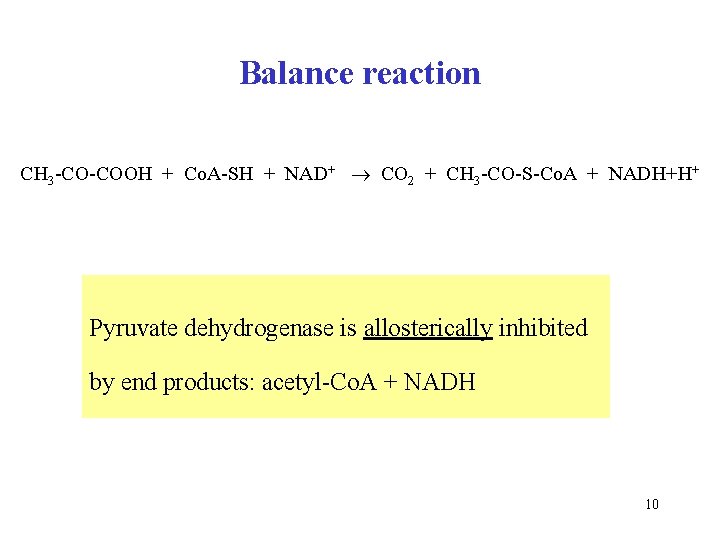
Balance reaction CH 3 -CO-COOH + Co. A-SH + NAD+ CO 2 + CH 3 -CO-S-Co. A + NADH+H+ Pyruvate dehydrogenase is allosterically inhibited by end products: acetyl-Co. A + NADH 10

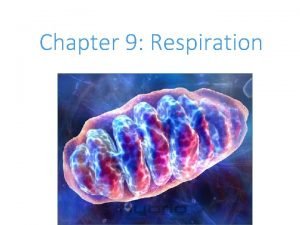
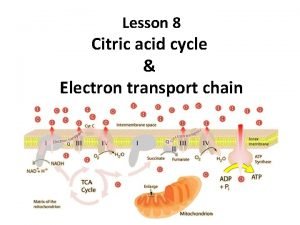
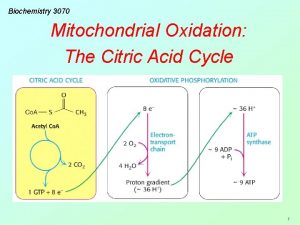
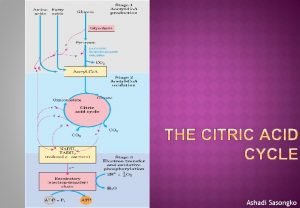
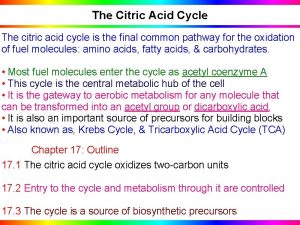
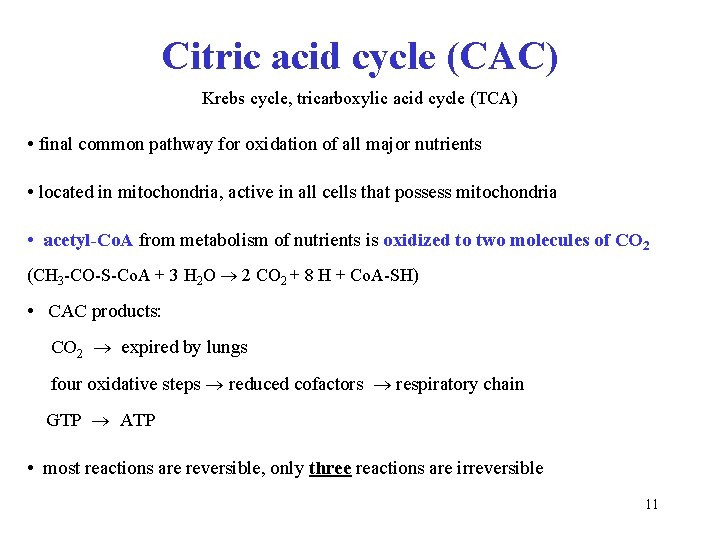
Citric acid cycle (CAC) Krebs cycle, tricarboxylic acid cycle (TCA) • final common pathway for oxidation of all major nutrients • located in mitochondria, active in all cells that possess mitochondria • acetyl-Co. A from metabolism of nutrients is oxidized to two molecules of CO 2 (CH 3 -CO-S-Co. A + 3 H 2 O 2 CO 2 + 8 H + Co. A-SH) • CAC products: CO 2 expired by lungs four oxidative steps reduced cofactors respiratory chain GTP ATP • most reactions are reversible, only three reactions are irreversible 11

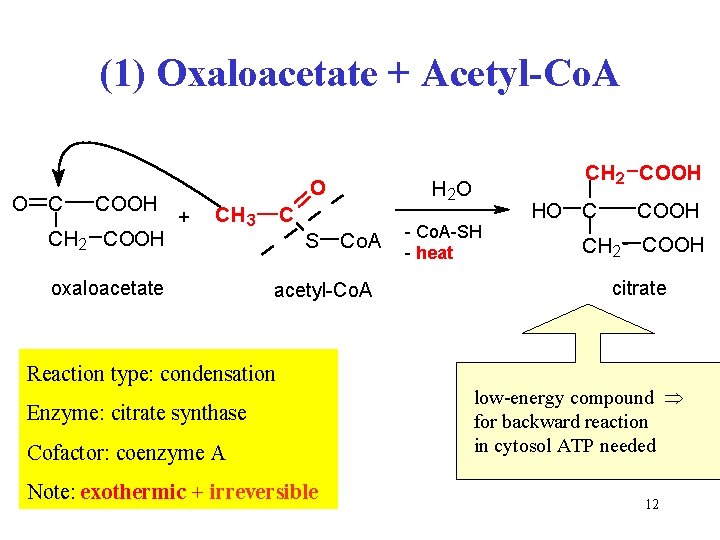
(1) Oxaloacetate + Acetyl-Co. A O C COOH CH 2 COOH O + CH 3 oxalacetate oxaloacetate C S Co. A acetyl-koenzym acetyl-Co. A A Reaction type: condensation Enzyme: citrate synthase Cofactor: coenzyme A Note: exothermic + irreversible CH 2 COOH H 2 O - Co. A-SH - heat HO C COOH CH 2 COOH citrate low-energy compound for backward reaction in cytosol ATP needed 12

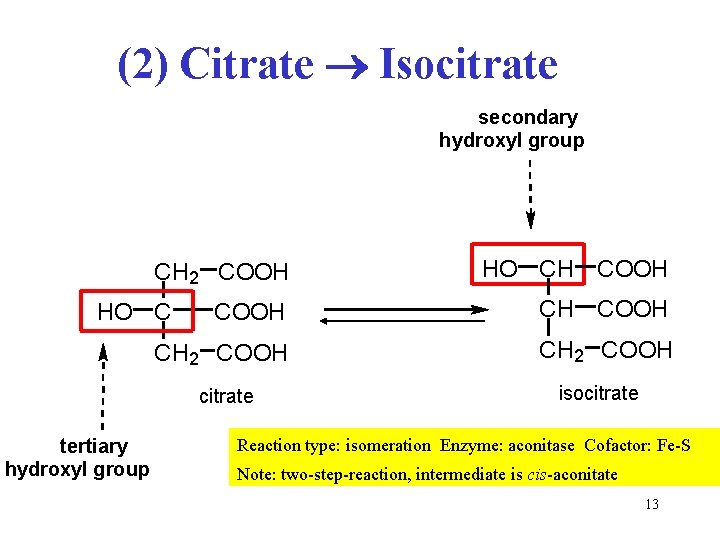
(2) Citrate Isocitrate secondary hydroxyl group CH 2 COOH CH 2 COOH HO C citrate tertiary hydroxyl group HO CH COOH isocitrate Reaction type: isomeration Enzyme: aconitase Cofactor: Fe-S Note: two-step-reaction, intermediate is cis-aconitate 13

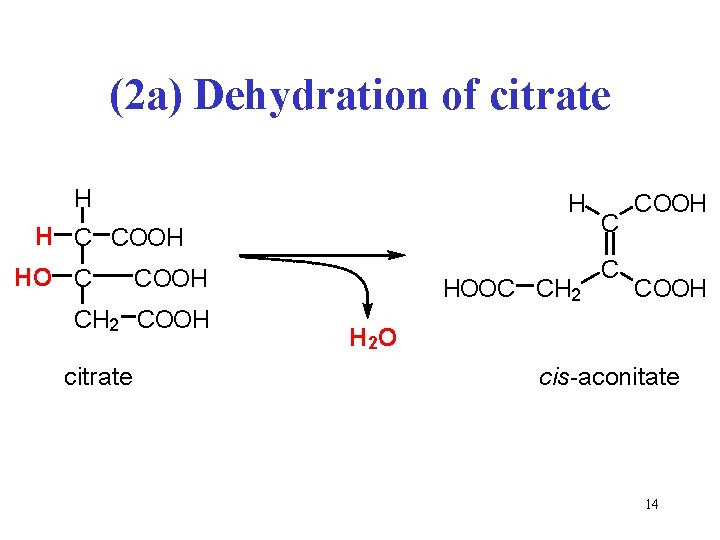
(2 a) Dehydration of citrate H H H C COOH HO C COOH CH 2 COOH citrate HOOC CH 2 C C COOH H 2 O cis-aconitate 14

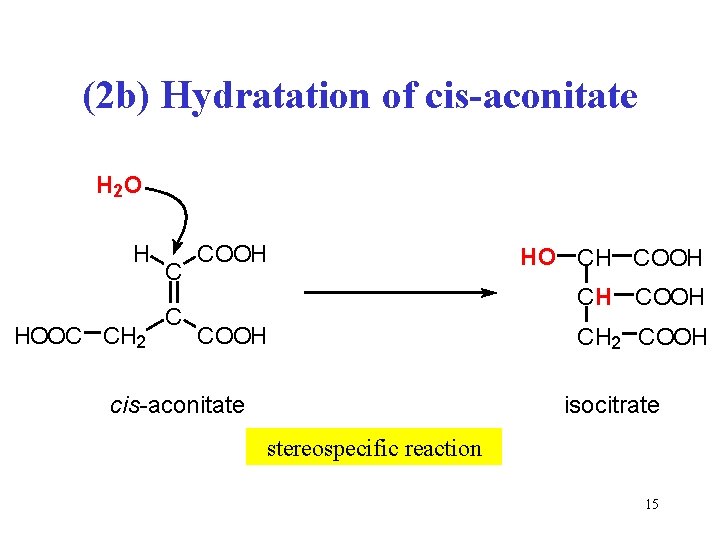
(2 b) Hydratation of cis-aconitate H 2 O H HOOC CH 2 C C COOH HO CH COOH cis-aconitate CH 2 COOH isocitrate stereospecific reaction 15

Aconitase is inhibited by fluoroacetate FCH 2 COOH reacts with oxaloacetate to give fluorocitrate CAC is stopped LD 50 for human is 1 mg/kg rat poison Dichapetalum cymosum (see also Med. Chem. II, p. 65) 16

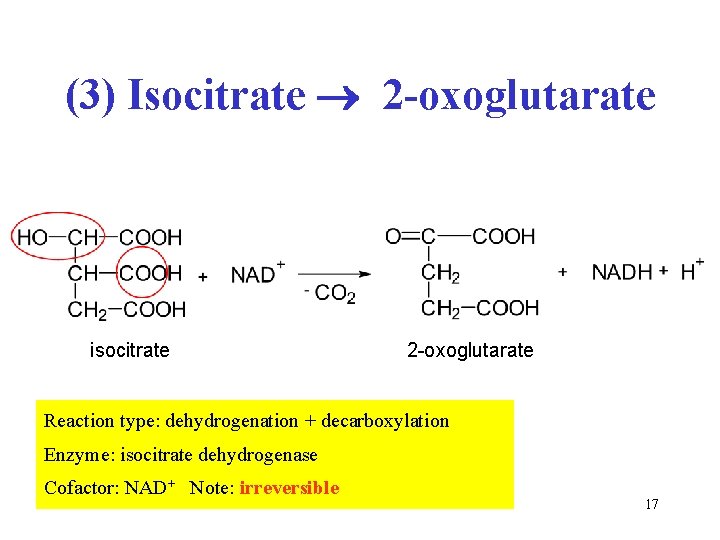
(3) Isocitrate 2 -oxoglutarate isocitrate 2 -oxoglutarate Reaction type: dehydrogenation + decarboxylation Enzyme: isocitrate dehydrogenase Cofactor: NAD+ Note: irreversible 17

