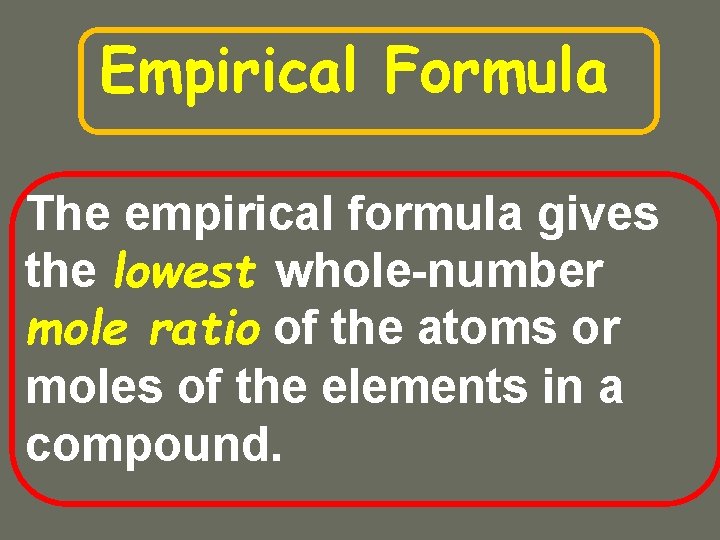
Empirical Formula The empirical formula gives the lowest


- Slides: 15

Empirical Formula The empirical formula gives the lowest whole-number mole ratio of the atoms or moles of the elements in a compound.

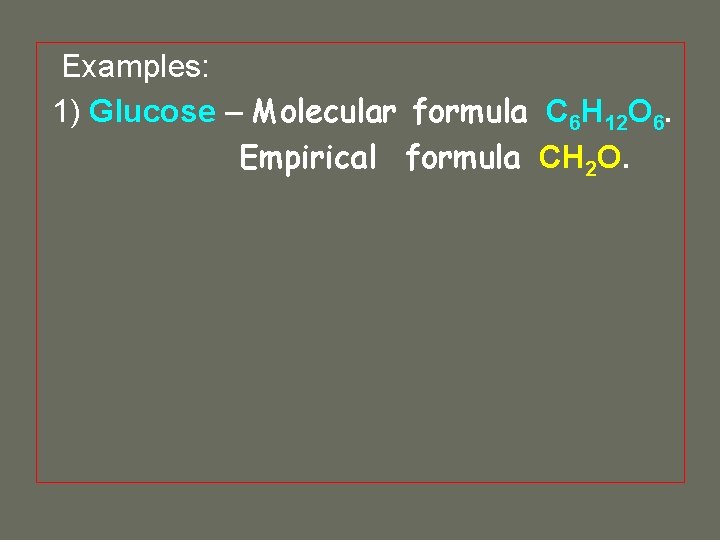
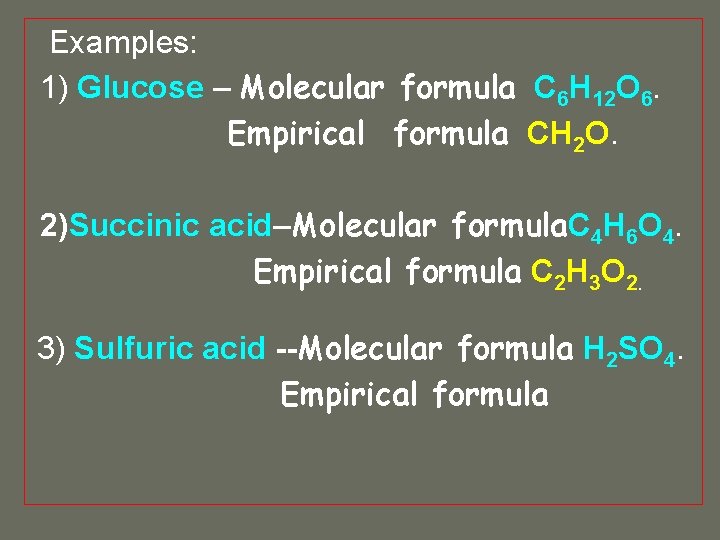
Examples: 1) Glucose – Molecular formula C 6 H 12 O 6. Empirical formula

Examples: 1) Glucose – Molecular formula C 6 H 12 O 6. Empirical formula CH 2 O.

