Atomic Structure Chapter 3 3 1 Atomic Theory































![3. 3 5) Which element has the electron configuration 6 2 [Ar] 3 d 3. 3 5) Which element has the electron configuration 6 2 [Ar] 3 d](https://slidetodoc.com/presentation_image_h2/72416944529f3cb16ab0ce273c7f2607/image-73.jpg)












- Slides: 88

Atomic Structure Chapter 3

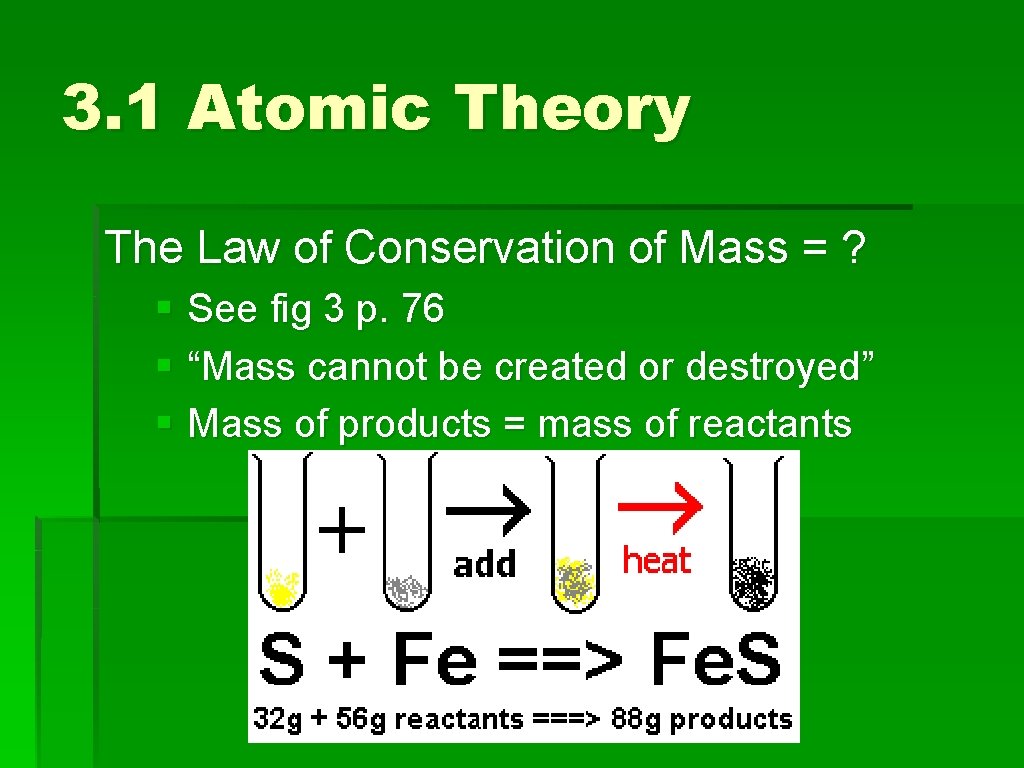
3. 1 Atomic Theory The Law of Conservation of Mass = ? § See fig 3 p. 76 § “Mass cannot be created or destroyed” § Mass of products = mass of reactants

3. 1 Atomic Theory 5. State the five principles of Dalton’s Atomic Theory: p. 78 a. All matter is composed of atoms b. Atoms of a given element are identical in their physical and chemical properties • One sodium atom is the same as all other sodium atoms c. Atoms of different elements differ in their physical and chemical properties. • A sodium atom is different from a potassium atom d. Atoms of different elements combine in simple, wholenumber ratios to form compounds. e. g water = H 20 = has two H atoms for every O atom e. In chemical reactions, atoms are rearranged but are never created or destroyed.

Dalton University of Manchester

3. 2 Learning Targets Students learn about the structure of the atom § Subatomic particles § Atomic number and mass number § Isotopes Think about it…. . What does the inside of an atom look like?

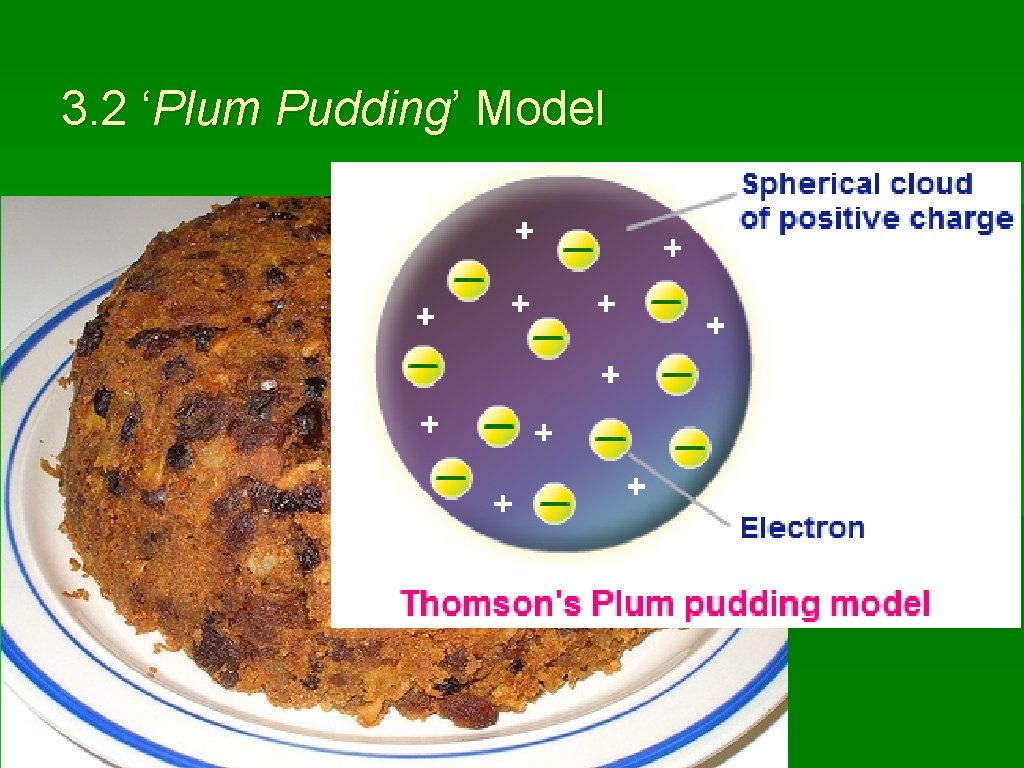
3. 2 ‘Plum Pudding’ Model

Think about it….

3. 2 The nucleus • Contains most of the mass of an atom th • Is only 1/10 000 of the size of the entire atom • The nucleus of made up of protons and neutrons. (p. 82)


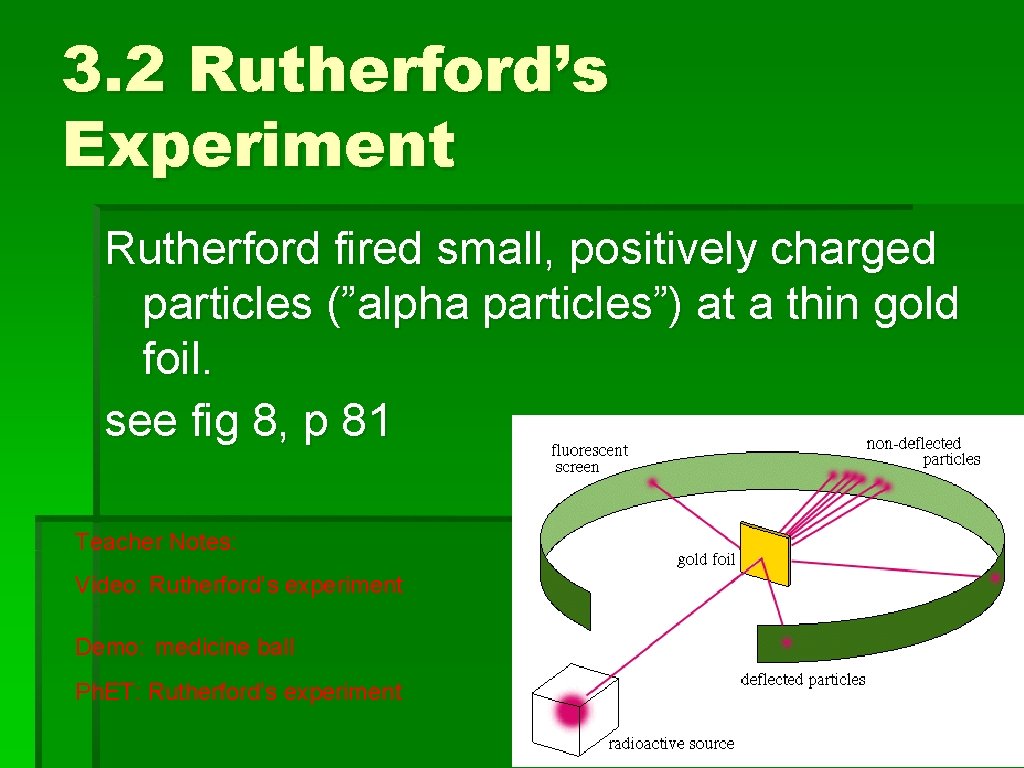
3. 2 Rutherford’s Experiment Rutherford fired small, positively charged particles (”alpha particles”) at a thin gold foil. see fig 8, p 81 Teacher Notes: Video: Rutherford’s experiment Demo: medicine ball Ph. ET: Rutherford’s experiment

3. 2 Rutherford’s Experiment 18. In the Rutherford gold foil experiment, positively charged alpha particles were directed at a thin gold foil. It was found that most of the particles passed through the foil undeflected. However, a small number of particles were deflected, some even backward. These two observations suggested that most of the volume of an atom is empty space but that there was a central core with a charge that repelled the positive particles. This core is a very small part of an atom. It contains most of the mass of the atom and is called the nucleus.

Electrostatic attraction What is the force that holds the atom together? Electrostatic force What happens when you rub a piece of fabric on a comb or a balloon? Check it out! It’s quite shocking Demo: Van der Graaf & Wimshurst generators Activity: electrostatic attraction Van der Graaf generator

3. 2 Electrostatic forces “Opposite charges attract”(attract/repel) “Like charges repel” (attract/repel) See Fig 10 Electrostatic Attraction Teacher Notes: Ph. ET balloon sim

3. 2 Atoms and isotopes Worksheet: Atoms and Their Isotopes # of protons = # of electrons = atomic # # of neutrons = mass # - atomic #

3. 2 Atoms and isotopes Concept Review p. 4 #19

3. 2 Atoms and Isotopes See Fig 14 p. 88 Isotopes of He Table 4 p. 88 Isotopes of Lead Practice (mass # and atomic #): P 86 #1 -4 Practice (isotopes): p. 89 #1, 2 Extras: p. 89 Section Review # 4 -5

3. 2 Atoms and Isotopes Practice: p. 108 # 30, 31, 32, 33 p. 108 # 34, 35, 36, 37

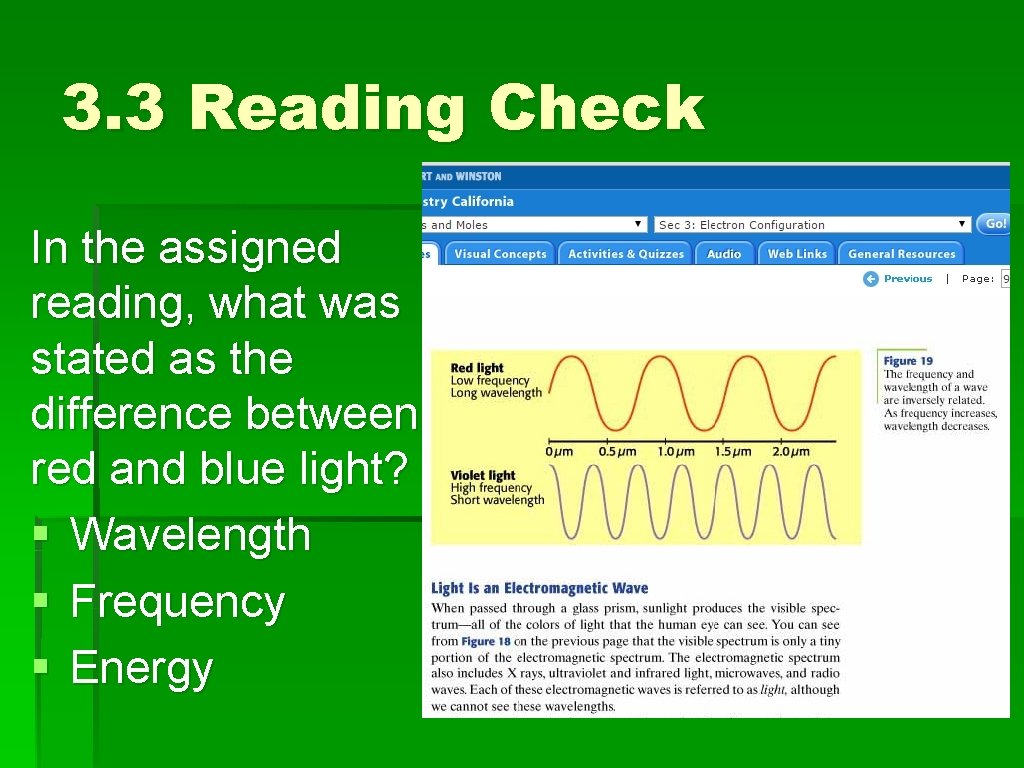
3. 3 Reading Check In the assigned reading, what was stated as the difference between red and blue light? § Wavelength § Frequency § Energy

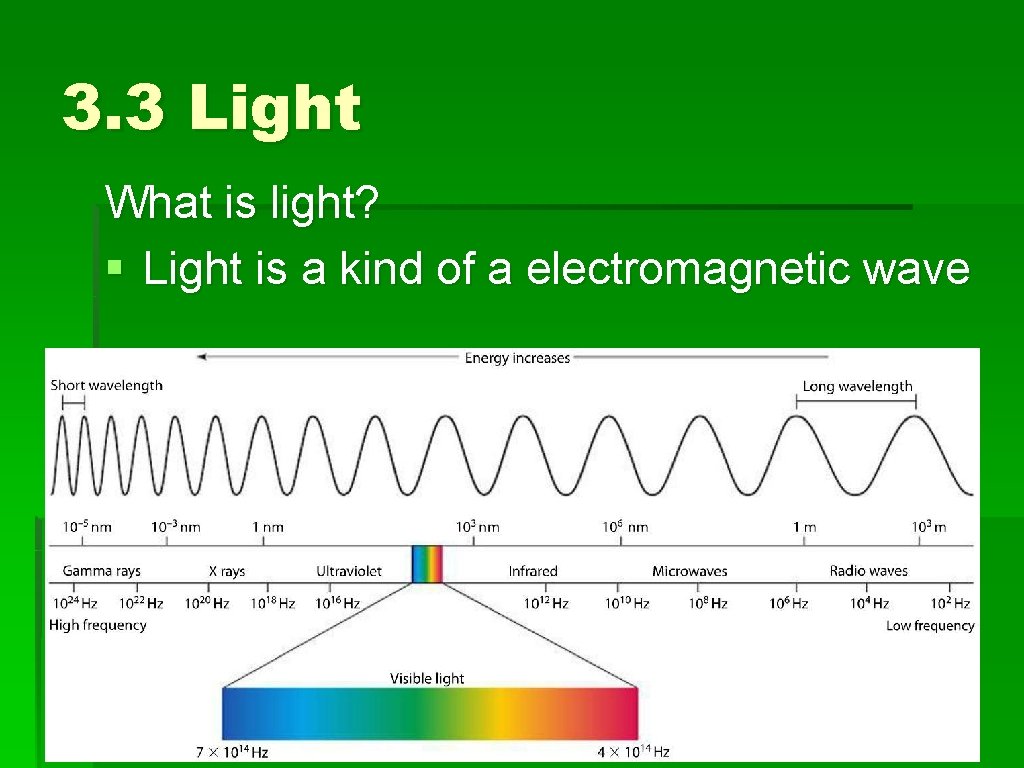
3. 3 Light What is light? § Light is a kind of a electromagnetic wave

3. 3 Learning Objectives Flame test lab: Why do different elements give different colors when we put them in a flame?