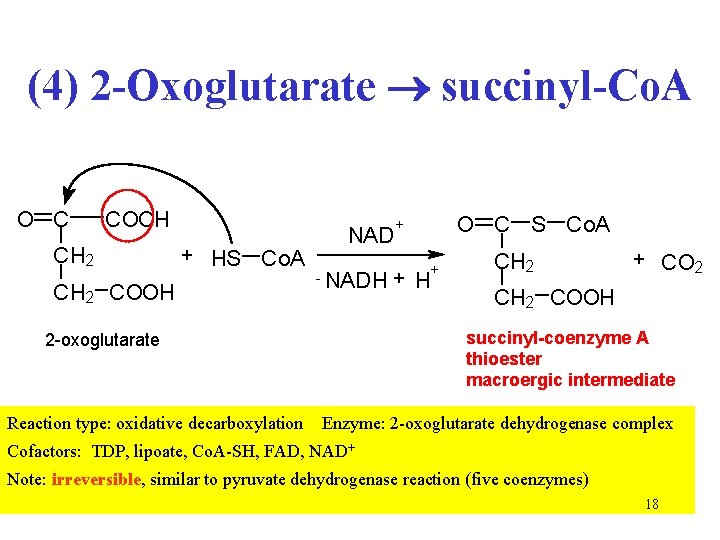
(4) 2 -Oxoglutarate succinyl-Co. A O C COOH CH 2 + HS Co. A CH 2 COOH NAD - + NADH + H CH 2 + CO 2 CH 2 COOH succinyl-coenzyme A thioester macroergic intermediate 2 -oxoglutarate Reaction type: oxidative decarboxylation O C S Co. A + Enzyme: 2 -oxoglutarate dehydrogenase complex Cofactors: TDP, lipoate, Co. A-SH, FAD, NAD+ Note: irreversible, similar to pyruvate dehydrogenase reaction (five coenzymes) 18

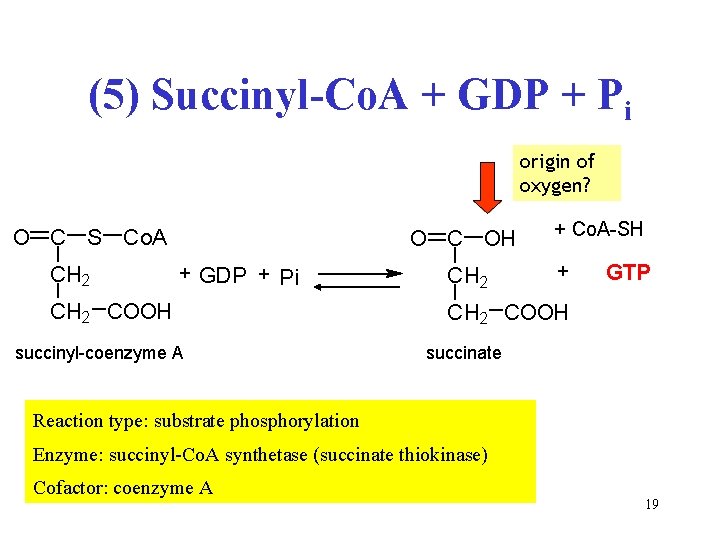
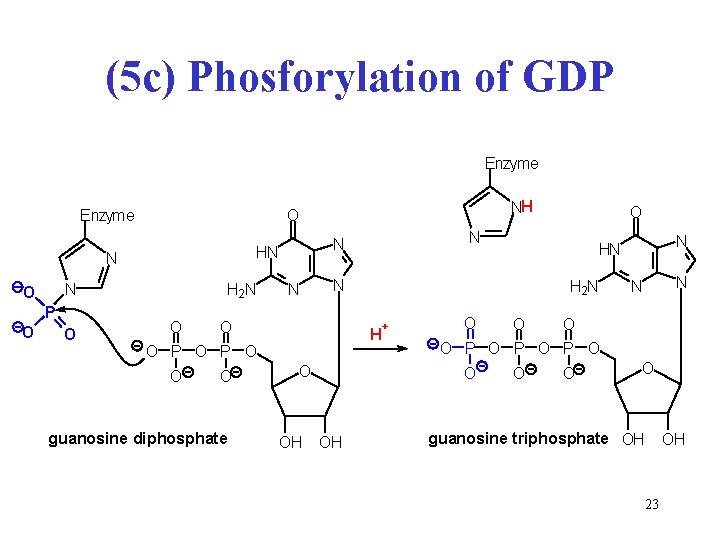
(5) Succinyl-Co. A + GDP + Pi origin of oxygen? O C S Co. A CH 2 O C OH + GDP + Pi CH 2 COOH succinyl-coenzyme A CH 2 + Co. A-SH + GTP CH 2 COOH succinate Reaction type: substrate phosphorylation Enzyme: succinyl-Co. A synthetase (succinate thiokinase) Cofactor: coenzyme A 19

GTP is formed in three-step reaction Chemical energy of macroergic succinyl-Co. A is gradually transformed into two macroergic intermediates and finally to macroergic GTP (Passing a hot potato) 20

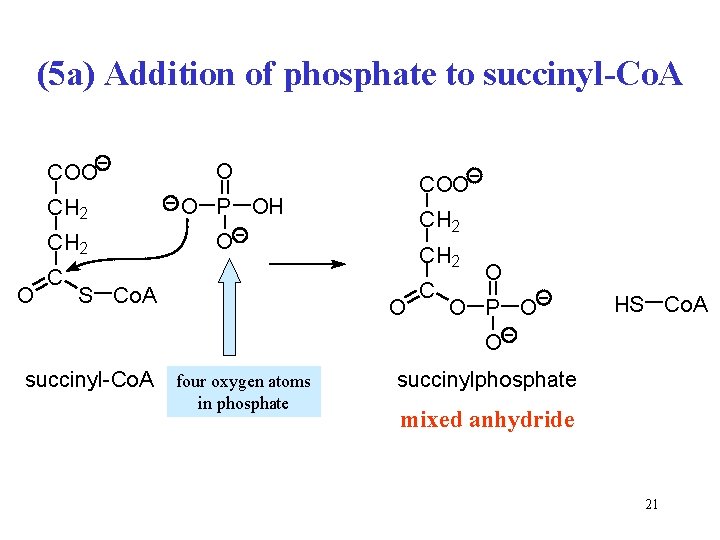
(5 a) Addition of phosphate to succinyl-Co. A COO CH 2 O COO O P OH CH 2 O S Co. A CH 2 O C O O P O HS Co. A O succinyl-Co. A four oxygen atoms in phosphate succinylphosphate mixed anhydride 21

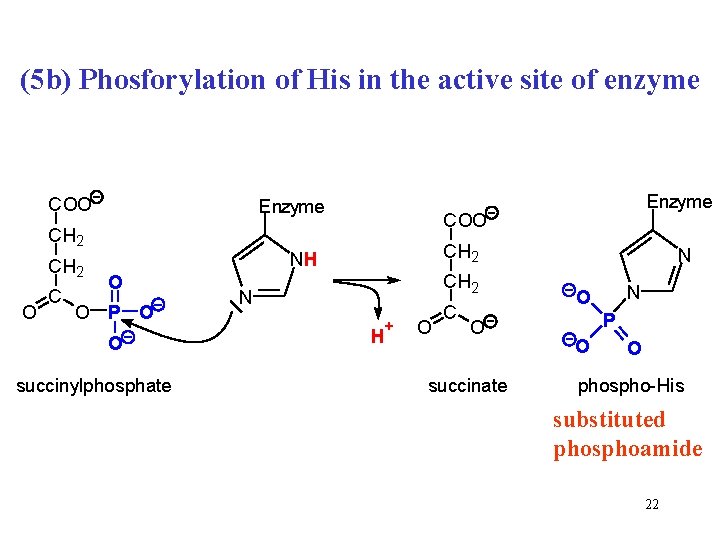
(5 b) Phosforylation of His in the active site of enzyme COO Enzyme CH 2 O C COO CH 2 NH O O P O O succinylphosphate Enzyme CH 2 N H + O C O succinate N N O P O O phospho-His substituted phosphoamide 22

(5 c) Phosforylation of GDP Enzyme N P O O H 2 N O P O O O guanosine diphosphate H 2 N H O OH + O O N N O O P O P O O OH N HN N O O N N HN N O NH O O guanosine triphosphate OH 23 OH

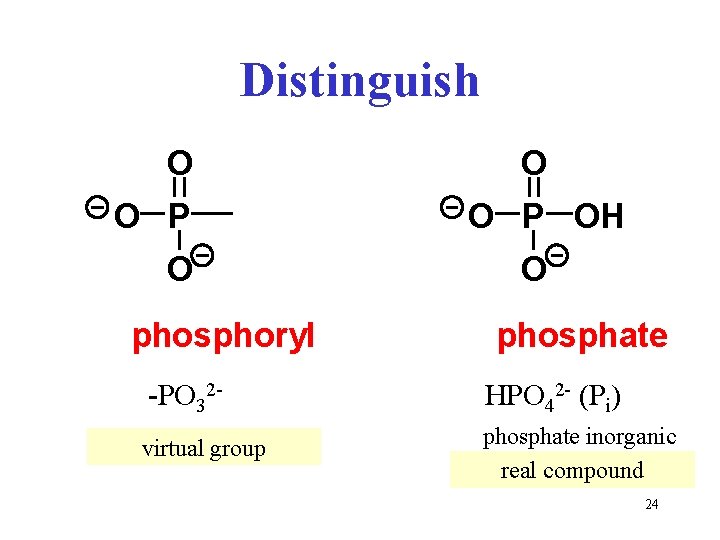
Distinguish O O P O phosphoryl O O P OH O phosphate -PO 32 - HPO 42 - (Pi) virtual group phosphate inorganic real compound 24

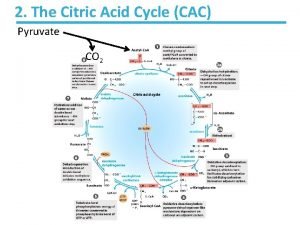
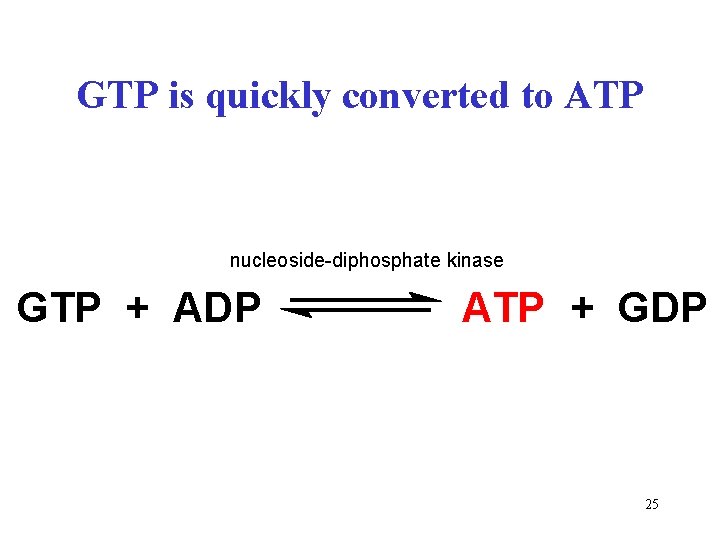
GTP is quickly converted to ATP nucleoside-diphosphate kinase GTP + ADP ATP + GDP 25

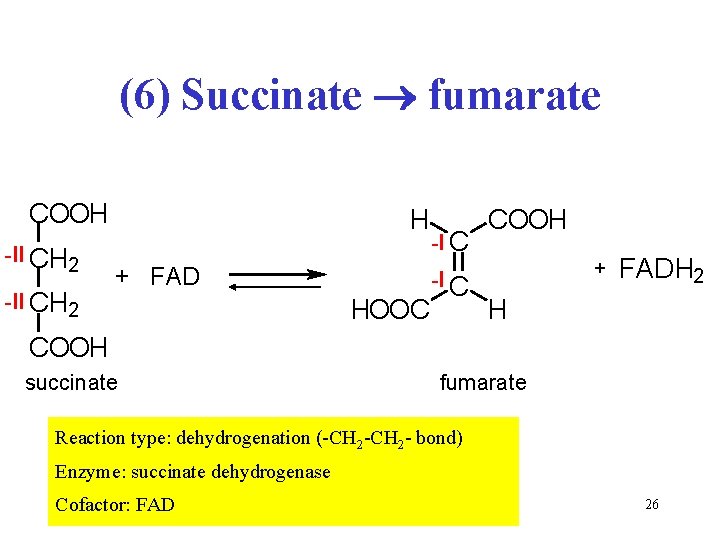
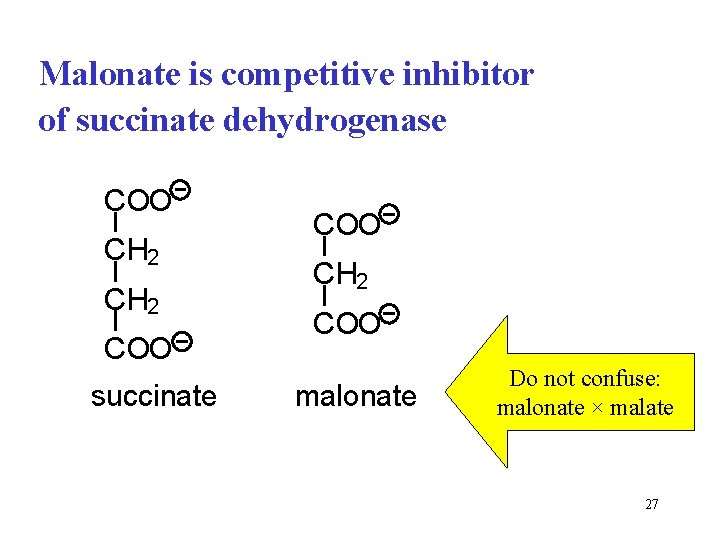
(6) Succinate fumarate COOH -II CH 2 H + FAD HOOC -I C COOH + FADH 2 H COOH succinate fumarate Reaction type: dehydrogenation (-CH 2 - bond) Enzyme: succinate dehydrogenase Cofactor: FAD 26