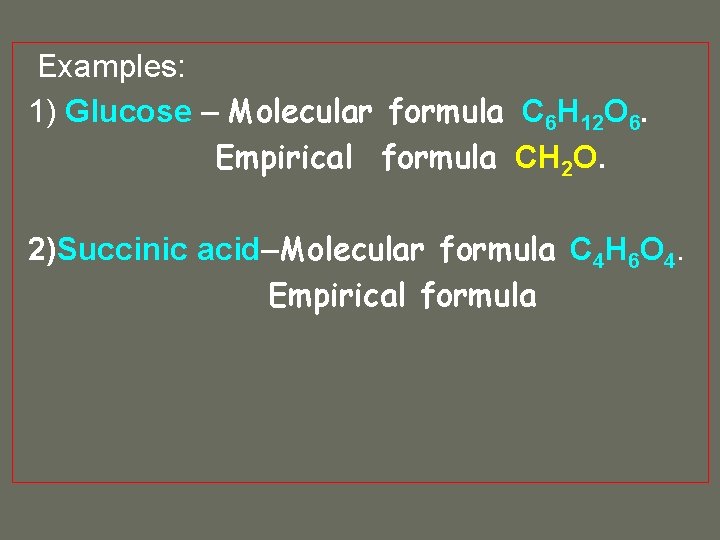
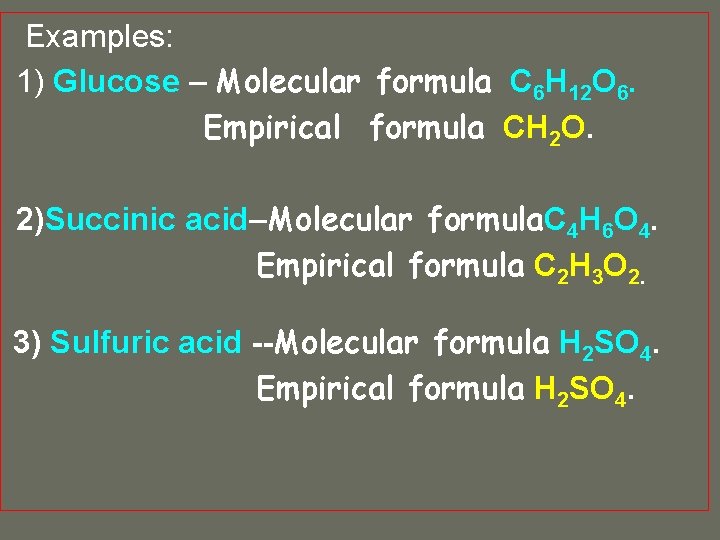
Examples: 1) Glucose – Molecular formula C 6 H 12 O 6. Empirical formula CH 2 O. 2)Succinic acid–Molecular formula C 4 H 6 O 4. Empirical formula

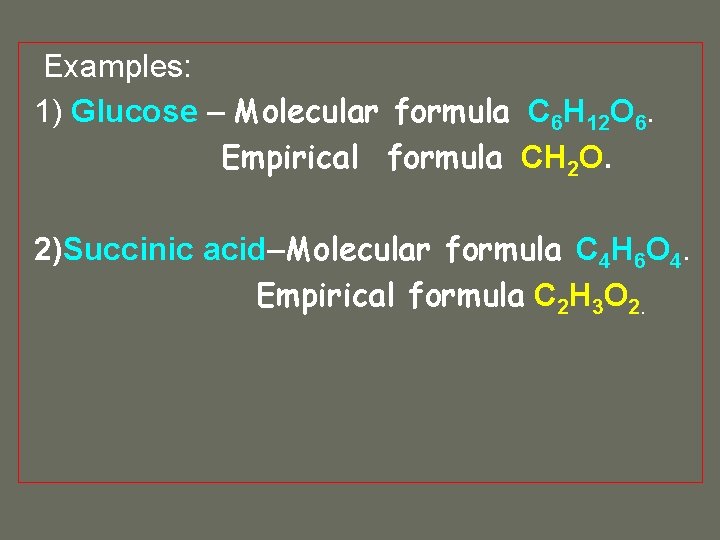
Examples: 1) Glucose – Molecular formula C 6 H 12 O 6. Empirical formula CH 2 O. 2)Succinic acid–Molecular formula C 4 H 6 O 4. Empirical formula C 2 H 3 O 2.