Frayer: Electromagnetic radiation Definition Electromagneticradiation(EMR)isaformofe nergythatisproducedbyavibratingelectrican dmagnetic field. Electromagnetic radiation (EMR) is a form of energy that is produced by a vibrating electric and magnetic field. http: //micro. magnet. fsu. edu/primer/java/electromagnetic/

3. 3 Electromagnetic Radiation Facts • Frequency: the number of waves that pass a point in one second. • unit = Hertz (Hz)

3. 3 Electromagnetic Radiation Example • Microwave frequency ~ GHz

3. 3 Electromagnetic Radiation Facts • The higher the frequency, the higher (higher/lower) the energy of a wave.

3. 3 Electromagnetic Radiation • speed of light = 299 792 458 m/s • rewrite in sci notn to 1 sig fig • = 3 x 108 m/s

Cute Baby Wabbit

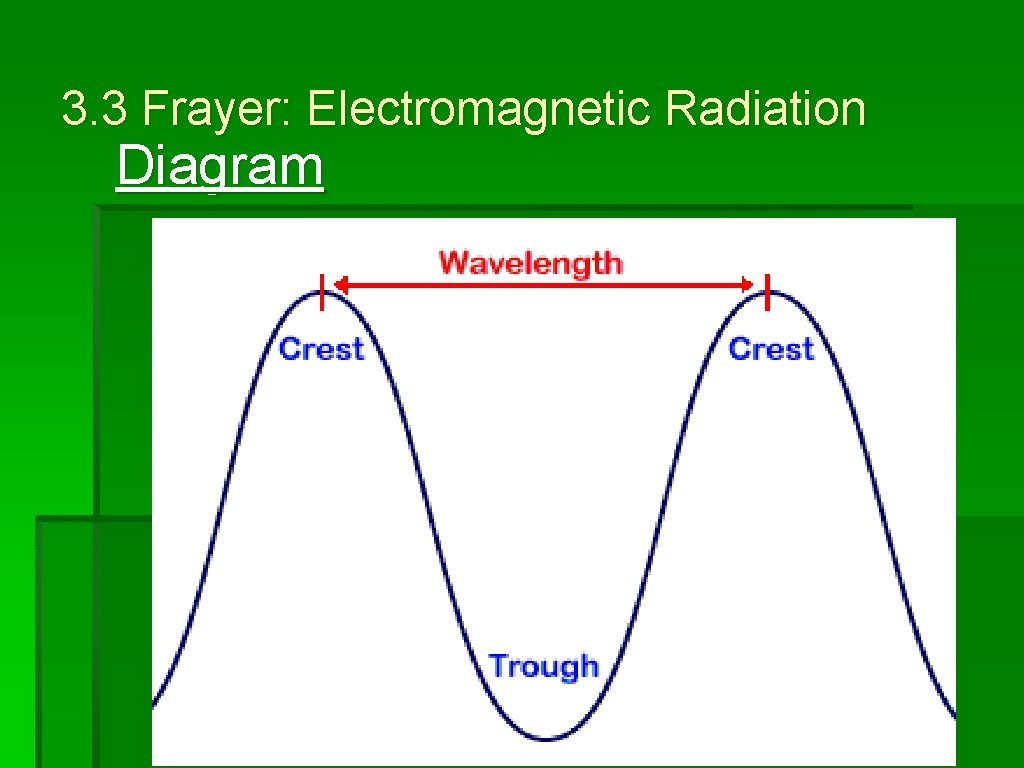
3. 3 Frayer: Electromagnetic Radiation Diagram

3. 3 Electromagnetic Radiation Facts: • Frequency and wavelength are inversely (directly/inversely) proportional. Teacher notes: Video: The electromagnetic spectrum (bestof. Science)

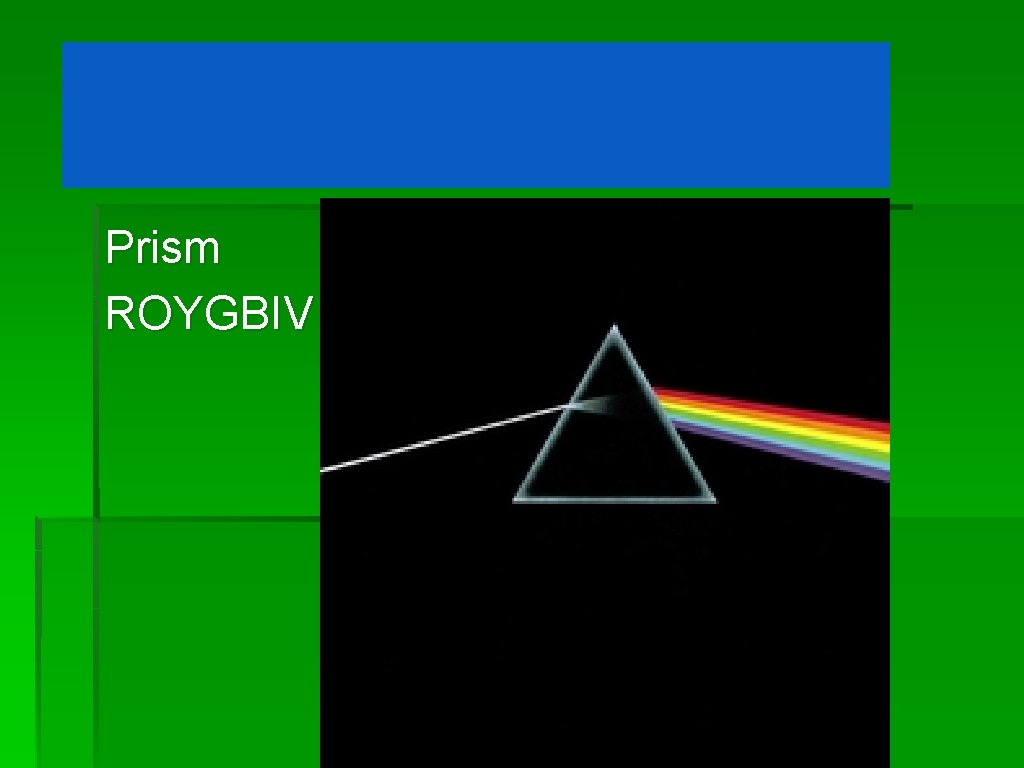
Dark Side of the Moon – Pink Floyd (1973) Prism ROYGBIV

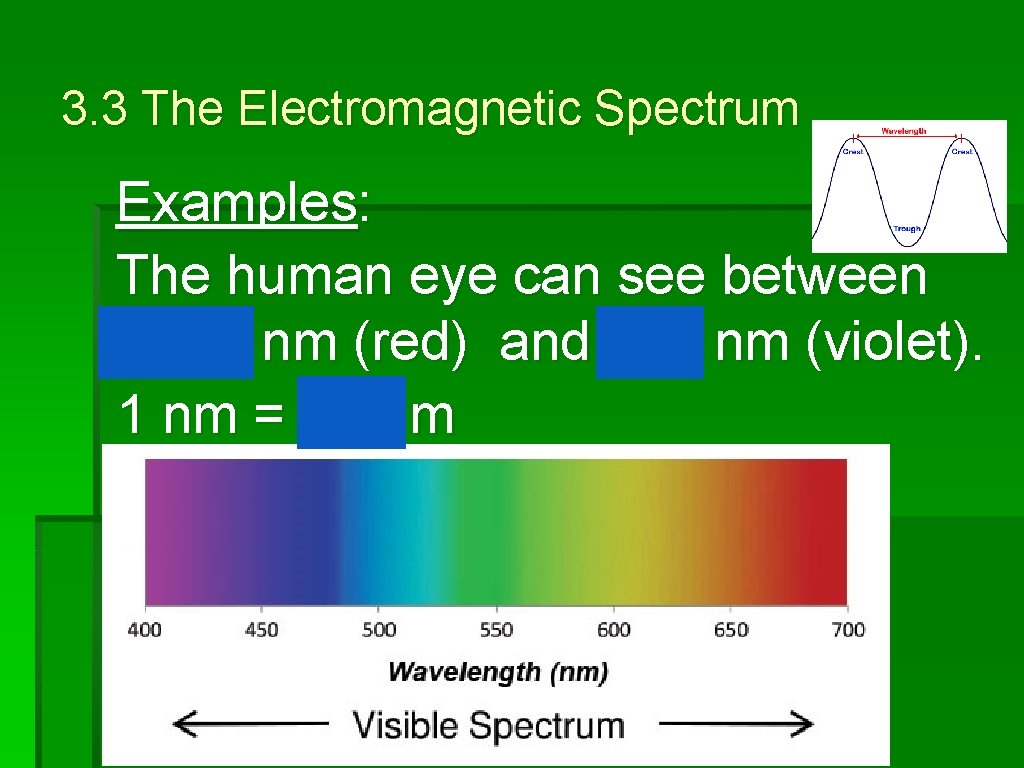
3. 3 The Electromagnetic Spectrum Examples: The human eye can see between 700 nm (red) and 400 nm (violet). -9 1 nm = 10 m

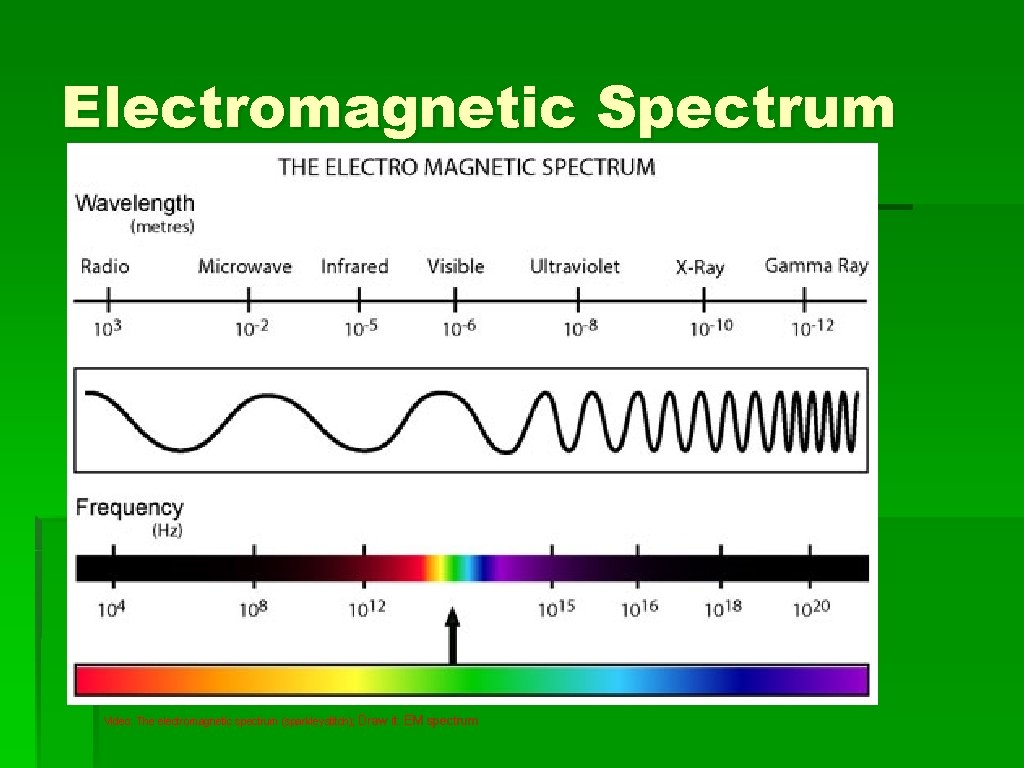
Electromagnetic Spectrum Video: The electromagnetic spectrum (sparkleystitch); Draw it: EM spectrum

3. 3 Learning Objectives § § The Bohr theory of the atomic orbits Electromagnetic spectrum Quantum numbers Writing electron configurations § 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6