Malonate is competitive inhibitor of succinate dehydrogenase COO CH 2 COO succinate COO CH 2 COO malonate Do not confuse: malonate × malate 27

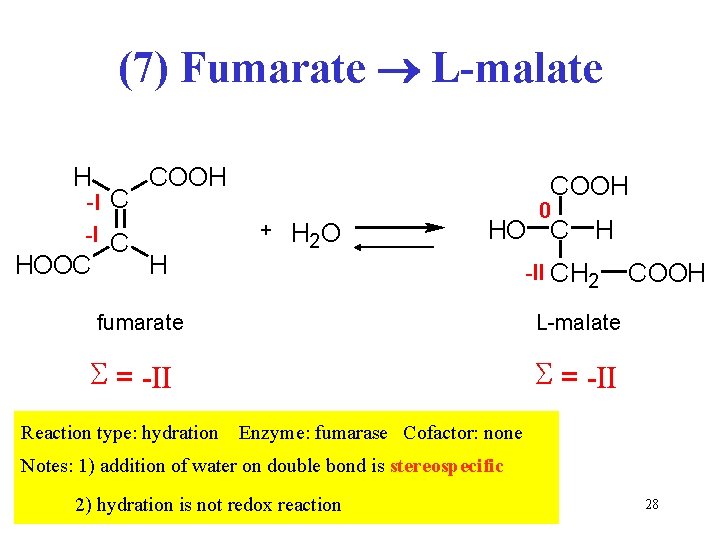
(7) Fumarate L-malate H -I C HOOC COOH H COOH + H 2 O HO C H -II CH 2 fumarate COOH L-malate S = -II Reaction type: hydration 0 S = -II Enzyme: fumarase Cofactor: none Notes: 1) addition of water on double bond is stereospecific 2) hydration is not redox reaction 28

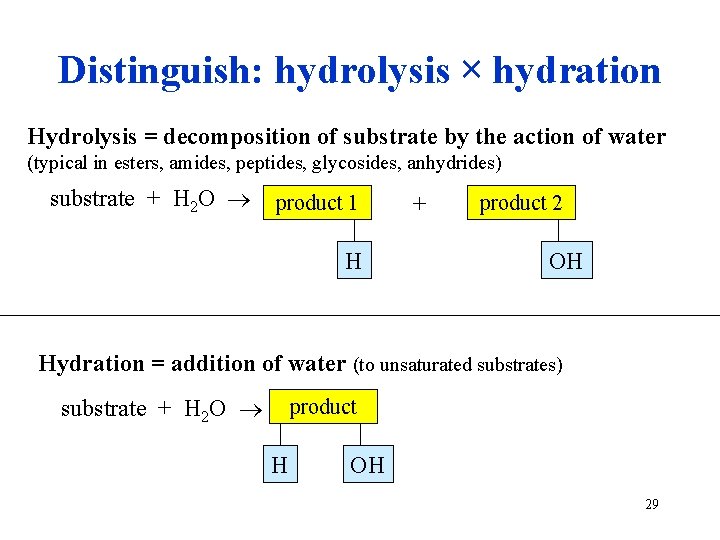
Distinguish: hydrolysis × hydration Hydrolysis = decomposition of substrate by the action of water (typical in esters, amides, peptides, glycosides, anhydrides) substrate + H 2 O product 1 H + product 2 OH Hydration = addition of water (to unsaturated substrates) substrate + H 2 O product H OH 29

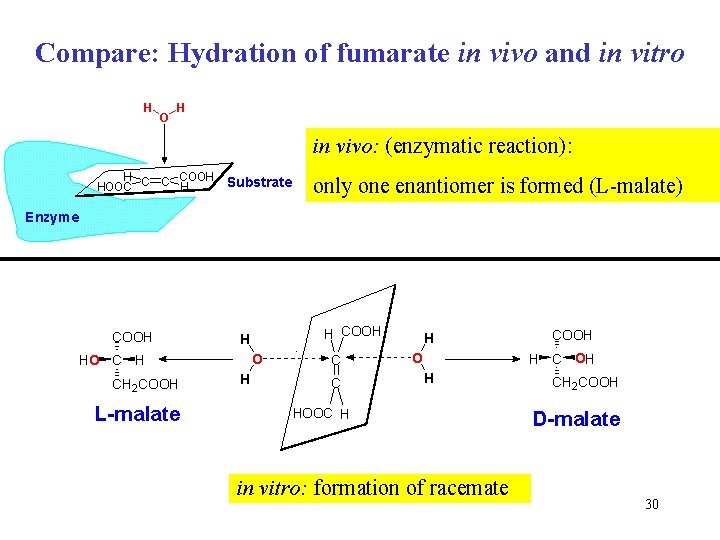
Compare: Hydration of fumarate in vivo and in vitro H O H in vivo: (enzymatic reaction): H COOH HOOC C C H Substrate only one enantiomer is formed (L-malate) Enzyme COOH O HO C H CH 2 COOH L-malate H COOH H H C C H O COOH H C OH H HOOC H in vitro: formation of racemate CH 2 COOH D-malate 30

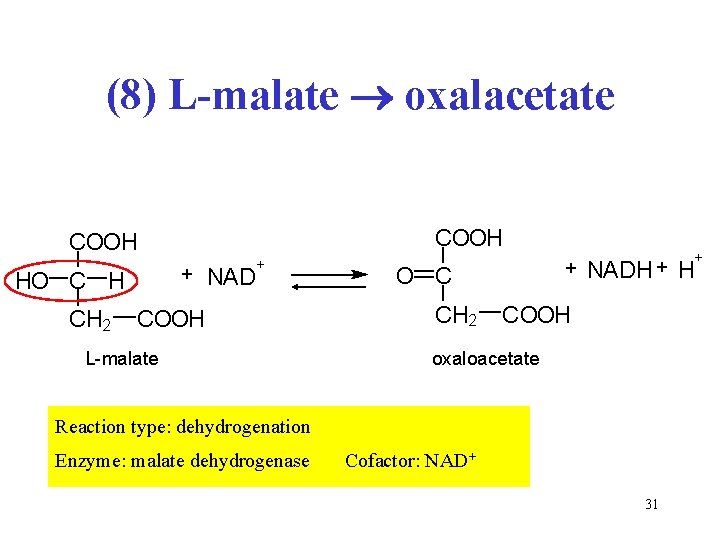
(8) L-malate oxalacetate COOH + HO C H CH 2 NAD + COOH L-malate + O C CH 2 + NADH + H COOH oxaloacetate Reaction type: dehydrogenation Enzyme: malate dehydrogenase Cofactor: NAD+ 31

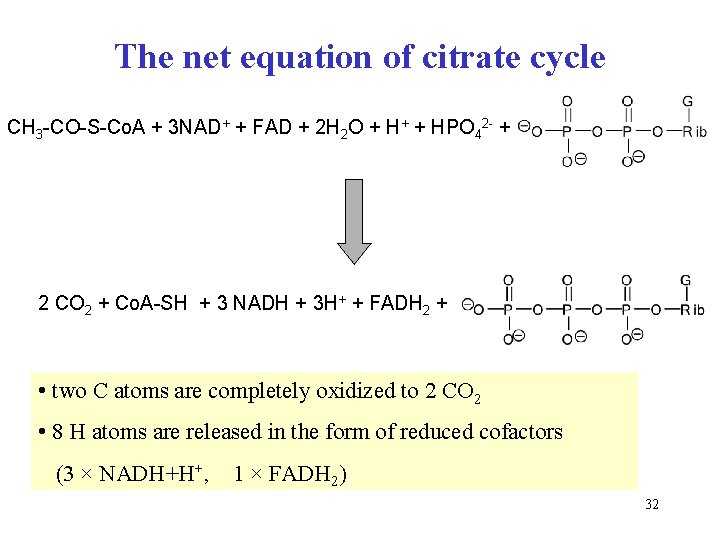
The net equation of citrate cycle CH 3 -CO-S-Co. A + 3 NAD+ + FAD + 2 H 2 O + H+ + HPO 42 - + 2 CO 2 + Co. A-SH + 3 NADH + 3 H+ + FADH 2 + • two C atoms are completely oxidized to 2 CO 2 • 8 H atoms are released in the form of reduced cofactors (3 × NADH+H+, 1 × FADH 2) 32

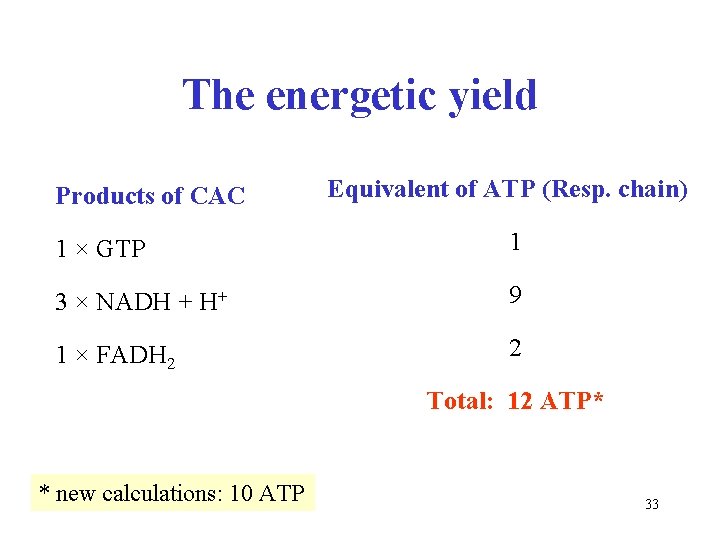
The energetic yield Products of CAC Equivalent of ATP (Resp. chain) 1 × GTP 1 3 × NADH + H+ 9 1 × FADH 2 2 Total: 12 ATP* * new calculations: 10 ATP 33

Factors affecting CAC • Energy charge of the cell: • ATP/ADP ratio and NADH/NAD+ ratio • Allosteric inhibition • Inhibition by products • Supply of oxygen - CAC can proceed only at aerobic conditions (reduced cofactors must be reoxidized in respiratory chain) 34

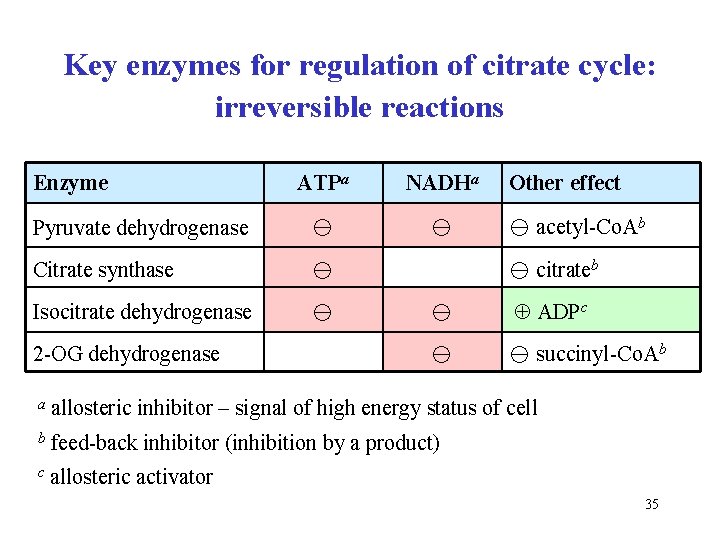
Key enzymes for regulation of citrate cycle: irreversible reactions Enzyme ATPa NADHa Pyruvate dehydrogenase Citrate synthase Isocitrate dehydrogenase 2 -OG dehydrogenase Other effect acetyl-Co. Ab citrateb ADPc succinyl-Co. Ab a allosteric inhibitor – signal of high energy status of cell b feed-back inhibitor (inhibition by a product) c allosteric activator 35