Examples: 1) Glucose – Molecular formula C 6 H 12 O 6. Empirical formula CH 2 O. 2)Succinic acid–Molecular formula. C 4 H 6 O 4. Empirical formula C 2 H 3 O 2. 3) Sulfuric acid --Molecular formula H 2 SO 4. Empirical formula

Examples: 1) Glucose – Molecular formula C 6 H 12 O 6. Empirical formula CH 2 O. 2)Succinic acid–Molecular formula. C 4 H 6 O 4. Empirical formula C 2 H 3 O 2. 3) Sulfuric acid --Molecular formula H 2 SO 4. Empirical formula H 2 SO 4.

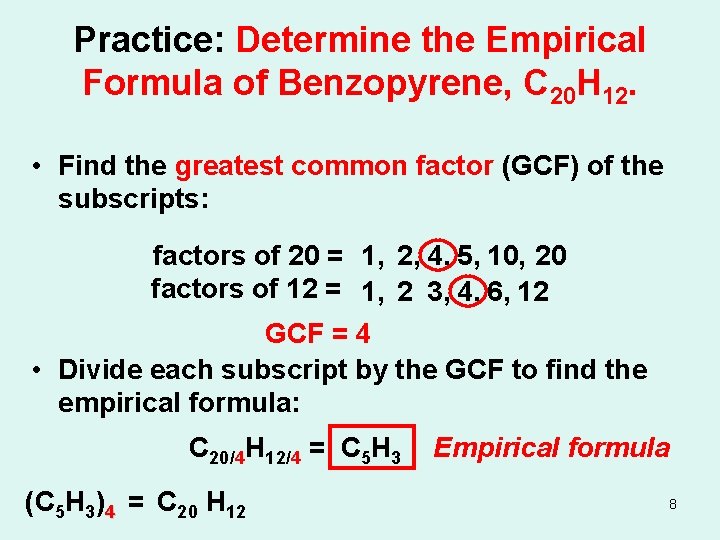
Practice: Determine the Empirical Formula of Benzopyrene, C 20 H 12. • Find the greatest common factor (GCF) of the subscripts: factors of 20 = 1, 2, 4, 5, 10, 20 factors of 12 = 1, 2 3, 4, 6, 12 GCF = 4 • Divide each subscript by the GCF to find the empirical formula: C 20/4 H 12/4 = C 5 H 3 (C 5 H 3)4 = C 20 H 12 Empirical formula 8

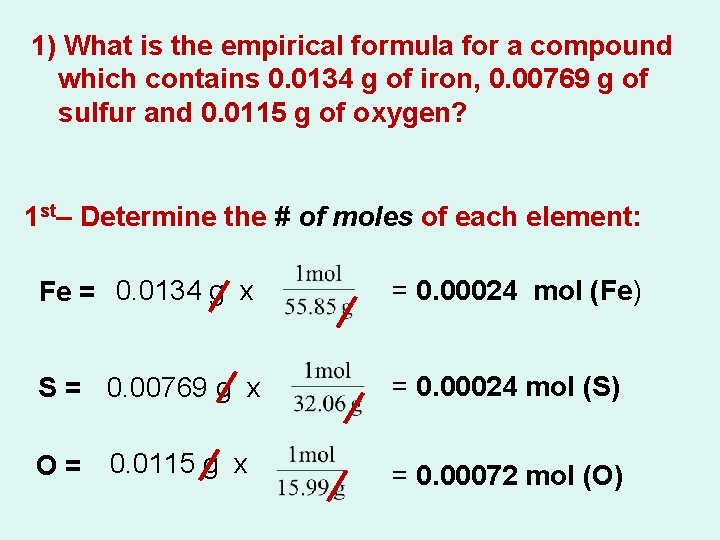
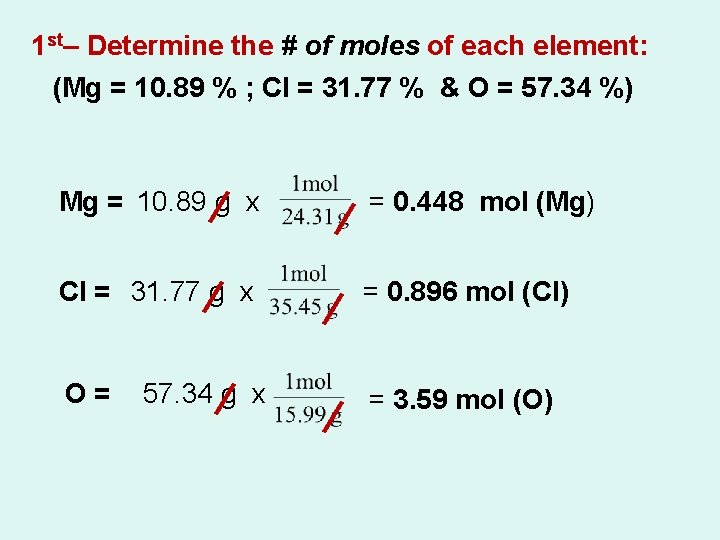
1) What is the empirical formula for a compound which contains 0. 0134 g of iron, 0. 00769 g of sulfur and 0. 0115 g of oxygen? 1 st– Determine the # of moles of each element: Fe = 0. 0134 g x = 0. 00024 mol (Fe) S = 0. 00769 g x = 0. 00024 mol (S) O= 0. 0115 g x = 0. 00072 mol (O)