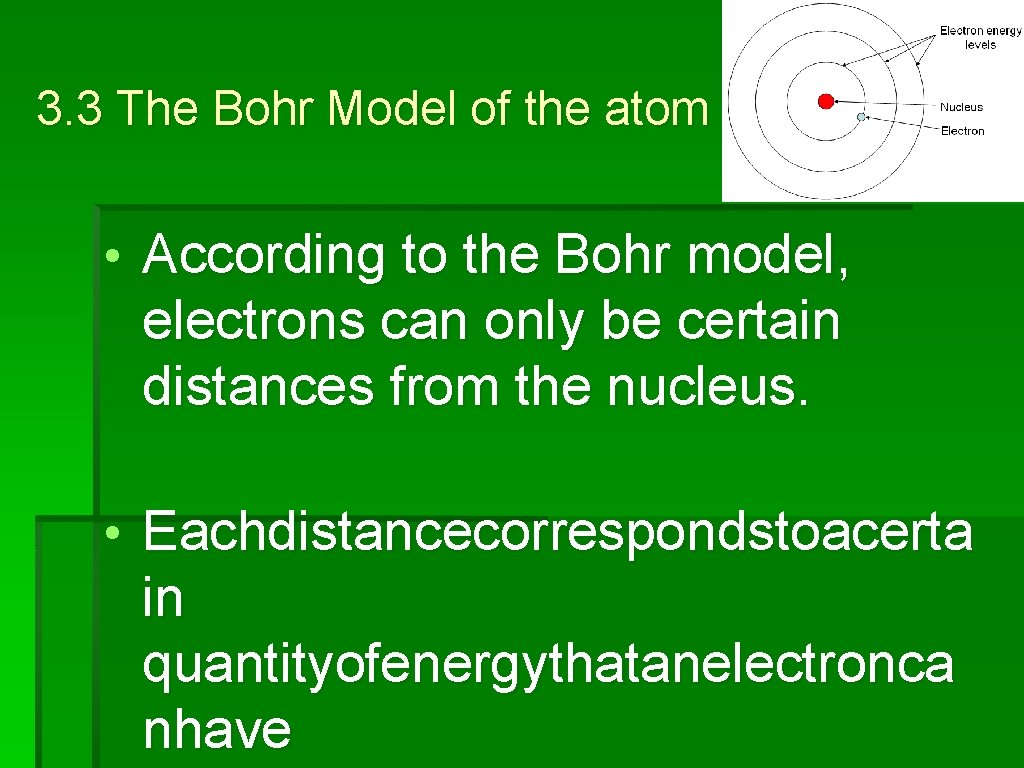
3. 3 The Bohr Model of the atom Notes: • Accordingtothe. Bohrmodelelectron s canonlybecertaindistancesfrom thenucleus Neils Bohr

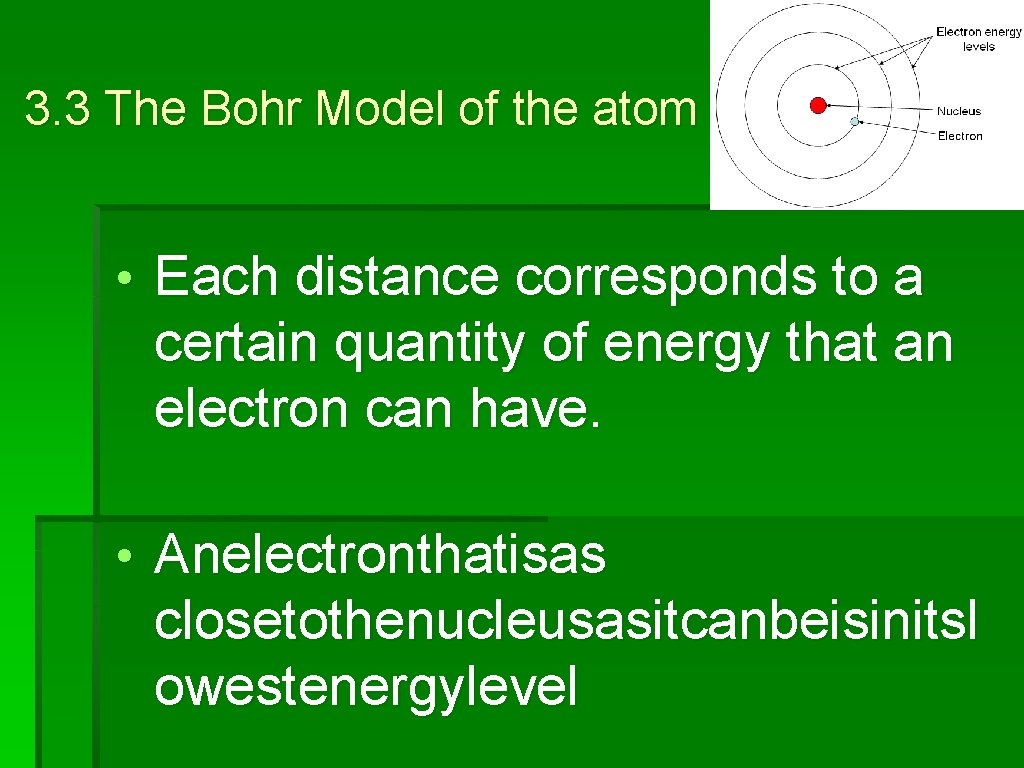
3. 3 The Bohr Model of the atom • According to the Bohr model, electrons can only be certain distances from the nucleus. • Eachdistancecorrespondstoacerta in quantityofenergythatanelectronca nhave

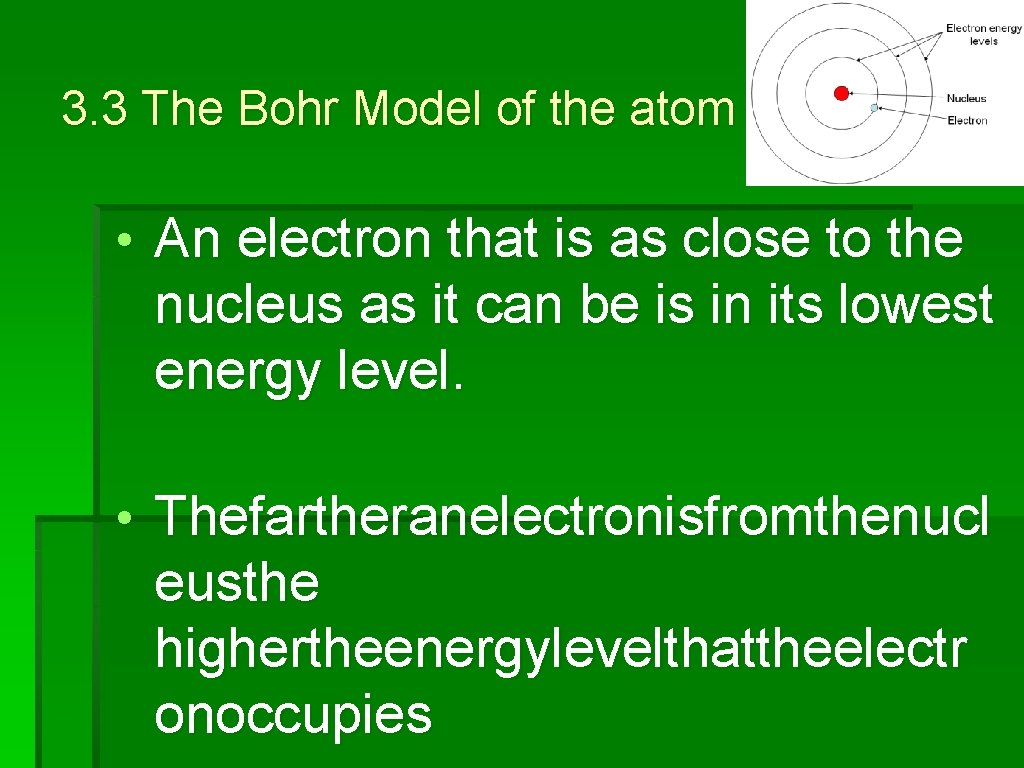
3. 3 The Bohr Model of the atom • Each distance corresponds to a certain quantity of energy that an electron can have. • Anelectronthatisas closetothenucleusasitcanbeisinitsl owestenergylevel

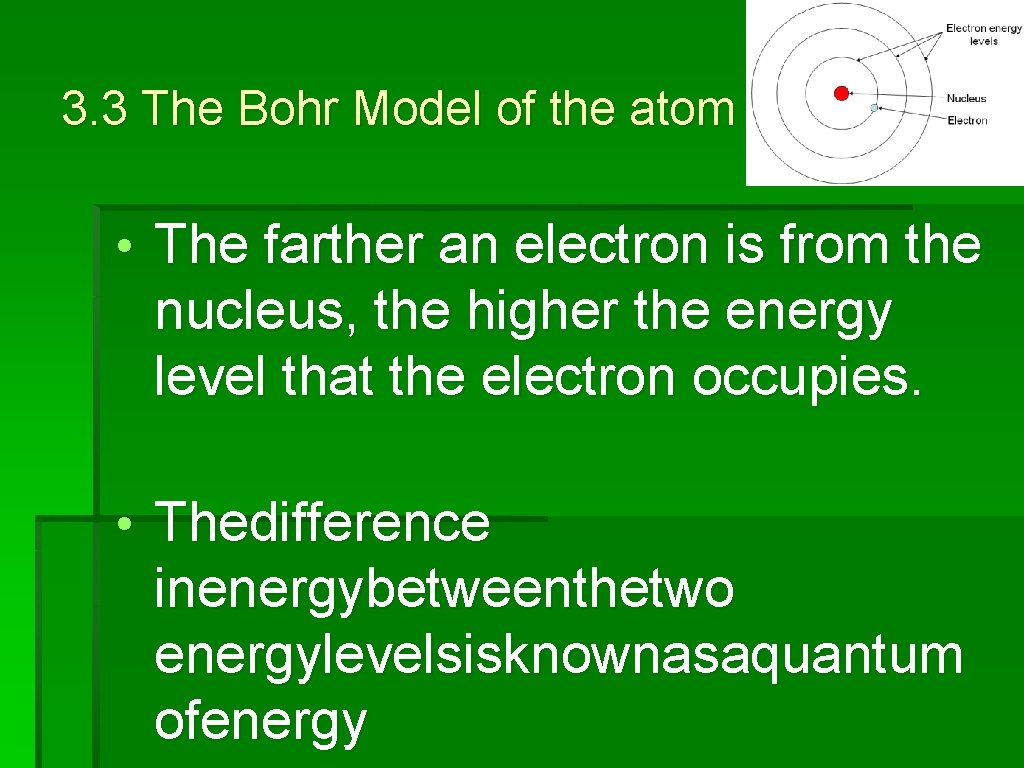
3. 3 The Bohr Model of the atom • An electron that is as close to the nucleus as it can be is in its lowest energy level. • Thefartheranelectronisfromthenucl eusthe highertheenergylevelthattheelectr onoccupies

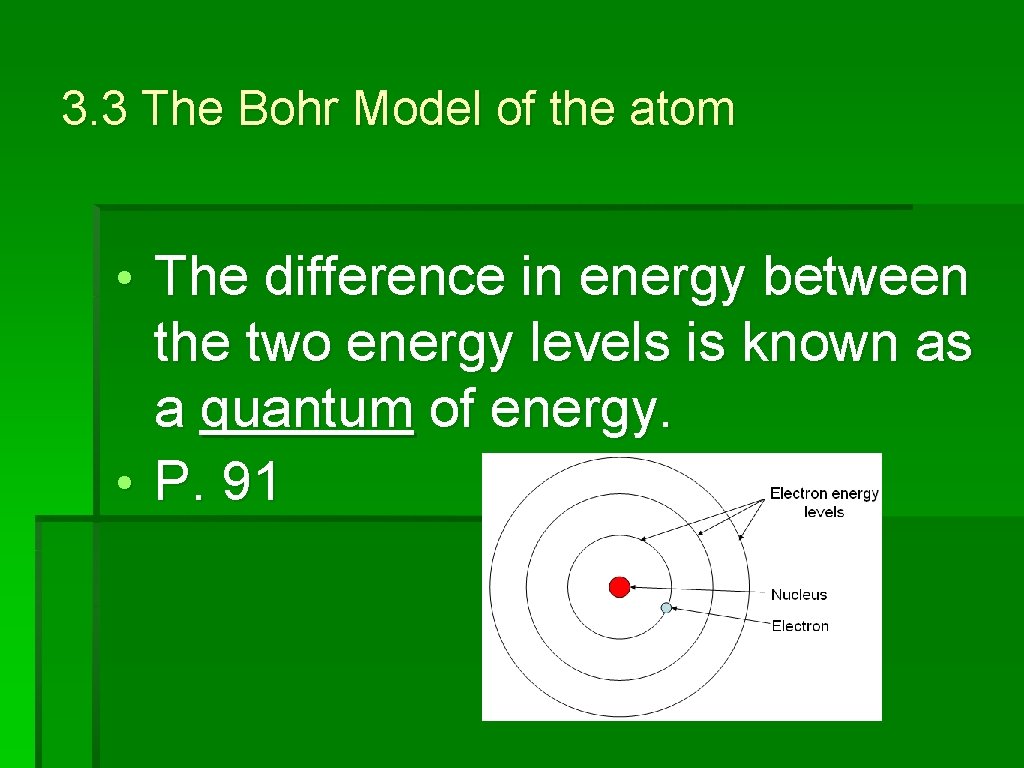
3. 3 The Bohr Model of the atom • The farther an electron is from the nucleus, the higher the energy level that the electron occupies. • Thedifference inenergybetweenthetwo energylevelsisknownasaquantum ofenergy

3. 3 The Bohr Model of the atom • The difference in energy between the two energy levels is known as a quantum of energy. • P. 91

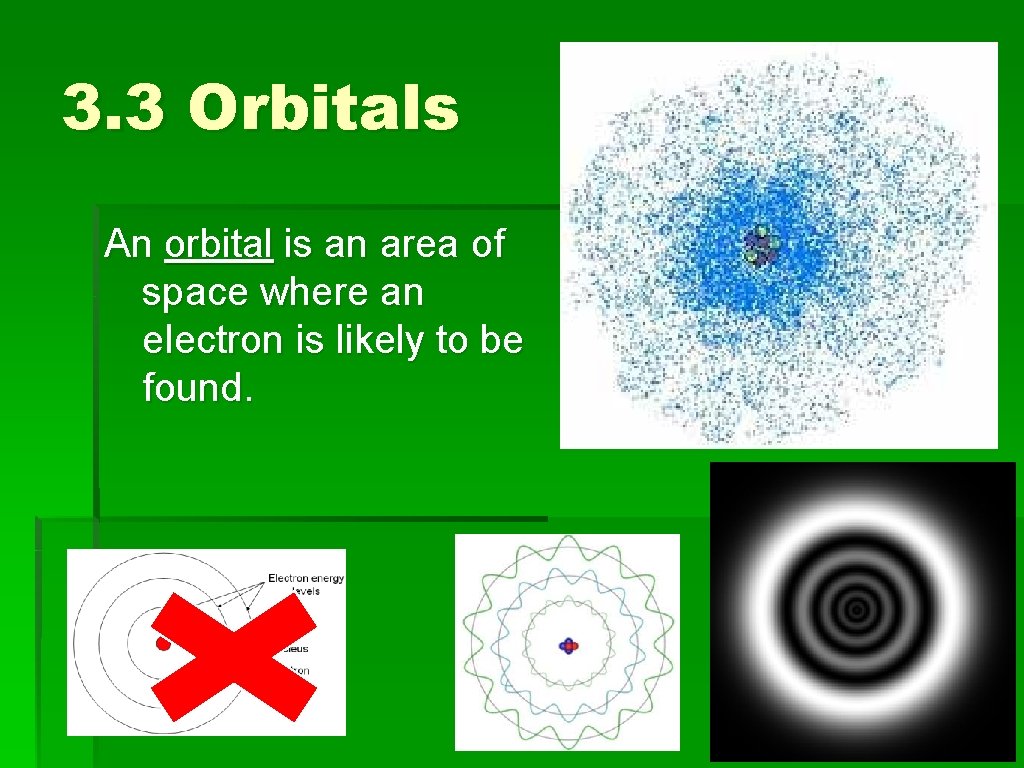
3. 3 Orbitals An orbital is an area of space where an electron is likely to be found.