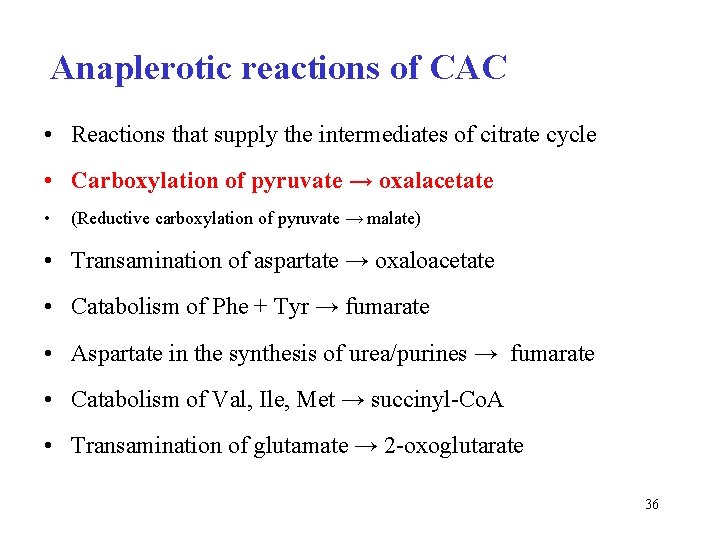
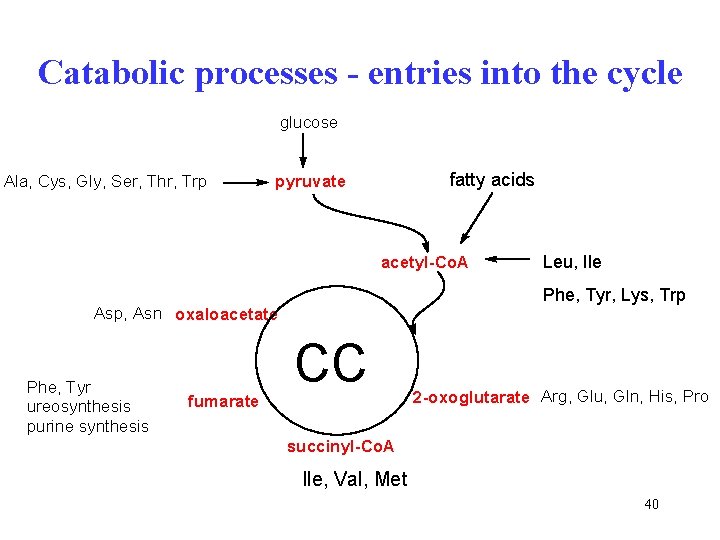
Anaplerotic reactions of CAC • Reactions that supply the intermediates of citrate cycle • Carboxylation of pyruvate → oxalacetate • (Reductive carboxylation of pyruvate → malate) • Transamination of aspartate → oxaloacetate • Catabolism of Phe + Tyr → fumarate • Aspartate in the synthesis of urea/purines → fumarate • Catabolism of Val, Ile, Met → succinyl-Co. A • Transamination of glutamate → 2 -oxoglutarate 36

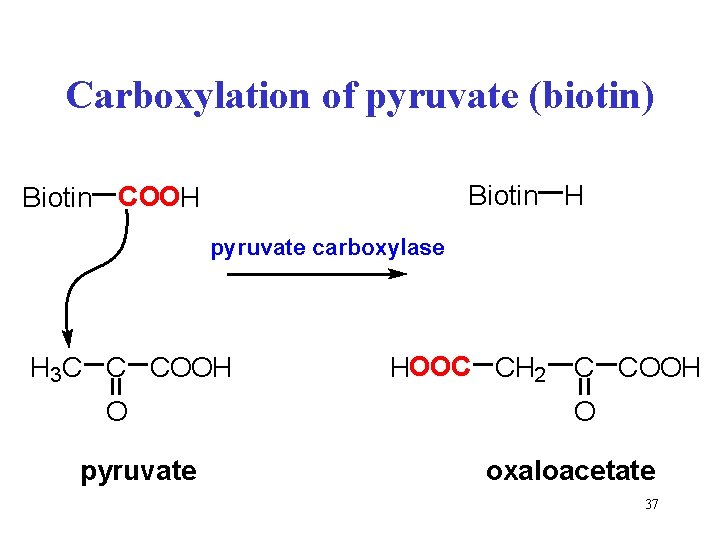
Carboxylation of pyruvate (biotin) Biotin H Biotin COOH pyruvate carboxylase H 3 C C COOH O pyruvate HOOC CH 2 C COOH O oxaloacetate 37

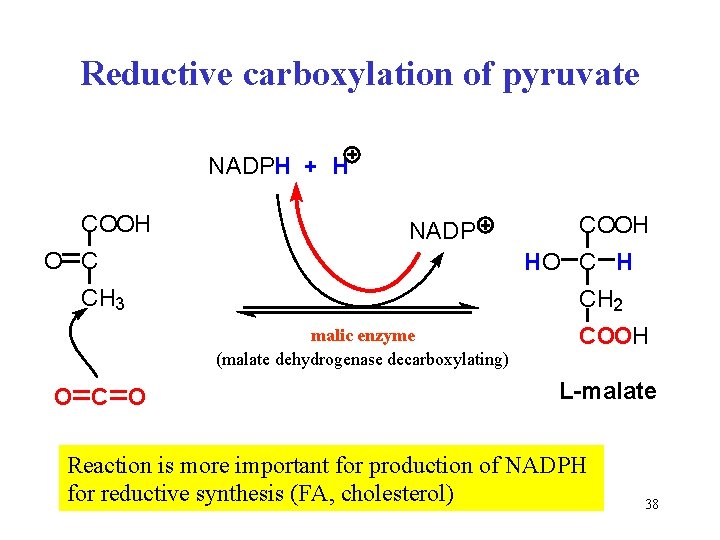
Reductive carboxylation of pyruvate NADPH + H COOH NADP O C H CH 3 CH 2 malic enzyme (malate dehydrogenase decarboxylating) O COOH L-malate Reaction is more important for production of NADPH for reductive synthesis (FA, cholesterol) 38

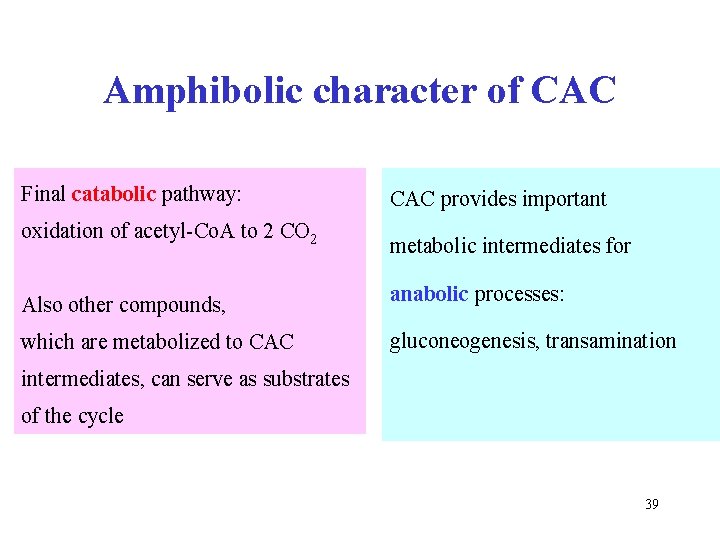
Amphibolic character of CAC Final catabolic pathway: CAC provides important oxidation of acetyl-Co. A to 2 CO 2 metabolic intermediates for Also other compounds, which are metabolized to CAC anabolic processes: gluconeogenesis, transamination intermediates, can serve as substrates of the cycle 39

Catabolic processes - entries into the cycle glucose Ala, Cys, Gly, Ser, Thr, Trp fatty acids pyruvate acetyl-Co. A Phe, Tyr, Lys, Trp Asp, Asn oxaloacetate Phe, Tyr ureosynthesis purine synthesis fumarate Leu, Ile CC 2 -oxoglutarate Arg, Glu, Gln, His, Pro succinyl-Co. A Ile, Val, Met, Met Thr 40

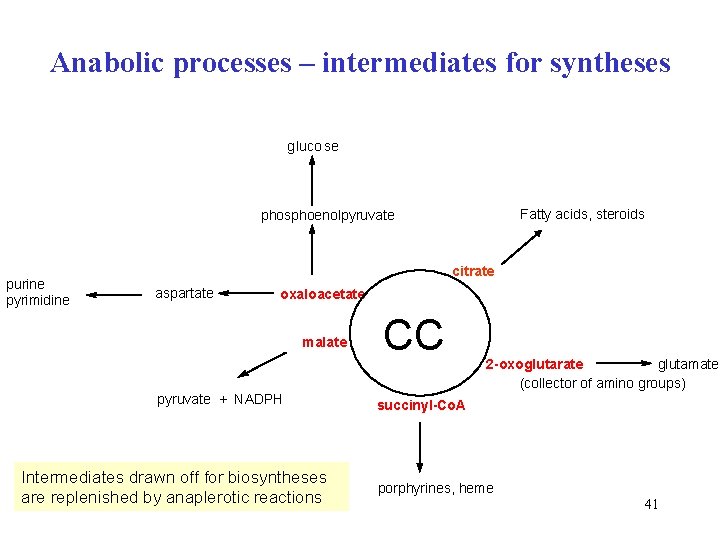
Anabolic processes – intermediates for syntheses gluco se Fatty acids, steroids phosphoenolpyruvate purine pyrimidine citrate aspartate oxaloacetate malate pyruvate + NADPH Intermediates drawn off for biosyntheses are replenished by anaplerotic reactions CC 2 -oxoglutarate glutamate (collector of amino groups) succinyl-Co. A porphyrines, heme 41

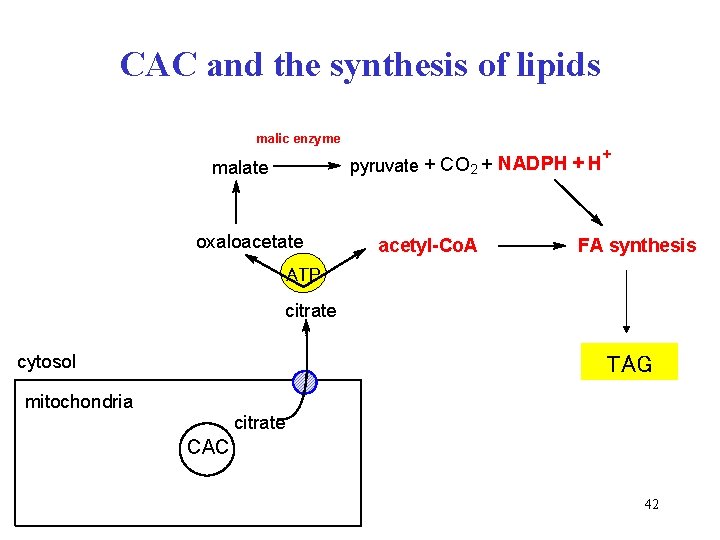
CAC and the synthesis of lipids malic enzyme pyruvate + CO 2 + NADPH + H malate oxaloacetate acetyl-Co. A + FA synthesis ATP citrate cytosol TAG mitochondria citrate CAC 42

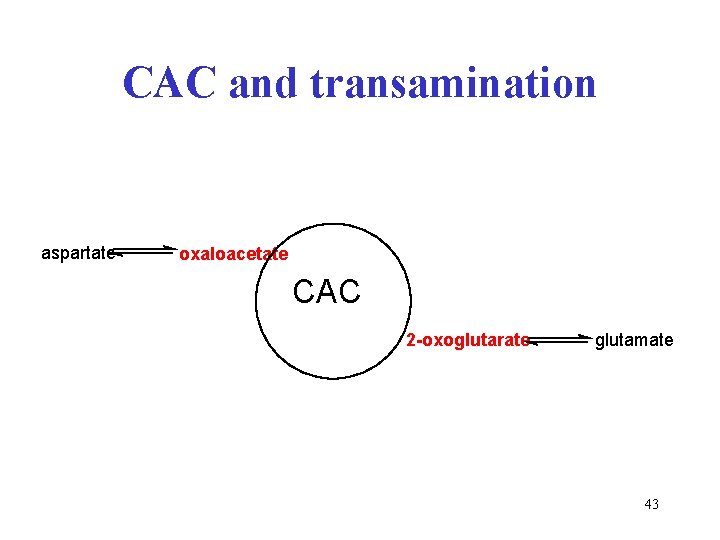
CAC and transamination aspartate oxaloacetate CAC 2 -oxoglutarate glutamate 43

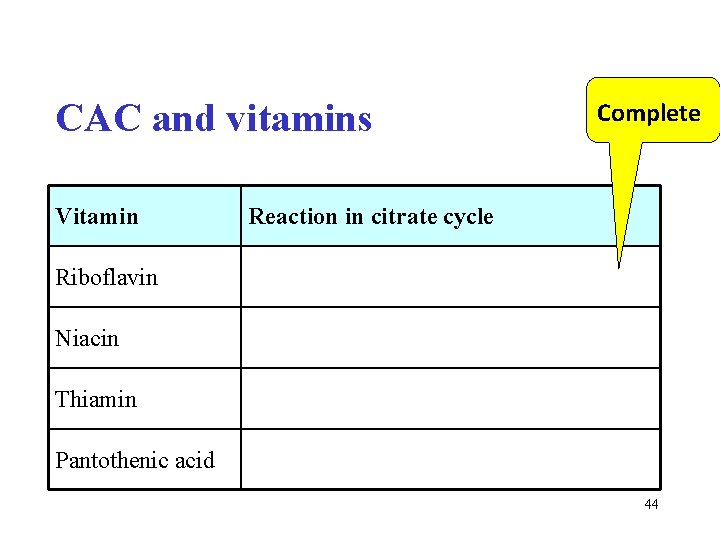
CAC and vitamins Vitamin Complete Reaction in citrate cycle Riboflavin Niacin Thiamin Pantothenic acid 44