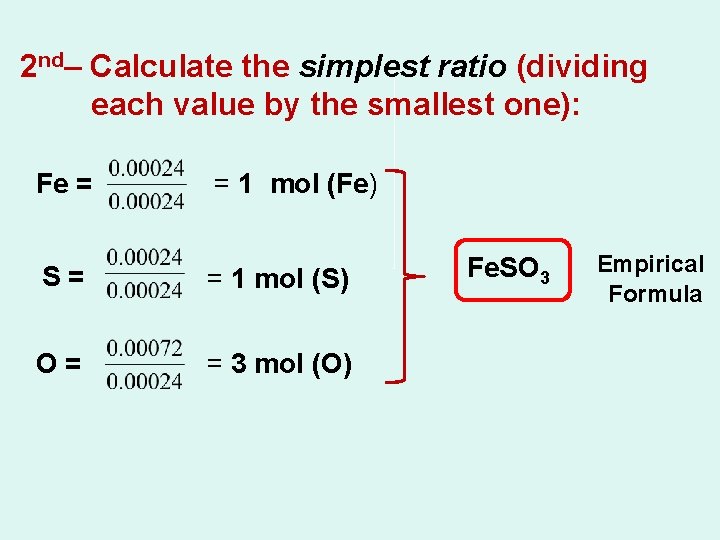
2 nd– Calculate the simplest ratio (dividing each value by the smallest one): Fe = = 1 mol (Fe) S= = 1 mol (S) O= = 3 mol (O) Fe. SO 3 Empirical Formula

2) Determine the empirical formula for a compound that contains 10. 89 % magnesium, 31. 77 % chlorine, and 57, 34 % oxygen?

1 st– Determine the # of moles of each element: (Mg = 10. 89 % ; Cl = 31. 77 % & O = 57. 34 %) Mg = 10. 89 g x = 0. 448 mol (Mg) Cl = 31. 77 g x = 0. 896 mol (Cl) O= = 3. 59 mol (O) 57. 34 g x

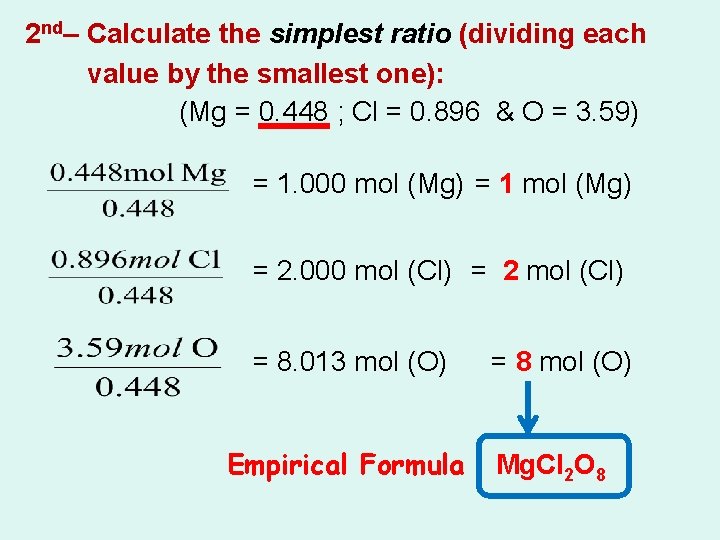
2 nd– Calculate the simplest ratio (dividing each value by the smallest one): (Mg = 0. 448 ; Cl = 0. 896 & O = 3. 59) = 1. 000 mol (Mg) = 1 mol (Mg) = 2. 000 mol (Cl) = 2 mol (Cl) = 8. 013 mol (O) Empirical Formula = 8 mol (O) Mg. Cl 2 O 8