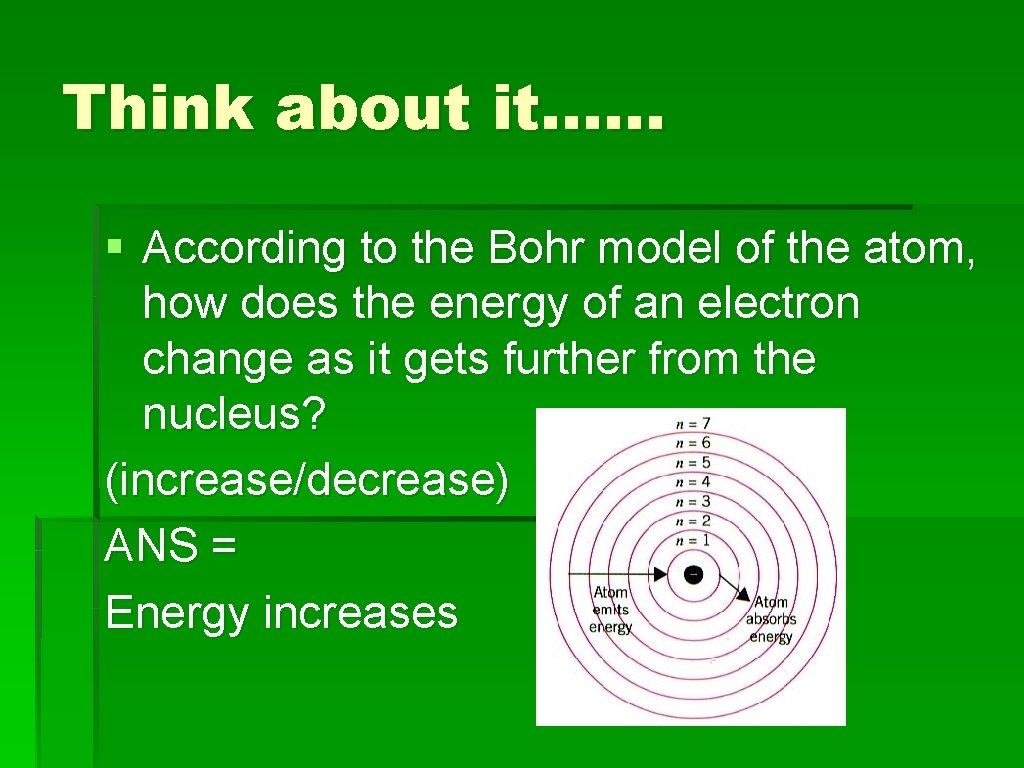
Think about it…… § According to the Bohr model of the atom, how does the energy of an electron change as it gets further from the nucleus? (increase/decrease) ANS = Energy increases

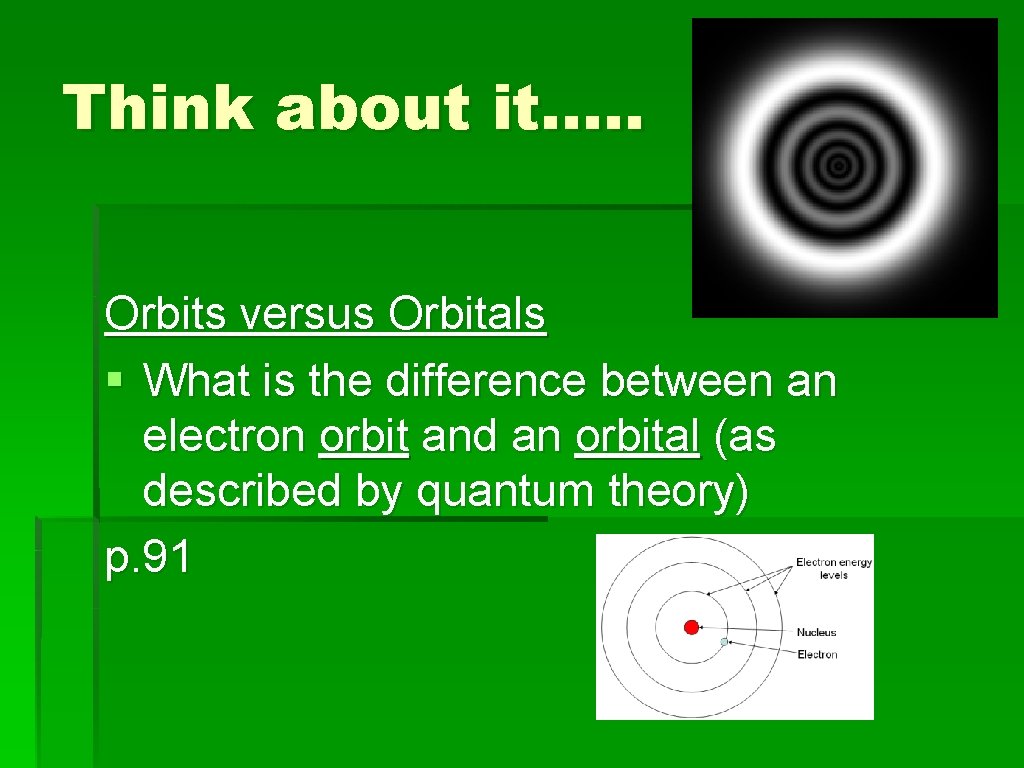
Think about it…. . Orbits versus Orbitals § What is the difference between an electron orbit and an orbital (as described by quantum theory) p. 91

3. 3 ground state and excited state § An electron can be excited by: § Electric current § Heating in a flame Read: p. 94 ground/excited state Vid: atomic emmision animation

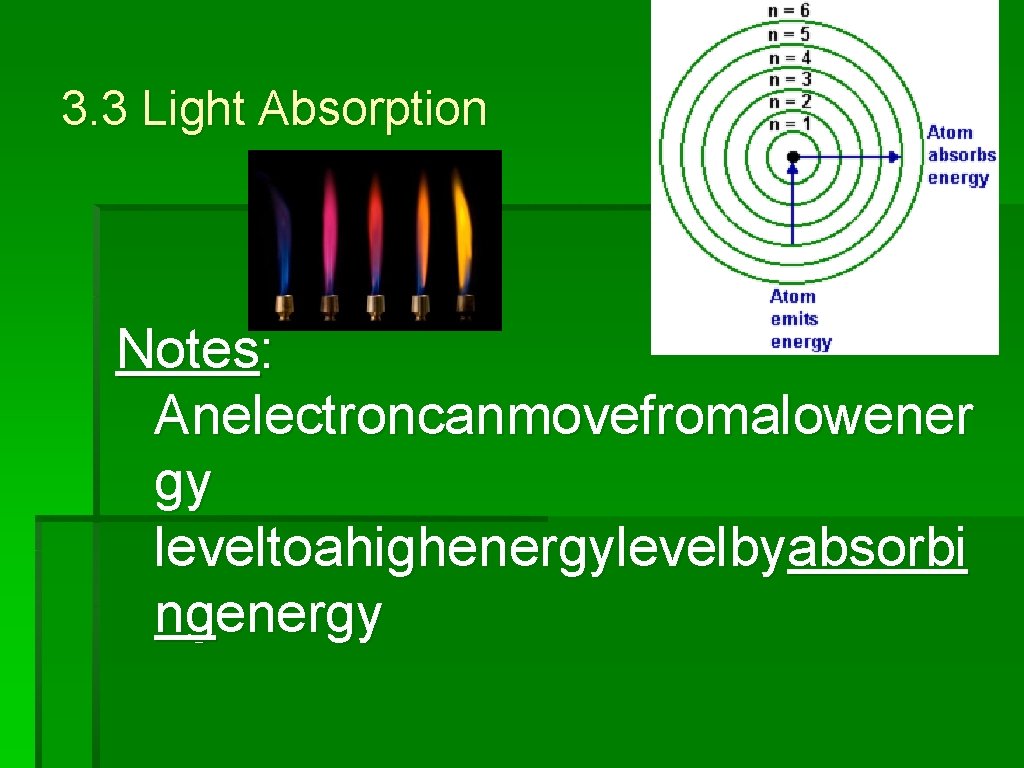
3. 3 Light Absorption Notes: Anelectroncanmovefromalowener gy leveltoahighenergylevelbyabsorbi ngenergy

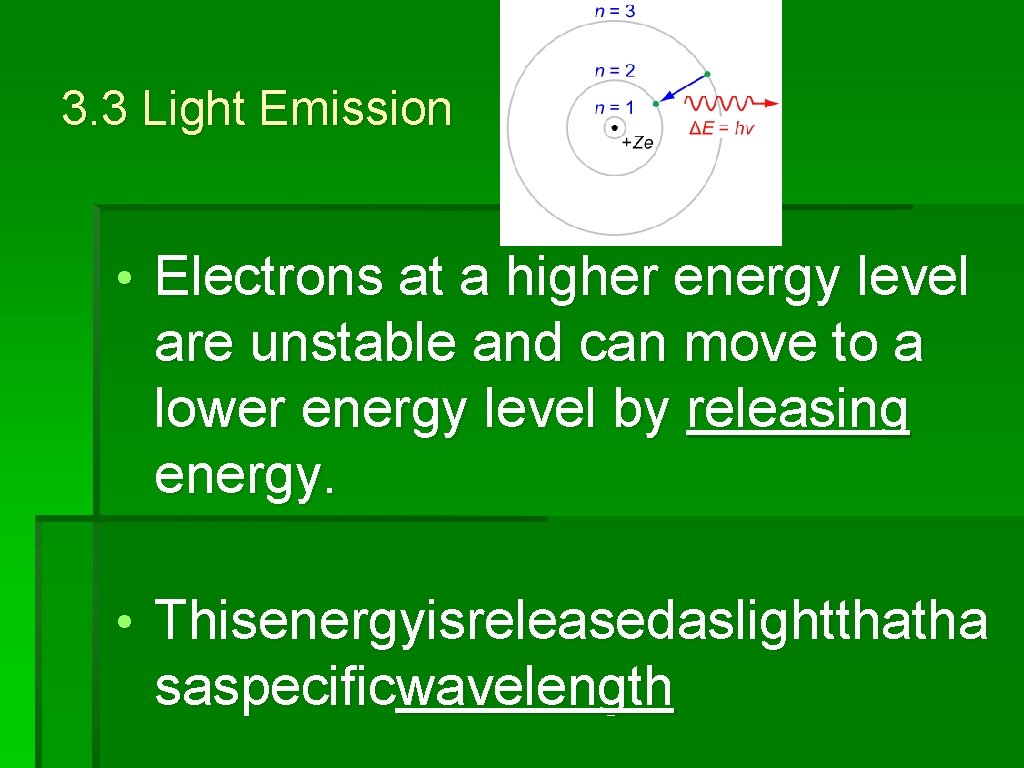
3. 3 Light Emission • An electron can move from a low energy level to a high energy level by absorbing energy. • Electronsatahigherenergylevelare unstableandcan movetoalowerenergylevelbyreleas ingenergy

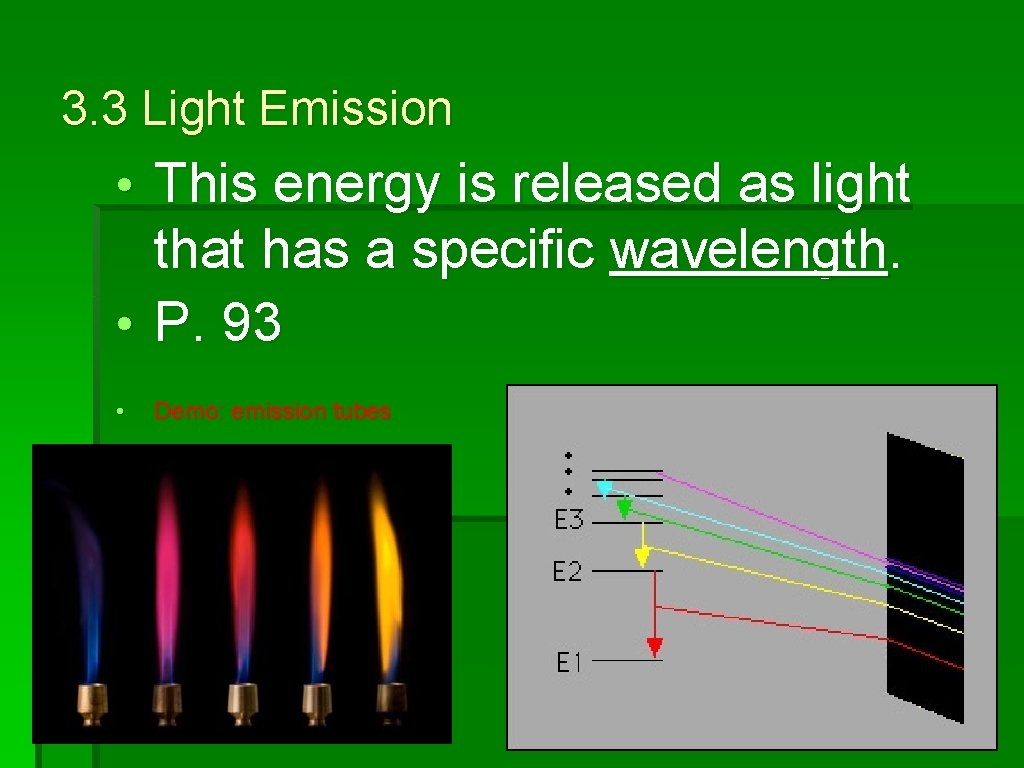
3. 3 Light Emission • Electrons at a higher energy level are unstable and can move to a lower energy level by releasing energy. • Thisenergyisreleasedaslightthatha saspecificwavelength