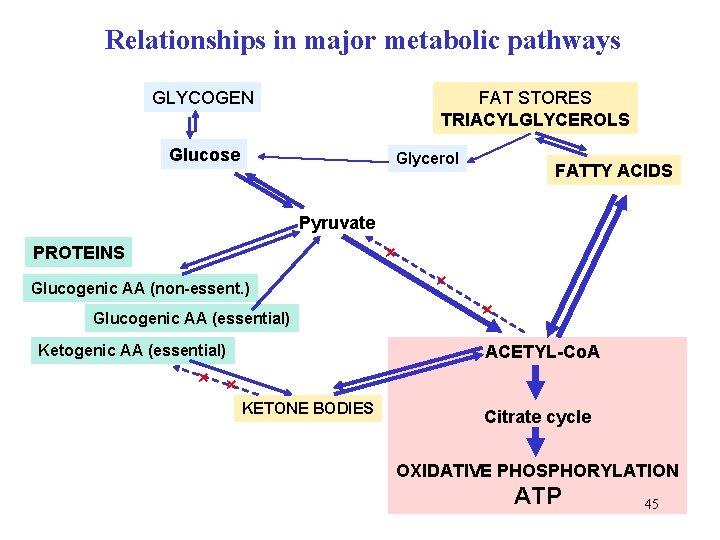
Relationships in major metabolic pathways GLYCOGEN FAT STORES TRIACYLGLYCEROLS Glucose Glycerol FATTY ACIDS Pyruvate × PROTEINS Glucogenic AA (non-essent. ) Glucogenic AA (essential) Ketogenic AA (essential) × × ACETYL-Co. A × × KETONE BODIES Citrate cycle OXIDATIVE PHOSPHORYLATION ATP 45

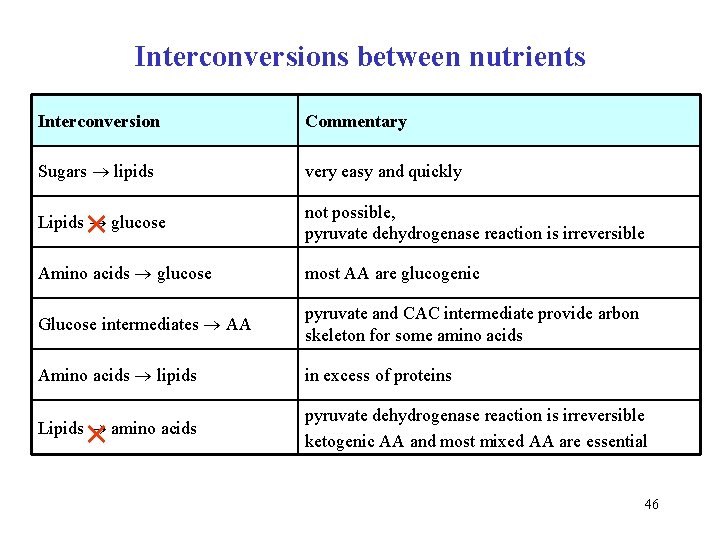
Interconversions between nutrients Interconversion Commentary Sugars lipids very easy and quickly Lipids glucose not possible, pyruvate dehydrogenase reaction is irreversible Amino acids glucose most AA are glucogenic Glucose intermediates AA pyruvate and CAC intermediate provide arbon skeleton for some amino acids Amino acids lipids in excess of proteins × × Lipids amino acids pyruvate dehydrogenase reaction is irreversible ketogenic AA and most mixed AA are essential 46

Saccharides are the most universal nutrients – the overdose is transformed in the fat stores, carbon skelet of non-essential amino acids can originate from saccharides. Triacylglycerols exhibit the highest energetic yield – but fatty acids cannot convert into saccharides or the skelet of amino acids. Amino acids represent the unique source of nitrogen for proteosynthesis that serves as fuel rather when the organism is lacking in other nutrients glucogenic amino acids can convert into glucose, a overdose of diet protein may be transformes in fat stores. The metabolism of nutrients is sophistically controlled with different mechanisms in the well-fed state (absorptive phase), short fasting (post-absorptive phase), and in prolonged starvation. It also depends on energy expenditure (predominantly muscular work) – either of maximal intensity (anaerobic, of short duration only) or aerobic work of much lower intensity (long duration). 47

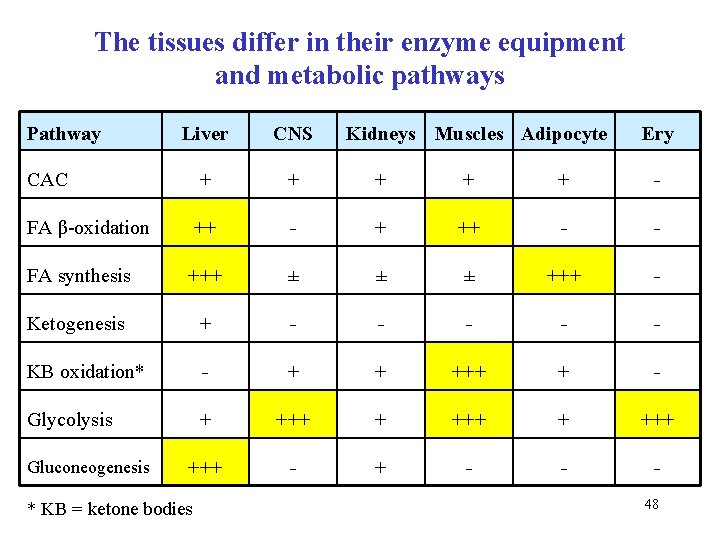
The tissues differ in their enzyme equipment and metabolic pathways Pathway Liver CNS CAC + + + - FA β-oxidation ++ - + ++ - - FA synthesis +++ ± ± ± +++ - Ketogenesis + - - - KB oxidation* - + + +++ + - Glycolysis + +++ - - - Gluconeogenesis * KB = ketone bodies Kidneys Muscles Adipocyte Ery 48

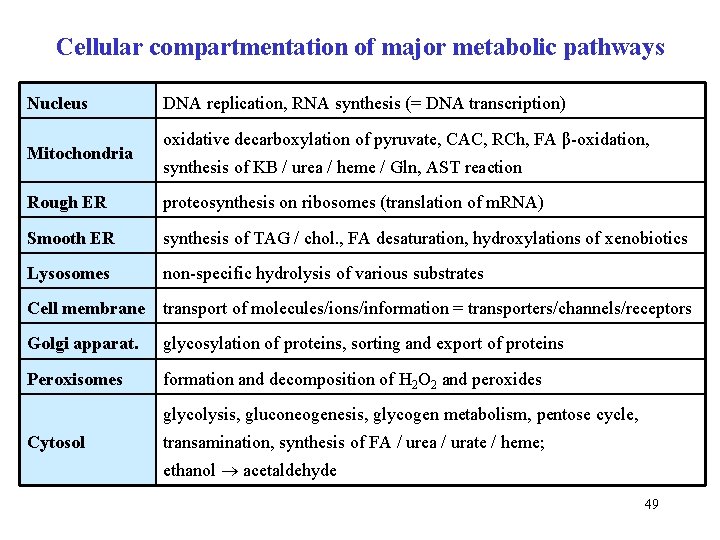
Cellular compartmentation of major metabolic pathways Nucleus Mitochondria DNA replication, RNA synthesis (= DNA transcription) oxidative decarboxylation of pyruvate, CAC, RCh, FA β-oxidation, synthesis of KB / urea / heme / Gln, AST reaction Rough ER proteosynthesis on ribosomes (translation of m. RNA) Smooth ER synthesis of TAG / chol. , FA desaturation, hydroxylations of xenobiotics Lysosomes non-specific hydrolysis of various substrates Cell membrane transport of molecules/ions/information = transporters/channels/receptors Golgi apparat. glycosylation of proteins, sorting and export of proteins Peroxisomes formation and decomposition of H 2 O 2 and peroxides glycolysis, gluconeogenesis, glycogen metabolism, pentose cycle, Cytosol transamination, synthesis of FA / urea / urate / heme; ethanol acetaldehyde 49

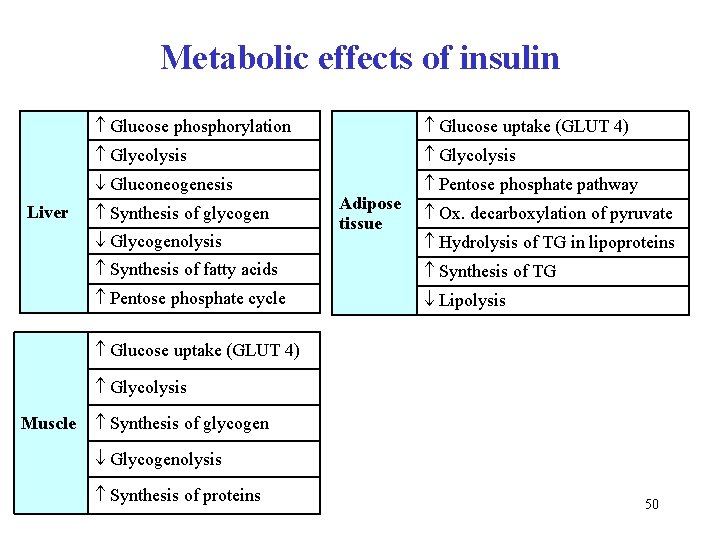
Metabolic effects of insulin Liver Glucose phosphorylation Glucose uptake (GLUT 4) Glycolysis Gluconeogenesis Pentose phosphate pathway Synthesis of glycogen Glycogenolysis Adipose tissue Ox. decarboxylation of pyruvate Hydrolysis of TG in lipoproteins Synthesis of fatty acids Synthesis of TG Pentose phosphate cycle Lipolysis Glucose uptake (GLUT 4) Glycolysis Muscle Synthesis of glycogen Glycogenolysis Synthesis of proteins 50
![Metabolic effects of glucagon not on muscles Glycolysis Gluconeogenesis Liver Synthesis of glycogen Glycogenolysis Metabolic effects of glucagon [not on muscles] Glycolysis Gluconeogenesis Liver Synthesis of glycogen Glycogenolysis](https://slidetodoc.com/presentation_image/3312bfae7af9d1911c66cf2c29acccb1/image-51.jpg)
Metabolic effects of glucagon [not on muscles] Glycolysis Gluconeogenesis Liver Synthesis of glycogen Glycogenolysis Synthesis of fatty acids Oxidation of fatty acids Adipocytes Lipolysis (HSL, hormone sensitive lipase) 51

Biosynthesis of heme ~ Hemoproteins 52

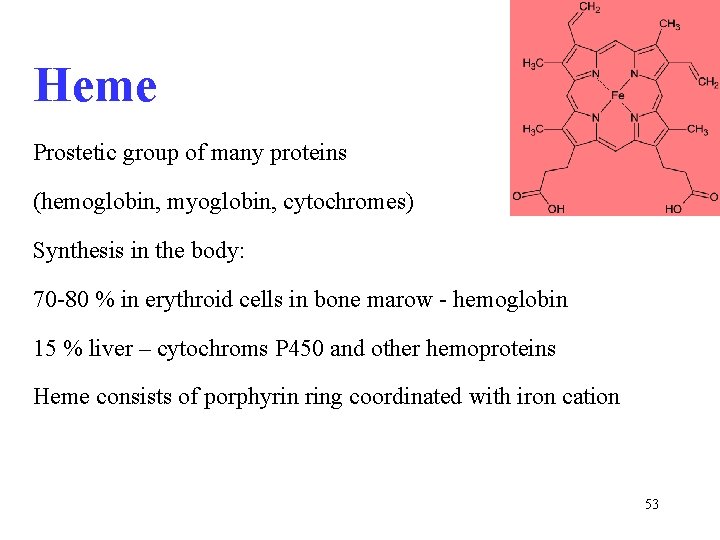
Heme Prostetic group of many proteins (hemoglobin, myoglobin, cytochromes) Synthesis in the body: 70 -80 % in erythroid cells in bone marow - hemoglobin 15 % liver – cytochroms P 450 and other hemoproteins Heme consists of porphyrin ring coordinated with iron cation 53

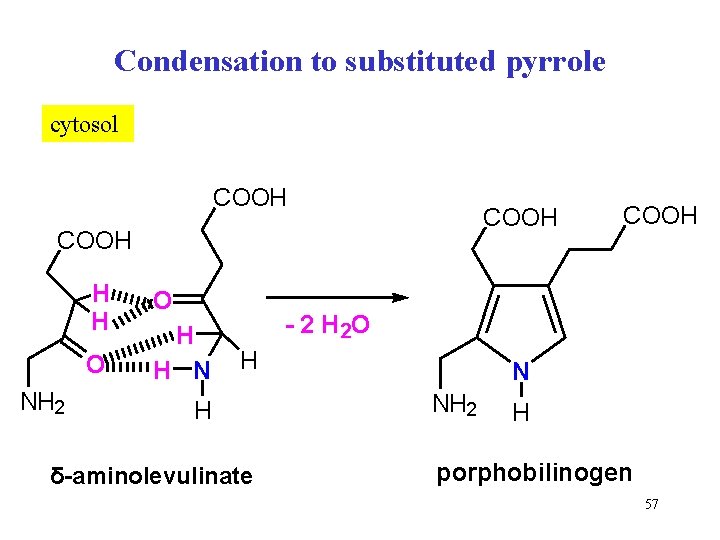
Biosynthesis of heme • initial compound for synthesis is succinyl-Co. A (intermediate of CAC) • source of nitrogen is glycine • reactions are located in mitochondria and cytosol • regulation: ALA-synthase mitochondria cytosol 54