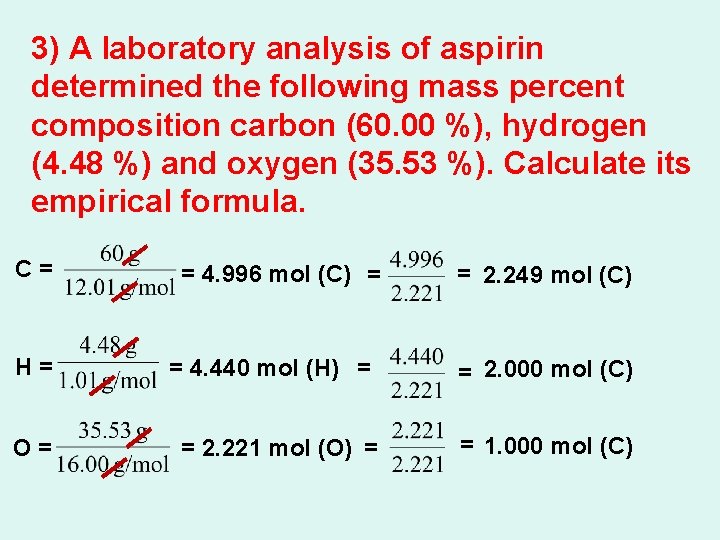
3) A laboratory analysis of aspirin determined the following mass percent composition carbon (60. 00 %), hydrogen (4. 48 %) and oxygen (35. 53 %). Calculate its empirical formula. C= = 4. 996 mol (C) = = 2. 249 mol (C) H= = 4. 440 mol (H) = = 2. 000 mol (C) O= = 2. 221 mol (O) = = 1. 000 mol (C)

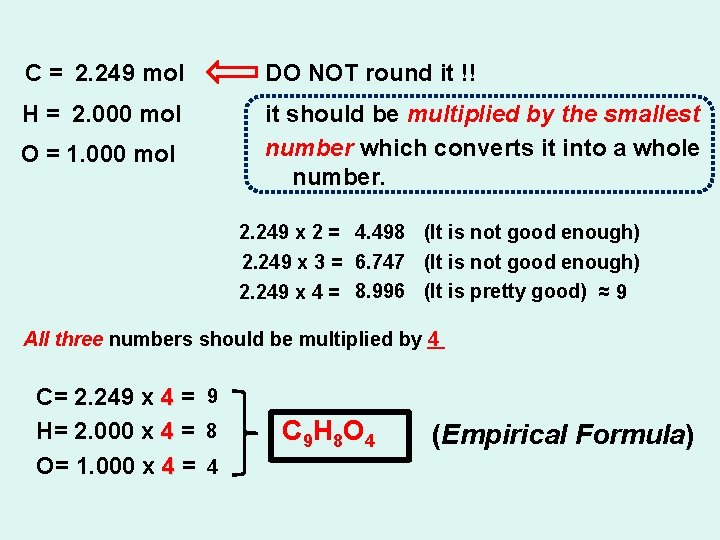
C = 2. 249 mol DO NOT round it !! H = 2. 000 mol it should be multiplied by the smallest number which converts it into a whole number. O = 1. 000 mol 2. 249 x 2 = 4. 498 (It is not good enough) 2. 249 x 3 = 6. 747 (It is not good enough) 2. 249 x 4 = 8. 996 (It is pretty good) ≈ 9 All three numbers should be multiplied by 4 C= 2. 249 x 4 = 9 H= 2. 000 x 4 = 8 O= 1. 000 x 4 = 4 C 9 H 8 O 4 (Empirical Formula)