3. 3 Light Emission • This energy is released as light that has a specific wavelength. • P. 93 • Demo: emission tubes

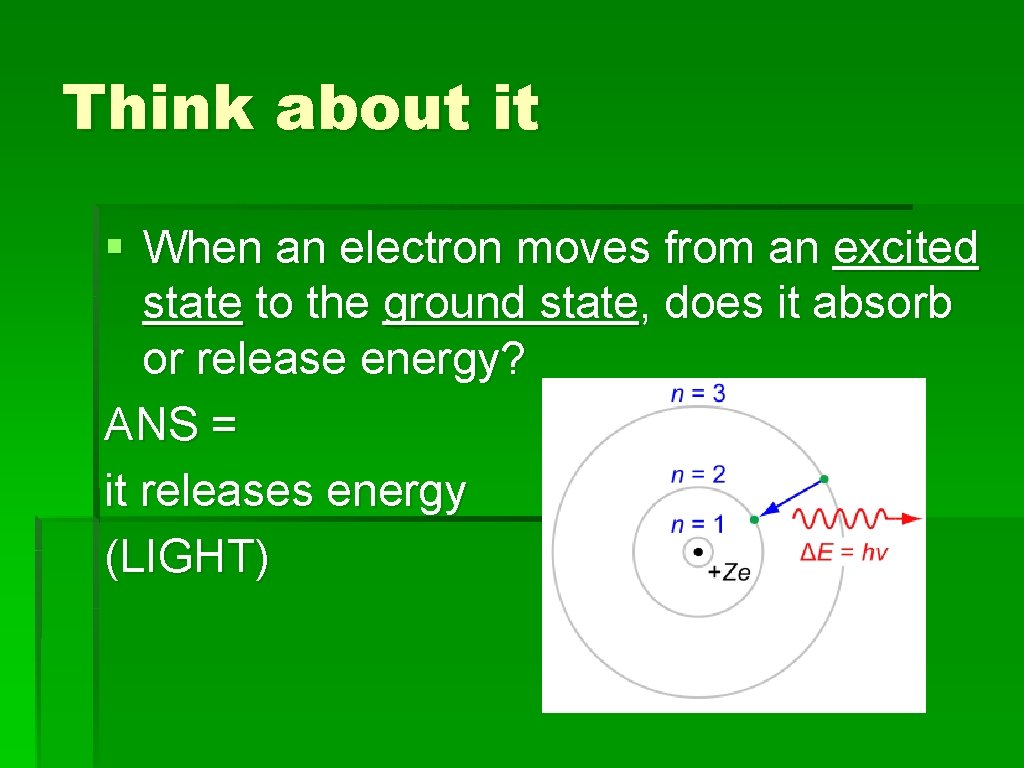
Think about it § When an electron moves from an excited state to the ground state, does it absorb or release energy? ANS = it releases energy (LIGHT)

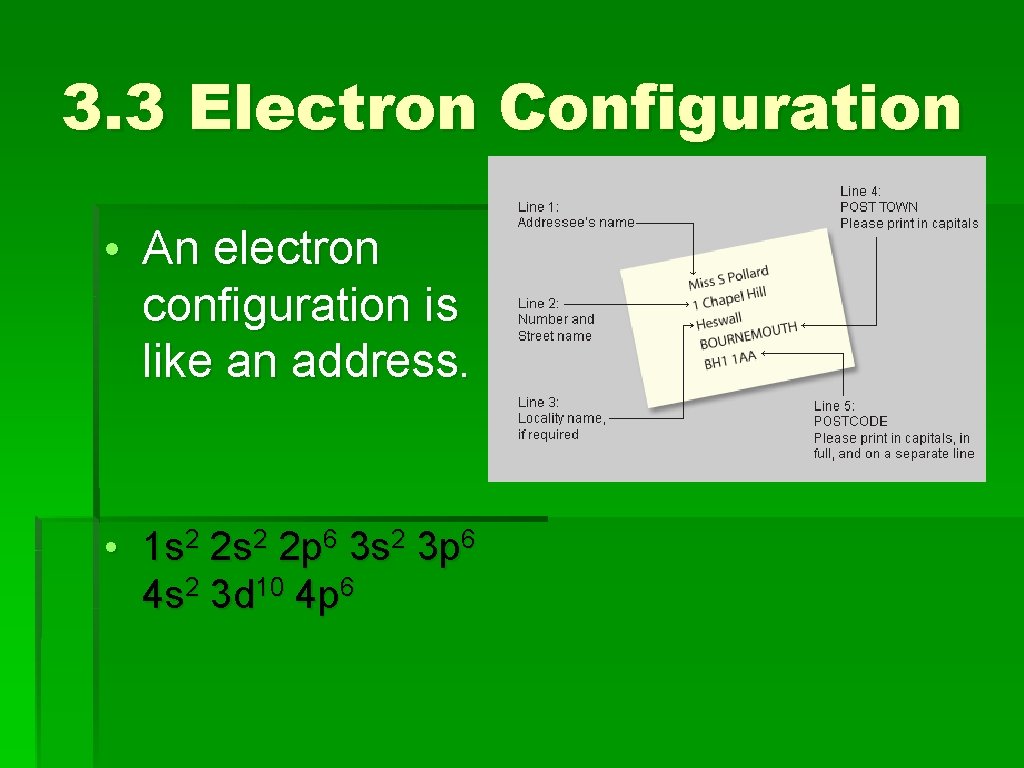
3. 3 Electron Configuration • An electron configuration is like an address. • 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6

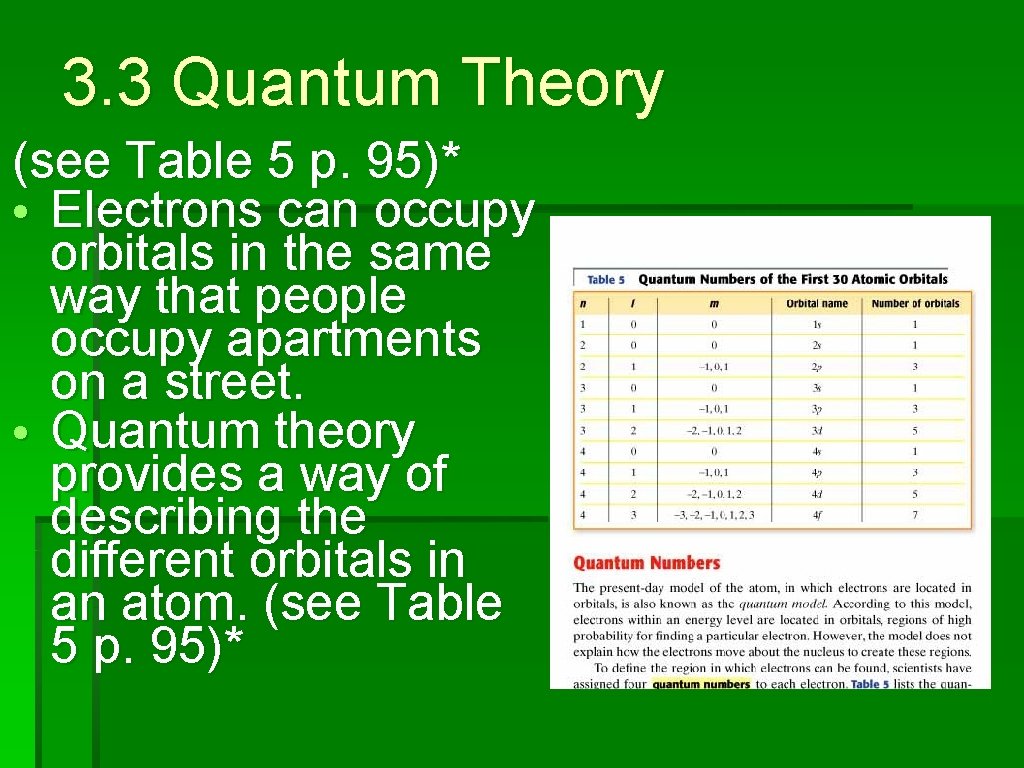
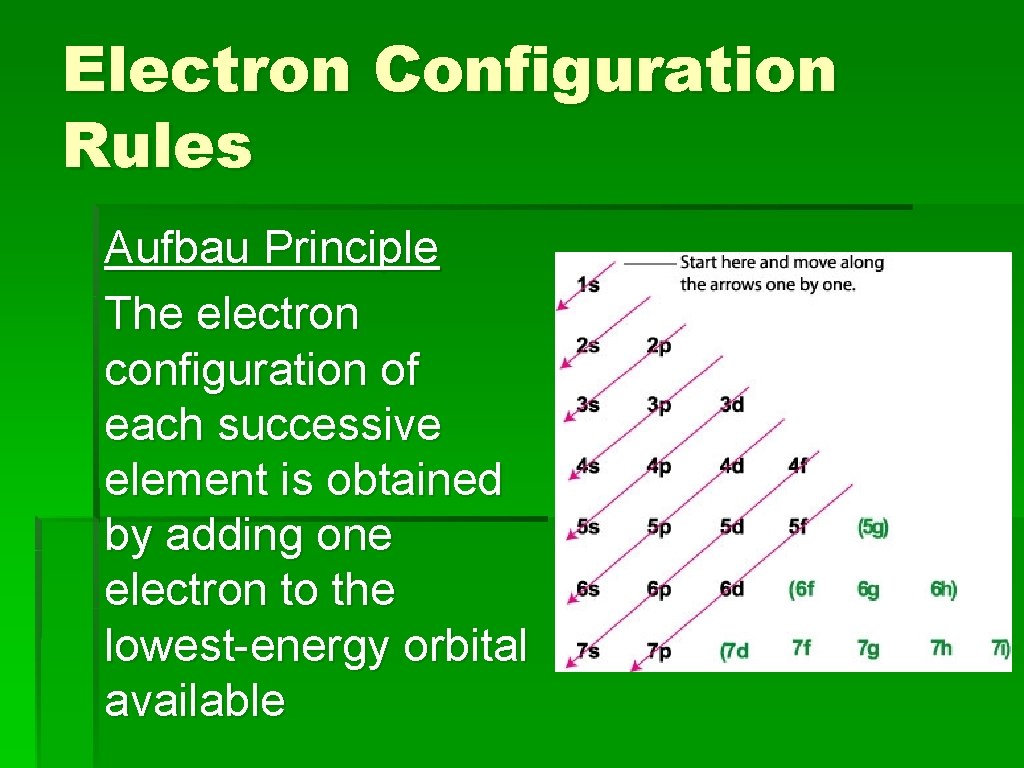
3. 3 Quantum Theory (see Table 5 p. 95)* • Electrons can occupy orbitals in the same way that people occupy apartments on a street. • Quantum theory provides a way of describing the different orbitals in an atom. (see Table 5 p. 95)*

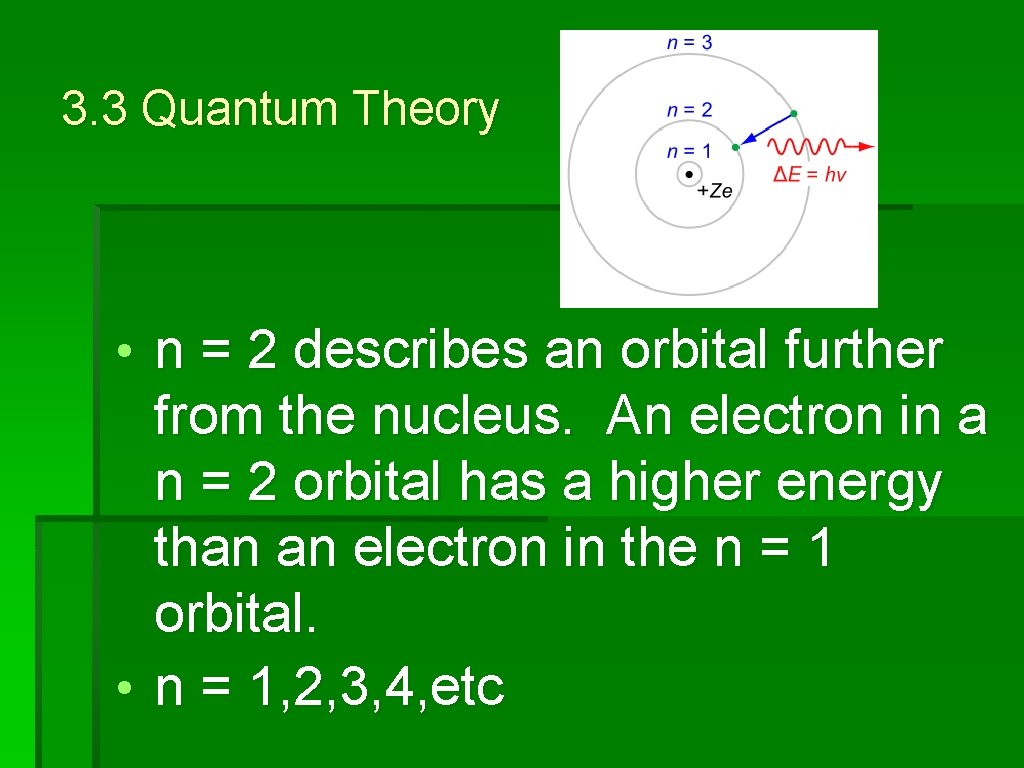
3. 3 Quantum Theory • The principal quantum number (n) tells us about the different energy levels of an atom. • Energy levels are sometimes called 'shells' • n = 1 describes an orbital closest to the nucleus. n = 1 has the lowest energy

3. 3 Quantum Theory • n = 2 describes an orbital further from the nucleus. An electron in a n = 2 orbital has a higher energy than an electron in the n = 1 orbital. • n = 1, 2, 3, 4, etc

3. 3 Quantum Theory § Fig. 22, p. 97 energy levels § Each energy level can contain a number of different types sublevels.