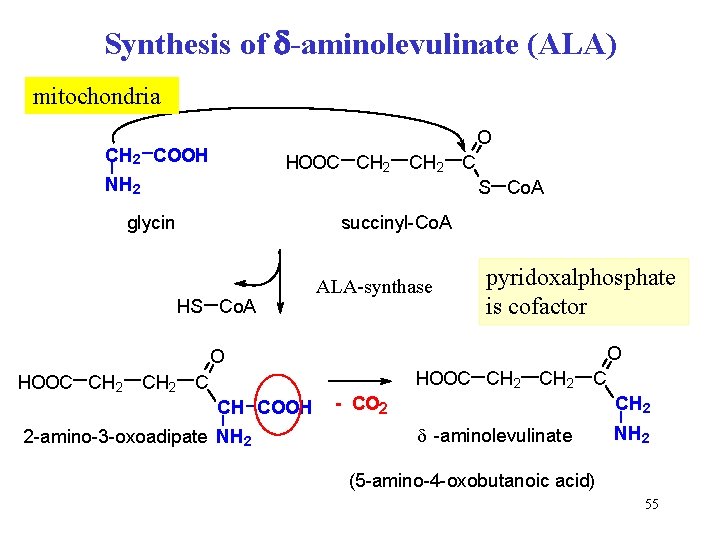
Synthesis of -aminolevulinate (ALA) mitochondria O CH 2 COOH HOOC CH 2 C NH 2 S Co. A glycin succinyl-Co. A HS Co. A ALA-synthase pyridoxalphosphate is cofactor O O HOOC CH 2 C CH COOH 2 -amino-3 -oxoadipate NH 2 - CO 2 CH 2 d -aminolevulinate NH 2 (5 -amino-4 -oxobutanoic acid) 55

ALA-synthase is the rate-controlling enzyme of porphyrine biosynthesis Half-life about 1 hour ALA-synthase - is inhibited by heme (allosteric inhibition) - synthesis of enzyme is repressed by heme - is induced by some drugs (barbiturates, phenytoin, griseofulvin) - cytochrome P-450 is needed for biotransformation of drugs/xenobiotics 56

Condensation to substituted pyrrole cytosol COOH H H O NH 2 O H H N COOH - 2 H 2 O H H δ-aminolevulinate N NH 2 H porphobilinogen 57

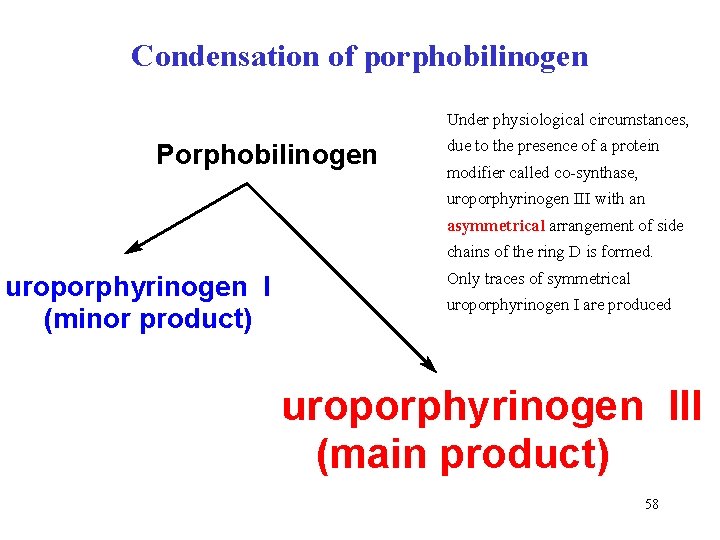
Condensation of porphobilinogen Under physiological circumstances, Porphobilinogen due to the presence of a protein modifier called co-synthase, uroporphyrinogen III with an asymmetrical arrangement of side chains of the ring D is formed. uroporphyrinogen I (minor product) Only traces of symmetrical uroporphyrinogen I are produced uroporphyrinogen III (main product) 58

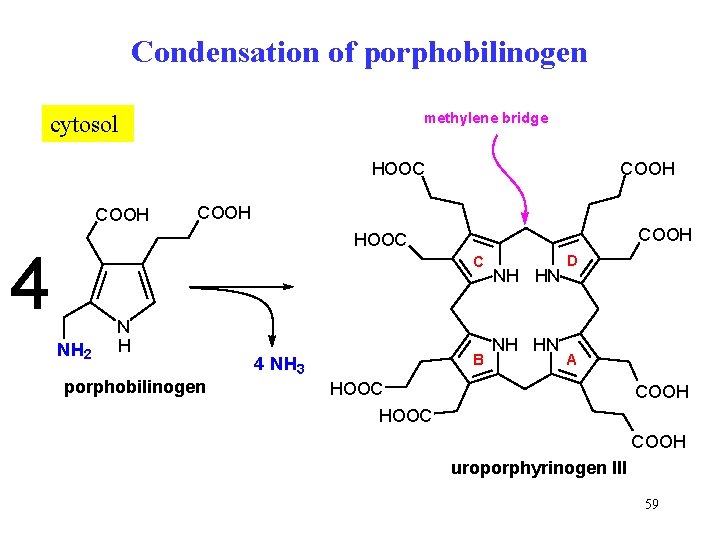
Condensation of porphobilinogen methylene bridge cytosol COOH HOOC COOH HOOC 4 C NH 2 N H porphobilinogen B 4 NH 3 NH HN D A HOOC COOH uroporphyrinogen III 59

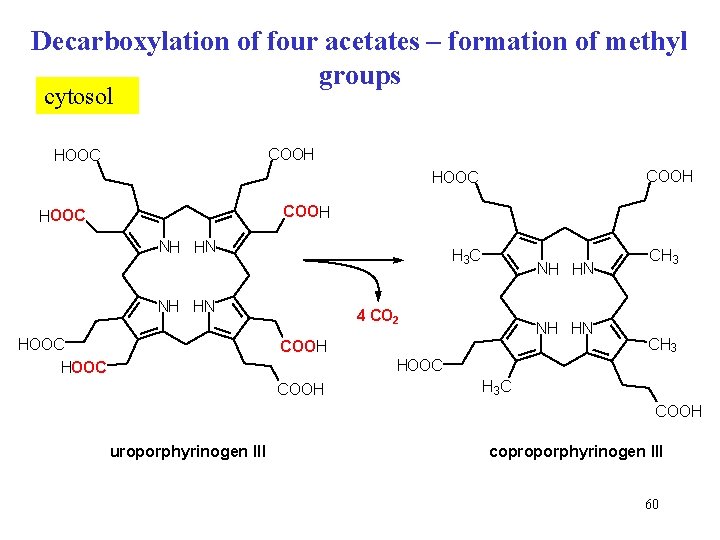
Decarboxylation of four acetates – formation of methyl groups cytosol COOH HOOC NH HN H 3 C NH HN HOOC NH HN 4 CO 2 NH HN COOH CH 3 HOOC COOH H 3 C COOH uroporphyrinogen III coproporphyrinogen III 60

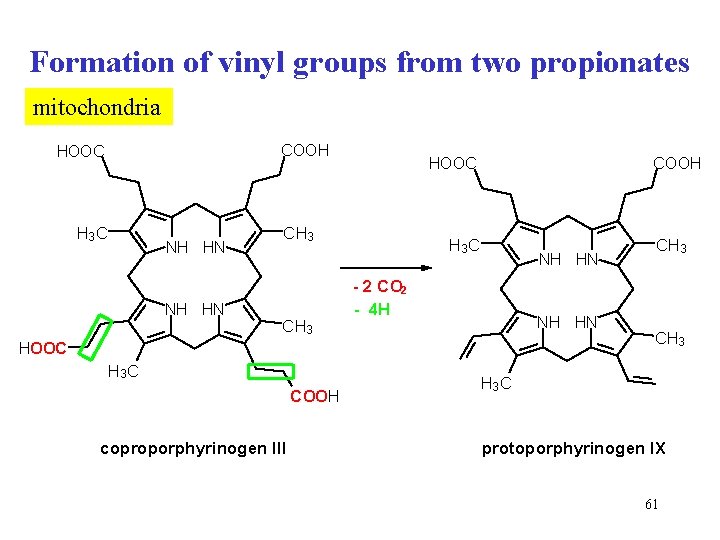
Formation of vinyl groups from two propionates mitochondria COOH HOOC H 3 C NH HN CH 3 COOH HOOC H 3 C NH HN - 2 CO 2 - 4 H NH HN HOOC H 3 C COOH coproporphyrinogen III CH 3 H 3 C protoporphyrinogen IX 61

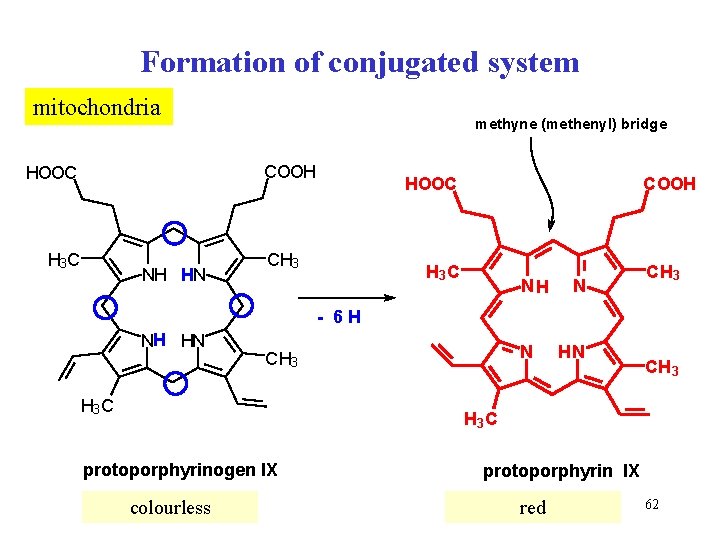
Formation of conjugated system mitochondria methyne (methenyl) bridge COOH HOOC H 3 C NH HN COOH HOOC CH 3 C NH N CH 3 - 6 H NH HN N CH 3 H 3 C HN CH 3 C protoporphyrinogen IX colourless protoporphyrin IX red 62

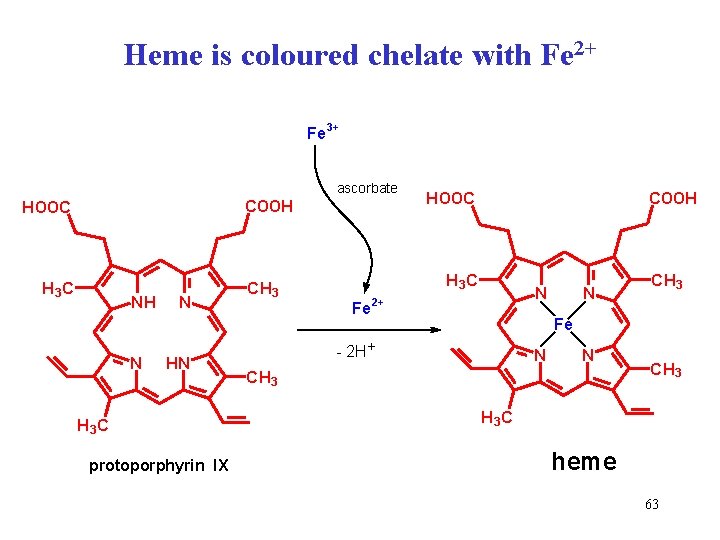
Heme is coloured chelate with Fe 2+ Fe 3+ ascorbate COOH HOOC H 3 C NH N N HN H 3 C protoporphyrin IX COOH HOOC H 3 C CH 3 Fe N 2+ N CH 3 Fe - 2 H+ N N CH 3 H 3 C heme 63

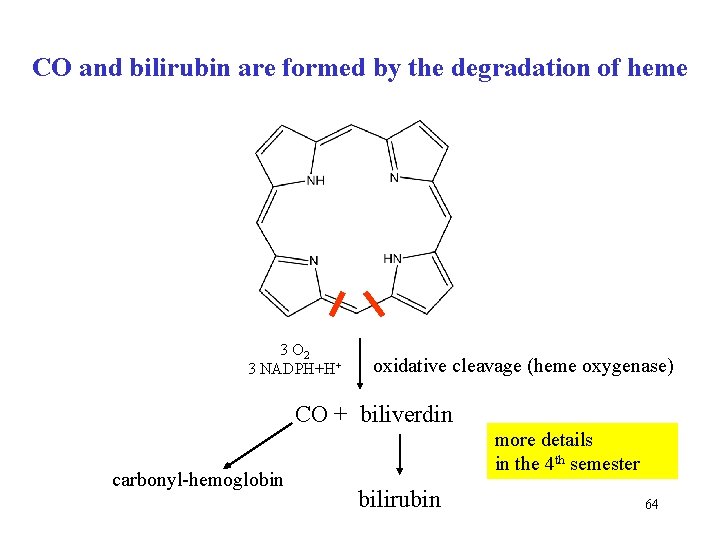
CO and bilirubin are formed by the degradation of heme 3 O 2 3 NADPH+H+ oxidative cleavage (heme oxygenase) CO + biliverdin carbonyl-hemoglobin more details in the 4 th semester bilirubin 64

