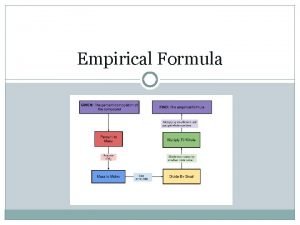
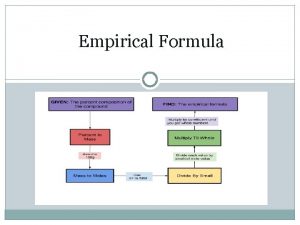
Determine the empirical formula of the compound containing
















- Slides: 17

Determine the empirical formula of the compound containing: 63. 50% silver 8. 25% nitrogen 28. 25% oxygen 1

Determine the empirical formula of the compound containing 52. 11% carbon 13. 14% hydrogen 34. 75% oxygen 2

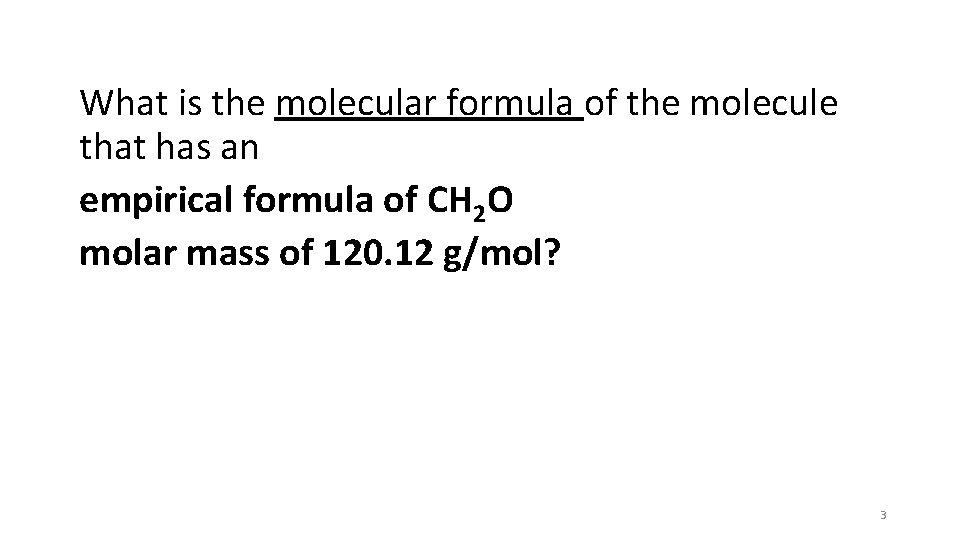
What is the molecular formula of the molecule that has an empirical formula of CH 2 O molar mass of 120. 12 g/mol? 3

A compound with a formula mass of 42. 08 amu is found to be 85. 64% carbon and 14. 36% hydrogen by mass. Find its molecular formula 4

Chemical analysis shows that citric acid contains 37. 51% C, 4. 20% H, and 58. 29% O. What is the empirical formula for citric acid? 5

The percent composition of diborane contain 78. 1% B and 21. 9% H. What is the empirical formula? 6

An unknown substance contained 0. 5219 g N, and 1. 4904 g O. Determine its empirical formula. 7

Styrene has an empirical formula of CH. It has a molecular weight equal to 104 amu. Determine it’s molecular formula. 8

What is the empirical formula if the compound consist 21. 2% N 6. 1% H 24. 2% S 48. 5% O 9

Empirical formula of a substance is CH 2 O. Molar mass is 180. What is the molecular formula? 10

Find the empirical formula for a substance with 88. 8% copper 11. 2% oxygen 11

Find the empirical formula for a substance with 40% carbon 6. 7% hydrogen 53. 3% oxygen 12

Find the empirical formula for a substance with 92. 3% carbon 7. 7% hydrogen 13

Find the empirical formula for a substance with 70. 0% iron 30. 0% oxygen 14

Find the empirical formula for a substance with 5. 88% hydrogen 94. 12% oxygen 15

A compound with empirical formula of NO 2 has a molar mass of 92 g/ml. Find molecular formula 16

A compound with molar mass of 60. 10 g/ml. 39. 97% C 13. 41% H 46. 62% N Find molecular formula 17
 Formula empirik
Formula empirik Determine the empirical formula
Determine the empirical formula What is the empirical formula
What is the empirical formula How to find the empirical formula
How to find the empirical formula What is the term for a compound containing two nonmetals?
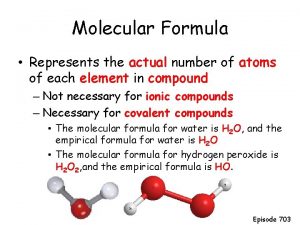
What is the term for a compound containing two nonmetals? Empirical formula vs molecular formula
Empirical formula vs molecular formula How to find the empirical formula
How to find the empirical formula Definition of molecular formula
Definition of molecular formula Empirical formula vs
Empirical formula vs Empirical formula
Empirical formula Empirical formula pogil
Empirical formula pogil Empirical formula to molecular formula
Empirical formula to molecular formula Empirical formula vs
Empirical formula vs Molecular formula
Molecular formula Simple compound complex and compound-complex sentences quiz
Simple compound complex and compound-complex sentences quiz Simple vs compound subject
Simple vs compound subject Compound complex simple sentences quiz
Compound complex simple sentences quiz Compound sentence with compound subject
Compound sentence with compound subject