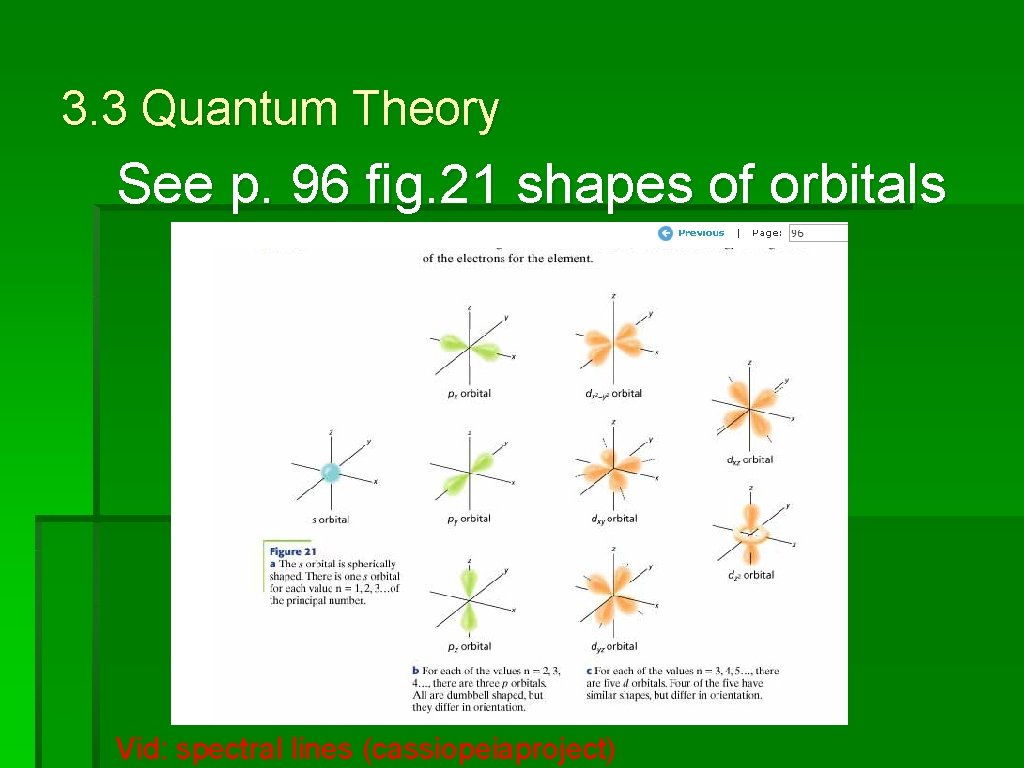
3. 3 Quantum Theory See p. 96 fig. 21 shapes of orbitals Vid: spectral lines (cassiopeiaproject)

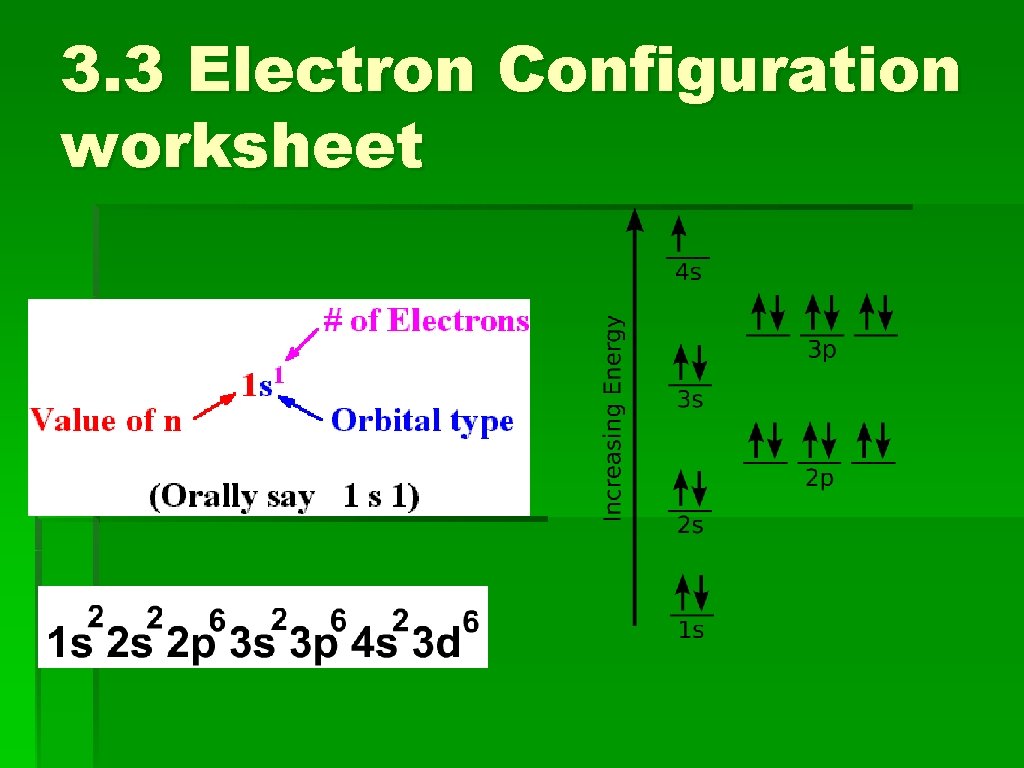
3. 3 Electron Configuration worksheet

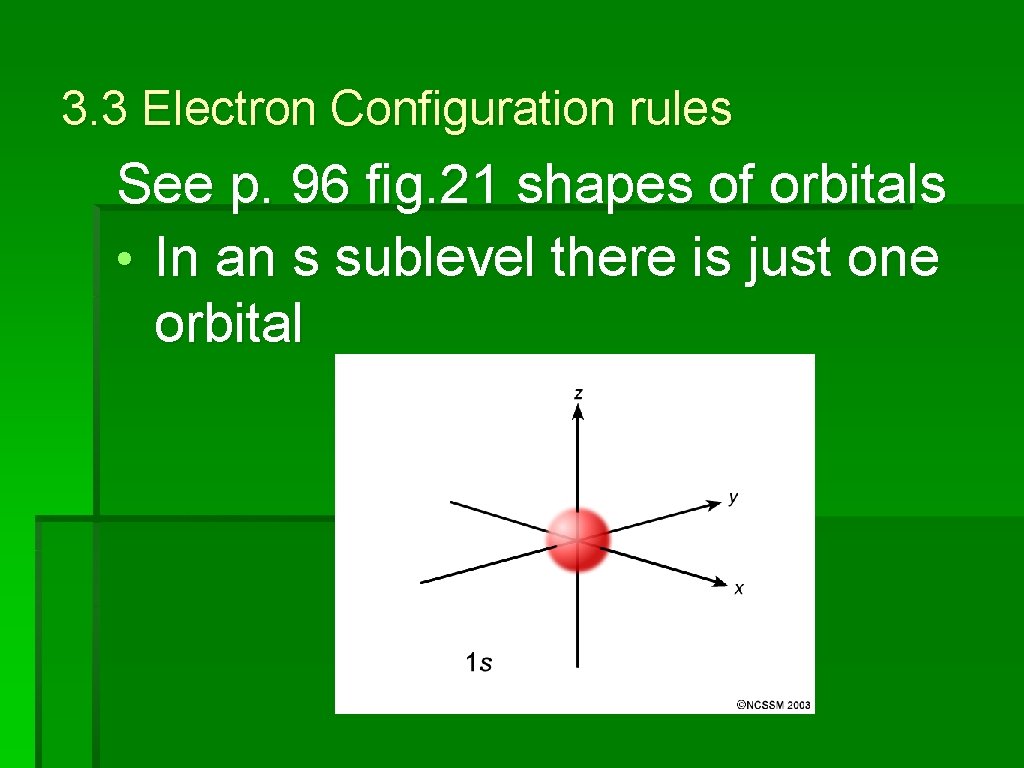
3. 3 Electron Configuration rules See p. 96 fig. 21 shapes of orbitals • In an s sublevel there is just one orbital

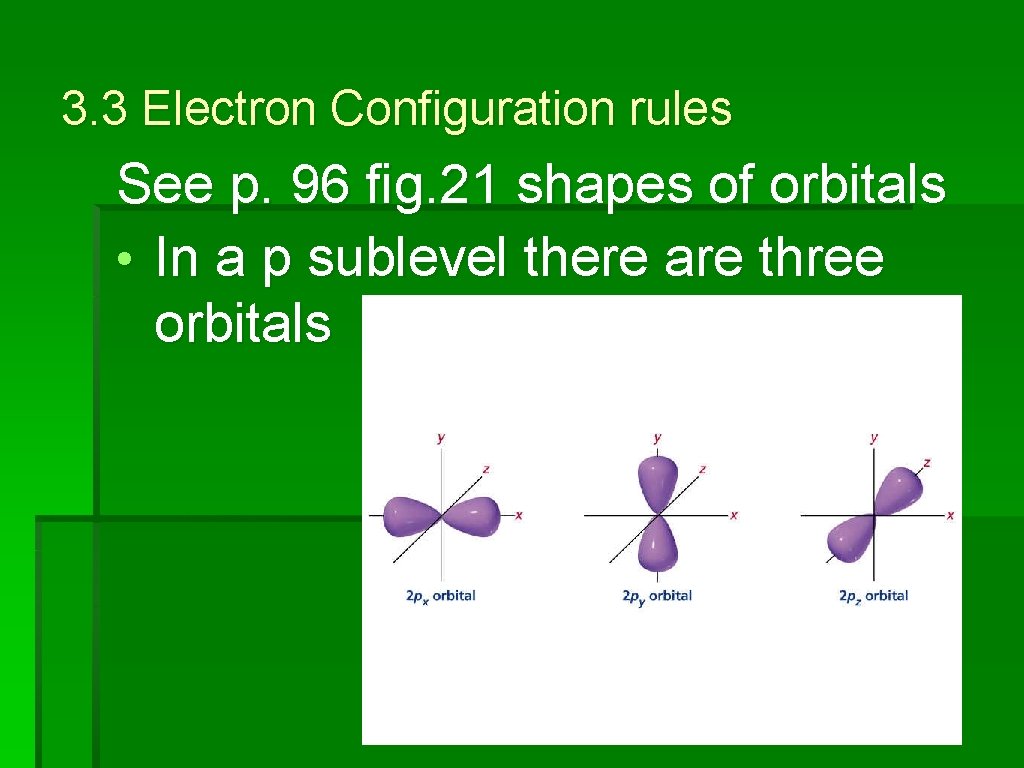
3. 3 Electron Configuration rules See p. 96 fig. 21 shapes of orbitals • In a p sublevel there are three orbitals

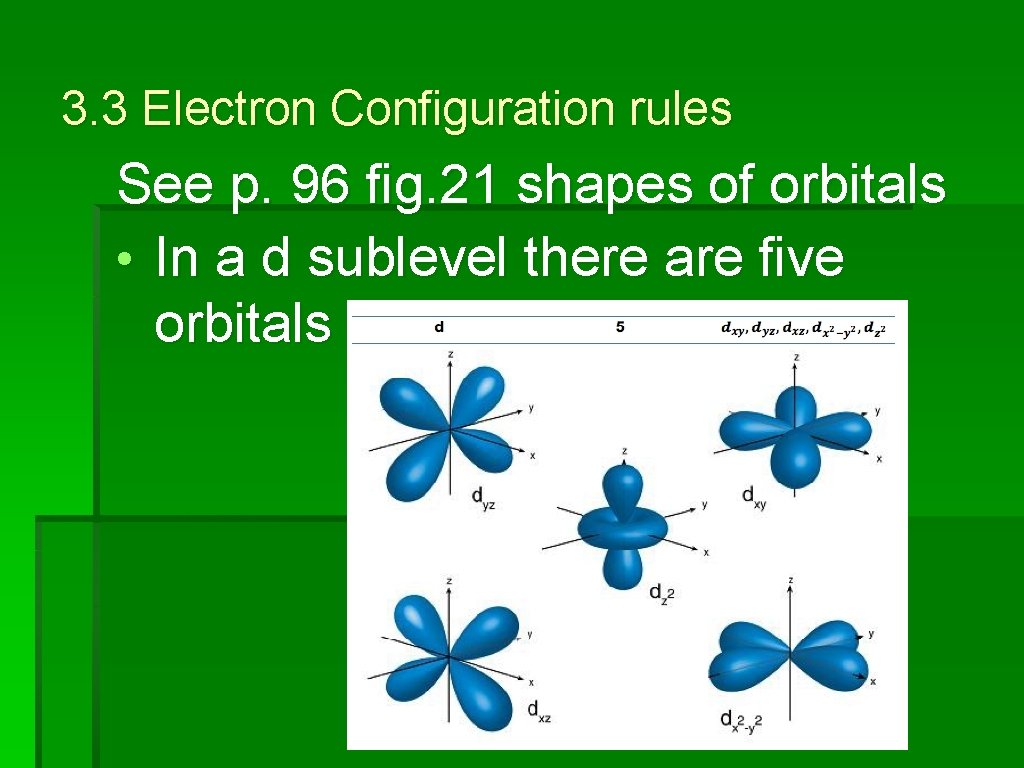
3. 3 Electron Configuration rules See p. 96 fig. 21 shapes of orbitals • In a d sublevel there are five orbitals