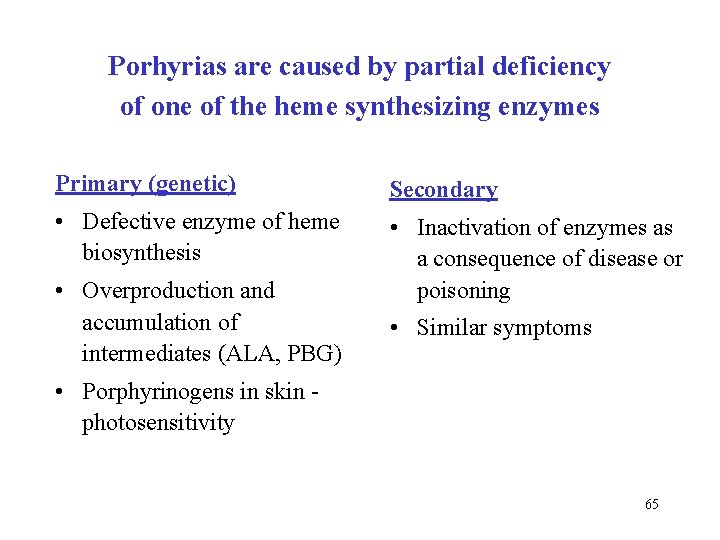
Porhyrias are caused by partial deficiency of one of the heme synthesizing enzymes Primary (genetic) Secondary • Defective enzyme of heme biosynthesis • Inactivation of enzymes as a consequence of disease or poisoning • Overproduction and accumulation of intermediates (ALA, PBG) • Similar symptoms • Porphyrinogens in skin photosensitivity 65

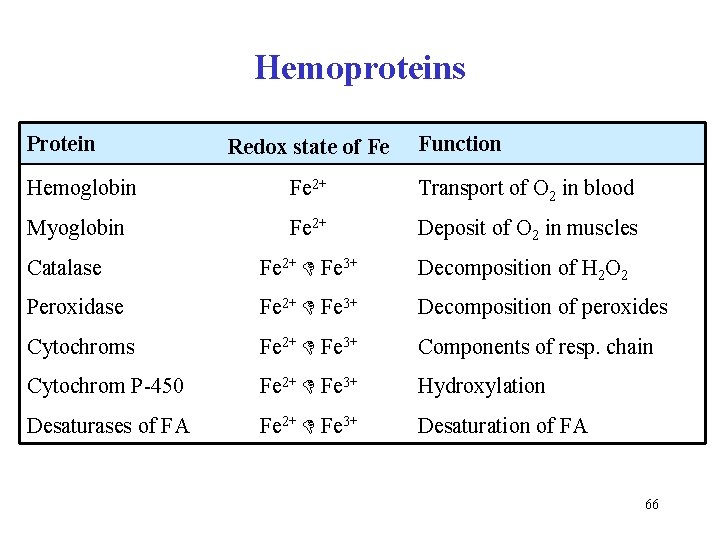
Hemoproteins Protein Redox state of Fe Function Hemoglobin Fe 2+ Transport of O 2 in blood Myoglobin Fe 2+ Deposit of O 2 in muscles Catalase Fe 2+ Fe 3+ Decomposition of H 2 O 2 Peroxidase Fe 2+ Fe 3+ Decomposition of peroxides Cytochroms Fe 2+ Fe 3+ Components of resp. chain Cytochrom P-450 Fe 2+ Fe 3+ Hydroxylation Desaturases of FA Fe 2+ Fe 3+ Desaturation of FA 66

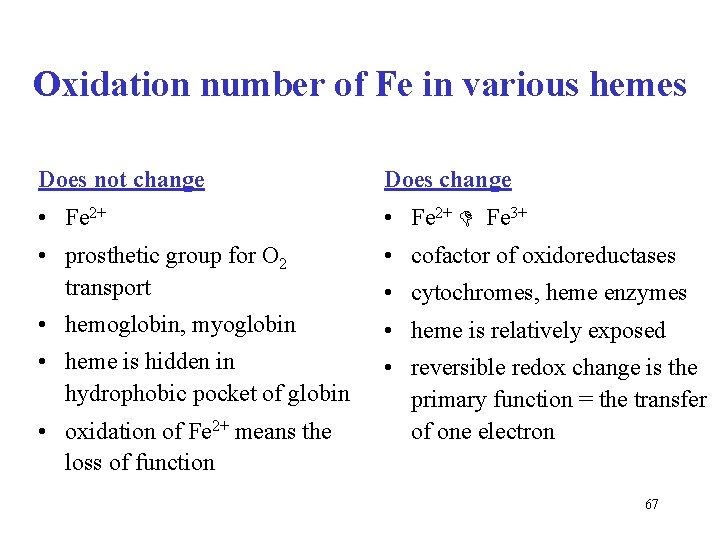
Oxidation number of Fe in various hemes Does not change Does change • Fe 2+ Fe 3+ • prosthetic group for O 2 transport • cofactor of oxidoreductases • hemoglobin, myoglobin • heme is relatively exposed • heme is hidden in hydrophobic pocket of globin • reversible redox change is the primary function = the transfer of one electron • oxidation of Fe 2+ means the loss of function • cytochromes, heme enzymes 67

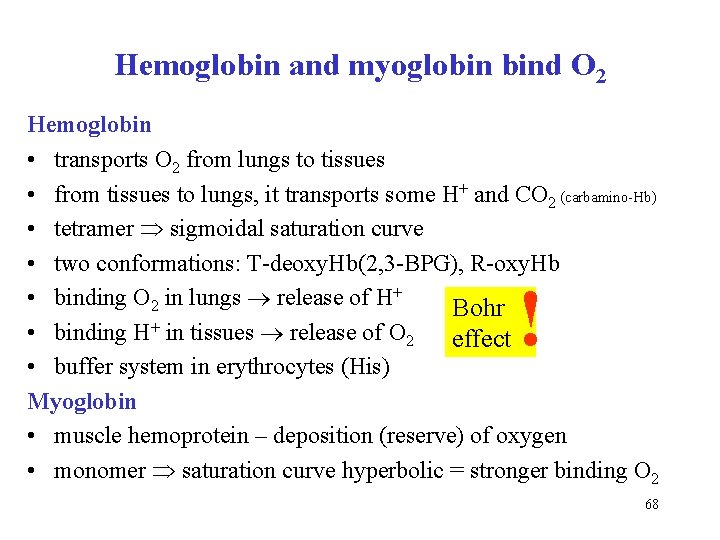
Hemoglobin and myoglobin bind O 2 Hemoglobin • transports O 2 from lungs to tissues • from tissues to lungs, it transports some H+ and CO 2 (carbamino-Hb) • tetramer sigmoidal saturation curve • two conformations: T-deoxy. Hb(2, 3 -BPG), R-oxy. Hb • binding O 2 in lungs release of H+ Bohr • binding H+ in tissues release of O 2 effect • buffer system in erythrocytes (His) Myoglobin • muscle hemoprotein – deposition (reserve) of oxygen • monomer saturation curve hyperbolic = stronger binding O 2 ! 68

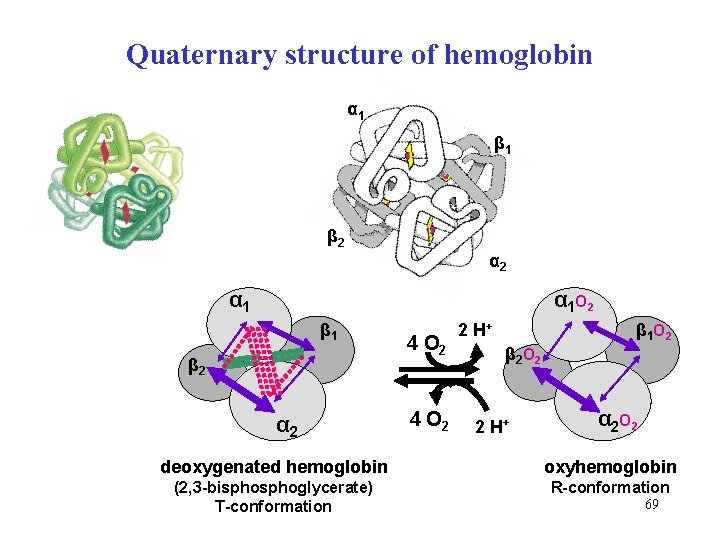
Quaternary structure of hemoglobin α 1 β 2 α 1 α 1 O 2 β 1 β 2 α 2 4 O 2 β 1 O 2 2 H+ β 2 O 2 2 H+ α 2 O 2 deoxygenated hemoglobin oxyhemoglobin (2, 3 -bisphoglycerate) T-conformation R-conformation 69

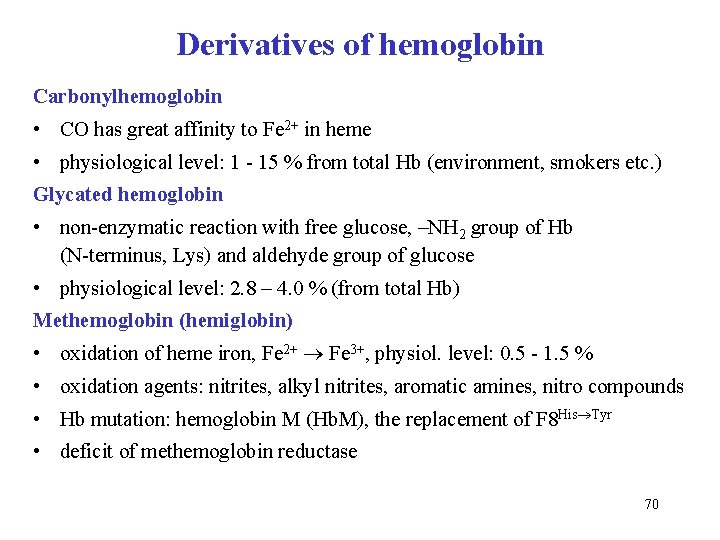
Derivatives of hemoglobin Carbonylhemoglobin • CO has great affinity to Fe 2+ in heme • physiological level: 1 - 15 % from total Hb (environment, smokers etc. ) Glycated hemoglobin • non-enzymatic reaction with free glucose, –NH 2 group of Hb (N-terminus, Lys) and aldehyde group of glucose • physiological level: 2. 8 – 4. 0 % (from total Hb) Methemoglobin (hemiglobin) • oxidation of heme iron, Fe 2+ Fe 3+, physiol. level: 0. 5 - 1. 5 % • oxidation agents: nitrites, alkyl nitrites, aromatic amines, nitro compounds • Hb mutation: hemoglobin M (Hb. M), the replacement of F 8 His Tyr • deficit of methemoglobin reductase 70

Language note: Methemoglobin • it has nothing to do with methyl group !!! • abbreviated from metahemoglobin • the prefix meta- (from Greek) indicates change, transformation, alteration • other examples with the prefix meta: metabolic (= catabolic + anabolic) metamorphosis, metazoan. . . 71

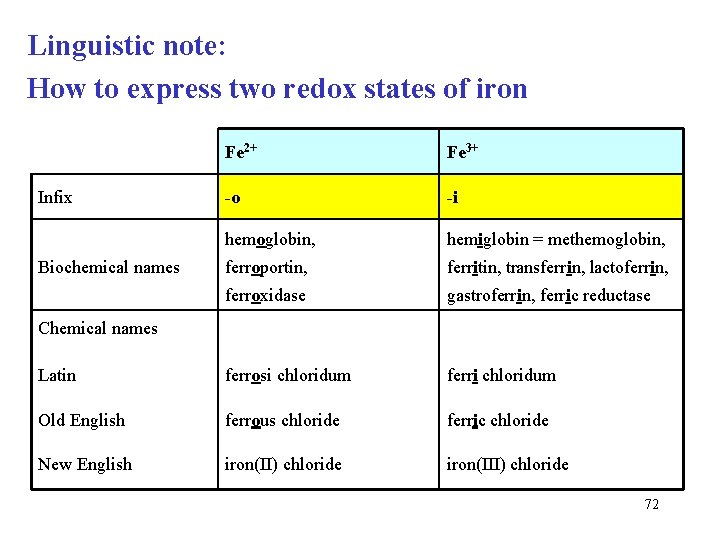
Linguistic note: How to express two redox states of iron Fe 2+ Fe 3+ -o -i hemoglobin, hemiglobin = methemoglobin, ferroportin, ferritin, transferrin, lactoferrin, ferroxidase gastroferrin, ferric reductase Latin ferrosi chloridum ferri chloridum Old English ferrous chloride ferric chloride New English iron(II) chloride iron(III) chloride Infix Biochemical names Chemical names 72