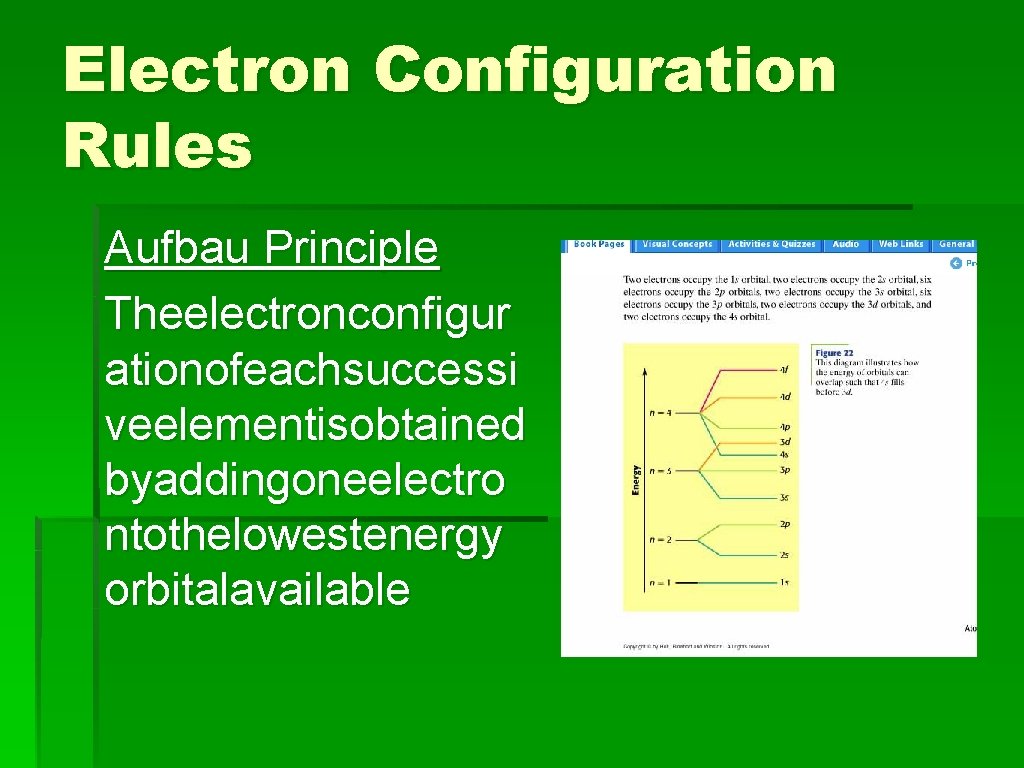
Electron Configuration Rules Aufbau Principle Theelectronconfigur ationofeachsuccessi veelementisobtained byaddingoneelectro ntothelowestenergy orbitalavailable

Electron Configuration Rules Aufbau Principle The electron configuration of each successive element is obtained by adding one electron to the lowest-energy orbital available

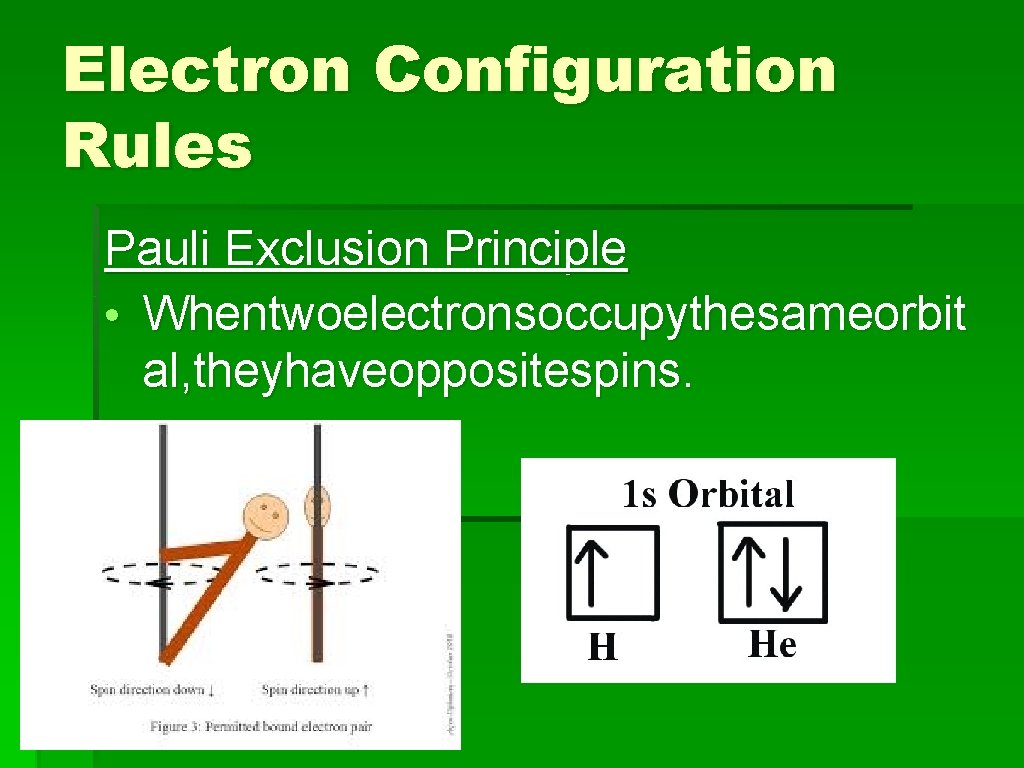
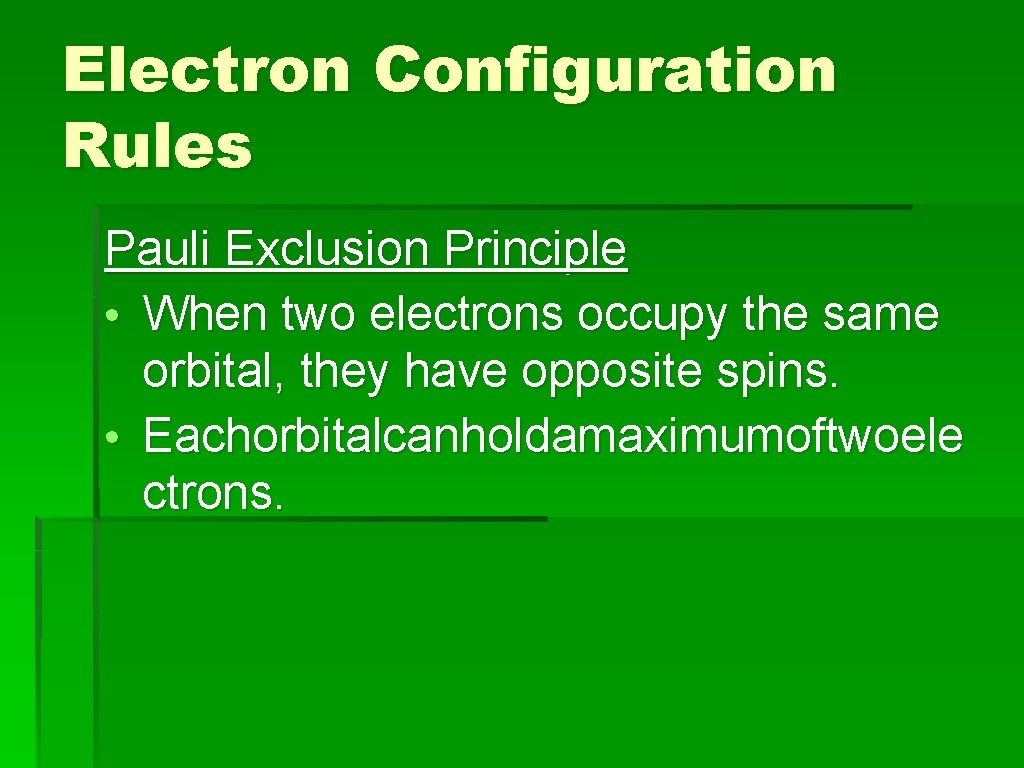
Electron Configuration Rules Pauli Exclusion Principle • Whentwoelectronsoccupythesameorbit al, theyhaveoppositespins.

Electron Configuration Rules Pauli Exclusion Principle • When two electrons occupy the same orbital, they have opposite spins. • Eachorbitalcanholdamaximumoftwoele ctrons.

Electron Configuration Rules Pauli Exclusion Principle • When two electrons occupy the same orbital, they have opposite spins. • Each orbital can hold a maximum of two electrons.

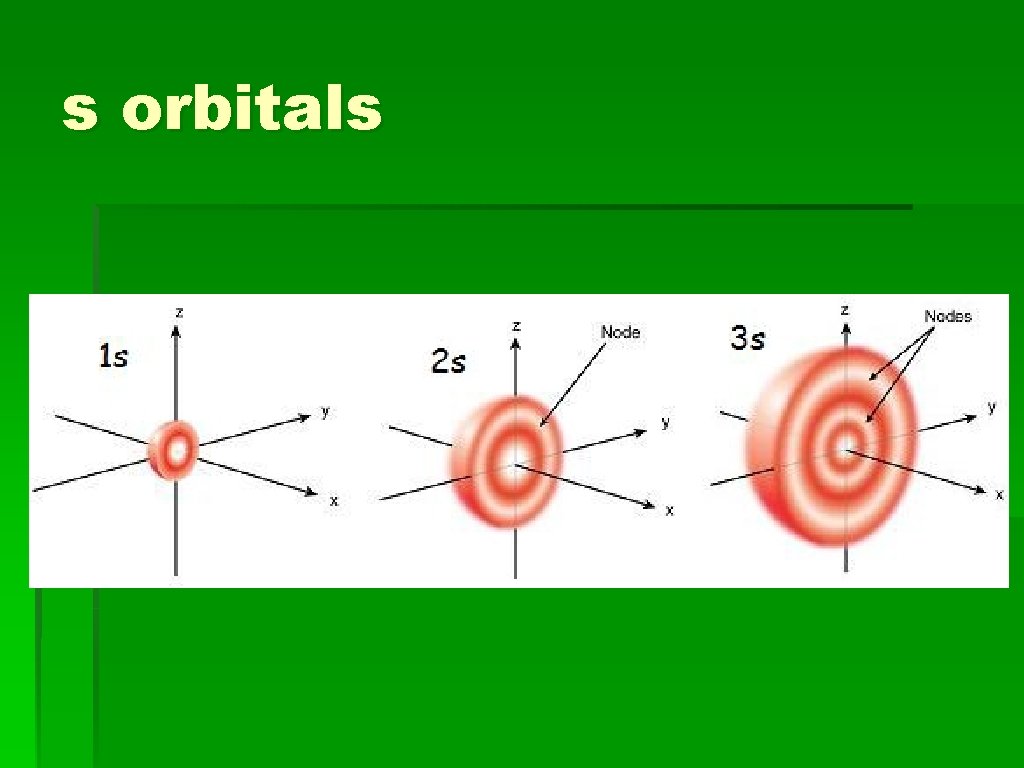
s orbitals

3. 3 Noble Gases 1. Noble gases are found at the end of each row in the periodic table 2. Noble gases = He, Ne, Ar, Kr, Xe 3. noble gases have full s and p orbitals 4. Noble gases have special properties

3. 3 Electron Configuration: Shorthand Notation Read paragraph 2 p. 98 § the element in square brackets should be the noble gas at the end of the previous row

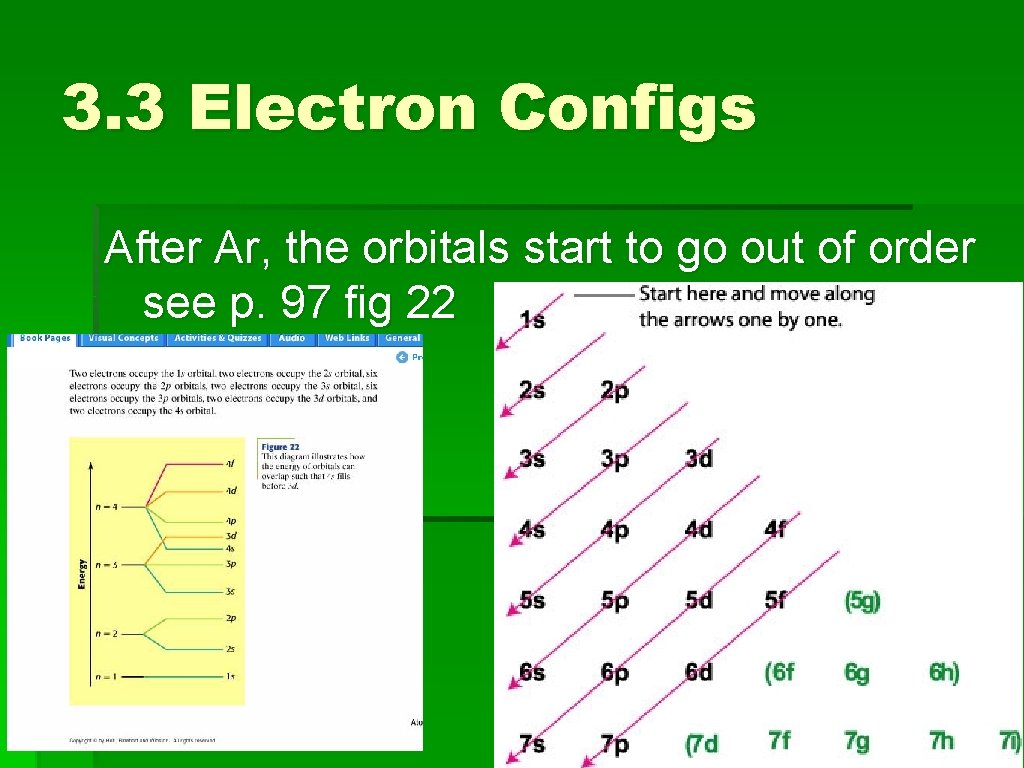
3. 3 Electron Configs After Ar, the orbitals start to go out of order see p. 97 fig 22

Think about it…. a. How many orbitals are there in a p sublevel? b. How many electrons can occupy a p sublevel?