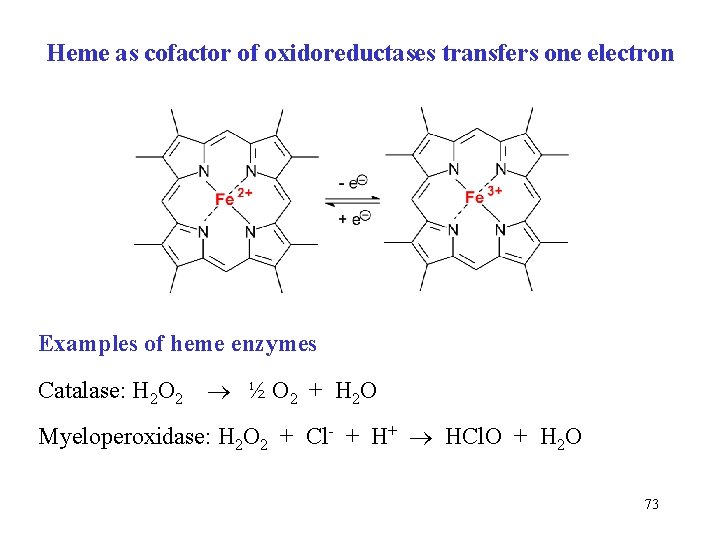
Heme as cofactor of oxidoreductases transfers one electron Examples of heme enzymes Catalase: H 2 O 2 ½ O 2 + H 2 O Myeloperoxidase: H 2 O 2 + Cl- + H+ HCl. O + H 2 O 73

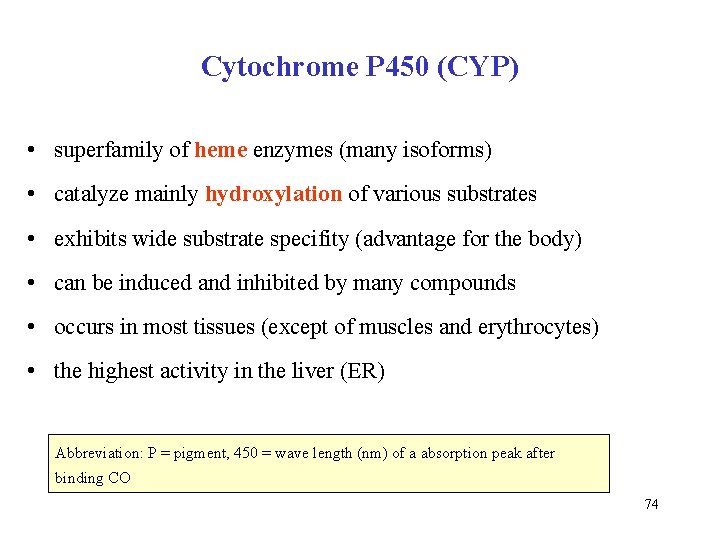
Cytochrome P 450 (CYP) • superfamily of heme enzymes (many isoforms) • catalyze mainly hydroxylation of various substrates • exhibits wide substrate specifity (advantage for the body) • can be induced and inhibited by many compounds • occurs in most tissues (except of muscles and erythrocytes) • the highest activity in the liver (ER) Abbreviation: P = pigment, 450 = wave length (nm) of a absorption peak after binding CO 74

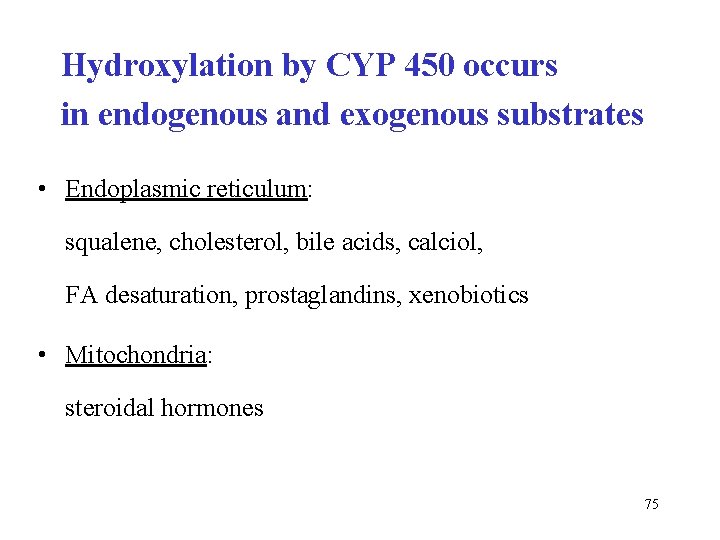
Hydroxylation by CYP 450 occurs in endogenous and exogenous substrates • Endoplasmic reticulum: squalene, cholesterol, bile acids, calciol, FA desaturation, prostaglandins, xenobiotics • Mitochondria: steroidal hormones 75

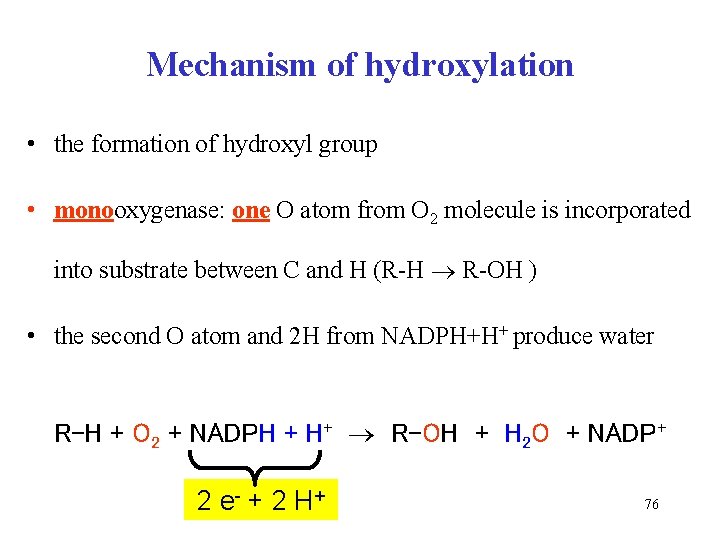
Mechanism of hydroxylation • the formation of hydroxyl group • monooxygenase: one O atom from O 2 molecule is incorporated into substrate between C and H (R-H R-OH ) • the second O atom and 2 H from NADPH+H+ produce water R-H + O 2 + NADPH + H+ R-OH + H 2 O + NADP+ 2 e- + 2 H + 76

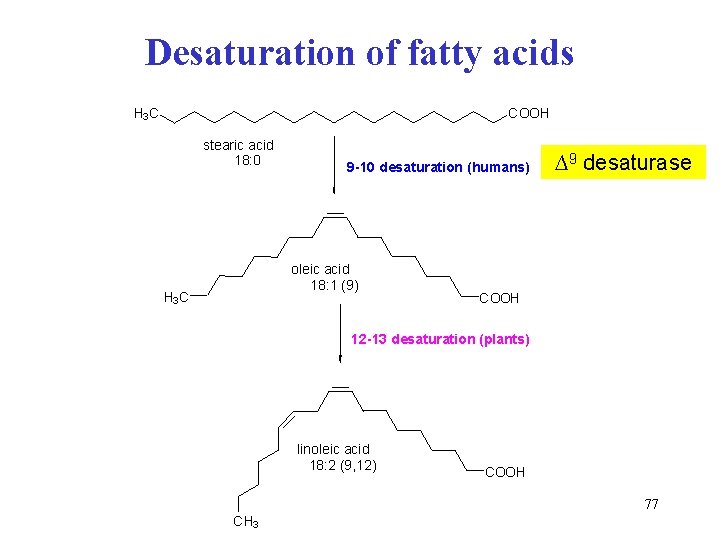
Desaturation of fatty acids H 3 C COOH stearic acid 18: 0 9 -10 desaturation (humans) oleic acid 18: 1 (9) H 3 C ∆9 desaturase COOH 12 -13 desaturation (plants) linoleic acid 18: 2 (9, 12) COOH 77 CH 3

Desaturation of FA requires cytochrome b 5 hydroxylation at C-10 dehydration 78
 Rate limiting step heme synthesis
Rate limiting step heme synthesis Krebs cycle main purpose
Krebs cycle main purpose Citric acid cycle also called
Citric acid cycle also called Citric acid cycle overall reaction
Citric acid cycle overall reaction Input of citric acid cycle
Input of citric acid cycle Net equation of citric acid cycle
Net equation of citric acid cycle Citric acid cycle definition
Citric acid cycle definition Where does the citric acid cycle occur
Where does the citric acid cycle occur Transamination of amino acids
Transamination of amino acids Net products of the citric acid cycle
Net products of the citric acid cycle Glycolysis overall reaction
Glycolysis overall reaction Definition of citric acid cycle
Definition of citric acid cycle Citric acid cycle
Citric acid cycle Citric acid cycle
Citric acid cycle Site:slidetodoc.com
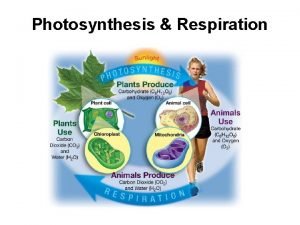
Site:slidetodoc.com Respiration diagram
Respiration diagram Photosynthesis citric acid cycle
Photosynthesis citric acid cycle Citric acid cycle and electron transport chain
Citric acid cycle and electron transport chain Photosynthesis citric acid cycle
Photosynthesis citric acid cycle Glyoxylate cycle
Glyoxylate cycle 2 methyl propyl butanoate
2 methyl propyl butanoate Who discovered citric acid
Who discovered citric acid Oxidation of citric acid
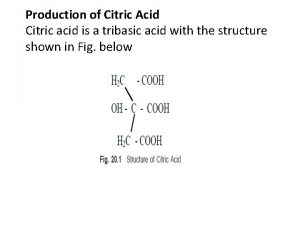
Oxidation of citric acid Citric acid formula
Citric acid formula Empirical formula of citric acid
Empirical formula of citric acid Citric acid
Citric acid Sixer toffee
Sixer toffee Chemical properties of citric acid
Chemical properties of citric acid Unc hematology and oncology
Unc hematology and oncology Heme breakdown
Heme breakdown Synthese de l'heme
Synthese de l'heme Heme group
Heme group Heme group
Heme group What color is bilirubin
What color is bilirubin Hypochromic
Hypochromic Skema metabolisme porfirin
Skema metabolisme porfirin Fatty acid synthesis
Fatty acid synthesis Fatty acid synthesis
Fatty acid synthesis Pyruvate amino acid synthesis
Pyruvate amino acid synthesis Fatty acid synthesis steps
Fatty acid synthesis steps Glycopeptide
Glycopeptide Cannizzaro reaction synthesis of benzoic acid
Cannizzaro reaction synthesis of benzoic acid Beta oxidation of fatty acids
Beta oxidation of fatty acids 9-which acid is not considered a strong acid?
9-which acid is not considered a strong acid? Example of acid-fast bacteria
Example of acid-fast bacteria Imides
Imides Acid fast and non acid fast bacteria
Acid fast and non acid fast bacteria Identifying lewis acids and bases practice
Identifying lewis acids and bases practice Lewis acid bronsted acid
Lewis acid bronsted acid 7 strong acids
7 strong acids Hcl lewis acid or base
Hcl lewis acid or base Is hcn acid or base
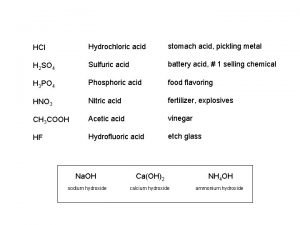
Is hcn acid or base Stomach acid vs battery acid
Stomach acid vs battery acid Hydrolysis of cyanide
Hydrolysis of cyanide Where can you find groundwater brainpop
Where can you find groundwater brainpop Phosphorus cycle pearson education
Phosphorus cycle pearson education Difference between phosphorus cycle and carbon cycle
Difference between phosphorus cycle and carbon cycle Difference between open cycle and closed cycle gas turbine
Difference between open cycle and closed cycle gas turbine Greedy cycle in computer architecture
Greedy cycle in computer architecture Chapter 5 principles of engine operation
Chapter 5 principles of engine operation Grille évaluation handball cycle 3
Grille évaluation handball cycle 3 Disadvantages of mhd generator
Disadvantages of mhd generator Cycle de consolidation
Cycle de consolidation Cycle 3 cycle de consolidation
Cycle 3 cycle de consolidation Water cycle the hydrologic cycle
Water cycle the hydrologic cycle Synthesis matrix template
Synthesis matrix template Activation-synthesis theory
Activation-synthesis theory Synthesis essay
Synthesis essay Experiencialism
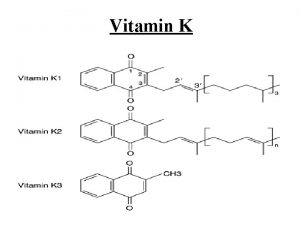
Experiencialism Vitamin k synthesis
Vitamin k synthesis Triacylglycerol synthesis
Triacylglycerol synthesis Bromine is
Bromine is Dehydration synthesis of amino acids
Dehydration synthesis of amino acids Xilinx synthesis tool
Xilinx synthesis tool Synthesis essay student samples
Synthesis essay student samples Synthesis essay definition
Synthesis essay definition What is a synthesis essay
What is a synthesis essay Paragraph synthesis
Paragraph synthesis Emerging technology synthesis
Emerging technology synthesis