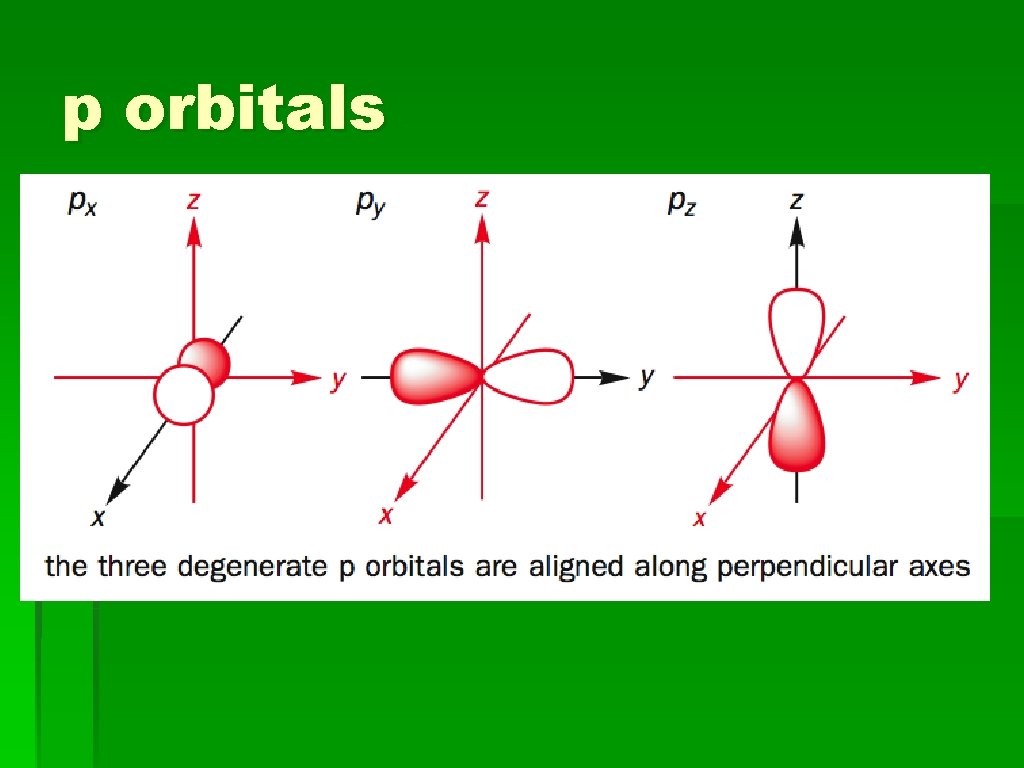
p orbitals

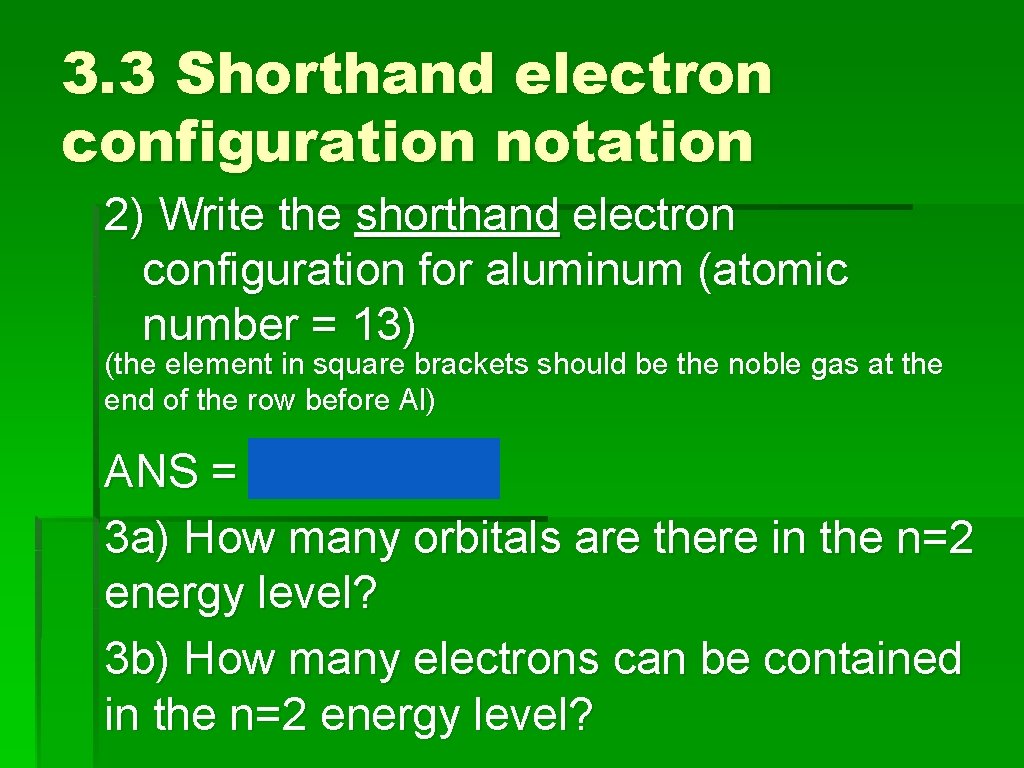
Practice Need scratch paper, periodic table and graphic organizer 1) Without referring to your notes, write the electron configuration for neon. ANS = 1 s 22 p 6 2) Write the shorthand electron configuration for aluminum (atomic number = 13)

3. 3 Shorthand electron configuration notation 2) Write the shorthand electron configuration for aluminum (atomic number = 13) (the element in square brackets should be the noble gas at the end of the row before Al) ANS = [Ne] 3 s 2 3 p 1 3 a) How many orbitals are there in the n=2 energy level? 3 b) How many electrons can be contained in the n=2 energy level?

Think about it…. . 3 a) How many orbitals are there in the n=3 energy level? 3 b) How many electrons can be contained in the n=3 energy level? ANS = 9 orbitals, 18 electrons 4) How many orbitals are completely filled for the element with an atomic number of 17?

Think about it…. . 4) How many orbitals are completely filled for the element with an atomic number of 17? ANS = 8 filled orbitals (chlorine) 5) Which element has the electron configuration [Ar] 4 s 2 3 d 6
![3 3 5 Which element has the electron configuration 6 2 Ar 3 d 3. 3 5) Which element has the electron configuration 6 2 [Ar] 3 d](https://slidetodoc.com/presentation_image_h2/72416944529f3cb16ab0ce273c7f2607/image-73.jpg)
3. 3 5) Which element has the electron configuration 6 2 [Ar] 3 d 4 s ANS = iron (Fe)

Electron Configuration Scratch paper, using PT and graphic organizer only Practice I p. 108 # 38, 39, 40, 41 Practice II p. 99 # 4 -11 (skip #9 ) (so that’s 7 questions) Beast-mode: * Write the electron configurations of: a) bromine b) iodine c) mercury

3. 3 Electron Configs Write the electron configuration of Bromine (atomic number = ? ) ANS = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5 OR [Ar] 2 10 5 4 s 3 d 4 p

3. 3 Electron Configs Write the electron configuration of Iodine (atomic number = 53) ANS = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 5 OR [Kr] 5 s 24 d 105 p 5

3. 3 Electron Configs Write the electron configuration of Mercury (atomic number = 80) ANS = [Xe] 6 s 2 4 f 14 5 d 10

3. 4 Beanz • What is the average mass of all of the beans in the baggie? • 1 black bean weighs 0. 26 g • 1 brown bean weighs 0. 45 g • 1 white bean weighs 1. 27 g

3. 4 Weighted average • What is the average mass of all the beans in the bag? • 1 black bean weighs 0. 26 g • # black beans = 15 • 1 brown bean weighs 0. 45 g • # of brown beans = 11 • 1 white bean weighs 1. 27 g • # of white beans = 8 • Average mass = 0. 56 g

3. 4 Atomic mass p. 100 • Atomic mass tells us what the mass of one atom of an element is. • Atomic mass is also known as ‘average atomic mass’ or ‘atomic weight’.

3. 4 Atomic mass p. 100 • Atomic mass is different from atomic number and mass number. • Copy the definitions of each of the three terms onto your Venn diagram

3. 4 Atomic mass • What are the units of atomic mass? • atomic mass unit (amu) • 1 atom of Cl = 35. 45 amu -27 • 1 amu = 1. 6 x 10 kg

3. 4 Atomic mass unit defined • How is the amu defined? C-12 is used as the standard…… -23 One C-12 atom = 2. 0 x 10 g Divide by 12…… -23 2. 0 x 10 g / 12 -24 = 1. 6 x 10 g

3. 4 Atomic mass unit defined • 1 amu = one twelfth of the mass of one atom of C-12 th • Why 1/12 ? • b/c the mass of a C-12 atom comes from it’s 12 protons & neutrons • 1 amu ~ 1 proton • 1 amu ~ 1 neutron

3. 4 Atomic mass • Think about it: why are the atomic masses listed on the periodic table not whole numbers? • b/c the elements found in nature are mixtures of different isotopes. • ‘Weighted average’

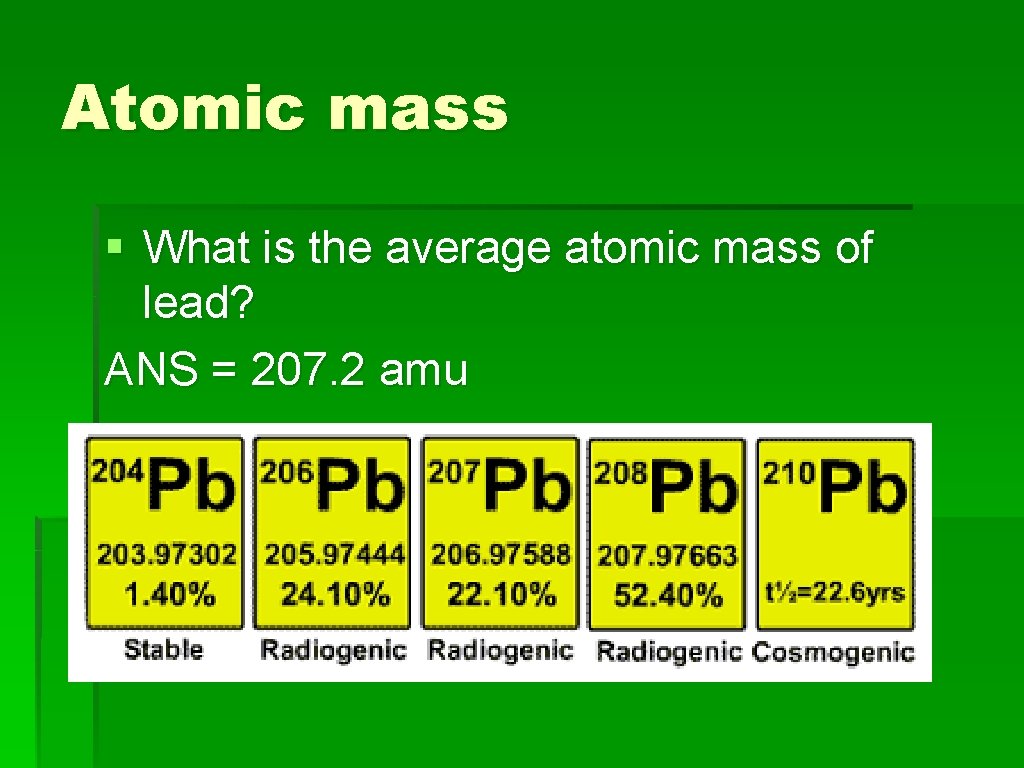
Atomic mass § What is the average atomic mass of lead? ANS = 207. 2 amu

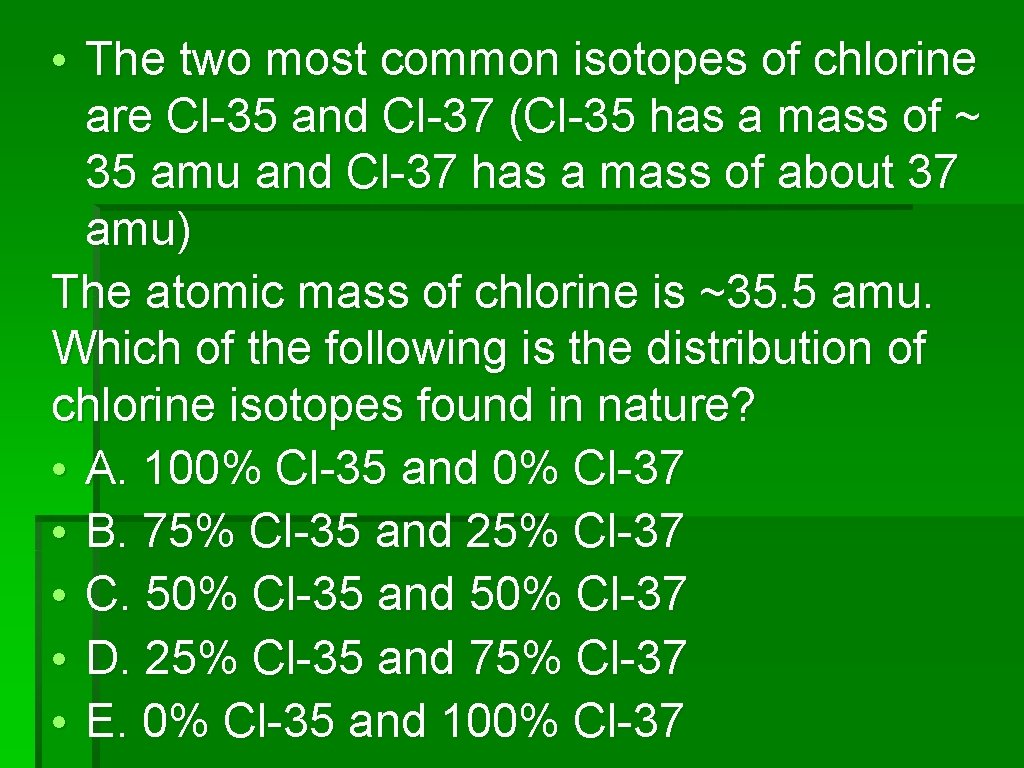
• The two most common isotopes of chlorine are Cl-35 and Cl-37 (Cl-35 has a mass of ~ 35 amu and Cl-37 has a mass of about 37 amu) The atomic mass of chlorine is ~35. 5 amu. Which of the following is the distribution of chlorine isotopes found in nature? • A. 100% Cl-35 and 0% Cl-37 • B. 75% Cl-35 and 25% Cl-37 • C. 50% Cl-35 and 50% Cl-37 • D. 25% Cl-35 and 75% Cl-37 • E. 0% Cl-35 and 100% Cl-37

Venn Diagram § § Atomic number Mass number Atomic mass Write in the definition of each of the terms § What do these three terms have in common with each other? § What is different about them?